3.1. Elastomers
The primary features of the poly(butadiene-
co-myrcene) copolymers are summarized in
Table 5. The synthesized polybutadienes exhibit a remarkably high
cis-1,4 microstructure exceeding 96%, along with elevated molecular weights conducive to elastomeric behavior. Sample B-1, a high molecular weight polybutadiene, was selected for ABS synthesis, whereas B-2 was chosen for vulcanized rubber formulations due to its comparable characteristics.
The copolymers containing terpenes, namely β-myrcene and trans-β-farnesene, retain a high 1,4-cis content exceeding 90%, a critical factor to achieve elastomeric properties similar to or approaching pure polybutadiene. Molecular weights vary between 397,000 and 735,000 g/mol, indicating successful control of polymerization conditions. The weight fraction of terpene monomers incorporated ranges from 8.6 to 29.2% for β-myrcene and from 16.8 to 45.2% for trans-β-farnesene copolymers, achieving significant renewable content while maintaining butadiene dominance for performance balance.
Maintaining a sufficiently high butadiene proportion is essential to ensuring optimal mechanical and processing properties in the resulting materials.
3.2. ABS Morphology and Performance
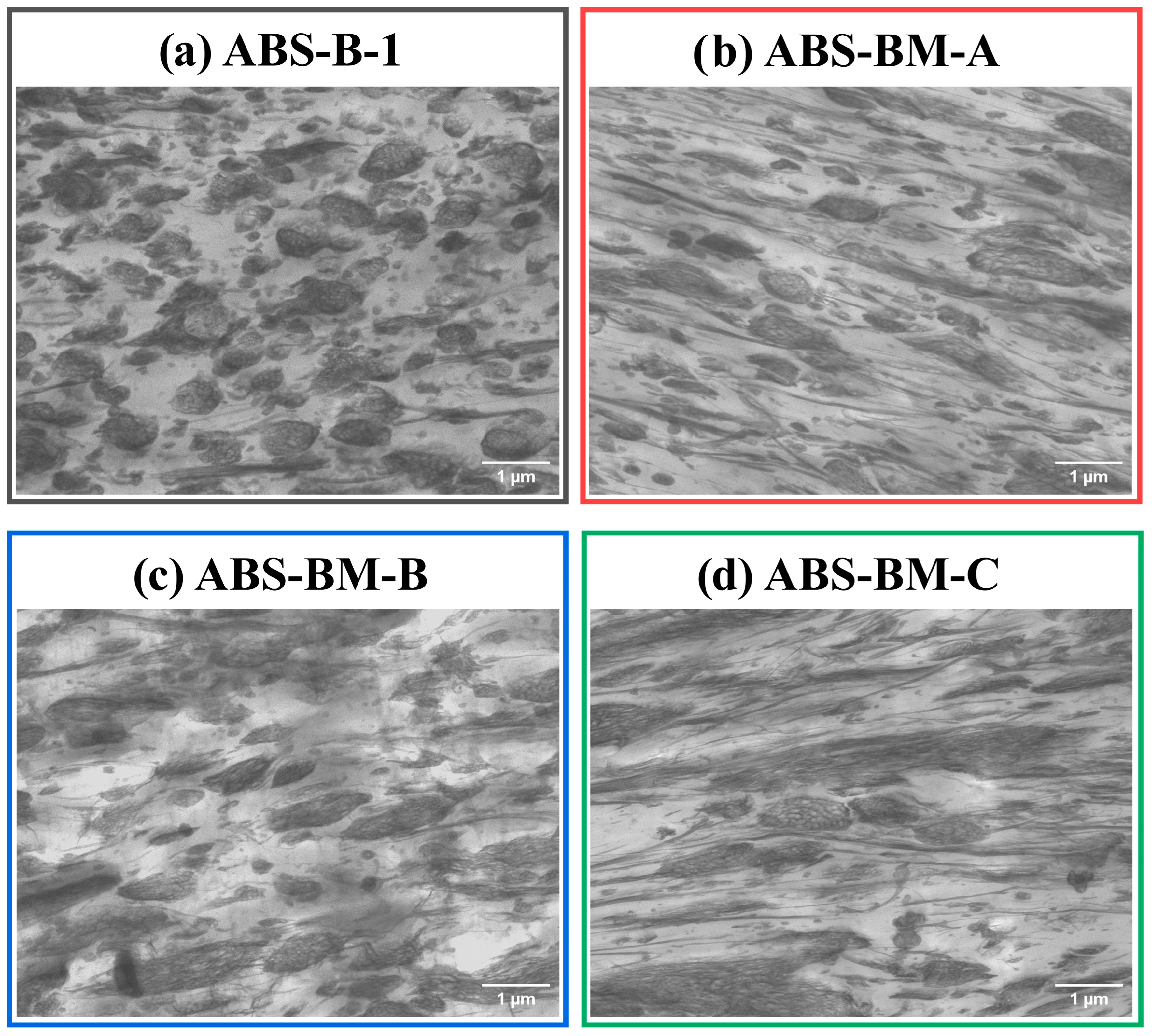
Figure 1 presents STEM micrographs illustrating the morphologies of ABS materials prepared with high-
cis PB (B-1) and poly(butadiene-
co-myrcene) copolymers. The dark regions correspond to the rubbery phase and PSAN-grafted rubber, whereas the light zones represent the continuous PSAN matrix and occluded PSAN fractions.
ABS formulated with high-
cis PB predominantly exhibits a classic salami-like morphology characterized by spherical rubber particles with embedded occlusions. In contrast, the ABS containing poly(butadiene-
co-myrcene) copolymers features a combination of salami-like particles alongside elongated and rod-shaped occlusions. This morphological evolution intensifies progressively with increasing myrcene content, reflecting the influence of terpene composition on phase development. The increased terpene fraction introduces a higher density of pendant double bonds along the polymer backbone, which promotes more extensive grafting reactions with the PSAN matrix. This enhanced grafting improves interfacial adhesion between the rubber phase and the matrix, stabilizing non-spherical morphologies such as elongated and rod-like particles. Consequently, both the particle diameter (D
p) and the rubber volume fraction (Φ
rubber) increase from 956 to 1187 nm and from 0.49 to 0.58, respectively, as detailed in
Table 6.
These morphological changes correlate with significant improvements in impact resistance and ductility, indicating that the altered topographies facilitate more effective energy absorption and stress dissipation [
27].
The increase in elongated morphologies further suggests that higher stirring speeds are necessary during phase inversion to achieve adequate particle dispersion due to the enhanced rubber–matrix interactions and viscosity changes imparted by the terpene segments. Consequently, optimal stirring speeds and reaction conditions must be carefully tuned to preserve desired morphology and maximize impact properties.
While the improved toughness of the ABS is mainly attributed to morphological changes and grafting, it is important to clarify the compatibilization mechanism. The terpene-derived bioelastomers maintain a high 1,4-cis polybutadiene microstructure, providing essential elastomeric properties. Interfacial compatibility in these systems arises from grafting reactions during processing, where unsaturated sites (e.g., pendent double bonds from terpene units) on the rubber phase chemically bond with the styrene-acrylonitrile (SAN) matrix. This grafting enhances interfacial adhesion, stabilizes the phase morphology, and improves dispersion, effectively functioning as an intrinsic compatibilization strategy.
Analyzing the type of morphology obtained from the copolymers, it shows a similarity to particles with occlusions, which is consistent with previous reports on ABS materials produced via bulk processes and using high-
cis polybutadiene. However, the elongated particles are a novel feature of this material. When combined with the occluded particles, the copolymers demonstrate superior impact resistance compared to materials reported in the literature [
5,
28].
Table 6 additionally details the properties of the continuous and dispersed phases. The continuous PSAN phase exhibits consistent molecular weights (236,000–250,000 g/mol) and dispersities (~2.3–2.6) regardless of rubber type, indicating that the bio-based copolymer does not significantly alter the matrix polymerization.
Regarding the rubber phase, gel content remains relatively constant across samples, whereas the swelling index exhibits a modest increase from 6.7 to 8.3 with higher myrcene incorporation. This increase likely reflects the presence of branched terpene units, which increase free volume and chain mobility in the rubber domains, leading to enhanced swelling capability.
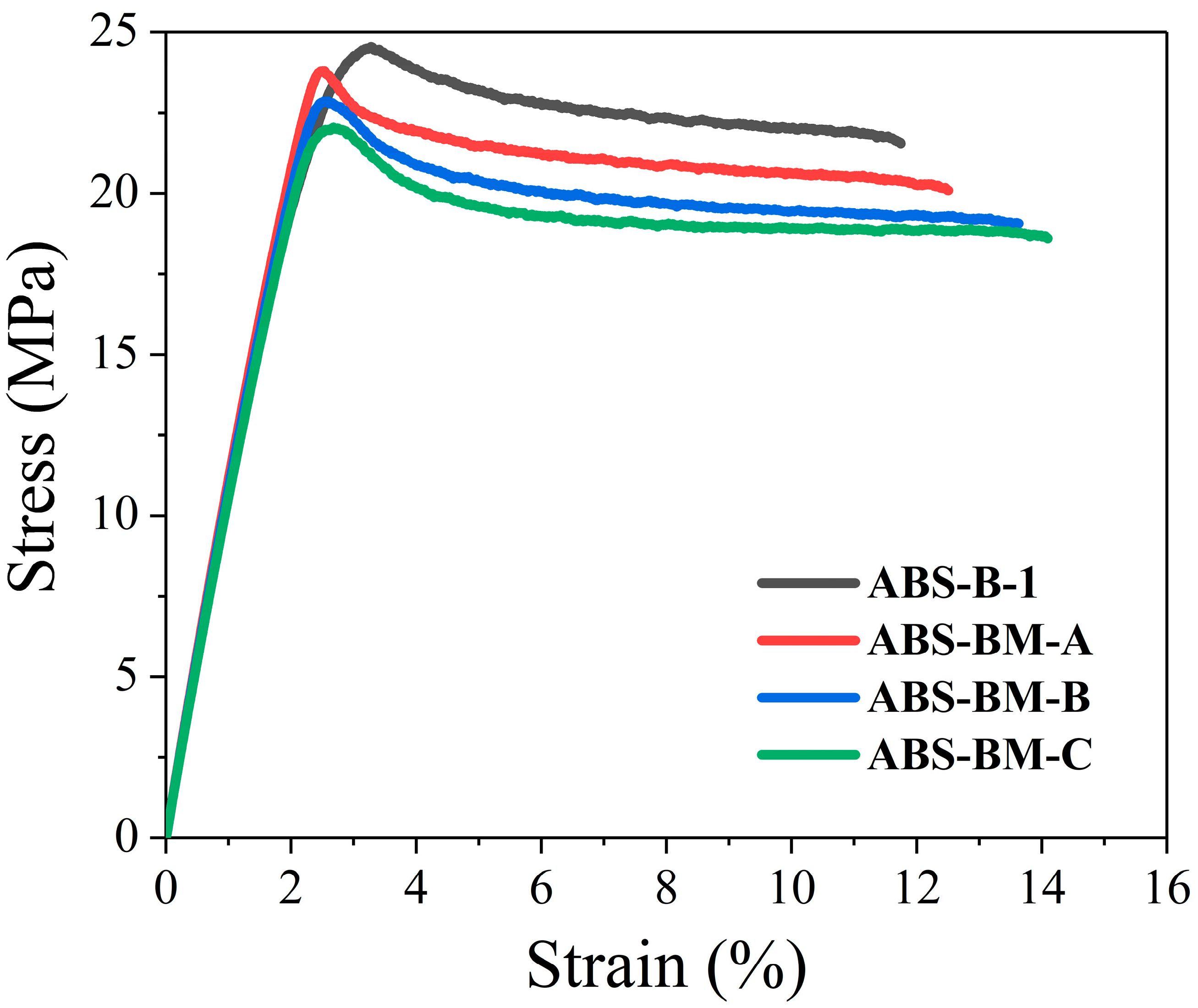
The mechanical behavior of the synthesized ABS materials is depicted in
Figure 2, and the quantitative results from tensile and impact testing along with melt flow index (MFI) values are summarized in
Table 7. The control ABS-B-1, based on high-
cis polybutadiene (PB), exhibits higher yield strength (24.45 MPa), ultimate tensile strength (21.58 MPa), and Young’s modulus (1202 MPa), while showing lower elongation at break (11.72%) compared to ABS samples containing poly(butadiene-
co-myrcene) copolymers.
A distinct trend is observed where increasing myrcene content in the copolymer correlates with a moderate decrease in yield and tensile strengths (from 23.78 to 22.22 MPa and from 19.26 to 18.62 MPa, respectively) but a corresponding increase from 12.66 to 14.09% in elongation at break, indicating enhanced ductility. This behavior is consistent with the modified rubber morphology and the increased rubber volume fraction (from 0.49 to 0.58), which effectively plasticize the PSAN matrix and promote greater deformability by facilitating stress transfer and energy dissipation during mechanical loading.
Impact resistance, a critical parameter for rubber-reinforced styrenic polymers, significantly improves with higher myrcene incorporation, surpassing 580 J/m at 29.2 wt% myrcene. This property depends mainly on the characteristics of the rubber phase, since it is responsible the absorption and dissipation of energy when a mechanical impact is applied [
29]. Therefore, the reported dramatic enhancement reflects the beneficial effects of the altered morphology—especially the presence of elongated and rod-like rubber particles—and increased rubber phase volume fraction [
30].
The swelling index (see
Table 6) suggests that crosslink density within the rubber phase slightly diminishes as myrcene content increases, leading to more flexible rubber domains. This balance between adequate crosslinking to maintain particle integrity and flexibility to facilitate energy dissipation is crucial for optimizing impact toughness. Over-crosslinked rigid particles would reduce toughness, whereas too little crosslinking may compromise reinforcement.
Melt flow indices remain within a narrow range (3.3–4.8 g/10 min), confirming effective removal of residual monomers and good processability for injection molding applications.
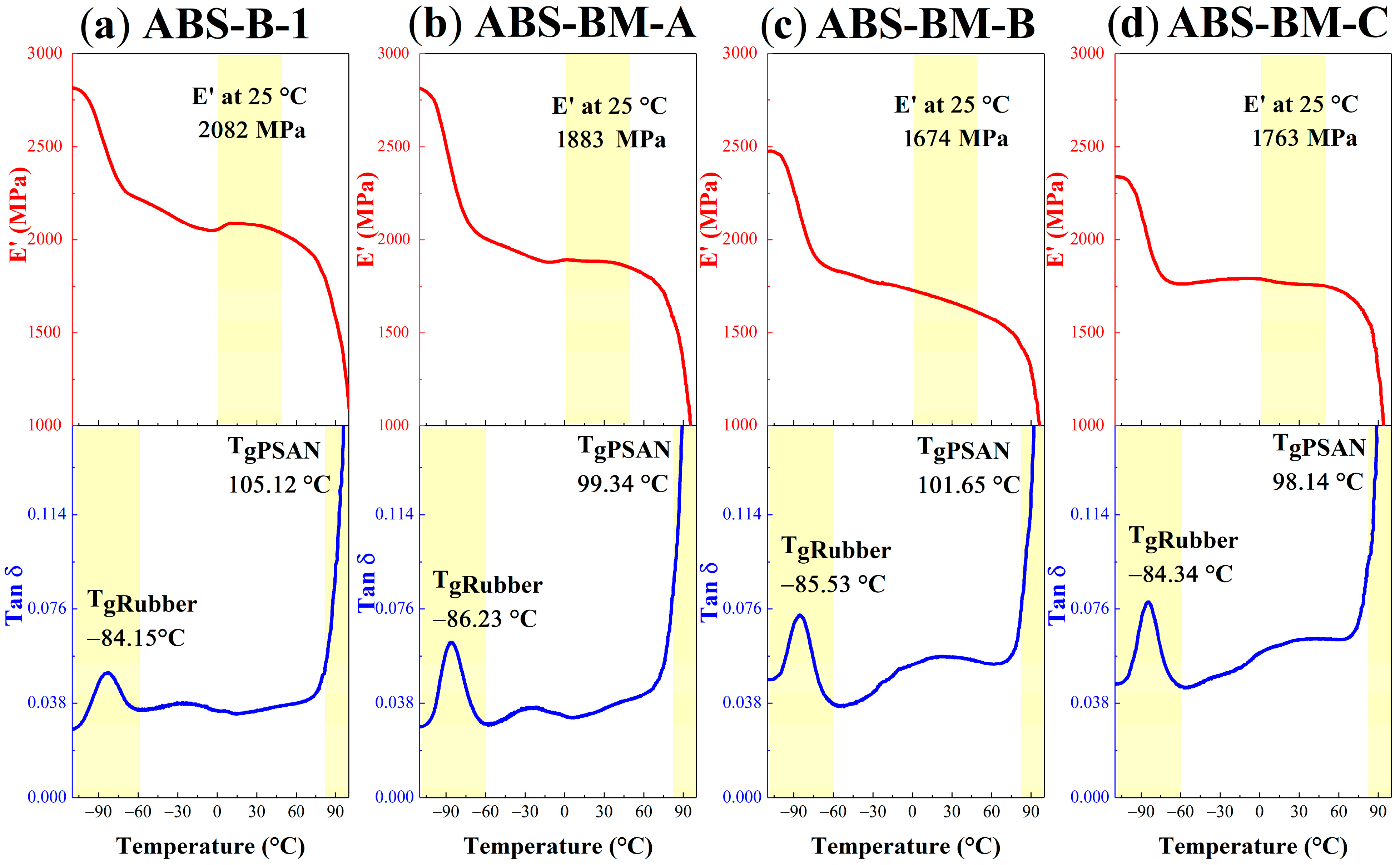
The dynamic-mechanical properties of the synthetized ABS are shown in
Figure 3. The behavior of the materials is similar; at low temperatures between −90 and −83 °C, there is a significant relaxation (maximum peak temperature in Tan δ) associated with the glass transition temperature (T
g) of the rubber phase. The occurrence of this relaxation results in a decrease in the storage modulus (E′). As the temperature increases, a secondary broad-range relaxation occurs at elevated temperatures (89–105 °C). This transition is associated with the T
g of the continuous PSAN phase.
ABS samples containing poly(butadiene-
co-myrcene) copolymers show a reduced storage modulus (E′) across the measured temperature range relative to the control ABS-B-1, consistent with the lowered stiffness from tensile testing results. The increased area under the rubber-phase T
g peak in the Tan δ curves corresponds to the elevated rubber volume fraction and suggests a higher fraction of rubber chains actively participating in relaxation processes [
31].
These observations confirm that the bio-based copolymers contribute to a more elastomeric and dynamically responsive rubber phase in ABS, enhancing energy absorption capabilities during mechanical deformation.
3.3. Rubbers
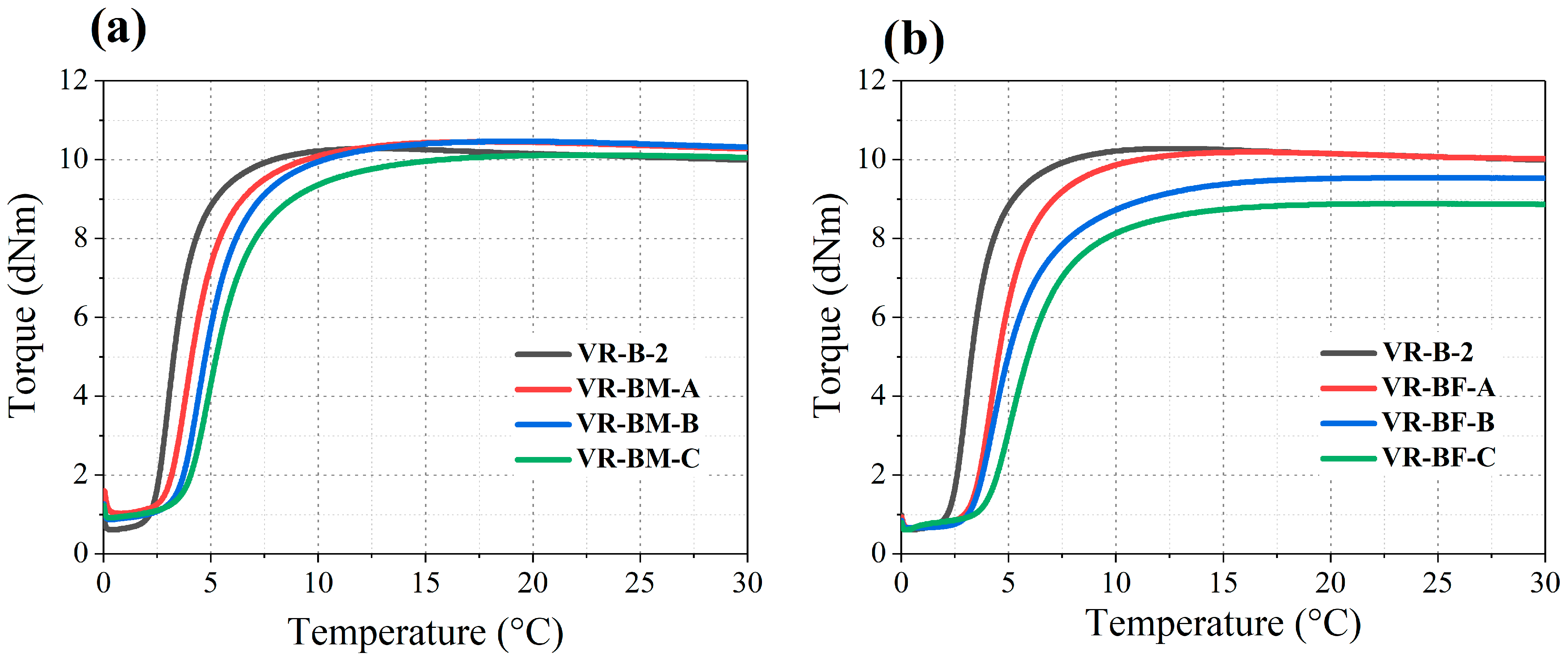
Concerning the copolymers used for vulcanization, curing tests were performed by monitoring the torque evolution at constant deformation under isothermal conditions at 145 °C. The resulting vulcanization curves are presented in
Figure 4, with summarized kinetic parameters listed in
Table 8.
The materials evaluated exhibited low minimum torque (ML) values, indicating no significant crosslinking prior to the initiation of vulcanization, which ensures proper handling and mixing stability. The scorch time (ts2), defined as the time before vulcanization onset, increases as the terpene content in the copolymer increases. This behavior can be explained by the steric and configurational influence of the double bonds in the pendant terpene units, which modulate the reactivity compared to pure polybutadiene chains.
Subsequent to the scorch time, the curing curves demonstrate an increment in torque, as the material’s stiffness rises proportionately with the density of elastically active crosslinks between the rubber chains [
32]. The maximum measured torque (M
H) is associated with the total contribution of elastically active bonds generated during the vulcanization process [
33]. Comparing the different systems studied, copolymers based on β-myrcene have M
H values similar to those obtained by the high-
cis PB (VR-B-2). However, in the case of copolymers containing
trans-β-farnesene above 10 wt%, there is a notable decrease in M
H. This is associated with a lower efficiency in consolidating crosslinks by this monomer. This reduction is likely due to steric hindrance or reduced accessibility of functional groups for crosslink formation, impairing network consolidation.
The delta torque (MH – ML) further reflects the extent of bond formation during vulcanization, including the contribution from double bonds in the copolymers. Despite the terpene units introducing more double bonds that could facilitate vulcanization, the overall crosslink density remains governed by polymer architecture and steric effects rather than double bond abundance alone. This is supported by the decreasing delta torque observed for trans-β-farnesene-containing rubbers with increasing terpene content.
Additionally, the curing rate index (CRI), representing vulcanization speed, declines with rising terpene incorporation, confirming that the presence of these bio-based monomers hinders crosslinking reactions, requiring longer times to reach full cure.
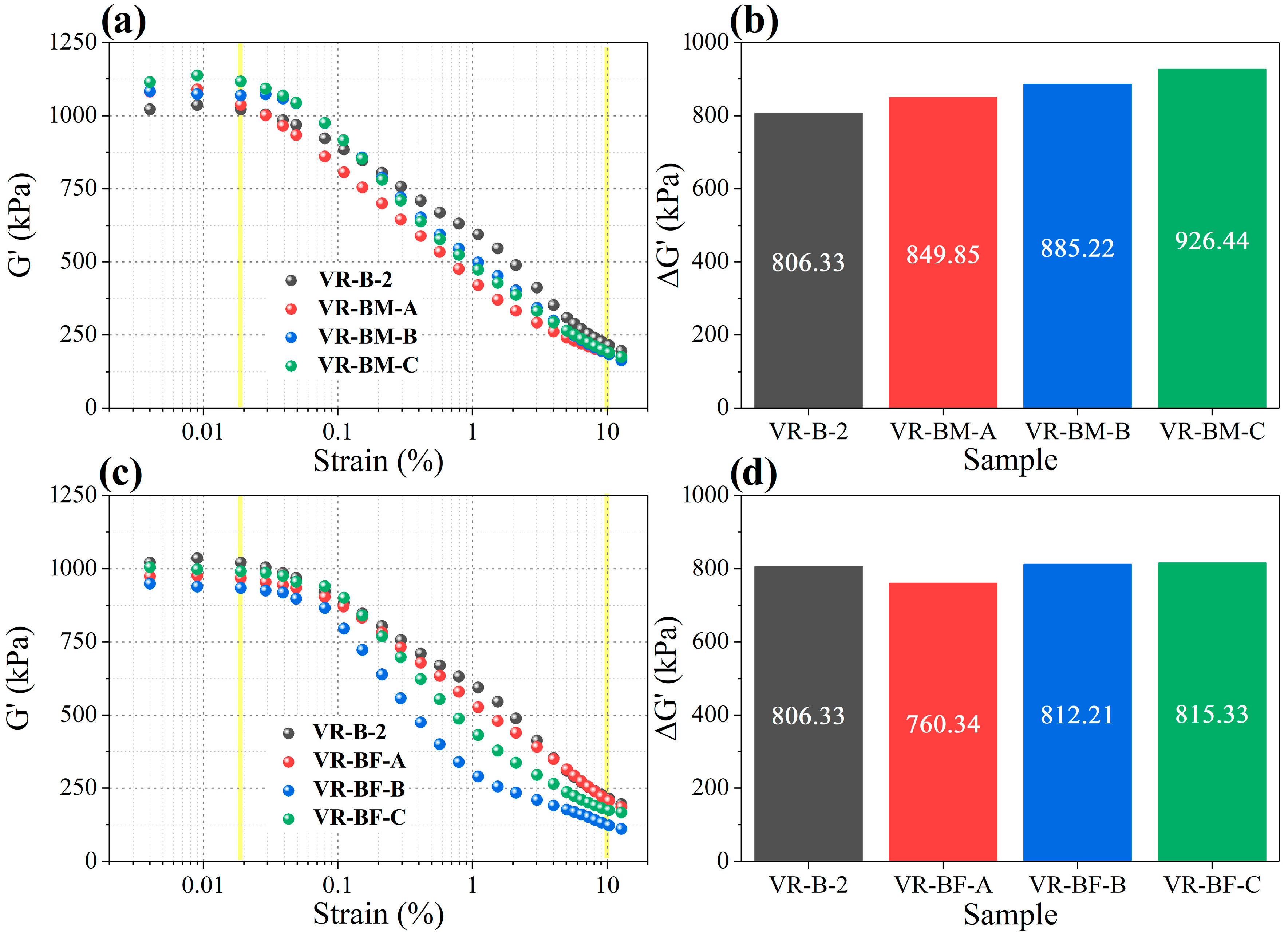
The Payne effect, which indicates filler–filler interactions and dispersion in the polymer matrix, was analyzed as shown in
Figure 5 along with corresponding ∆G′ values. Vulcanized rubbers containing β-myrcene exhibit higher ∆G′ (926.44 kPa) than the control VR-B-2 (806.33 kPa), implying stronger filler–filler networking and thus less homogeneous filler dispersion. This trend intensifies with increasing β-myrcene content, suggesting that the lower crosslink density and altered polymer–filler interactions promote carbon black aggregation.
Conversely, rubbers with trans-β-farnesene maintain ∆G′ values close to the control (between 806 and 815 kPa), indicating better filler dispersion. This effect is attributed to the larger size and flexible nature of the farnesene side groups, which inhibit filler–filler interactions and promote improved polymer chain mobility, reducing filler agglomeration.
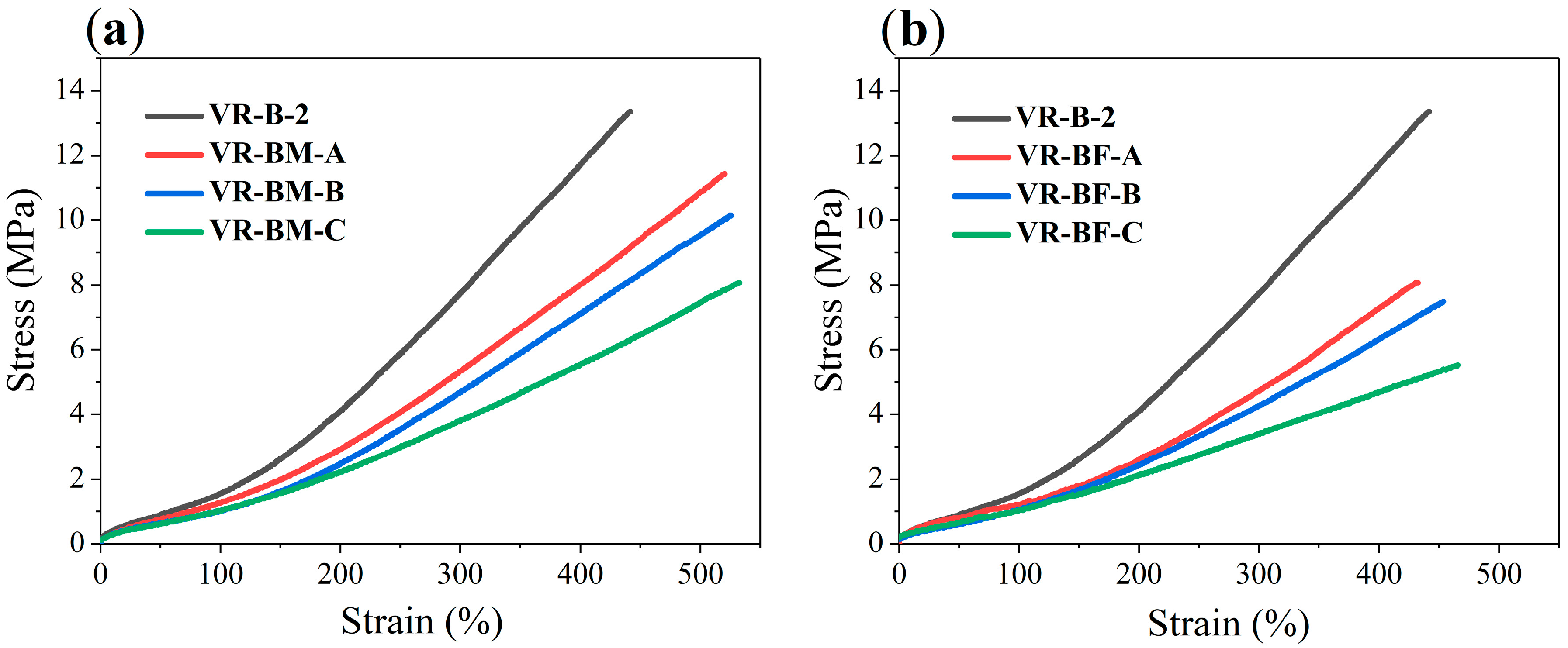
Stress–strain behavior of vulcanized compounds is depicted in
Figure 6, with mechanical parameters detailed in
Table 9. β-myrcene copolymers show reduced tensile strength (<12 MPa) and modulus (8.03–1.26) relative to the high-
cis polybutadiene (VR-B-2) but exhibit significantly increased elongation at break (up to ~530%). This enhanced extensibility correlates with decreased crosslink density and more flexible network structures, as supported by vulcanization data and swelling tests.
In contrast, trans-β-farnesene-based copolymers demonstrate further lowered stress (<8.5 MPa) and modulus values (7.29–1.20 MPa) and moderate elongations (~400–465%). This confirms the notion of softer elastomeric behavior with lower crosslink density and suggests the need to optimize vulcanization formulations to improve polymer–polymer and filler–polymer interactions for enhanced mechanical properties.
The Shore A hardness values (
Figure 7) decrease with terpene content, dropping from 51 in controls to approximately 47 and 49 for β-myrcene and farnesene copolymers, respectively, reflecting the softer nature of these materials due to their reduced crosslink densities (<1.20 × 10
−4 mol/mL).
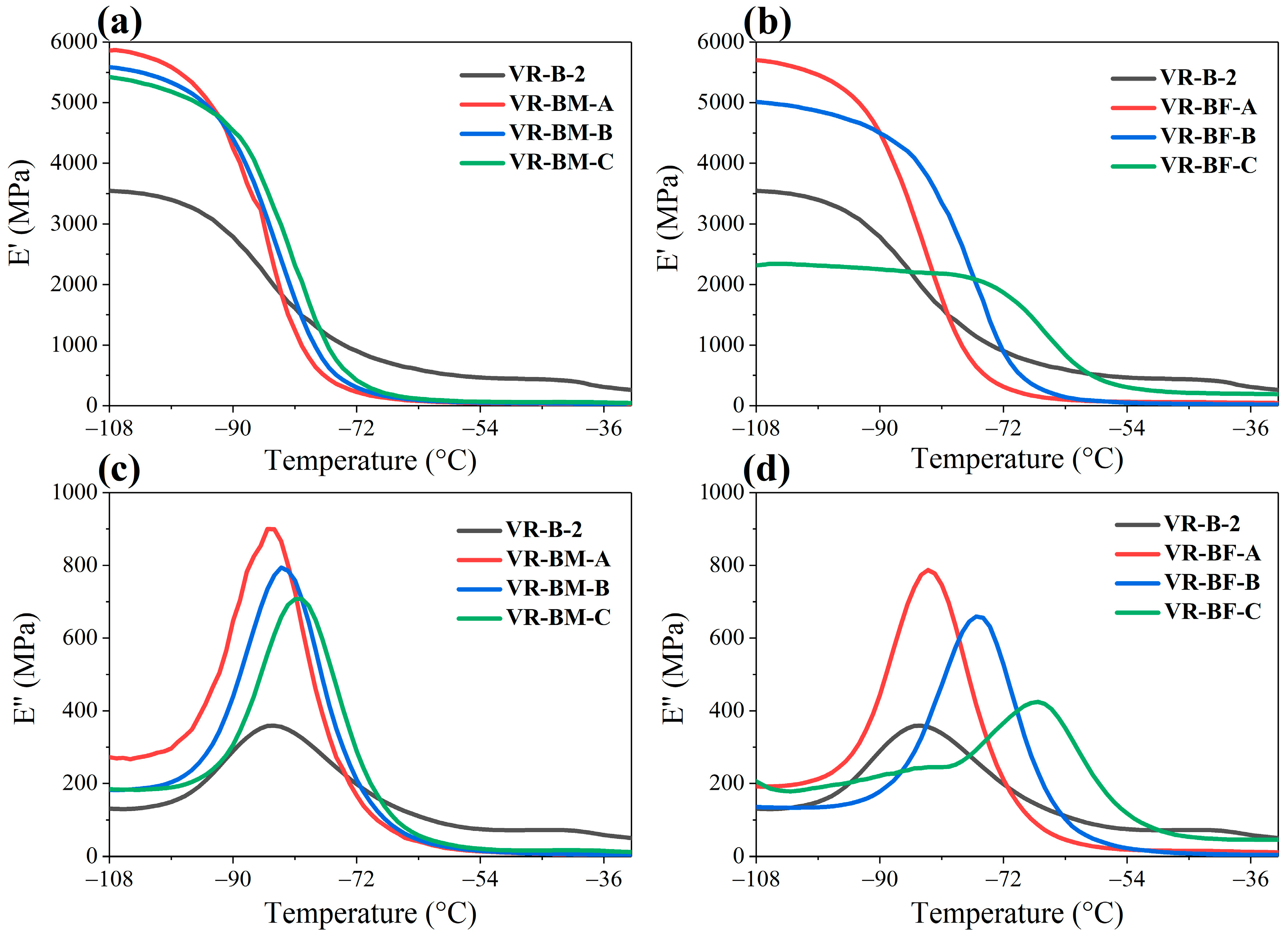
The dynamic-mechanical behavior of the vulcanized compounds as a function of temperature is shown in
Figure 8. As temperature increases, the storage modulus (E′) decreases in all samples, reflecting the increased flexibility of the vulcanized materials at elevated temperatures. This phenomenon is related to the T
g of the vulcanized compounds, where the material changes from a glassy (rigid) state to an elastic or viscous state.
In the loss modulus (E″), the magnitude of the peak progressively increases with temperature until reaching a maximum at Tg, beyond which it declines. This loss peak signifies the material’s maximum energy dissipation capability during segmental chain motion. At temperatures below the Tg, all vulcanized compounds exhibited relatively low loss modulus values, indicating that energy dissipation is lower in the glassy state. However, when relaxation occurs, copolymers exhibit greater energy dissipation, which is attributed to decreased mobility restriction given by a lower crosslinking density. Tg of the copolymer increases with increasing terpene content. Notably, when comparing copolymers with equivalent terpene concentration (~30 wt%) of β-myrcene and trans-β-farnesene, the farnesene-containing system displays a Tg shift toward higher temperatures. This shift is consistent with greater molecular restriction caused by the larger side group size of farnesene.