Selective Recovery of Ce and La from Coal Ash Leachates by Stepwise pH-Controlled Precipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Real Leachate
2.3. Preparation of Model Leachate
2.4. Precipitation of Metals
2.5. Characterization of Liquid and Solid Samples
3. Results and Discussion
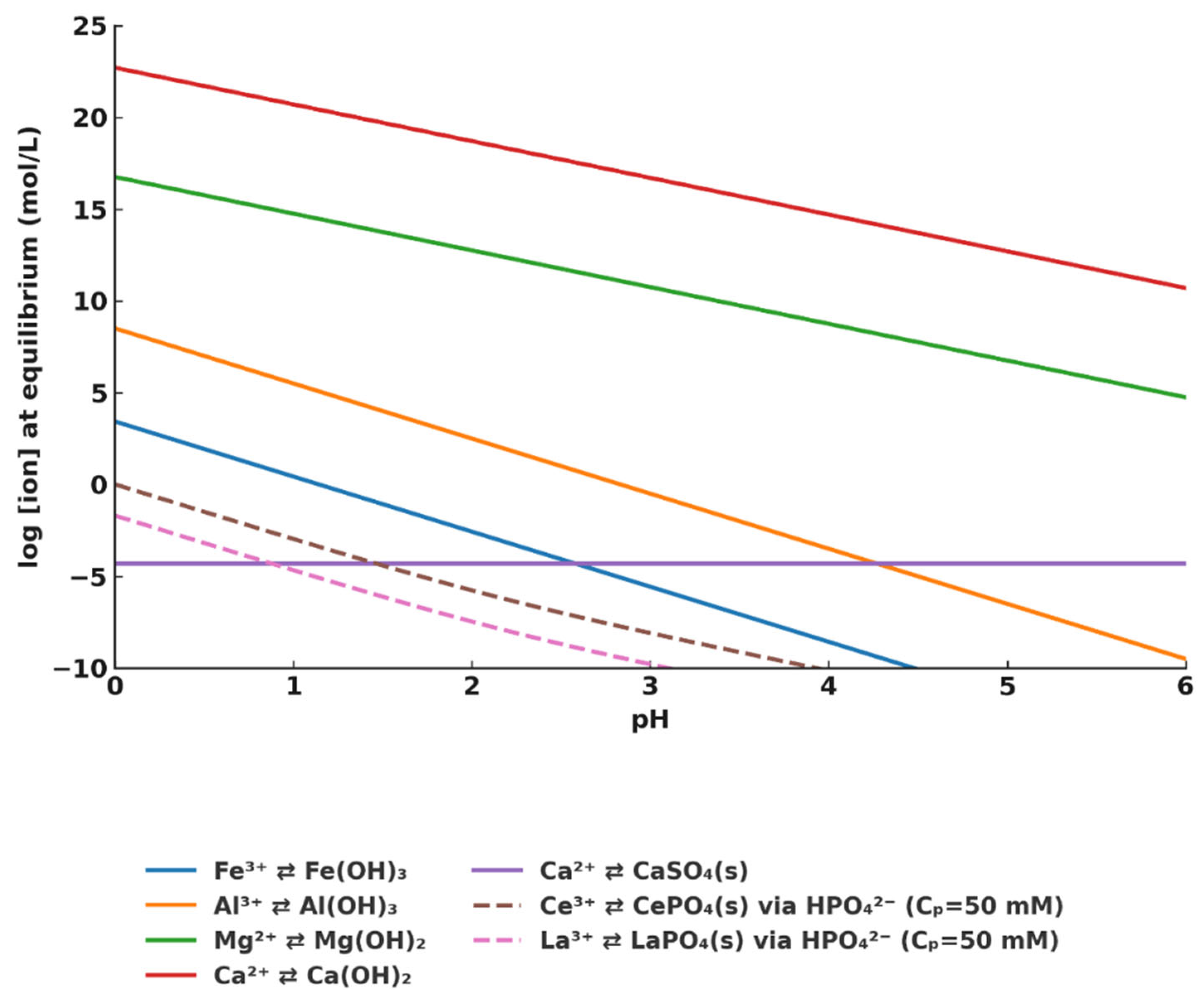
3.1. Thermodynamic Modeling and Precipitation Strategy
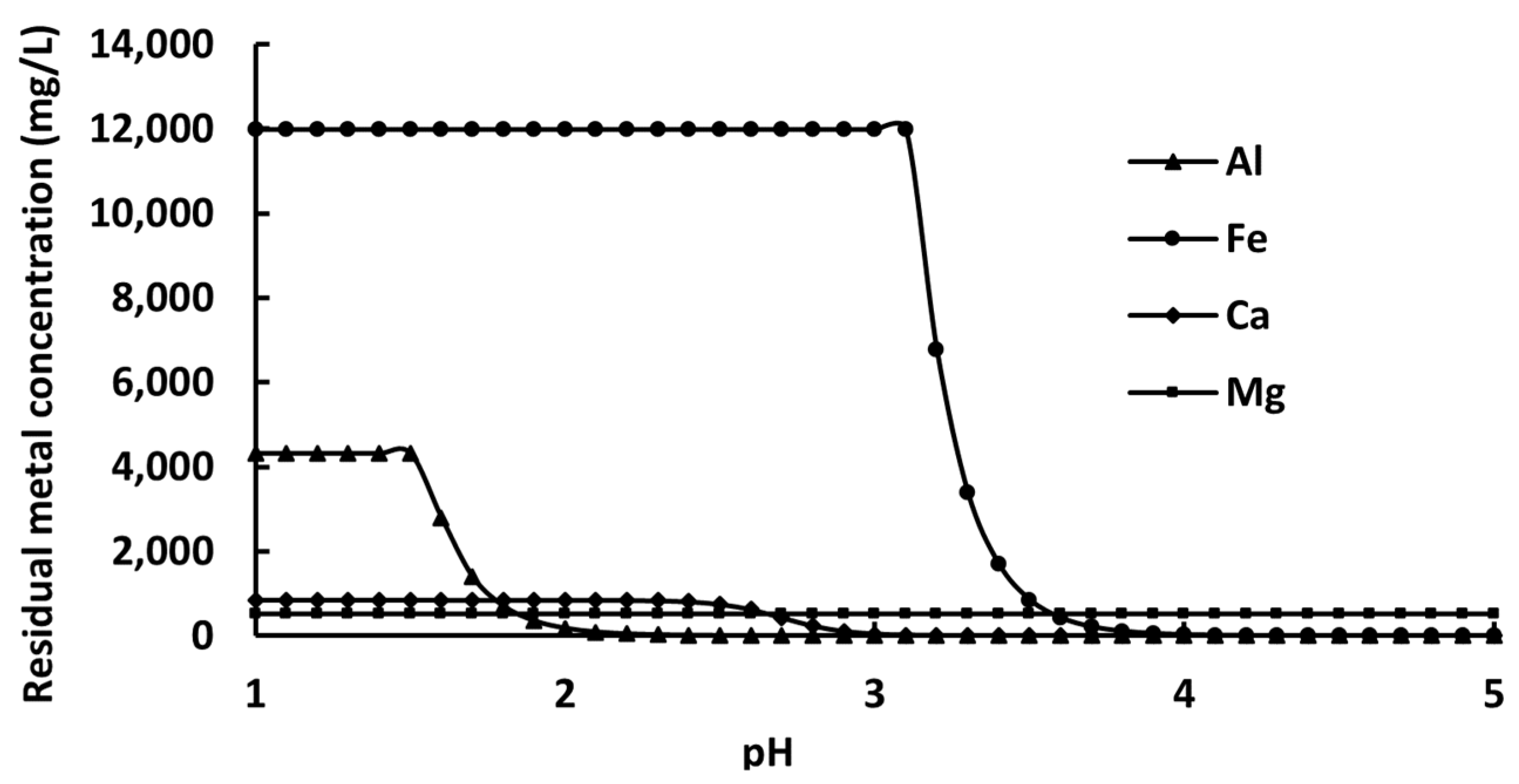
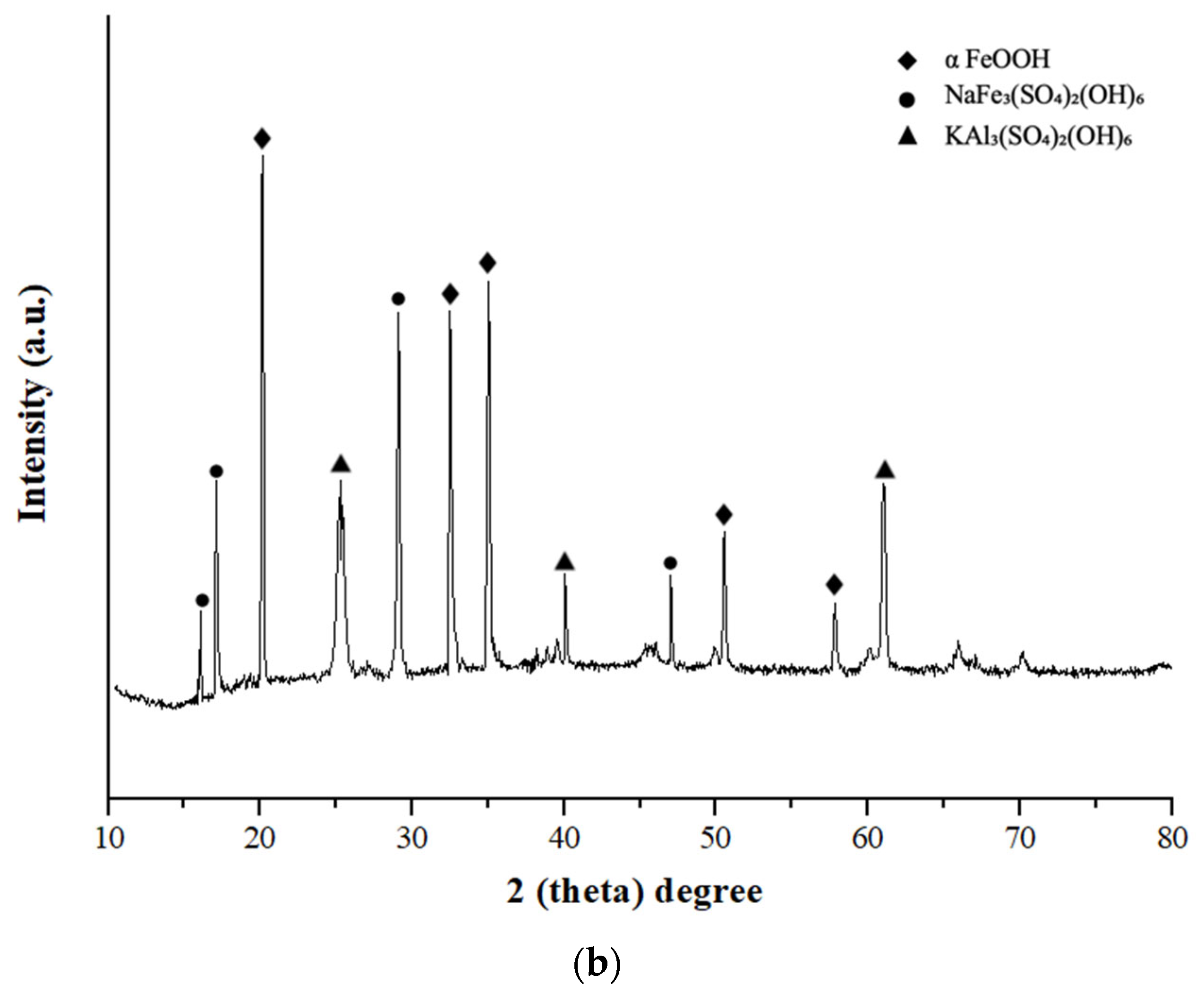
3.2. Precipitation Behavior of Interfering Elements
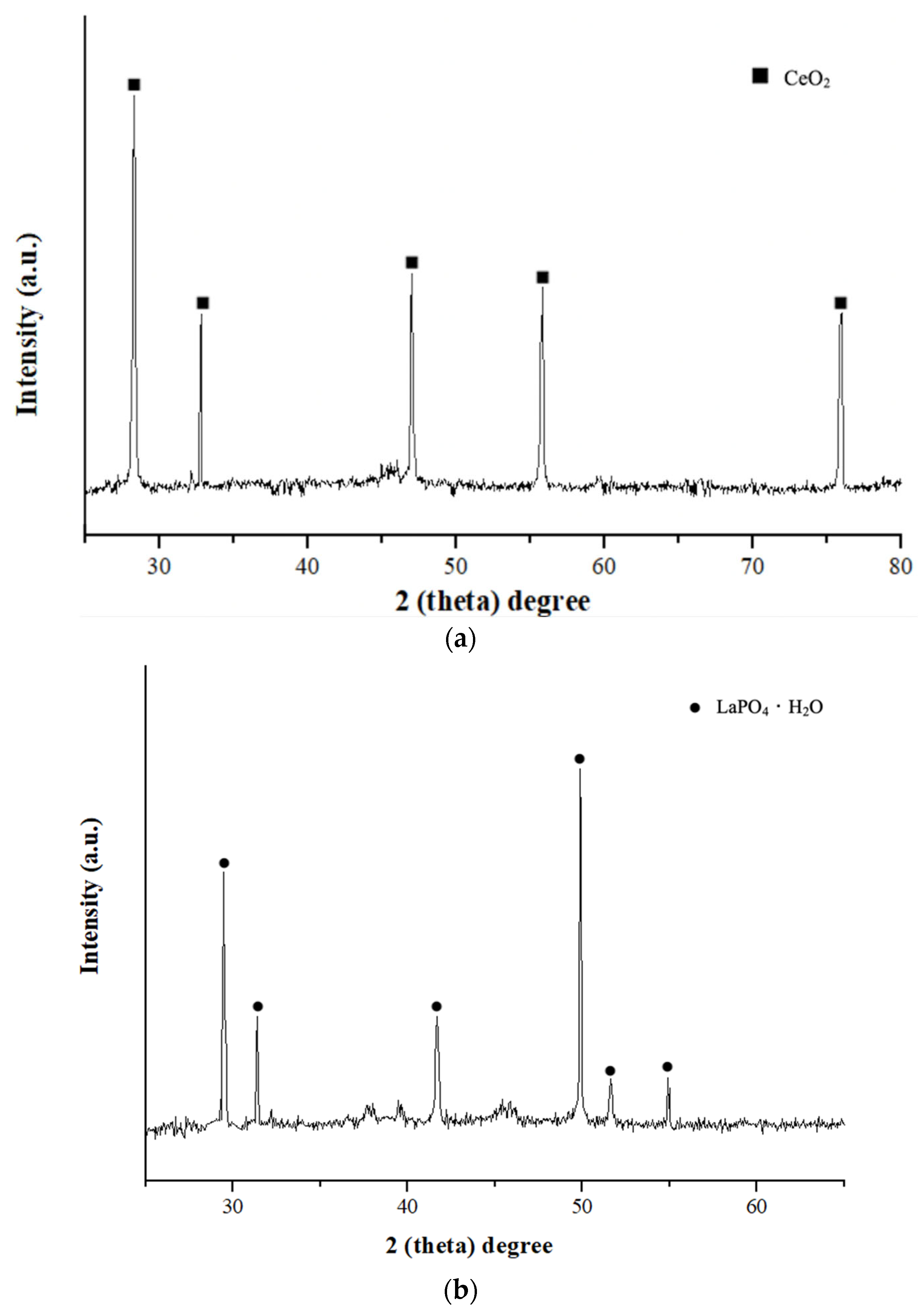
3.3. Precipitation of La and Ce from the Purified Leachate
- (1)
- Cerium step. The pH was adjusted to 2.7–2.8 with 1 M NaOH added dropwise (≤1 mL/min) at 25 ± 2 °C under stirring at 400 rpm. No phosphate was added at this point. A single dose of 30% H2O2 was introduced in ~1000-fold molar excess relative to the dissolved Ce. The suspension was stirred for 30 min while maintaining the pH within 2.7–2.8, followed by an additional 30 min of aging; a persistent pale-yellow coloration indicated oxidation of Ce3+ to Ce4+. The precipitate was separated by filtration, washed with 0.01 M H2SO4 and distilled water, and dried at 60 °C.
- (2)
- Lanthanum step. The filtrate obtained after Ce removal was (optionally) re-acidified to pH 2.0–2.2 to standardize the starting conditions. Orthophosphoric acid was then added to achieve a total phosphate concentration of 25–50 mM. The pH was gradually raised to 3.4–3.5 using 1 M NaOH (≤0.5 mL/min) over 30–60 min while stirring at 25 ± 2 °C and 400 rpm. The suspension was held at this pH for 60 min to complete precipitation, with pH stability verified, after which the solid was collected by filtration, washed with distilled water, and dried at 60 °C.
3.4. Material Balance and Metals Distribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.K.; Hossain, S.; Ahmed, M.H.; Khan, M.I.; Haque, N.; Raihan, G.A. A review on optical applications, prospects, and challenges of rare-earth oxides. ACS Appl. Electron. Mater. 2021, 3, 3715–3746. [Google Scholar] [CrossRef]
- Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding rare earth elements as critical raw materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Balaram, V. Potential future alternative resources for rare earth elements: Opportunities and challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Gkika, D.A.; Chalaris, M.; Kyzas, G.Z. Review of methods for obtaining rare earth elements from recycling and their impact on the environment and human health. Processes 2024, 12, 1235. [Google Scholar] [CrossRef]
- Wang, S.; Huang, W.; Ao, W. The Distribution of Rare Earth Elements in Coal Fly Ash Determined by LA-ICP-MS and Implications for Its Economic Significance. Sustainability 2025, 17, 275. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q. A review study of rare Earth, Cobalt, Lithium, and Manganese in Coal-based sources and process development for their recovery. Miner. Eng. 2022, 189, 107897. [Google Scholar] [CrossRef]
- Thomas, B.S.; Dimitriadis, P.; Kundu, C.; Vuppaladadiyam, S.S.V.; Raman, R.S.; Bhattacharya, S. Extraction and separation of rare earth elements from coal and coal fly ash: A review on fundamental understanding and on-going engineering advancements. J. Environ. Chem. Eng. 2024, 12, 112769. [Google Scholar] [CrossRef]
- Agrawal, R.; Ragauskas, A.J. Sustainable recovery of Rare Earth Elements (REEs) from coal and coal ash through urban mining: A Nature Based Solution (NBS) for circular economy. J. Environ. Manag. 2025, 384, 125411. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Pan, J.; Yang, F.; Long, X.; Yang, Y.; Zhou, C. Rare earth elements recovery and mechanisms from coal fly ash by column leaching using citric acid. Sep. Purif. Technol. 2025, 353, 128471. [Google Scholar] [CrossRef]
- Nadirov, R.; Kamunur, K.; Mussapyrova, L.; Batkal, A.; Tyumentseva, O.; Karagulanova, A. Integrated Compositional Modeling and Machine Learning Analysis of REE-Bearing Coal Ash from a Weathered Dumpsite. Minerals 2025, 15, 734. [Google Scholar] [CrossRef]
- Palozzi, J.; Bailey, J.G.; Tran, Q.A.; Stanger, R. A characterization of rare earth elements in coal ash generated during the utilization of Australian coals. Int. J. Coal Prep. Util. 2023, 43, 2106–2135. [Google Scholar] [CrossRef]
- Reedy, R.C.; Scanlon, B.R.; Bagdonas, D.A.; Hower, J.C.; James, D.; Kyle, J.R.; Uhlman, K. Coal ash resources and potential for rare earth element production in the United States. Int. J. Coal Sci. Technol. 2024, 11, 74. [Google Scholar] [CrossRef]
- Kuppusamy, V.K.; Holuszko, M. Sulfuric acid baking and water leaching of rare earth elements from coal tailings. Fuel 2022, 319, 123738. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Zhang, W.; Werner, J. Acid leaching of rare earth elements from coal and coal ash: Implications for using fluidized bed combustion to assist in the recovery of critical materials. Energy Fuels 2019, 33, 5971–5980. [Google Scholar] [CrossRef]
- van Wyk, P.; Bradshaw, S.; Dorfling, C.; Ghosh, T.; Akdogan, G. Characterisation and hydrochloric acid leaching of rare earth elements in discard coal and coal fly ash. Minerals 2024, 14, 1070. [Google Scholar] [CrossRef]
- Mokoena, K.; Mokhahlane, L.S.; Clarke, S. Effects of acid concentration on the recovery of rare earth elements from coal fly ash. Int. J. Coal Geol. 2022, 259, 104037. [Google Scholar] [CrossRef]
- Chen, H.; Wen, Z.; Pan, J.; Zhang, L.; He, X.; Valeev, D.; Zhou, C. Study on leaching behavior differences of rare earth elements from coal fly ash during microwave-assisted HCl leaching. Int. J. Coal Prep. Util. 2023, 43, 1993–2015. [Google Scholar] [CrossRef]
- Peiravi, M.; Ackah, L.; Guru, R.; Mohanty, M.; Liu, J.; Xu, B.; Zhu, X.; Chen, L. Chemical extraction of rare earth elements from coal ash. Miner. Metall. Process. 2017, 34, 170–177. [Google Scholar] [CrossRef]
- Znamenackova, I.; Dolinska, S.; Hredzak, S.; Cablik, V.; Lovas, M.; Gesperova, D. Study of extraction of rare earth elements from hard coal fly ash. Inżynieria Miner. 2020, 2, 229–232. [Google Scholar] [CrossRef]
- Ketegenov, T.; Kamunur, K.; Mussapyrova, L.; Batkal, A.; Nadirov, R. Enhancing Rare Earth Element Recovery from Coal Ash Using High-Voltage Electrical Pulses and Citric Acid Leaching. Minerals 2024, 14, 693. [Google Scholar] [CrossRef]
- Banerjee, R.; Mohanty, A.; Chakravarty, S.; Chakladar, S.; Biswas, P. A single-step process to leach out rare earth elements from coal ash using organic carboxylic acids. Hydrometallurgy 2021, 201, 105575. [Google Scholar] [CrossRef]
- Banerjee, R.; Chakladar, S.; Mohanty, A.; Chakravarty, S.; Chattopadhyay, S.K.; Jha, M.K. Review on the environment friendly leaching of rare earth elements from the secondary resources using organic acids. Geosystem Eng. 2022, 25, 95–115. [Google Scholar] [CrossRef]
- Karan, R.; Sreenivas, T.; Kumar, M.A.; Singh, D.K. Recovery of rare earth elements from coal flyash using deep eutectic solvents as leachants and precipitating as oxalate or fluoride. Hydrometallurgy 2022, 214, 105952. [Google Scholar] [CrossRef]
- Dodbiba, G.; Fujita, T. Trends in extraction of rare earth elements from coal ashes: A review. Recycling 2023, 8, 17. [Google Scholar] [CrossRef]
- Tian, G.; Liu, H. Review on the mineral processing in ionic liquids and deep eutectic solvents. Miner. Process. Extr. Metall. Rev. 2024, 45, 130–153. [Google Scholar] [CrossRef]
- Park, S.; Liang, Y. Bioleaching of trace elements and rare earth elements from coal fly ash. Int. J. Coal Sci. Technol. 2019, 6, 74–83. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Wang, J.; Jiang, S.; Panchal, B.; Sun, Y. Bioleaching rare earth elements from coal fly ash by Aspergillus niger. Fuel 2023, 354, 129387. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Oliveira, É.D. Selective precipitation of rare earth from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate. Miner. Eng. 2019, 134, 402–416. [Google Scholar] [CrossRef]
- Masmoudi-Soussi, A.; Hammas-Nasri, I.; Horchani-Naifer, K.; Ferid, M. Rare earths recovery by fractional precipitation from a sulfuric leach liquor obtained after phosphogypsum processing. Hydrometallurgy 2020, 191, 105253. [Google Scholar] [CrossRef]
- Xing, M.; Wu, X.; Li, Z.; Zhang, F.; Wang, Y.; Zhao, L. Rare earth element recovery and aluminum-rich residue production from high alumina fly ash by alkali pre-desilication enhance the mechanochemical extraction process. Process Saf. Environ. Prot. 2023, 175, 60–69. [Google Scholar] [CrossRef]
- Praneeth, S.; Sakr, A.K.; Dardona, M.; Tummala, C.M.; Roy, P.K.; Dittrich, T.M. Selective separation and recovery of rare-earth elements (REEs) from acidic solutions and coal fly ash leachate by novel TODGA-Impregnated organosilica media. Chem. Eng. J. 2024, 500, 156849. [Google Scholar] [CrossRef]
- Hassas, B.V.; Rezaee, M. Selective precipitation of rare earth and critical elements from acid mine drainage-Part II: Mechanistic effect of ligands in staged precipitation process. Resour. Conserv. Recycl. 2023, 188, 106655. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, S.; Xie, N.; Yang, L.; Wang, Q.; Wen, Y.; Chen, H.; Tang, Y. Green approach for rare earth element (REE) recovery from coal fly ash. Environ. Sci. Technol. 2023, 57, 5414–5423. [Google Scholar] [CrossRef]
- Farrah, H.E.; Lawrance, G.A.; Wanless, E.J. Solubility of calcium sulfate salts in acidic manganese sulfate solutions from 30 to 105 °C. Hydrometallurgy 2007, 86, 13–21. [Google Scholar] [CrossRef]
- Firsching, F.H.; Brune, S.N. Solubility products of the trivalent rare-earth phosphates. J. Chem. Eng. Data 1991, 36, 93–95. [Google Scholar] [CrossRef]
- Cetiner, Z.S.; Wood, S.A.; Gammons, C.H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150 °C. Chem. Geol. 2005, 217, 147–169. [Google Scholar] [CrossRef]
- Gausse, C.; Szenknect, S.; Qin, D.W.; Mesbah, A.; Clavier, N.; Neumeier, S.; Bosbach, D.; Dacheux, N. Determination of the solubility of rhabdophanes LnPO4·0.667H2O (Ln = La to Dy). Eur. J. Inorg. Chem. 2016, 2016, 4615–4630. [Google Scholar] [CrossRef]
- Stefánsson, A. Iron (III) hydrolysis and solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef]
- Zou, Y.; Chernyaev, A.; Seisko, S.; Sainio, J.; Lundström, M. Removal of iron and aluminum from hydrometallurgical NMC-LFP recycling process through precipitation. Miner. Eng. 2024, 218, 109037. [Google Scholar] [CrossRef]
- Destefani, T.A.; Onaga, G.L.; de Farias, M.A.; Percebom, A.M.; Sabadini, E. Stabilization of spherical nanoparticles of iron (III) hydroxides in aqueous solution by wormlike micelles. J. Colloid Interface Sci. 2018, 513, 527–535. [Google Scholar] [CrossRef]
- De Hek, H.; Stol, R.J.; De Bruyn, P.L. Hydrolysis-precipitation studies of aluminum (III) solutions. 3. The role of the sulfate ion. J. Colloid Interface Sci. 1978, 64, 72–89. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Gadipelly, C.R.; Marathe, K.V.; Rathod, V.K. Treatment of fluoride concentrates from membrane unit using salt solutions. J. Water Process Eng. 2014, 2, 31–36. [Google Scholar] [CrossRef]
- Senevirathna, H.L.; Lee, W.C.; Wu, S.; Bai, K.; Wu, P. Transforming desalination brine into highly reactive magnesium oxide and life cycle analysis. Watershed Ecol. Environ. 2025, 7, 36–46. [Google Scholar] [CrossRef]
- Beltrami, D.; Deblonde, G.J.P.; Bélair, S.; Weigel, V. Recovery of yttrium and lanthanides from sulfate solutions with high concentration of iron and low rare earth content. Hydrometallurgy 2015, 157, 356–362. [Google Scholar] [CrossRef]



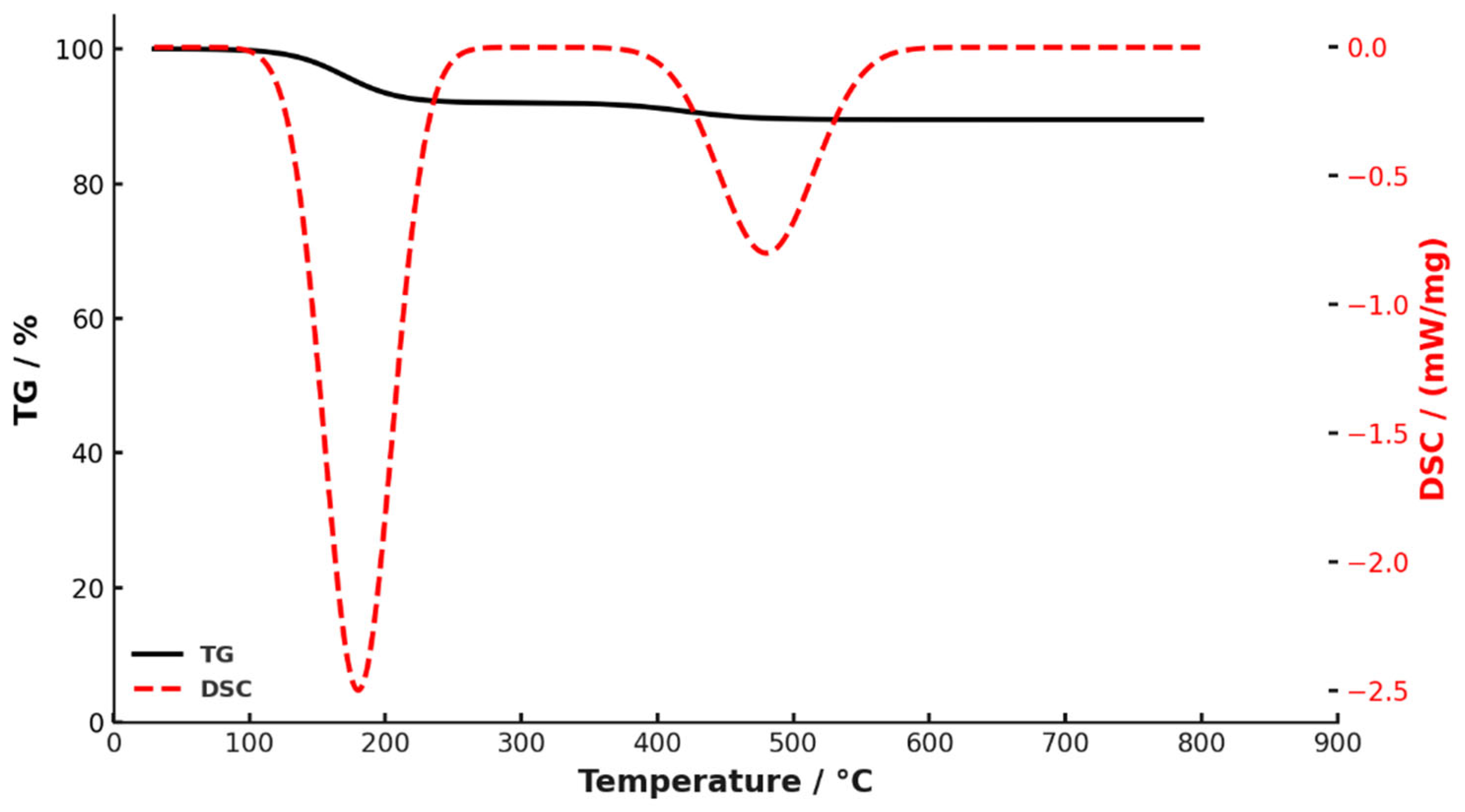
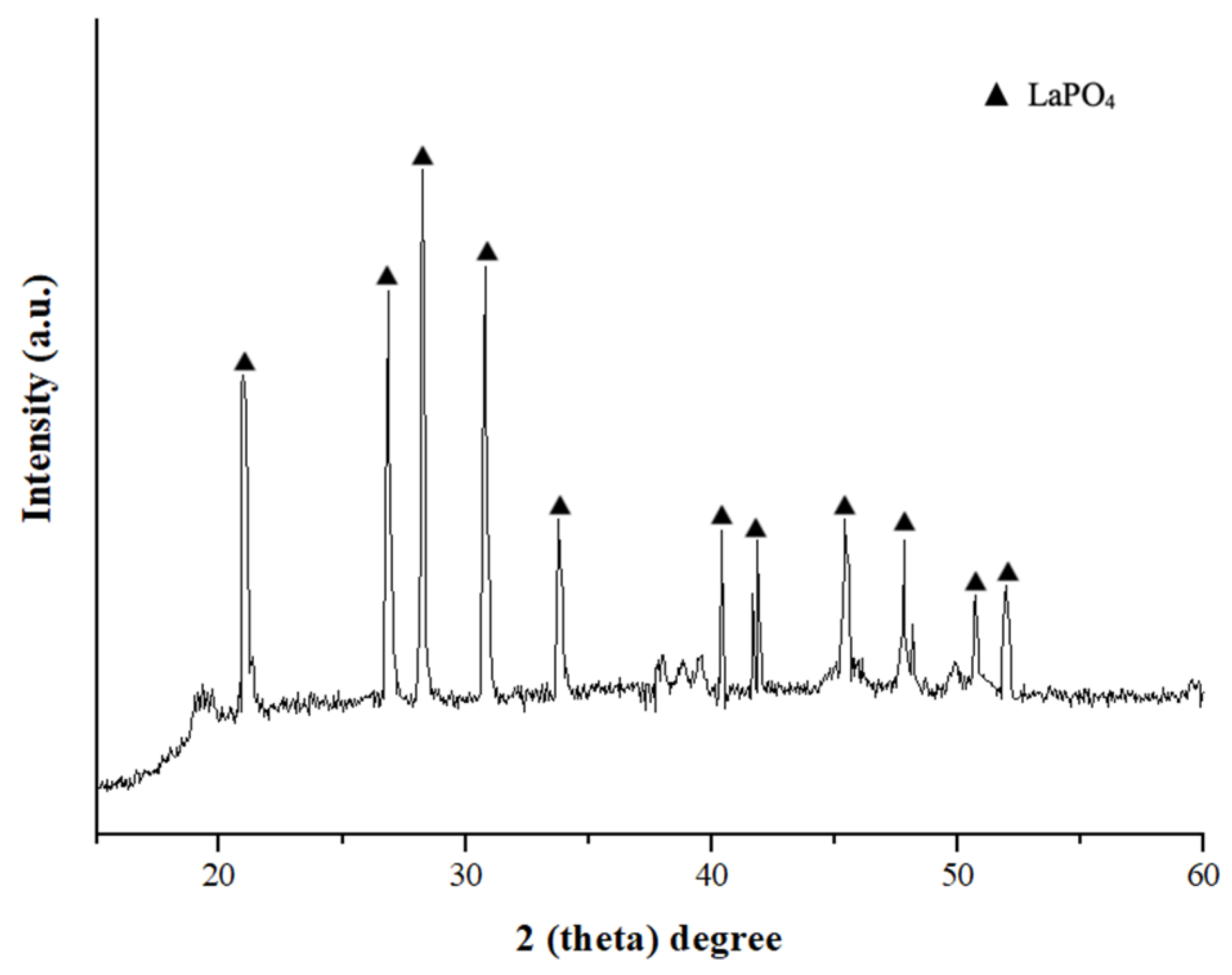
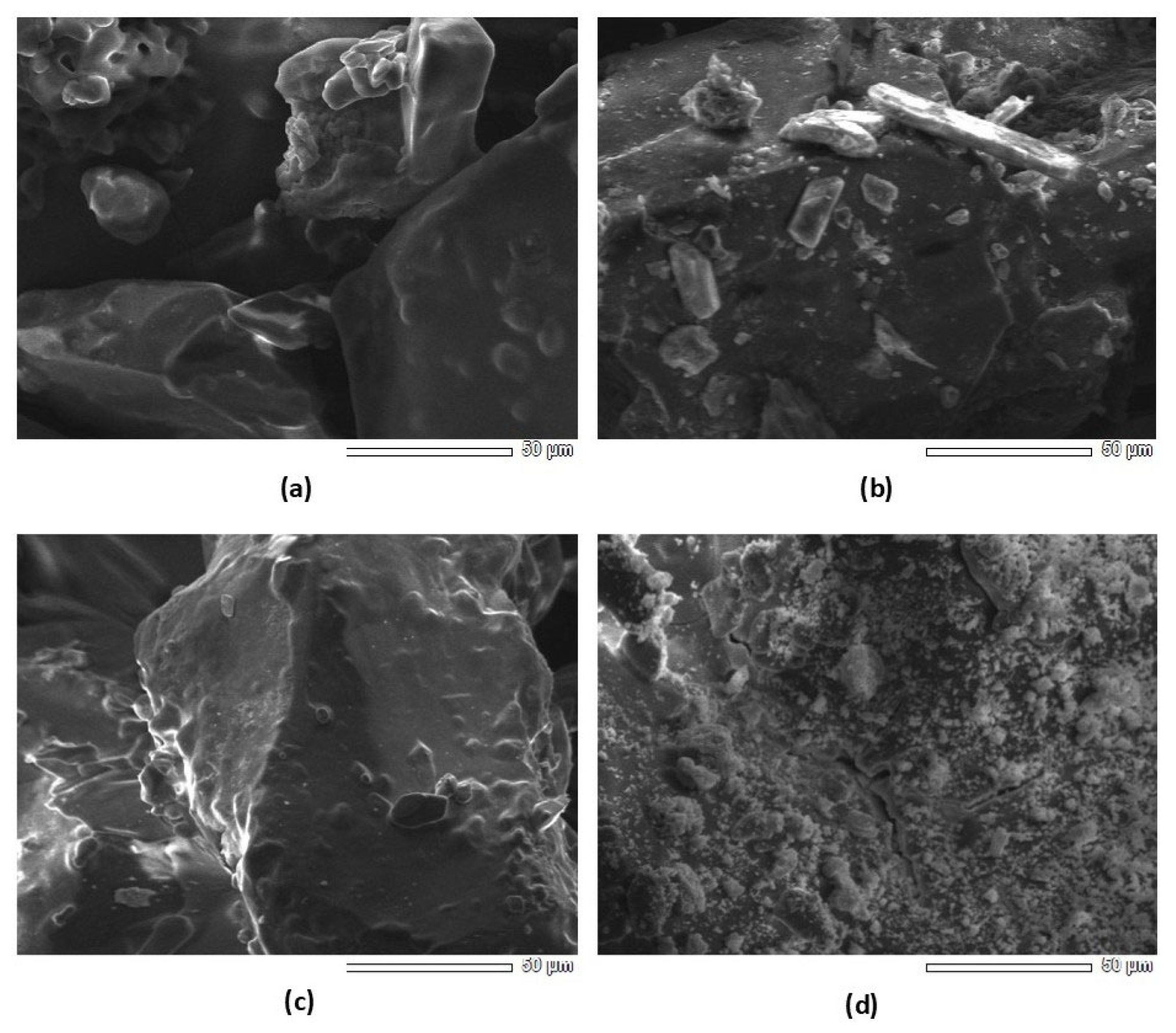
| Al, mg/L | Fe, mg/L | Ca, mg/L | Mg, mg/L | P, mg/L | Na+K, mg/L | Si, mg/L | Ce, mg/L | La, mg/L |
|---|---|---|---|---|---|---|---|---|
| 11,977 | 4320 | 850 | 514 | 117 | 149 | 64 | 4.77 | 1.81 |
| Stream | Al, mg | Fe, mg | Ca, mg | Mg, mg | Si, mg | Ce, mg | La, mg |
|---|---|---|---|---|---|---|---|
| S0 | 11,977.00 | 4320.00 | 850.00 | 514.00 | 64.0 | 4.77 | 1.81 |
| P1 | 598.85 | 4233.60 | 833.00 | 1.54 | 14.3 | - | - |
| S1 | 11,378.15 | 86.40 | 17.00 | 512.46 | 49.4 | 4.77 | 1.81 |
| P2 | 11,378.12 | 86.39 | 15.08 | 1.46 | 10.1 | - | - |
| S2 | 0.03 | 0.01 | 1.92 | 511.00 | 38.9 | 4.77 | 1.81 |
| Stream | Ce, mg | La, mg | Ca, mg | Si, mg | Mg, mg | Al, mg | Fe, mg |
|---|---|---|---|---|---|---|---|
| S2 | 4.77 | 1.81 | 1.92 | 38.2 | 511.00 | 0.03 | 0.01 |
| P3 | 4.50 | - | <0.01 | - | - | <0.01 | - |
| S3 | 0.20 | 1.81 | 1.92 | 38.2 | 511.00 | 0.03 | 0.01 |
| P4 | 0.10 | 1.70 | 0.01 | - | 0.00 | <0.01 | <0.01 |
| S4 | 0.05 | 0.05 | 1.90 | 38.2 | 510.50 | 0.02 | 0.01 |
| Precipitate | Solid, mg | Ce | La | Ca | Al | Fe |
|---|---|---|---|---|---|---|
| P3 | 5.50 | 4.50 | - | <0.01 | <0.01 | <0.01 |
| P4 | 3.20 | 0.10 | 1.70 | 0.01 | 0.01 | 0.01 |
| Closure | - | 0.15 | 0.06 | 0.02 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamunur, K.; Tyumentseva, O.; Mussapyrova, L.; Batkal, A.; Karagulanova, A.; Nadirov, R. Selective Recovery of Ce and La from Coal Ash Leachates by Stepwise pH-Controlled Precipitation. Processes 2025, 13, 3203. https://doi.org/10.3390/pr13103203
Kamunur K, Tyumentseva O, Mussapyrova L, Batkal A, Karagulanova A, Nadirov R. Selective Recovery of Ce and La from Coal Ash Leachates by Stepwise pH-Controlled Precipitation. Processes. 2025; 13(10):3203. https://doi.org/10.3390/pr13103203
Chicago/Turabian StyleKamunur, Kaster, Olesya Tyumentseva, Lyazzat Mussapyrova, Aisulu Batkal, Ardak Karagulanova, and Rashid Nadirov. 2025. "Selective Recovery of Ce and La from Coal Ash Leachates by Stepwise pH-Controlled Precipitation" Processes 13, no. 10: 3203. https://doi.org/10.3390/pr13103203
APA StyleKamunur, K., Tyumentseva, O., Mussapyrova, L., Batkal, A., Karagulanova, A., & Nadirov, R. (2025). Selective Recovery of Ce and La from Coal Ash Leachates by Stepwise pH-Controlled Precipitation. Processes, 13(10), 3203. https://doi.org/10.3390/pr13103203





