Destruction Mechanism of Laser Melted Layers of AISI 321 Austenitic Stainless Steel After Electrochemical Corrosion in Ringer’s Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Electrochemical Corrosion Test
2.3. Sample Characterization
- For OM, the Leica M 80 (Leica Microsystems, Manheim, Germany) with the Leica IC 90 E digital camera was used.
- For SEM, the Zeiss Evo 10 SEM (Jena, Germany) was used. The micrographs were taken in variable pressure mode using secondary electron detection. The chemical composition on the surface of the samples was registered with a Zeiss SmartEDX energy-dispersive X-ray probe.
- For SEM, the Gemini SEM 460 (Carl Zeiss Microscopy, Cambourne, UK), equipped with the Ultim Max EDS detector and CMOS EBSD detector, was used. The EDS and EBSD data were analyzed using AZtecCrystal 3.3 software from Oxford Instruments (Oxford Instruments Nanotechnology Limited, Oxford, UK).
3. Results
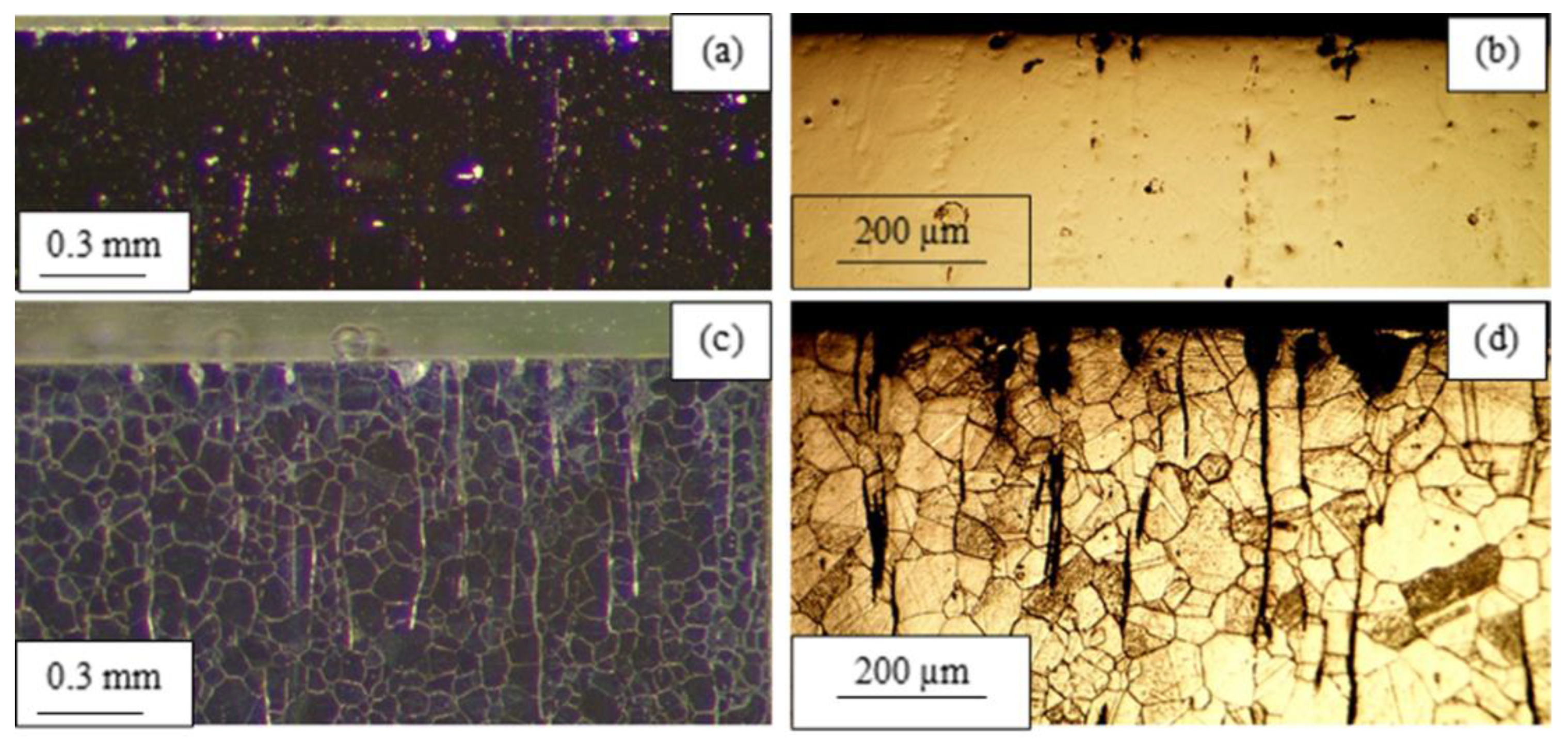
3.1. Microstructure and Chemical Composition
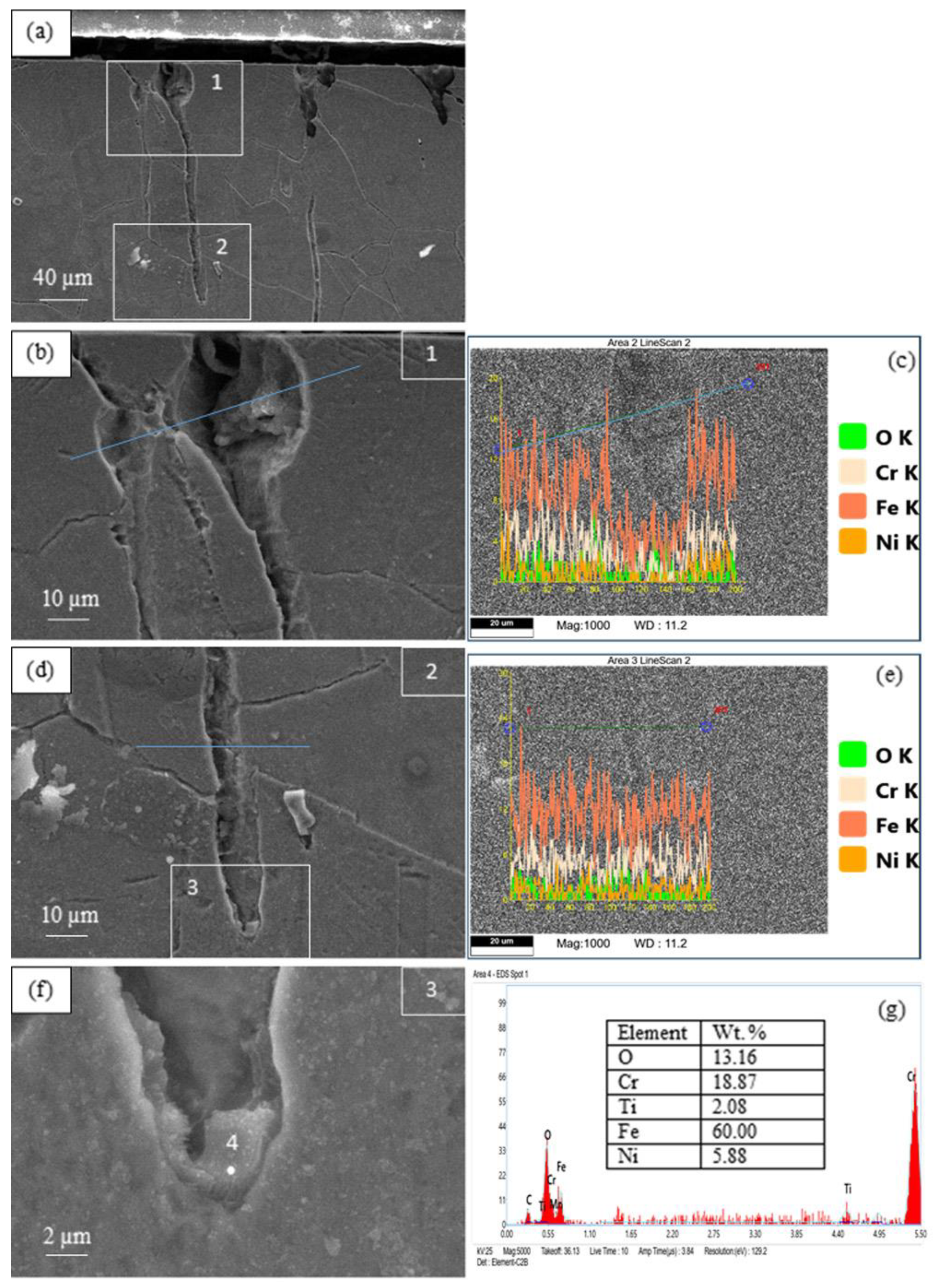
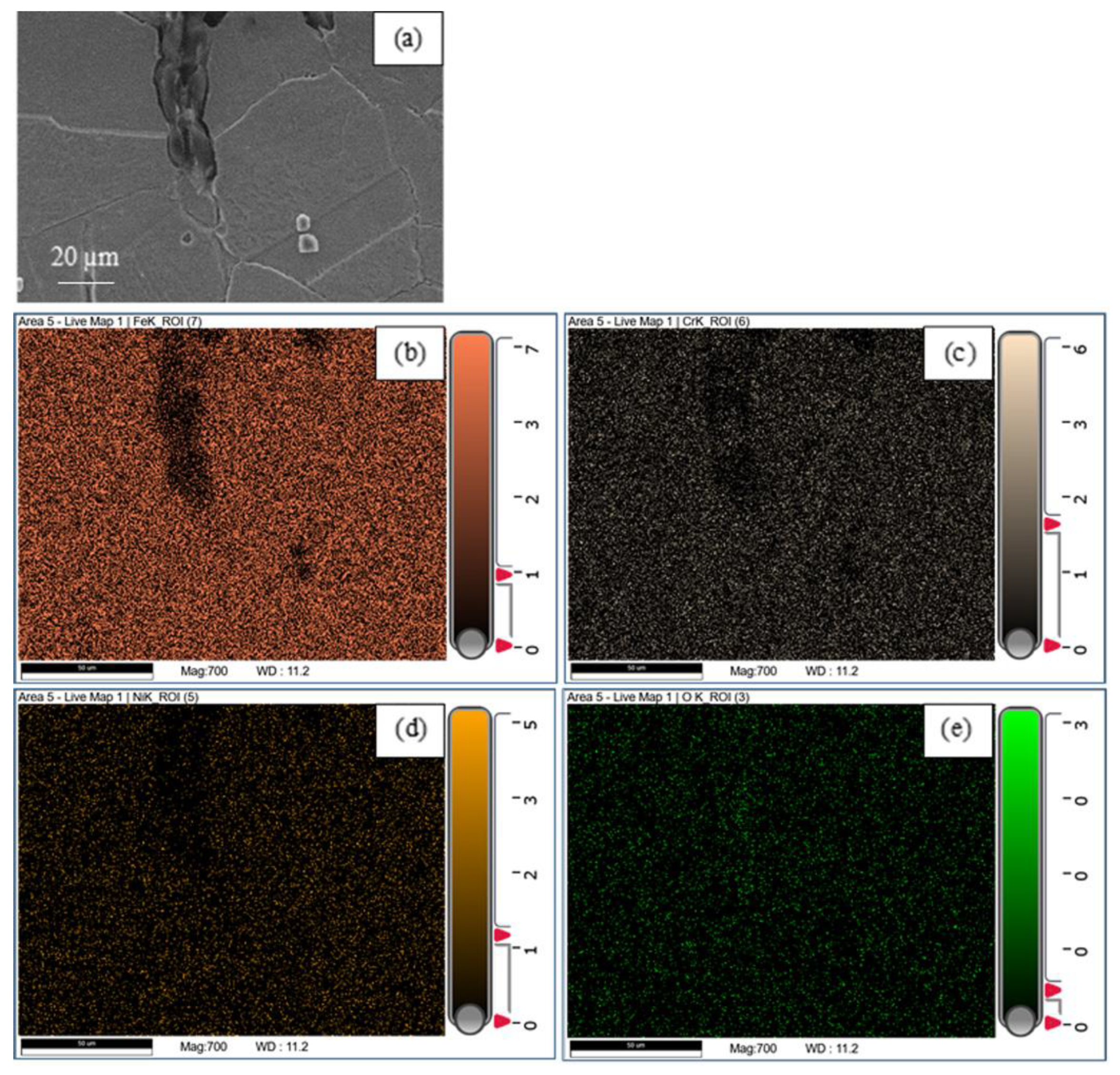
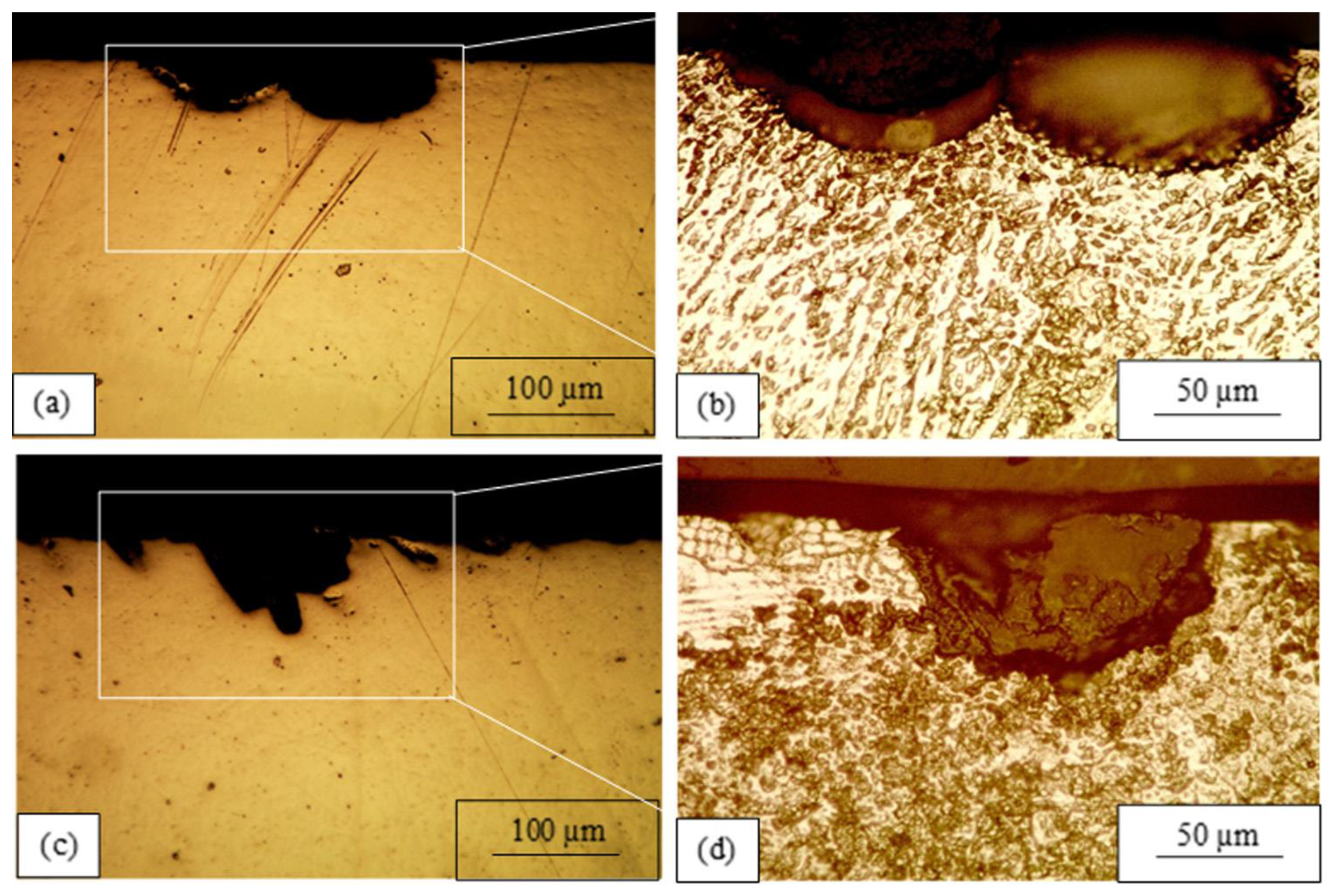
3.2. Corrosion Destruction
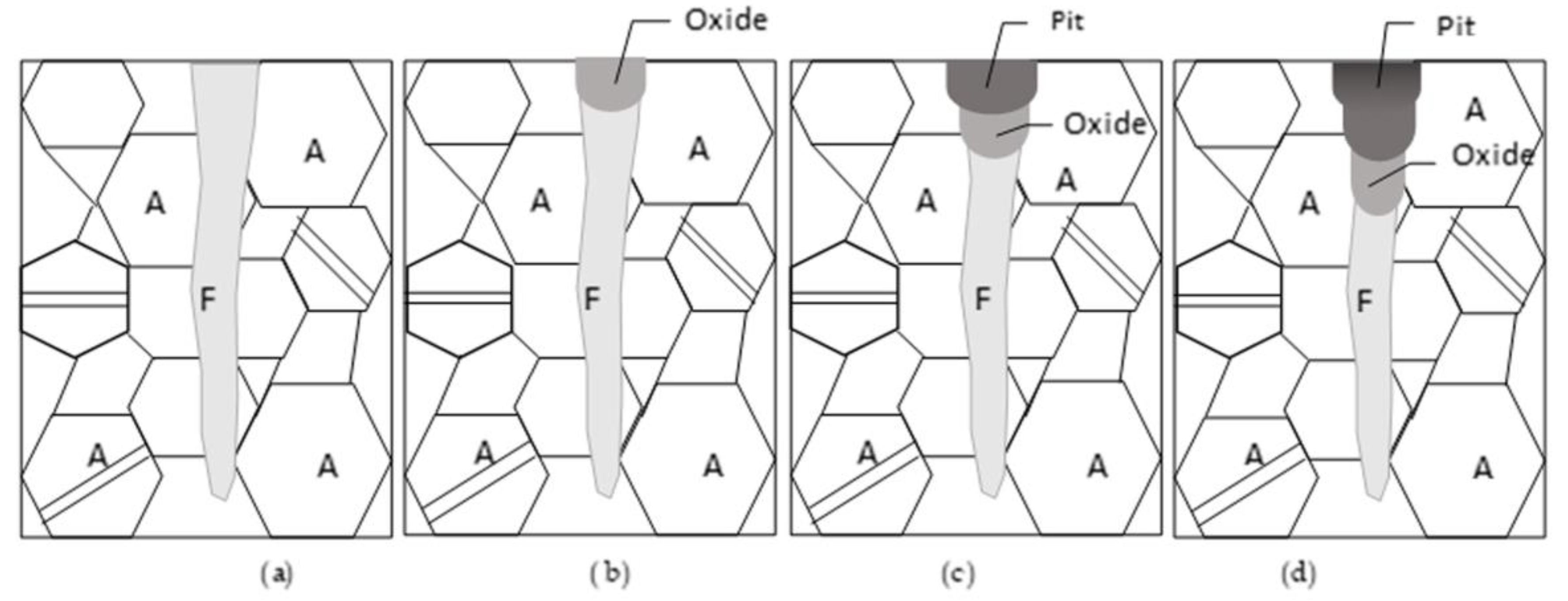
4. Discussion
5. Conclusions
- The mechanism behind corrosion destruction in laser-melted layers of AISI 321 steel in Ringer’s solution is proposed and proven through our investigation. It consists of the following stages: (1) the initial destruction of the δ-ferrite; (2) the formation of an austenitic dendrite network; (3) the mechanical fracture of the austenitic dendrites and pit formation; and (4) the growth of the pit inside the grain following the previous steps.
- A relationship between corrosion pit development and dendrite orientation in the laser-melted layers is observed: (1) In the MZ, where the dendrite axes are perpendicular to or inclined toward the surface, the corrosion pit grows within the grain. (2) At the MZ/BM boundary, the dendrite axes are parallel to the surface and the corrosion pit develops through the HAZ along the MZ/BM boundary.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Austenite |
| AMT | Additive manufacturing technologies |
| AS | Artificial saliva |
| BM | Base metal |
| CW | Continuous wave |
| DLD | Direct laser deposition |
| DMLS | Direct metal laser sintering |
| EBSD | Evaluated by electron back-scattered diffraction |
| EDS | Energy dispersive spectroscopy |
| HAZ | Heat affected zone |
| LMD | Laser metal deposition |
| LML | Laser melted layers |
| LOF | Lack of fusion |
| LRM | Laser rapid manufacturing |
| MZ | Melted zone |
| OM | Optical microscopy |
| SBF | Simulated body fluids |
| SEM | Scanning electron microscopes |
| SLM | Selective laser melting |
References
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Introduction to Biomaterials: Basic Theory with Engineering Applications; Cambridge University Press: Cambridge, UK, 2014; p. 402. [Google Scholar]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Liu, T.; Kong, D.; Wang, D.; Li, X. The enhancement of microstructure on the passive and pitting behaviors of selective laser melting 316L SS in simulated body fluid. Appl. Surf. Sci. 2019, 467, 193–205. [Google Scholar] [CrossRef]
- Ko, G.; Kim, W.; Kwon, K.; Lee, T.K. The corrosion of stainless steel made by additive manufacturing: A review. Metals 2021, 11, 516. [Google Scholar] [CrossRef]
- Kathuria, Y.P. Laser microprocessing of metallic stent for medical therapy. J. Mater. Process. Technol. 2005, 170, 545–550. [Google Scholar] [CrossRef]
- Meng, H.; Liao, J.; Zhou, Y.; Zhang, Q. Laser micro-processing of cardiovascular stent with fiber laser cutting system. Opt. Laser Technol. 2009, 41, 300–302. [Google Scholar]
- Muhammad, N.; Whitehead, D.; Boor, A.; Li, L. Comparison of dry and wet fibre laser profile cutting of thin 316L stainless steel tubes for medical device applications. J. Mater. Process. Technol. 2010, 210, 2261–2267. [Google Scholar] [CrossRef]
- Alhajhamoud, M.; Candan, L.; Ilgaz, M.A.; Cinar, I.; Ozbey, S.; Čorović, S.; Kayahan, E. Laser welding of 316L austenitic stainless steel in an air and a water environment. Materials 2022, 15, 2248. [Google Scholar] [CrossRef]
- Baldissin, D.; Baricco, M.; Battezzati, L. Microstructures in rapidly solidified AISI 304 interpreted according to phase selection theory. Mater. Sci. Eng. A 2007, 449, 999–1002. [Google Scholar] [CrossRef]
- Chikarakara, E.; Naher, S.; Brabazon, D. Analysis of microstructural changes during pulsed CO2 laser surface processing of AISI 316L stainless steel. Adv. Mater. Res. 2011, 264, 1401–1408. [Google Scholar] [CrossRef]
- Khalfallah, I.Y.; Rahoma, M.N.; Abboud, J.H.; Benyounis, K.Y. Microstructure and corrosion behavior of austenitic stainless steel treated with laser. Opt. Laser Technol. 2011, 43, 806–813. [Google Scholar] [CrossRef]
- Ahmed, N.; Barsoum, I.; Haidemenopoulos, G.; Al-Rub, R.A. Process parameter selection and optimization of laser powder bed fusion for 316L stainless steel: A review. J. Manuf. Process. 2022, 75, 415–434. [Google Scholar] [CrossRef]
- Panova, N.K.; Nikolova, K.T.; Dikova, T.D. Application of lasers and laser processing technologies in modern dentistry: A review. J. Chem. Technol. Metall. 2023, 58, 1116–1127. [Google Scholar] [CrossRef]
- Revilla, R.I.; Van Calster, M.; Raes, M.; Arroud, G.; Andreatta, F.; Pyl, L.; De Graeve, I. Microstructure and corrosion behavior of 316L stainless steel prepared using different additive manufacturing methods: A comparative study bringing insights into the impact of microstructure on their passivity. Corros. Sci. 2020, 176, 108914. [Google Scholar] [CrossRef]
- Liu, S.; Lee, M.; Choi, C.; Shin, K. Effect of additive manufacturing of SUS316L using selective laser melting. J. Mater. Res. Technol. 2023, 24, 9824–9833. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Adijāns, I.; Lazov, L.; Ilieva, M.; Nikolova, M.P. Investigation of the change in wettability properties and corrosion behavior of AISI 304 after laser surface texturing. J. Phys. Conf. Ser. 2023, 2487, 012040. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z. Physical-mechanical and electrochemical corrosion behaviors of additively manufactured Cr-Ni-based stainless steel formed by laser cladding. Mater. Des. 2016, 100, 254–262. [Google Scholar] [CrossRef]
- Stavrev, D.; Dikova, T.D.; Shtarbakov, V.; Milkov, M. Laser surface melting of austenitic Cr-Ni stainless steel. Adv. Mater. Res. 2011, 264, 1287–1292. [Google Scholar] [CrossRef]
- Dikova, T.; Tsaneva, D.; Ilieva, M.; Panova, N. Electrochemical corrosion of laser melted layers of stainless steel in Ringer solution. In Proceedings of the VI-th International Metallurgical Congress “Metallurgy, Materials, Environmental (MME), Ohrid, North Macedonia, 29 May–1 June 2014. [Google Scholar]
- Dikova, T.; Tsaneva, D.; Ilieva, M.; Panova, N.; Galunska, B. Investigation of the electro-chemical corrosion of laser-melted layers of stainless steel in artificial saliva. Adv. Mater. Process. Technol. 2015, 1, 115–123. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Haghdadi, N.; Wang, K.; Cizek, P.; Tan, M.Y. On the unusual intergranular corrosion resistance of 316L stainless steel additively manufactured by selective laser melting. Corros. Sci. 2019, 161, 108189. [Google Scholar] [CrossRef]
- Tarasov, S.Y.; Filippov, A.V.; Shamarin, N.N.; Fortuna, S.V.; Maier, G.G.; Kolubaev, E.A. Microstructural evolution and chemical corrosion of electron beam wire-feed additively manufactured AISI 304 stainless steel. J. Alloys Compd. 2019, 803, 364–370. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Yao, J.; Man, C.; Li, X. Mechanical properties and corrosion behavior of selective laser melted 316L stainless steel after different heat treatment processes. J. Mater. Sci. Technol. 2019, 35, 1499–1507. [Google Scholar]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Luo, H.; Li, R.; Li, X. The passivity of selective laser melted 316L stainless steel. Appl. Surf. Sci. 2020, 504, 144495. [Google Scholar] [CrossRef]
- Li, B.; Wang, T.; Li, P.; Wang, S.; Wang, L. Selective laser melting of 316L stainless steel: Influence of Co-Cr-Mo-W addition on corrosion resistance. Metals 2021, 11, 597. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, Y.; Zhang, Y.; Wang, X.; Xue, J.M.; Wang, H. Influence of scanning strategy and building direction on microstructure and corrosion behaviour of selective laser melted 316L stainless steel. Mater. Des. 2021, 209, 109999. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Zhao, Z.; Li, X.; Liu, B.; Bai, P. Electrochemical noise comparative study of pitting corrosion of 316L stainless steel fabricated by selective laser melting and wrought. J. Electroanal. Chem. 2021, 894, 115351. [Google Scholar] [CrossRef]
- Hu, H.; Wen, S.; Duan, L.; Wang, C.; Chen, K.; Wei, Q.; Shi, Y. Enhanced corrosion behavior of selective laser melting S136 mould steel reinforced with nano-TiB2. Opt. Laser Technol. 2019, 119, 105588. [Google Scholar] [CrossRef]
- Dikova, T.; Panova, N. Corrosion failure in biological fluids of laser melted surface of AISI 321 stainless steel. Procedia Struct. Integr. 2025, 68, 99–105. [Google Scholar] [CrossRef]
- Dikova, T.D.; Panova, N.K.; Parushev, I.D. Investigation of the microstructure of AISI 321 stainless steel after laser surface melting. J. Chem. Technol. Metall. 2024, 59, 207–214. [Google Scholar] [CrossRef]
- ASTM E2627; Standard Practice for Determining Average Grain Size Using Electron Backscatter Diffraction (EBSD) in Fully Recrystallized Polycrystalline Materials. ASTM International: West Conshohocken, PA, USA, 2019.
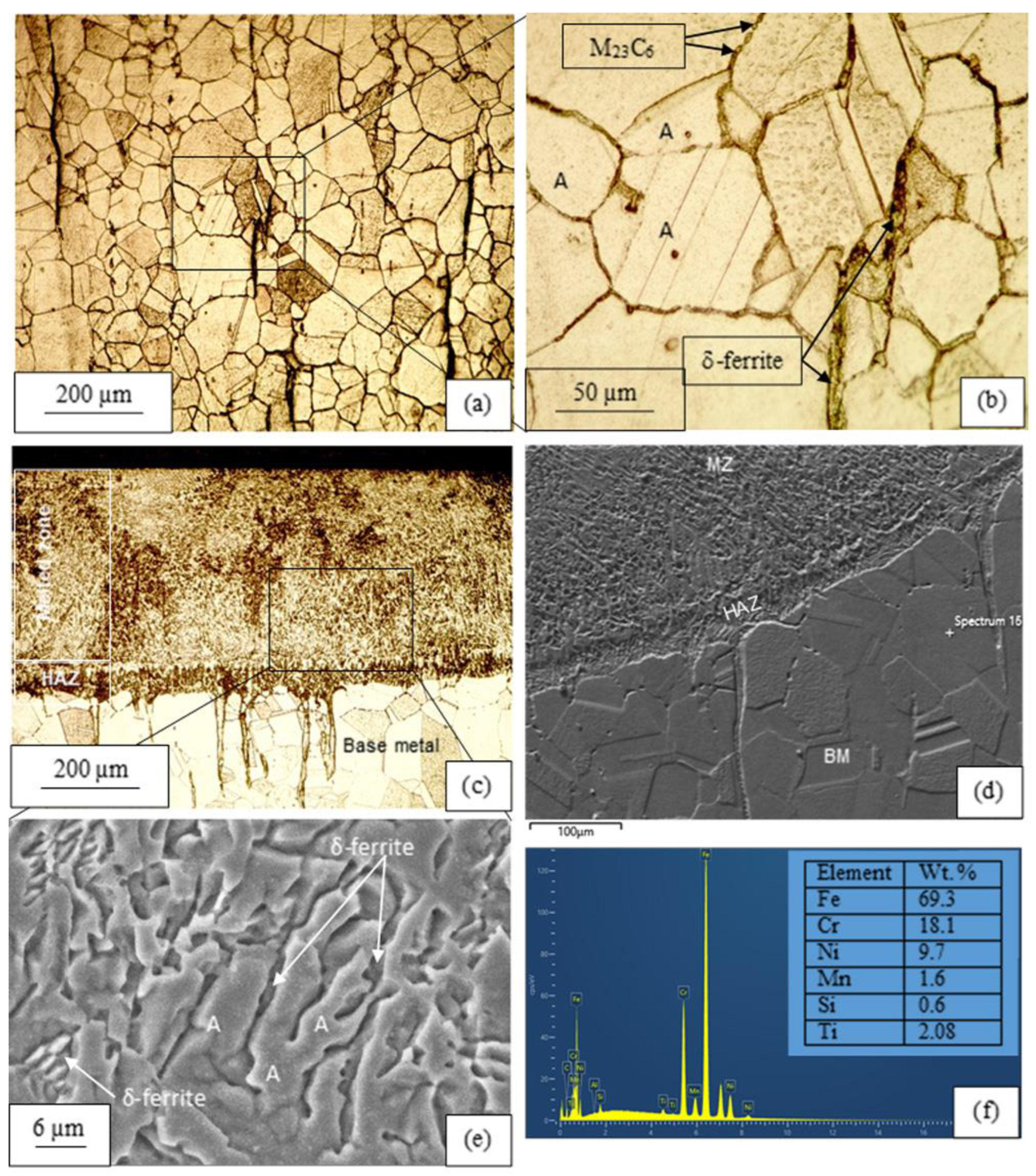
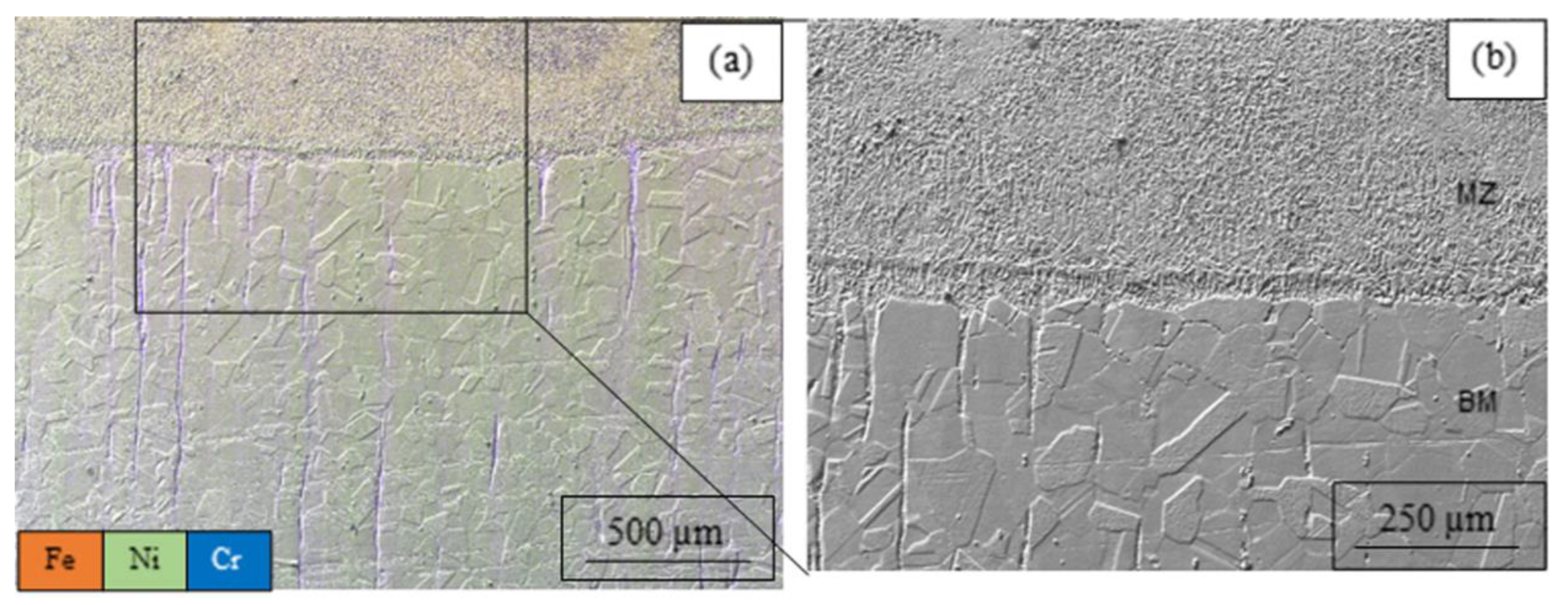


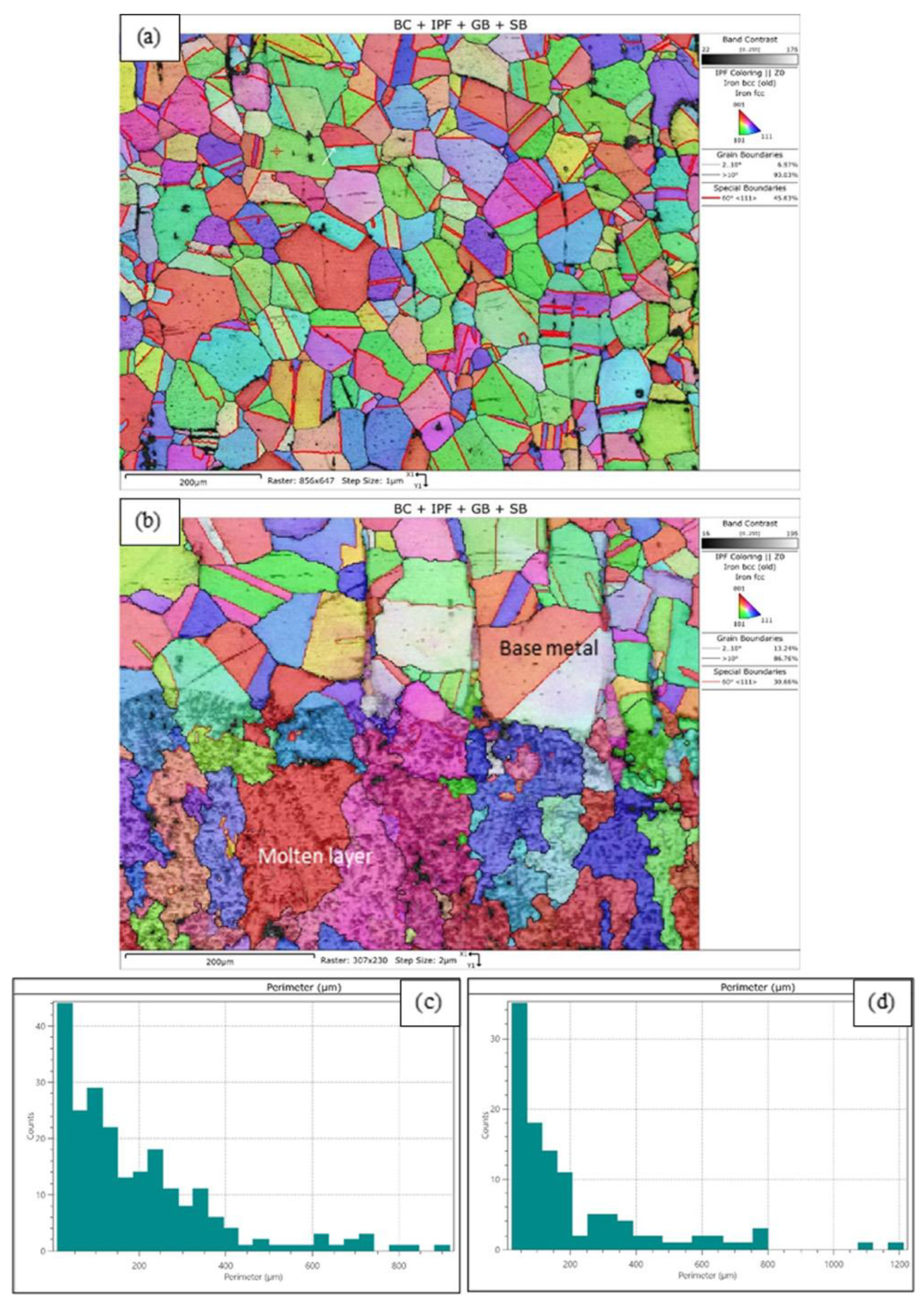
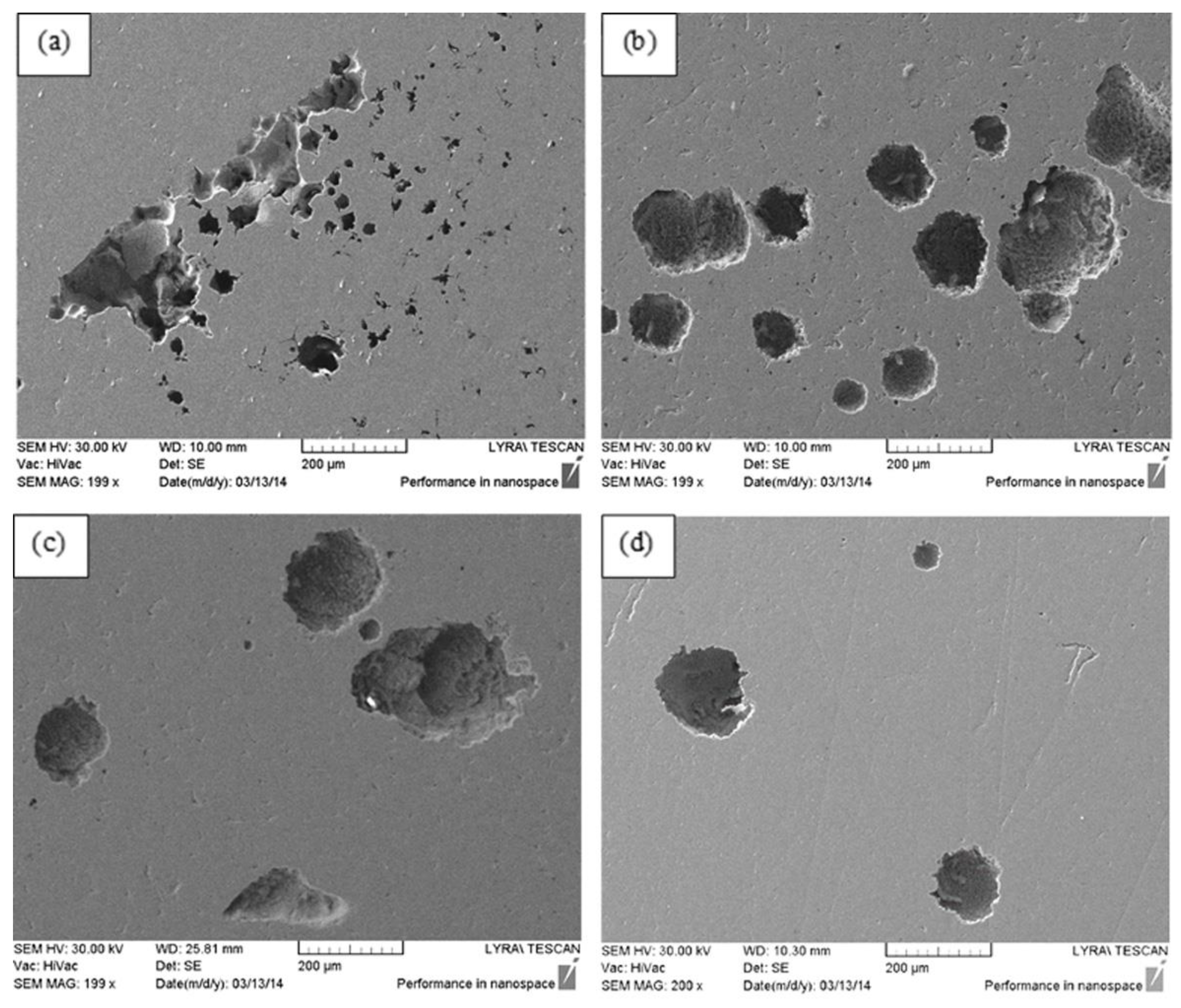

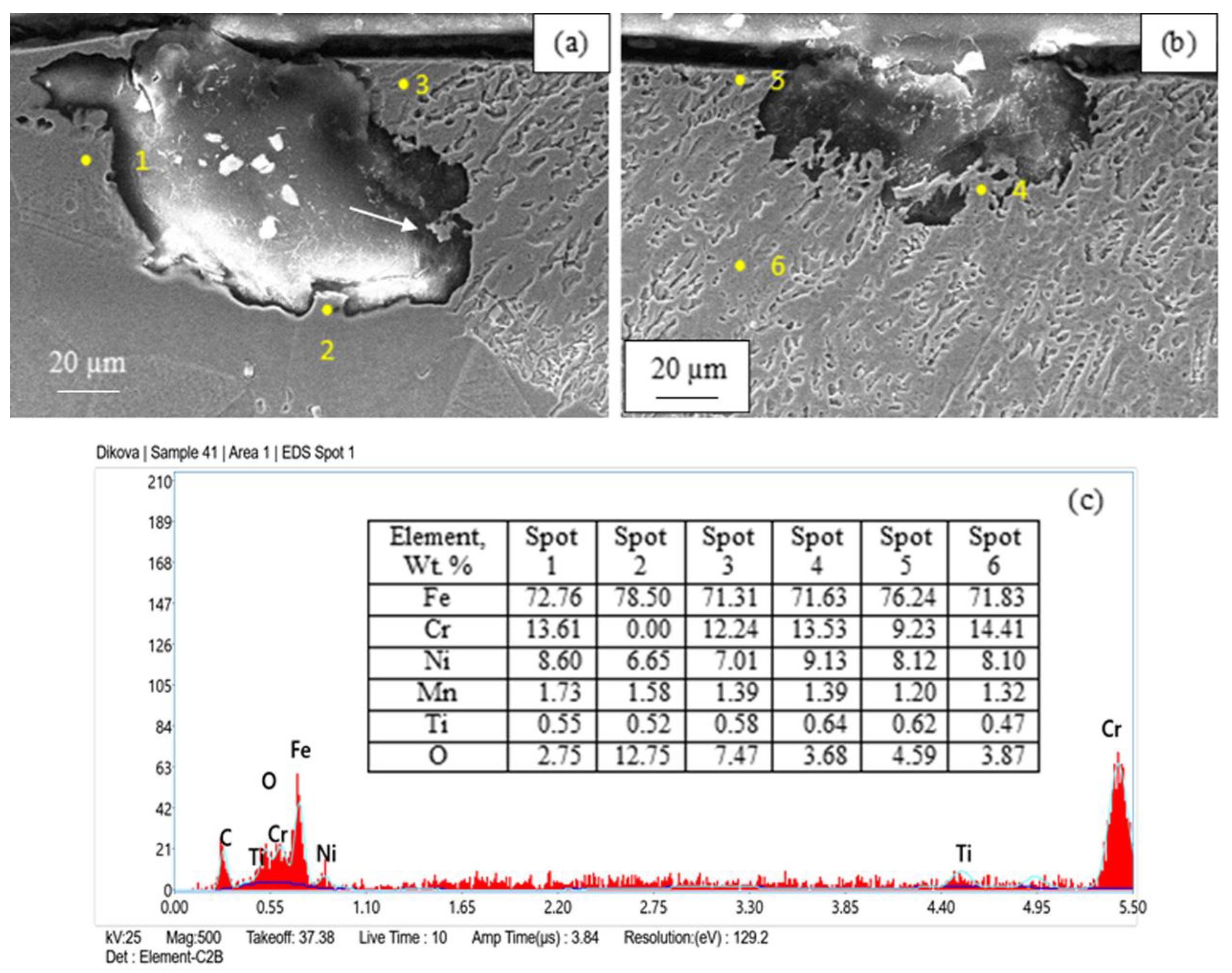

| Sample’s Number | Regime Parameters in Treatment by CW CO2 Laser | Surface Finish | |||
|---|---|---|---|---|---|
| d (cm) | V (cm/s) | Ns × 103 (W/cm2) | Ev × 103 (J/cm3) | ||
| 0 | - | - | - | - | ground |
| 1 | 0.4 | 0.3 | 9.5 | 31.7 | ground |
| 4 | 0.3 | 0.5 | 17.0 | 34.0 | ground |
| 6 | 0.3 | 0.6 | 17.0 | 28.3 | polished |
| No. | Investigated Area | Grain Count | Area-Weight, µm | Grain Perimeter, µm | Grain Size, ASTM 2627 [32] | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Min. | Max. | St. Dev. | ||||
| 1. | Base metal | 233 | 423.22 | 190.33 | 11.05 | 881.99 | 174.51 | 5.7 |
| 2. | Transitional zone | 111 | 545.73 | 208.99 | 23.21 | 1165.32 | 227.02 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikova, T.; Panova, N. Destruction Mechanism of Laser Melted Layers of AISI 321 Austenitic Stainless Steel After Electrochemical Corrosion in Ringer’s Solution. Processes 2025, 13, 3116. https://doi.org/10.3390/pr13103116
Dikova T, Panova N. Destruction Mechanism of Laser Melted Layers of AISI 321 Austenitic Stainless Steel After Electrochemical Corrosion in Ringer’s Solution. Processes. 2025; 13(10):3116. https://doi.org/10.3390/pr13103116
Chicago/Turabian StyleDikova, Tsanka, and Natalina Panova. 2025. "Destruction Mechanism of Laser Melted Layers of AISI 321 Austenitic Stainless Steel After Electrochemical Corrosion in Ringer’s Solution" Processes 13, no. 10: 3116. https://doi.org/10.3390/pr13103116
APA StyleDikova, T., & Panova, N. (2025). Destruction Mechanism of Laser Melted Layers of AISI 321 Austenitic Stainless Steel After Electrochemical Corrosion in Ringer’s Solution. Processes, 13(10), 3116. https://doi.org/10.3390/pr13103116







