Polymerization Reaction Kinetics of Poly α-Olefin and Numerical Simulation of a Continuous Polymerization Reactor
Abstract
1. Introduction
2. Polymerization Reactor System
3. Numerical Simulation Method
3.1. Numerical Method
3.2. Numerical Setup
4. Results and Discussion
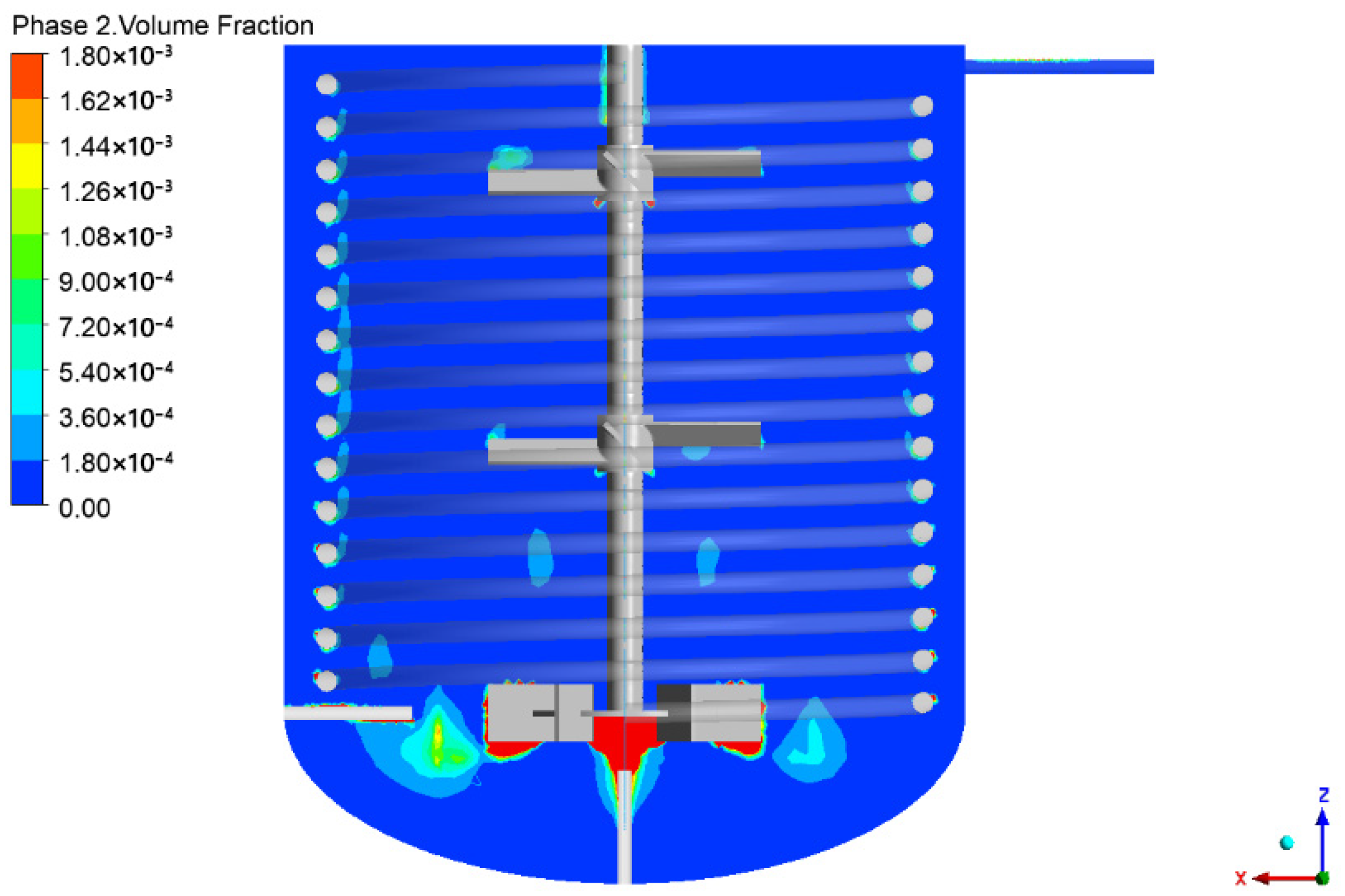
4.1. Flow Field in the Reactor
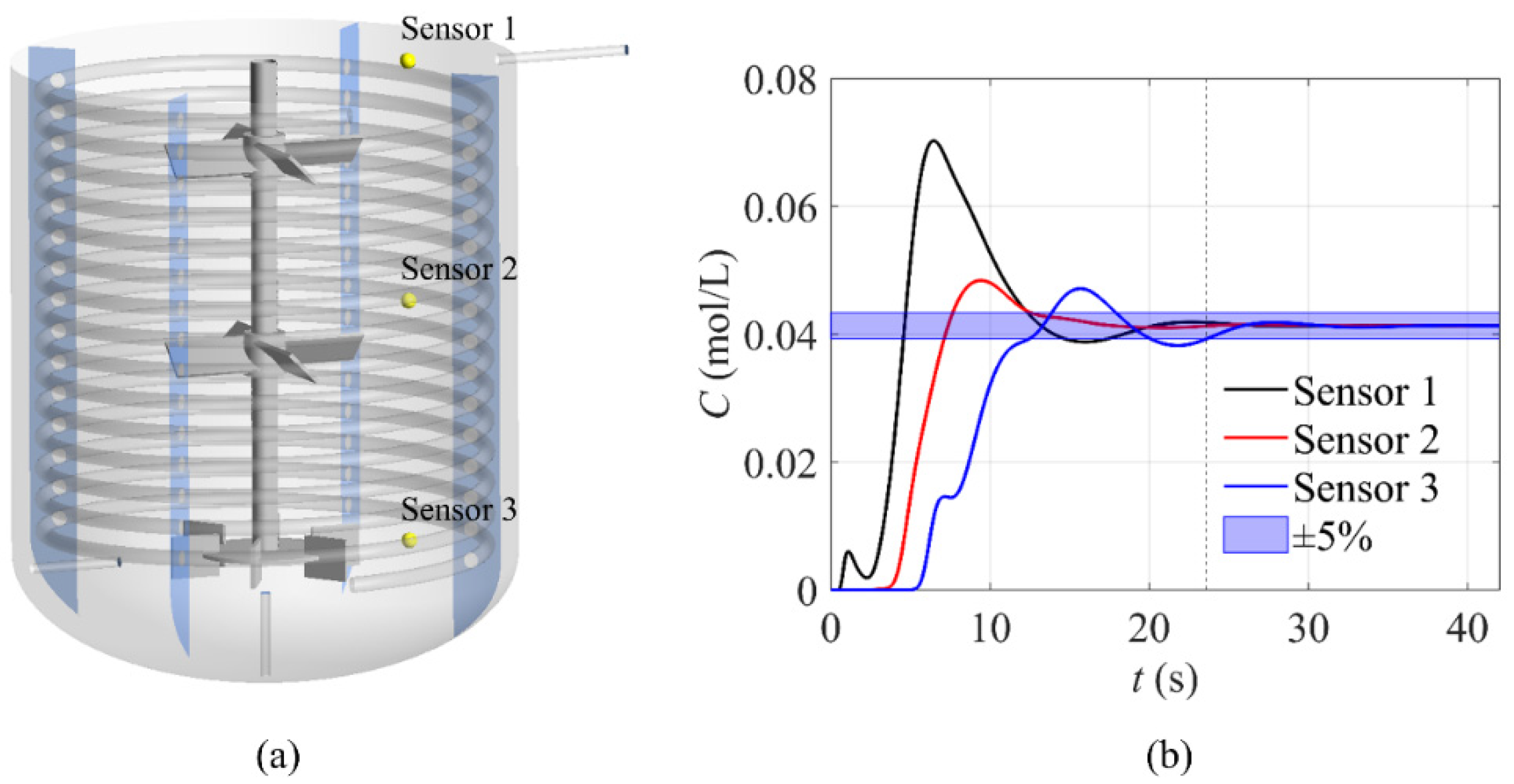
4.2. Analysis of Reaction and the Mixing Time in the Reactor
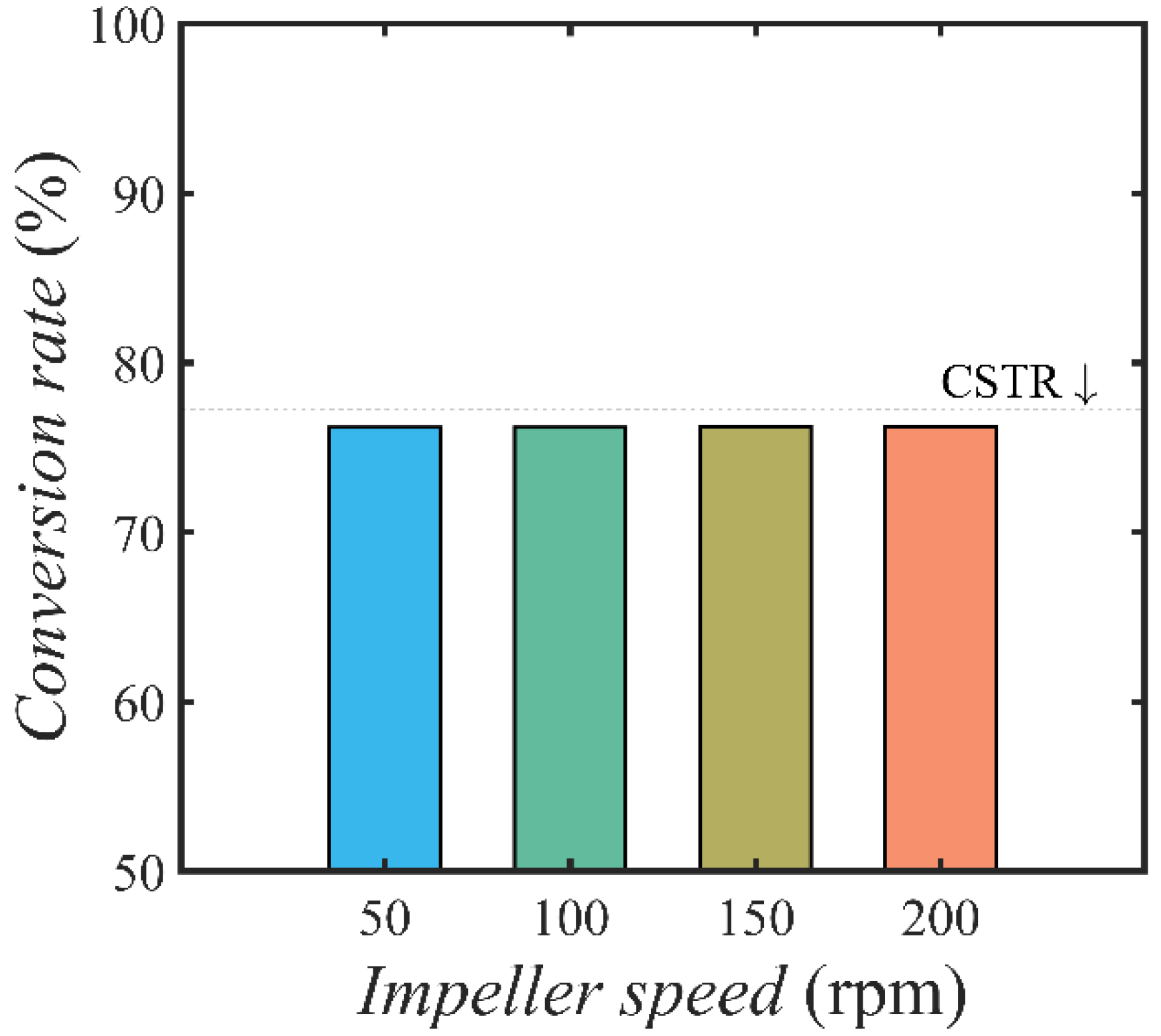
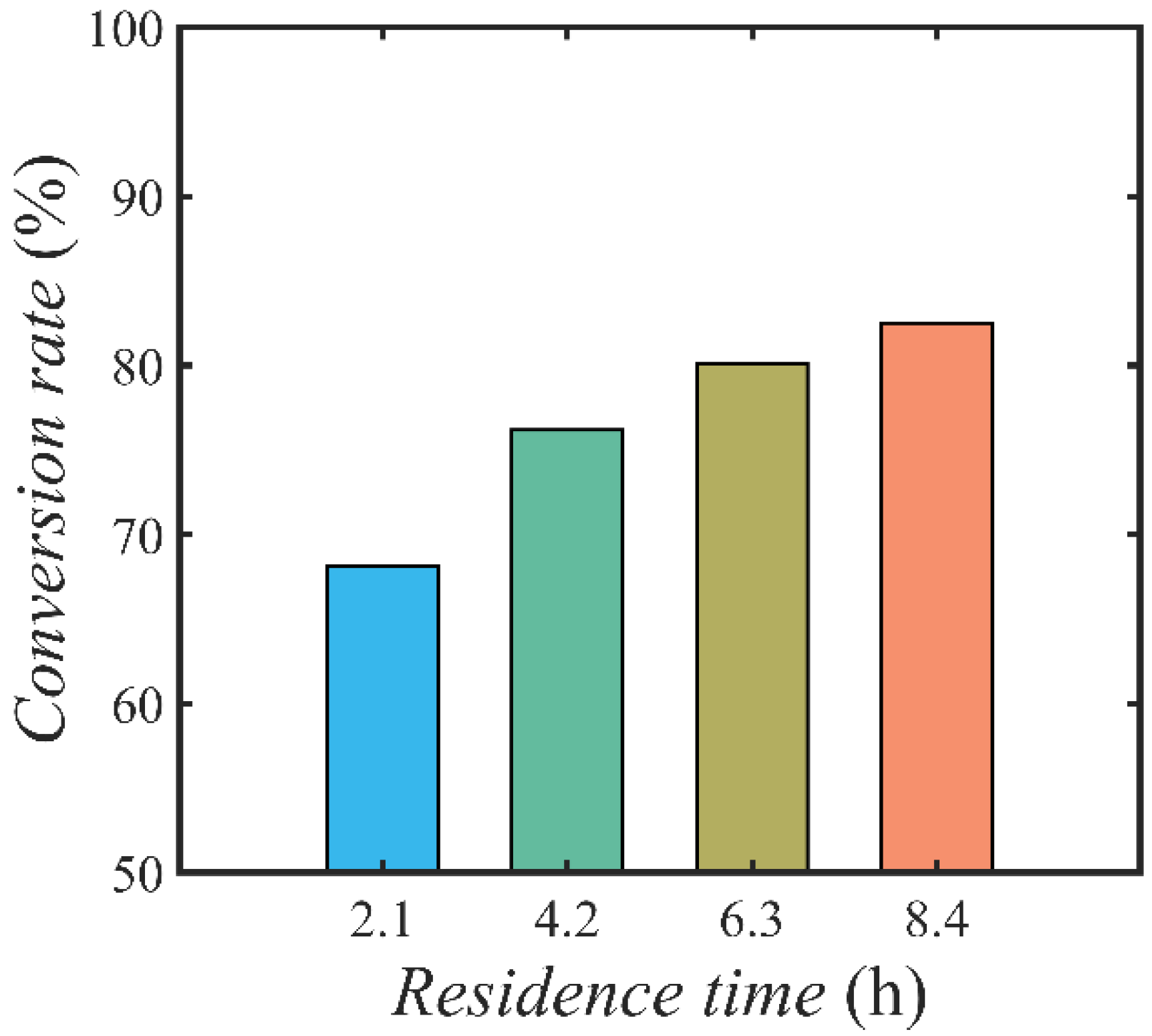
4.3. Effect of Flow Condition and Reactor Configuration on Conversion Rate
4.3.1. Effect of Temperature on Conversion Rate
4.3.2. Effect of Space Time on Conversion Rate
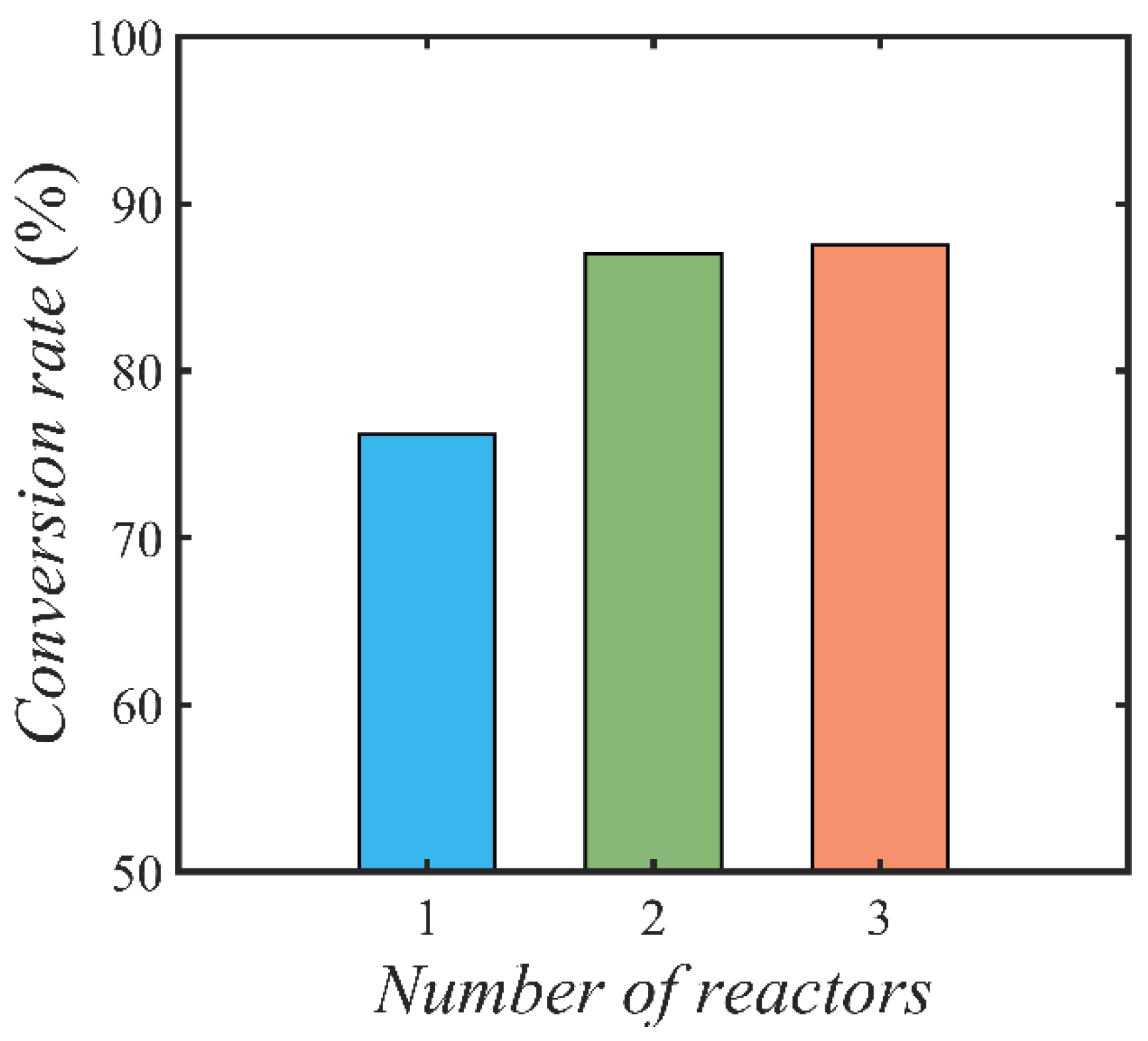
4.3.3. Effect of Reactor Number on Conversion Rate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fathi Roudsari, S.; Dhib, R.; Ein-Mozaffari, F. Mixing effect on emulsion polymerization in a batch reactor. Polym. Eng. Sci. 2015, 55, 945–956. [Google Scholar] [CrossRef]
- Inglès, X.; Pallares, J.; Larre, M.T.; Méndez, L.; Grau, F.X. Experimental and numerical study of turbulent mixing in a model of a polymerization reactor. J. Ind. Eng. Chem. 2013, 19, 1251–1256. [Google Scholar] [CrossRef]
- Read, N.K.; Zhang, S.X.; Ray, W.H. Simulations of a LDPE reactor using computational fluid dynamics. AlChE J. 1997, 43, 104–117. [Google Scholar] [CrossRef]
- Tosun, G. A mathematical model of mixing and polymerization in a semibatch stirred-tank reactor. AlChE J. 1992, 38, 425–437. [Google Scholar] [CrossRef]
- Javad, H.; Nayef, M.G.; Wan. Effects of the temperature and mixing rate on foaming in a polymerization reaction to produce fatty polyamides in the presence of catalyst. Ind. Eng. Chem. Res. 2004, 43, 6048–6054. [Google Scholar] [CrossRef]
- Kemmere, M.; Meuldijk, J.; Drinkenburg, A.; German, A. Emulsification in batch-emulsion polymerization of styrene and vinyl acetate: A reaction calorimetric study. J. Appl. Polym. Sci. 2001, 79, 944–957. [Google Scholar] [CrossRef]
- Patel, H.; Ein-Mozaffari, F.; Dhib, R. CFD analysis of mixing in thermal polymerization of styrene. Comput. Chem. Eng. 2010, 34, 421–429. [Google Scholar] [CrossRef]
- Ameur, H. Some modifications in the Scaba 6SRGT impeller to enhance the mixing characteristics of Hershel–Bulkley fluids. Food Bioprod. Process. 2019, 117, 302–309. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, Z.; Liao, Z.; Wang, J.; Yang, Y.; Wang, Y. Computational fluid dynamics simulations and experimental validation of macromixing and flow characteristics in low-density polyethylene autoclave reactors. Ind. Eng. Chem. Res. 2014, 53, 14865–14875. [Google Scholar] [CrossRef]
- Cherbański, R.; Milewska, A.; Molga, E. Safety aspects in batch reactors for styrene suspension polymerization. Ind. Eng. Chem. Res. 2007, 46, 5898–5906. [Google Scholar] [CrossRef]
- Pohn, J.; Cunningham, M.; McKenna, T.F. Scale-Up of Emulsion Polymerization Reactors Part II—Simulations and Interpretations. Macromol. React. Eng. 2013, 7, 393–408. [Google Scholar] [CrossRef]
- Xu, C.-Z.; Wang, J.-J.; Gu, X.-P.; Feng, L.-F. CFD modeling of styrene polymerization in a CSTR. Chem. Eng. Res. Des. 2017, 125, 46–56. [Google Scholar] [CrossRef]
- Zhou, W.; Marshall, E.; Oshinowo, L. Modeling LDPE tubular and autoclave reactors. Ind. Eng. Chem. Res. 2001, 40, 5533–5542. [Google Scholar] [CrossRef]
- Fathi Roudsari, S.; Dhib, R.; Ein-Mozaffari, F. Using a novel CFD model to assess the effect of mixing parameters on emulsion polymerization. Macromol. React. Eng. 2016, 10, 108–122. [Google Scholar] [CrossRef]
- Xu, C.Z.; Wang, J.J.; Gu, X.P.; Feng, L.F. Modeling of molecular weight and copolymer composition distributions for ethylene-propylene solution copolymerization. AlChE J. 2019, 65, e16632. [Google Scholar] [CrossRef]
- Schmitt, S.; Fleckenstein, F.; Hasse, H.; Stephan, S. Comparison of force fields for the prediction of thermophysical properties of long linear and branched alkanes. J. Phys. Chem. B 2023, 127, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, B.; Ma, A.; Liu, D.; Yang, L.; Zhang, A.; Pan, S.; Yu, H.; Li, H.; Gui, J. Low-viscosity poly-α-olefin production from Fischer-Tropsch mixed α-olefins: Synthesis and kinetics. Fuel 2025, 386, 134322. [Google Scholar] [CrossRef]
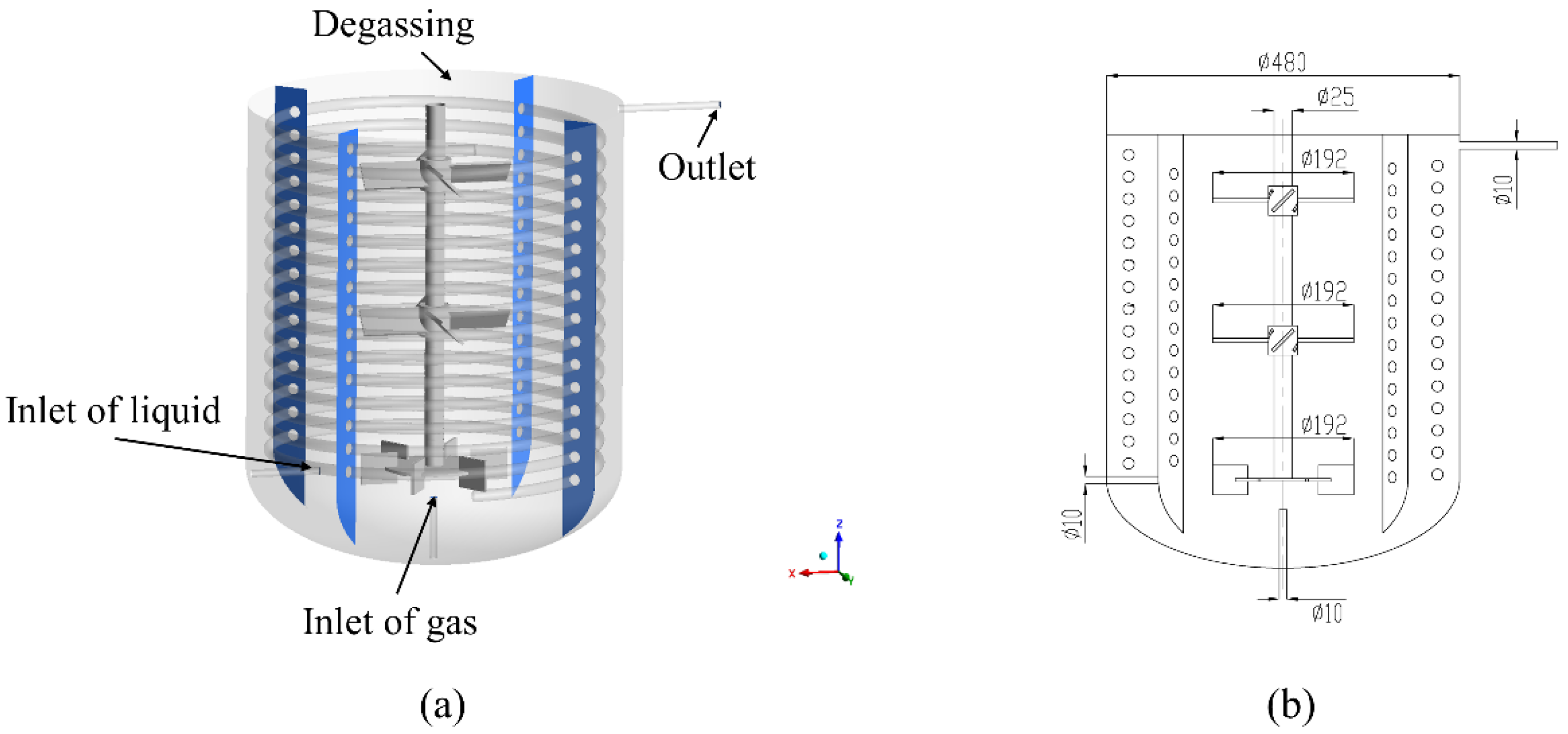
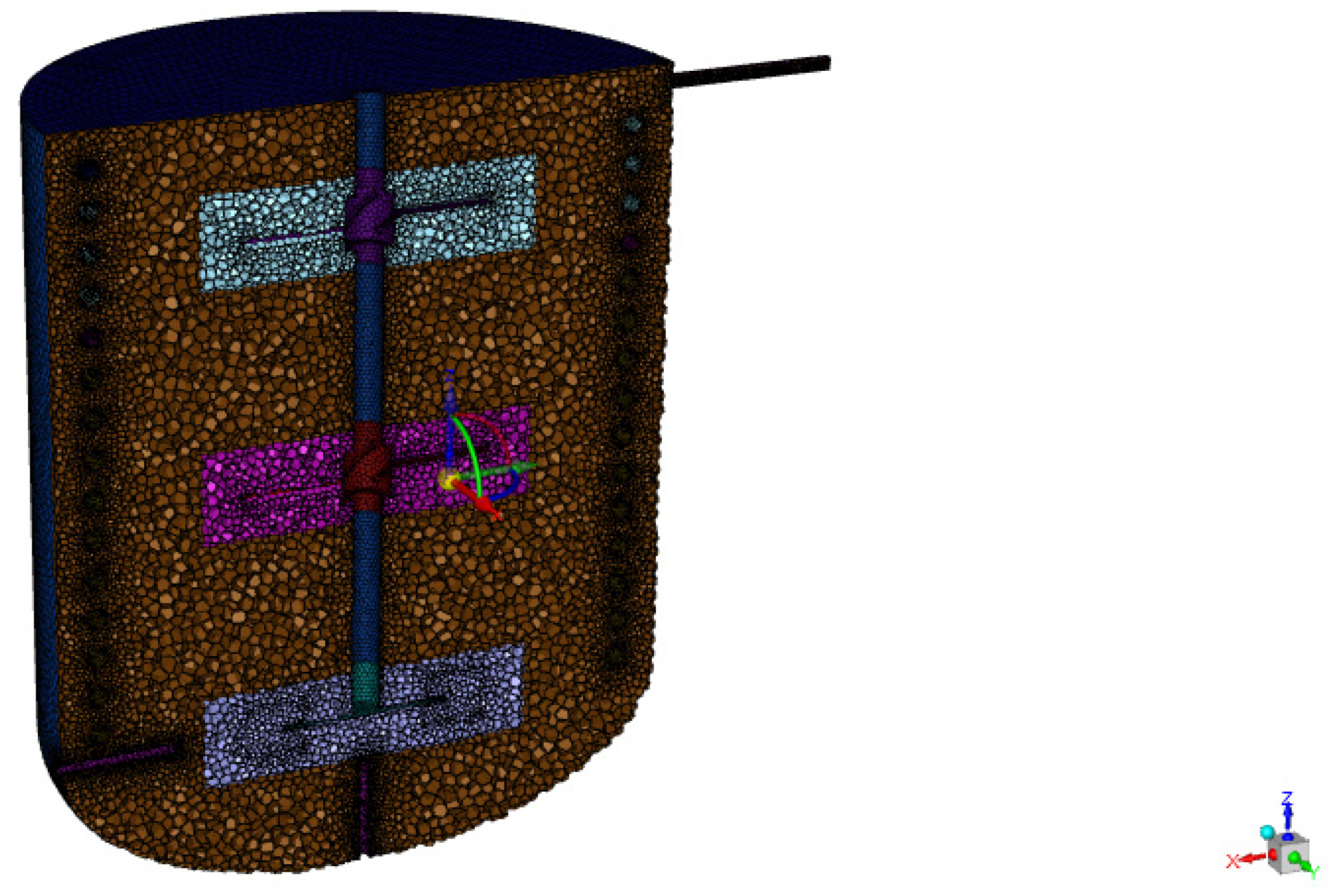
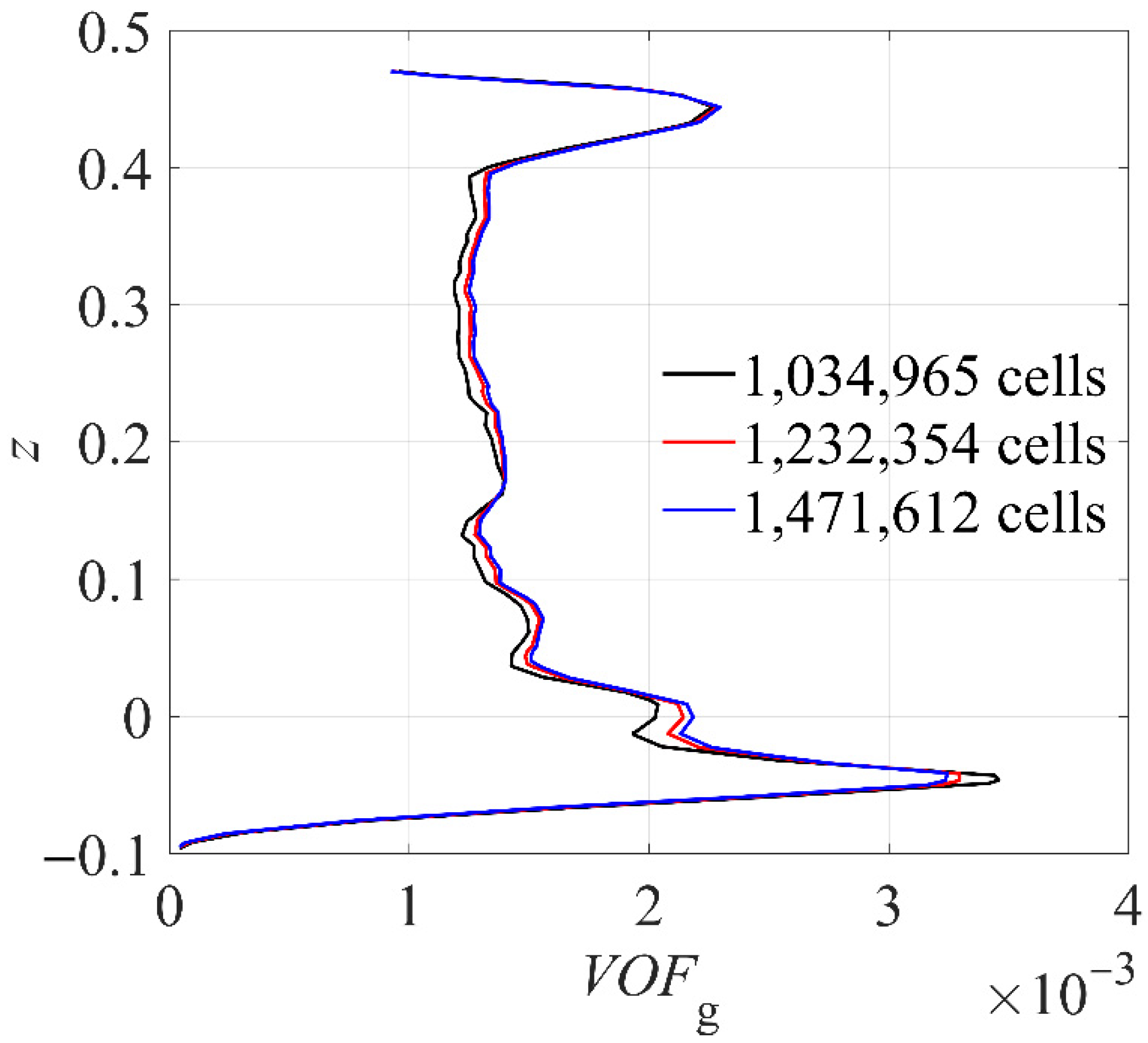
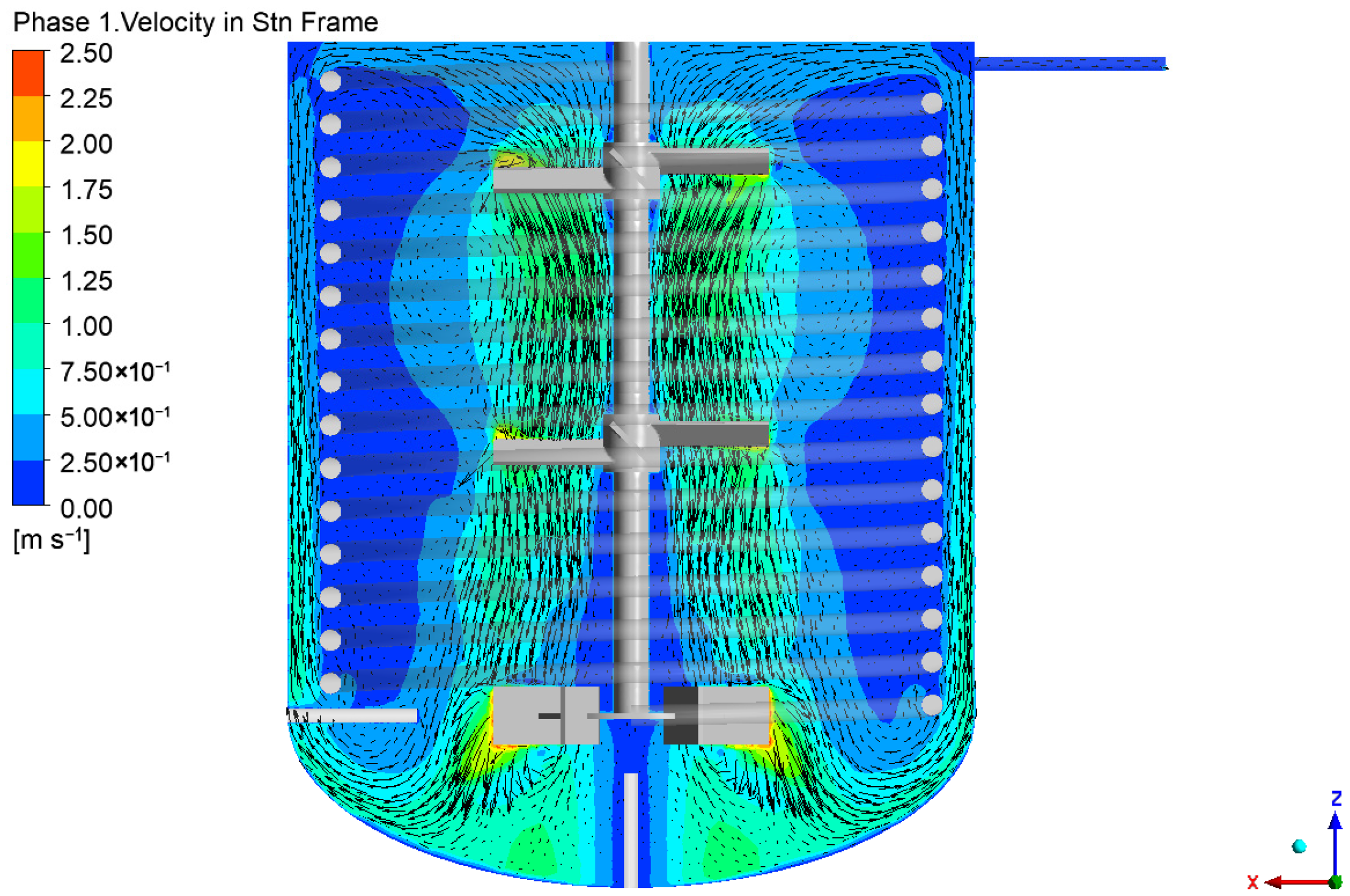

| Case | Temperature (°C) | Impeller Speed (rpm) | Number of Reactors (-) | Liquid Space Time (h) |
|---|---|---|---|---|
| 1 | 30 | 50 | 1 | 4.2 |
| 2 | 30 | 100 | 1 | 4.2 |
| 3 | 30 | 150 | 1 | 4.2 |
| 4 | 30 | 200 | 1 | 4.2 |
| 5 | 10 | 100 | 1 | 4.2 |
| 6 | 50 | 100 | 1 | 4.2 |
| 7 | 70 | 100 | 1 | 4.2 |
| 8 | 30 | 100 | 1 | 2.1 |
| 9 | 30 | 100 | 1 | 6.3 |
| 10 | 30 | 100 | 1 | 8.4 |
| 11 | 30 | 100 | 2 | 4.2 |
| 12 | 30 | 100 | 3 | 4.2 |
| Material | Density/kg m−3 | Viscosity/Pa s |
|---|---|---|
| BF3 | 6.575 | 1.789 × 10−5 |
| 1-decene | 740 | 9 × 10−4 |
| PAO | 830 | 8.4 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; He, J.; Yao, Q.; Li, R.; Liu, D.; Liang, X.; Wang, L. Polymerization Reaction Kinetics of Poly α-Olefin and Numerical Simulation of a Continuous Polymerization Reactor. Processes 2025, 13, 3375. https://doi.org/10.3390/pr13113375
Shi J, He J, Yao Q, Li R, Liu D, Liang X, Wang L. Polymerization Reaction Kinetics of Poly α-Olefin and Numerical Simulation of a Continuous Polymerization Reactor. Processes. 2025; 13(11):3375. https://doi.org/10.3390/pr13113375
Chicago/Turabian StyleShi, Jianxin, Jinxue He, Qiang Yao, Ruilong Li, Dan Liu, Xuemei Liang, and Lin Wang. 2025. "Polymerization Reaction Kinetics of Poly α-Olefin and Numerical Simulation of a Continuous Polymerization Reactor" Processes 13, no. 11: 3375. https://doi.org/10.3390/pr13113375
APA StyleShi, J., He, J., Yao, Q., Li, R., Liu, D., Liang, X., & Wang, L. (2025). Polymerization Reaction Kinetics of Poly α-Olefin and Numerical Simulation of a Continuous Polymerization Reactor. Processes, 13(11), 3375. https://doi.org/10.3390/pr13113375






