Abstract
Macroalgae-derived polyphenols have been considered as a potential source of food supplements that can enhance the nutritional value and extend the shelf life of foods. However, thermal treatment during food processing as well as storage might induce the degradation of some bioactive compounds in the extract. In the present study, the stability of the extract from the edible brown algae Ascophyllum nodosum was evaluated under thermal treatment (40–90 °C). Significant differences in TPC, RSC, and antioxidant activity were found during all treatments. The total phenol content (TPC) and antioxidant activity (DPPH scavenging activity) decreased up to 5% and 10%, respectively, after 6 h of thermal treatment, while the reducing sugar content (RSC) increased from 8 to 35% as the temperature increased from 40 to 90 °C. The stability of the extract during storage with or without exposure to air was evaluated at room temperature (25 °C) and low temperature (4 °C) for 108 h, and the influence of the solvent used to contain the extract has been investigated by studying both concentrated and non-concentrated extracts. It was found that the extract stored at 4 °C without exposure to air had a negligible TPC change, while RSC increased in the extract exposed to air, suggesting that oxygen in the air might accelerate polysaccharide degradation during storage. Antioxidant activity of extracts remained constant at both 4 and 25 °C, regardless of exposure to air.
1. Introduction
Macroalgae, also known as seaweed, have been widely consumed as a food since ancient times in Asia, being less consumed in Europe and America [1]. They are often used for extraction of phytocolloids such as agar and carrageenan, which are used as thickening agents in the food industry [2]. Edible algae are good sources of protein, dietary fiber, and bioactive compounds, e.g., polysaccharides, polyphenols, fatty acids, peptides, and vitamins [3]. Ascophyllum nodosum belongs to the class Phaeophyceae and mainly grows in the rocky intertidal zones of the North Atlantic Ocean. According to the authors’ previous research [4], the antioxidant activity of A. nodosum extract obtained at optimized extraction procedure was 74.01 mL/mg (DPPH scavenging activity) with a yield of 53.80 mg extract/g algae, which made it a promising source of natural antioxidants that deserves further development. The extract of A. nodosum has a wide use in food and agricultural applications. For example, the addition of the extract could effectively inhibit the oxidation of canola oil, and its antioxidant effect was significantly higher than that of butylated hydroxytoluene (BHT) [2]. The extract of A. nodosum has also been utilized as a biostimulant to enhance lettuce growth under potassium deficiency [5]. Research has also been performed on the pharmaceutical activities of A. nodosum extract, such as its anti-hyperglycemic [6], anti-inflammatory, anti-senescence [7], antidiabetic, antithrombotic [1], and anticancer [8] activities. Based on the authors’ previous research, the extract showed a strong antibacterial effect against fish spoilage bacteria and postponed the spoilage of cold-storage tilapia [9]. Because of its various bioactivities, bioactive compounds from A. nodosum have become a potential source of natural antioxidants, antimicrobial agents, and preservatives that can be incorporated into foods, nutraceuticals, and other health-benefiting products [1,8].
Thermal processing is an important processing technique that can ensure the safety of foods and extend their shelf life [10]. However, thermal processes induce undesired chemical reactions such as the Maillard reaction, oxidation of polyphenol, and degradation of polysaccharides, especially in plant-based foods or food incorporated with natural antioxidants originated from plants [10]. Therefore, evaluating the thermal stability of natural antioxidants and preservatives is crucial to ensure they withstand processing temperatures and retain their bioactivity. Among all components in plant extracts, polyphenols suffer from poorer stability than other active compounds [10,11,12] towards high temperature, depending on the phenolic compound content, pH, temperature, light intensity, and oxygen [13]. In addition, the solvent used to dissolve the extract might also affect the bioactivity of the extract by altering its oxygen content. Polyphenol contents are usually adversely affected by thermal process, while the antioxidant activity of plant food and extract is mainly related to their polyphenol content [14,15]. Research has been performed to investigate the influence of thermal processing on physicochemical properties such as antioxidant activity, total phenol content, pH, and viscosity of extracts from Mengkudu [16], elderberry [13], Aloe vera [17], and grape marc [18] and found that thermal treatment had a negative effect on the phenolic content and antioxidant activity on these extracts. However, the influence of thermal processing on algae extract, especially A. nodosum extract, has not been fully studied. Further investigation is needed to explore the thermal stability of this extract as a potential antioxidant and to evaluate its bioactivity after thermal treatment, thereby supporting its potential application in the food industry.
As consumers’ demand for less processed and mildly preserved foods has increased, novel food preservation techniques such as edible coating have drawn great attention due to their safe and biodegradable nature [19]. Incorporation of algae extracts into edible coating as a bioactive preservative could enhance the preservative functions of the coating and prolong the shelf life of foods during storage [20]. However, the phytochemical properties of polyphenols could also be diminished during storage because the unsaturated bonds and hydroxyl groups in the phenolic compounds can be oxidized [21]. For instance, introduction of anthocyanins into foods has been considered difficult due to their low stability during storage [22]. Yin et al. [23] reported that tea polyphenols were sensitive to storage temperature, light, pH, and oxygen, as these factors could accelerate the degradation of polyphenols during long-term storage. The total phenol content and antioxidant activity of phenolic extracts obtained from grape pomace showed a fluctuating and declining trend during storage [21]. Based on these studies, it may be concluded that the storage temperature and oxygen are two major factors influencing the bioactivity of the phenolic extracts. However, investigations on the physicochemical properties of algae extract during storage and how storage conditions (e.g., temperature and oxygen exposure) could influence these properties are still insufficient, therefore hindering the application of algae extracts as food preservatives.
The objective of the present study was to evaluate the stability of extracts from the edible brown algae A. nodosum under the influence of thermal treatment (e.g., heating temperature and solvent used to dissolve the extract) and storage conditions including storage temperature and exposure to air. The total phenol content (TPC), reducing sugar content (RSC), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the extract were studied.
2. Materials and Methods
2.1. Brown Algae Extract Preparation
The phylum of A. nodosum was obtained from Maine Coast Sea Vegetables (Franklin, ME, USA), washed with tap water, dried at room temperature, and ground into powder. Dried powders of A. nodosum were extracted using the method reported in the authors’ previous research [4]. Briefly, the powder was extracted at 20 °C, 70 mL/g using 80% ethanol for 30 min on a Thermo Scientific MaxQ shaker (Thermo Scientific, Waltham, MA, USA). Then the extract was filtered through No. 1 Whatman filter paper to separate supernatant from algae debris. Part of the supernatant was collected as non-concentrated extract, and the other part was concentrated under reduced pressure at 55 °C using a rotary evaporator (BUCHI Type V-850, BUCHI, Flawil, Switzerland) to remove ethanol from the sample. In this study, the term “concentrated extract” refers to the extract with the solvent removed, while the “non-concentrated extract” retains the solvent. The rationale for investigating both forms is that the concentrated extract represents the actual form used in applications where solvent removal is required, whereas the non-concentrated extract serves as a potential method for long-term storage in solvent prior to use. Both the non-concentrated and concentrated extracts were stored in sealed containers in an −20 °C refrigerator before testing.
2.2. Treatment of the Extract
Thermal treatment of the algae extract was performed based on the method of Sólyom et al. [18] and Yu et al. [24] with minor modifications. Briefly, a 1.0 mL aliquot of concentrated extract was placed in a sealed centrifuge tube to prevent evaporation. Sealed tubes were incubated in a water bath of 40, 50, 60, 70, 80, or 90 °C for 6 h. The TPC, RSC, and DPPH radical scavenging activity were analyzed every hour. The non-concentrated extract was also placed in a sealed centrifuge tube and incubated in a water bath of 50 or 60 °C for 6 h to evaluate its thermal stability. Temperatures higher than 60 °C were not considered for the non-concentrated extract because the ethanol–water solvent system applied was unstable at temperatures higher than 60 °C. After thermal treatment, the tubes were cooled immediately in an ice–water mixture to prevent further degradation.
The influence of storage conditions on extract stability was also investigated. Four groups of concentrated extracts were placed in sealed centrifuge tubes to avoid direct contact with air, stored in a 4 °C refrigerator (closed low temperature, CLT), at room temperature (closed room temperature, CRT), or placed in open tubes, stored at 4 °C (open low temperature, OLT) and at room temperature (open room temperature, ORT) for 108 h. In each test, 1 mL extract was placed in a 1.5 mL centrifuge tube, and the tubes were placed in varying conditions as previously described. In all treatments, the extracts were not dried before stability testing because the intended use of the extracts was mainly for fresh seafood storage, which usually involves high water activity. Because fresh seafoods usually have a shelf life of 3–4 days at 4 °C, the storage stability of the extract was tested for a maximum of 108 h. The volume of the groups OLT and ORT was adjusted by adding deionized water to compensate for liquid evaporation during storage. The results are expressed as At/A0, where At is the bioassay (TPC, RSC, or DPPH scavenging capacity) at treatment time t and A0 is the bioassay at the very beginning of thermal treatment.
2.3. Total Phenol Content (TPC) Test
TPC was determined using a modified version of the Folin–Ciocalteu method, using phloroglucinol (PHG) as the standard based on the authors’ previous research [25]. A 0.04 mL aliquot of the sample was mixed in a 1.5 mL centrifuge tube with 0.4 mL 1 N Folin–Ciocalteu reagent and 0.8 mL 20% Na2CO3. After standing for 3 min, the sample was incubated in the dark at room temperature for 45 min and centrifuged at 1600× g for 8 min. The absorbance of the supernatant was measured at 730 nm using a BioTek 96-well microplate reader (Winooski, VT, USA). The calibration equation obtained was Y = 0.472 × X + 0.1124 (r2 = 0.99), where X is the OD at 730 nm and Y is the concentration of phloroglucinol (mg PHG/mL).
2.4. Reducing Sugar Content (RSC) Test
RSC is positively correlated with the degradation of polysaccharide in the sample [26]. RSC was determined by the DNS (3,5-dinitrosalicylic acid) method using L-rhamnose (RHA) as the standard. The DNS reagent was prepared by mixing two solutions. Solution A was prepared by dissolving 1 g of DNS in 20 mL 2 M NaOH and solution B was made by dissolving 30 g of sodium and potassium tartrate tetrahydrate in 50 mL distilled water. The two solutions were mixed and completed to 100 mL with distilled water. The reagent was stored at 4 °C before use.
The experiment was performed following the procedure used by Garriga et al. [26]. Briefly, 0.1 mL algae extract and 0.1 mL DNS reagent were placed in a 1.5 mL tube. Then the tubes were incubated in a 100 °C bath for 5 min and cooled to room temperature. Then the volume was completed with distilled water to 1 mL. Finally, the mixture was homogenized and read at 540 nm using a BioTek 96-well microplate reader (Winooski, VT, USA). The calibration equation obtained was Y = 4.529 × X − 0.160 (r2 = 0.99), where X is the OD at 540 nm and Y is the concentration of L-rhamnose (mg RHA/mL).
2.5. DPPH Radical Scavenging Activity Test
DPPH radical scavenging activity was applied to evaluate the antioxidant activity of the extract using the method developed by the authors previously [4]. An aliquot of 0.1 mL 152 μM DPPH radical solution was added to 0.1 mL treated extract. The reaction mixtures were incubated in the dark for 30 min at room temperature, and the absorbance was measured at 517 nm using a BioTek 96-well microplate reader (Winooski, VT, USA). The DPPH test was performed in triplicate and the result was expressed as scavenging capacity (percentage radical scavenged), which was calculated with the following equations:
where Acontrol is the OD of the DPPH solution only, Asample is the OD of the DPPH solution with sample, Asampleblank is the OD value of the sample only. The DPPH radicals were purchased from Sigma-Aldrich CO. LLC. (St. Louis, MO, USA). Folin–Ciocalteu reagent, ethanol, 3,5-dinitrosalicylic acid, sodium and potassium tartrate tetrahydrate, sodium hydroxide, and L-rhamnose were purchased from Thermo Fisher Scientific (Hampton, NH, USA).
Scavenging capacity (%) = 1 − (Asample − Asampleblank)/Acontrol
2.6. Statistical Analysis
Statistical analysis was performed using one-way ANOVA and Tukey test using SAS 9.4M9 (Cary, NC, USA). A p-value of 0.05 or less was considered statistically significant. All results are expressed as the mean ± standard deviation and the standard deviations are presented as error bars in the figures. All assays were performed in triplicate.
3. Results and Discussion
3.1. Influence of Thermal Treatment on the Concentrated Extract
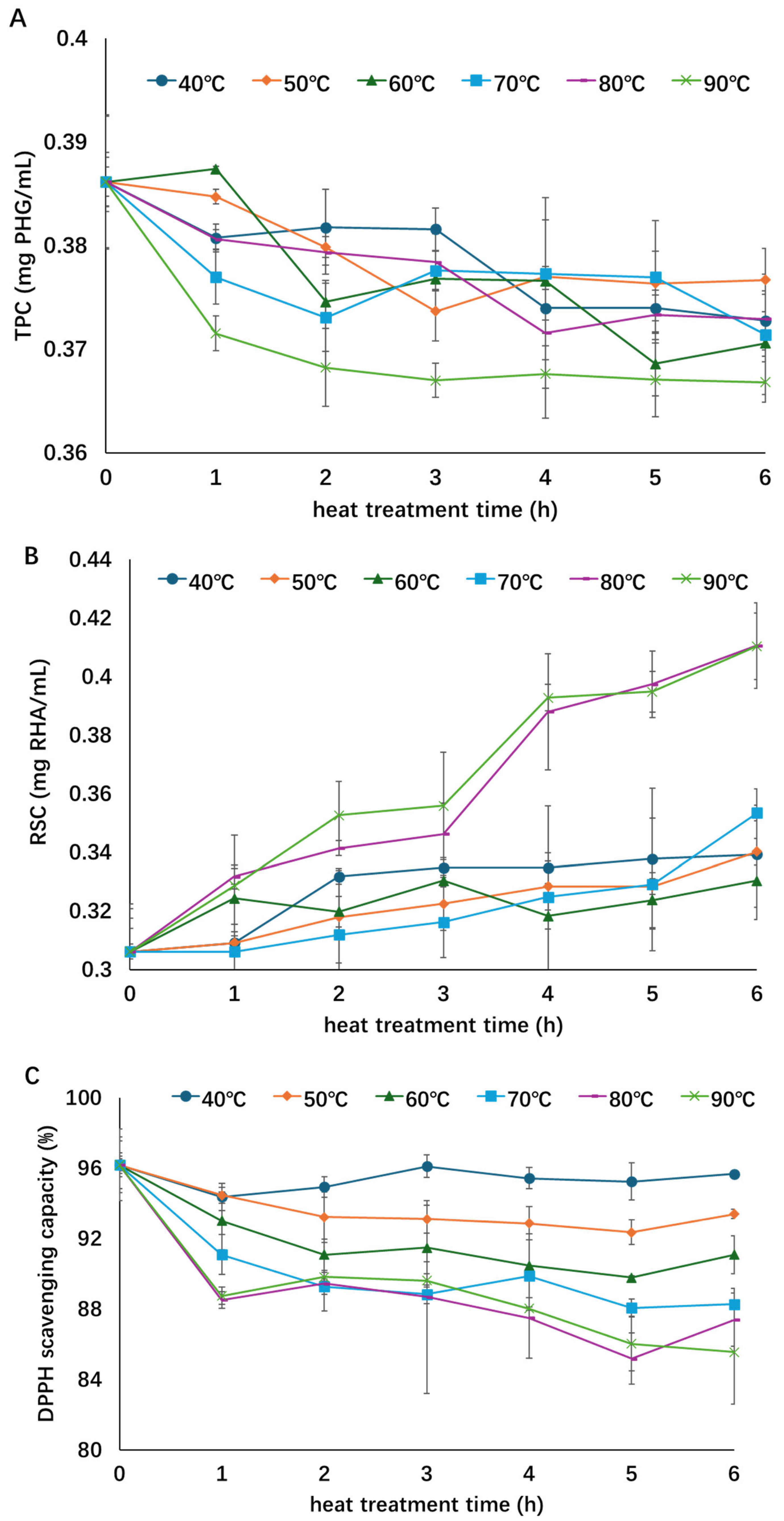
The influence of thermal treatment on the TPC, RSC, and DPPH radical scavenging activity of the concentrated extract is shown in Figure 1. As treatment time and temperature increased, the TPC and antioxidant activity showed a declining trend while the RSC increased. The TPC decreased by about 5% at the highest temperature of 90 °C, suggesting degradation of phenolic compounds during thermal treatment [11,14]. Although steady decreases in TPC were observed during all thermal treatments, no significant differences were found among most treatments. Only the highest temperature treatment (90 °C) showed significant differences from the other treatments. Meanwhile, the degradation of TPC was the fastest at 90 °C, as the lowest TPC was always found in the 90 °C treatment. A longer treatment time could expose more phenolic compounds to oxygen and light that resulted in the degradation of these compounds and decline of TPC as the heat treatment continued [11]. The RSCs of samples treated at a relatively low temperature (<80 °C) increased by approximately 7–15%, while the RSCs of samples treated with a higher temperature (80 or 90 °C) increased by approximately 35%, indicating that more polysaccharides degraded under higher heating temperatures. Significant differences in the RSC during the treatment were observed between higher temperatures (80 and 90 °C) and lower temperatures (40–70 °C), while no significant differences were found within the two temperature groups. Although the RSC of the extracts increased during thermal processing, the antioxidant activity decreased by 0.5~10% as the treatment temperature increased from 40 to 90 °C, which suggests that the antioxidants in the A. nodosum extract are thermally sensitive and their activity could be dependent on phenolic content [15,27]. Similar to that of RSC, significant differences were observed between lower-temperature (40–60 °C) and higher-temperature groups (70–90 °C), but not within each group.
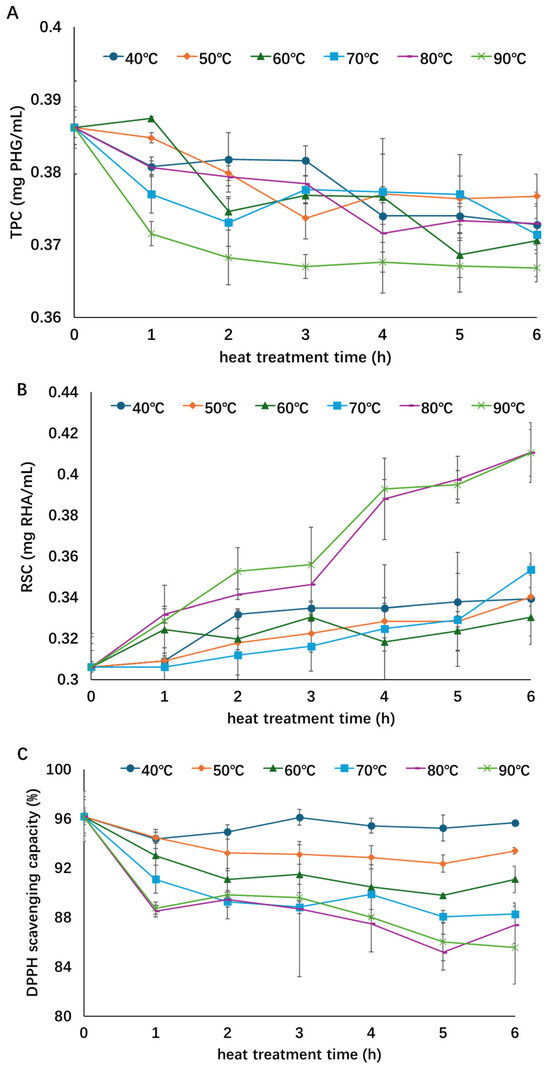
Figure 1.
Influence of thermal treatment on total phenol content (A), reducing sugar content (B), and antioxidant activity (C) of the concentrated extract. Different symbols and color indicate different treatment temperatures (○—40 °C; ◇—50 °C; Δ—60 °C; □—70 °C; -—80 °C; ╳—90 °C). This figure was adopted from the lead author’s thesis [28].
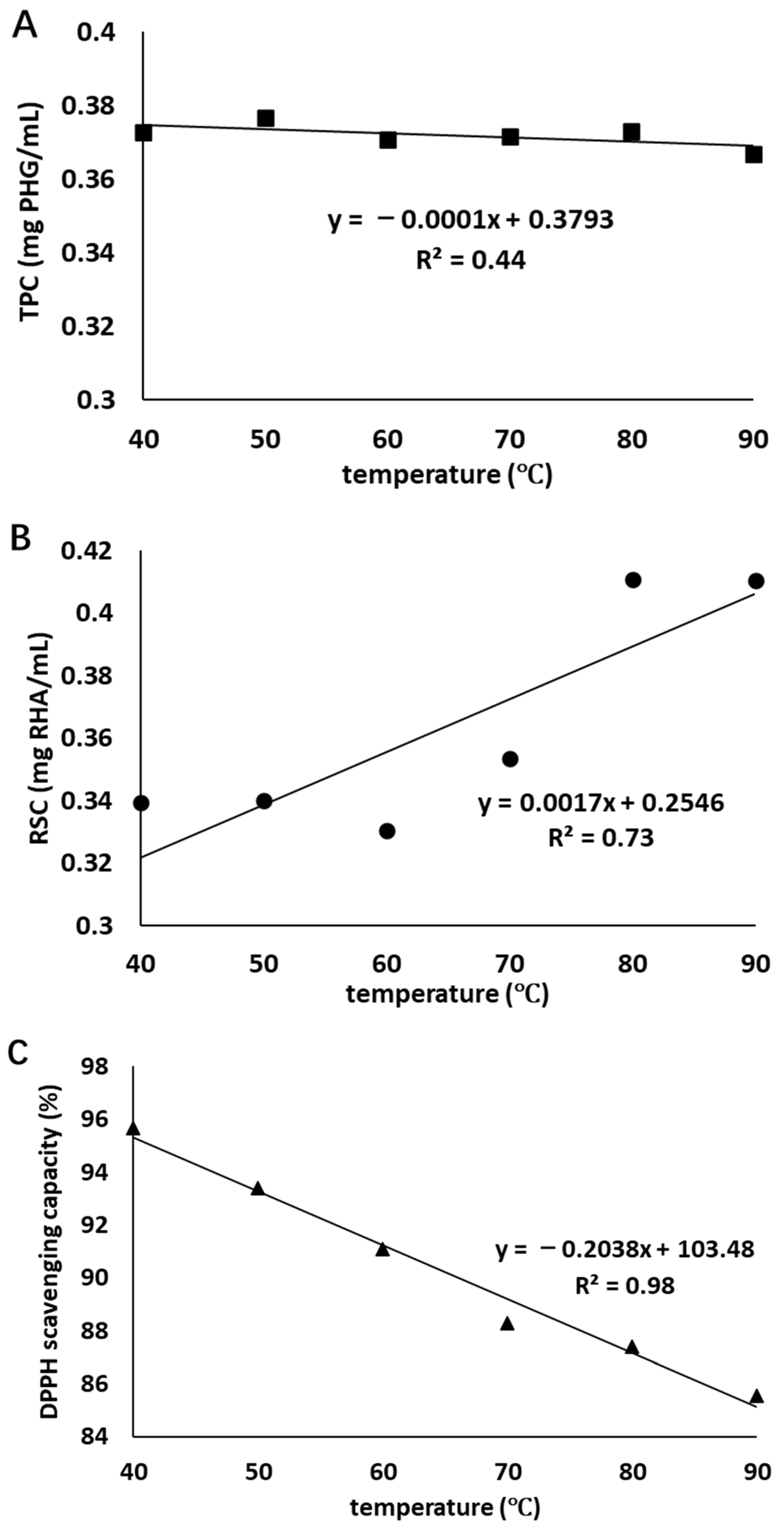
The correlations between the TPC, RSC, and antioxidant activity at the end of heat treatment and heating temperature can be observed in Figure 2. There is a strong negative correlation (R2 = 0.98) between antioxidant activity and heating temperature, a moderate positive correlation (R2 = 0.73) between RSC and heating temperature, and a weak negative correlation (R2 = 0.44) between TPC and heating temperature. These indicated that increasing temperature could adversely affect the antioxidant activity of the extract and this influence was strongly dependent on the temperature, while the positive influence on RSC was moderate. A negative influence on TPC was found in heat treatment; however, this influence was weak.
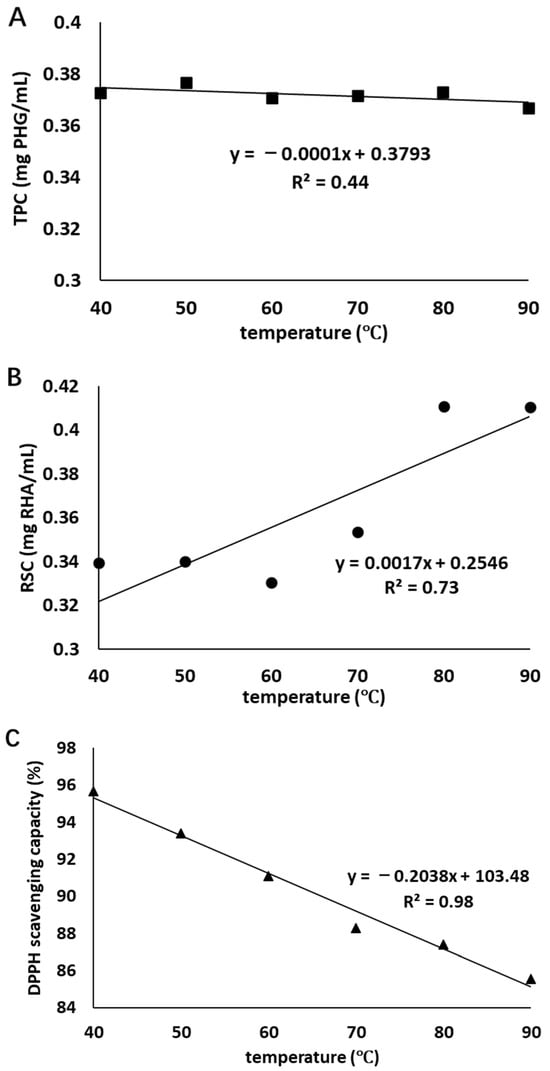
Figure 2.
Correlations between TPC (■, graph (A)), RSC (●, graph (B)), and antioxidant activity (▲, graph (C)) at the end of heat treatment and treatment temperature.
Thermal degradation of bioactive components, especially polysaccharides and polyphenols, has been studied in various plant materials and extracts. Alginate is one of the major bioactive compounds and polysaccharides in brown algae [29]. The thermal degradation of alginate, carrageenan, and carboxymethyl cellulose has been studied, and alginate was much less stable than the other two polysaccharides. In addition, the activation energy for the depolymerization of alginate and carrageenan was 50 and 100 kJ/mol, respectively, indicating less energy needed for alginate degradation [30], therefore explaining the increasing RSC as the product of alginate degradation under thermal treatments. They also concluded that high temperature could accelerate the degradation of high-molecular-weight polysaccharides, which explains the higher RSC at 80–90 °C treatments than <80 °C treatments. It has also been reported that during the thermal treatment of black garlic processing, the higher treatment temperature would cause polysaccharides to degrade into reducing sugars sooner than a lower treatment temperature [31], which agrees with the observation in the present study that a higher treatment temperature resulted in a higher RSC of the algae extract. The phenolic compounds were also sensitive to thermal treatment; for instance, Maskat and Tan [16] reported that increasing the temperature from 30 to 90 °C had a negative influence on the TPC of Mengkudu extract, which agrees with the findings of the present study that the TPC of A. nodosum extract decreased during thermal treatment. Reduced TPC was also observed in elderberry extract [13], grape marc extract [18], roselle extract [32], navel orange peel extract [24], and sea buckthorn extract [15] during thermal treatment. In addition to the treatment temperature, the solvent used for extraction can also affect the thermal stability of phenolic compounds. Yu et al. [24] found that the TPC of navel orange peel extract obtained using organic solvents was more stable than that in the aqueous phase, with no significant changes observed in the organic phase at temperatures lower than 60 °C, while a significant decrease of 10% was observed at 70 °C. Antony and Farid [11] concluded that the polyphenol yield in plant extract decreases at higher extraction temperatures (>80 °C) in traditional solvent extraction, and this conclusion has been confirmed in the present research where heating the algae extract at 90 °C resulted in the lowest TPC. Changes in the content of polyphenols and polysaccharides, especially the polyphenols, might alter the antioxidant activity of the extract as well [11]. Reduction in DPPH scavenging activity of elderberry extract was reported to increase from 21 to 49% as treatment temperature raised from 100 to 150 °C for 90 min [13]. Heat treatment ranging from 70 to 110 °C induced a reduction of 3–12% in DPPH radical scavenging activity when heating plum extract for 5 min [14]. It has been found that the TPC of plum extract decreased under the same treatment conditions and therefore concluded that the loss or degradation of certain phenolic components in plum extracts was responsible for the reduction in DPPH radical scavenging activity, which agreed with the present research that the antioxidant activity might depend on the content of phenolic compounds of the extract. As heating temperature increased from 50 to 120 °C, the TPC and DPPH radical scavenging activities of sweet cherry extract were also reduced, and higher temperature resulted in a greater loss of both TPC and DPPH radical scavenging capacity [33]. Compared with the abovementioned studies, the highest treatment temperature in the present study was lower (90 °C) but the treatment time was longer (up to 6 h), and the algae extract showed a relatively good thermal stability, with a reduction in DPPH radical scavenging activity and TPC of up to 10 and 5% at the highest temperature, respectively. Thermal degradation of phenic compounds, e.g., flavonoid glycosides, might also lead to a reduction in the antioxidant activity of the extract. For example, Ye et al. [34] found that the flavonol glycosides in the Ginkgo biloba extract were sensitive to a heating temperature of 70 °C. Further investigation into the chemical composition and degradation kinetics of algae extracts is essential to fully assess their utility, stability, and safety in real-world applications.
3.2. Influence of Thermal Treatment on the Non-Concentrated Extract
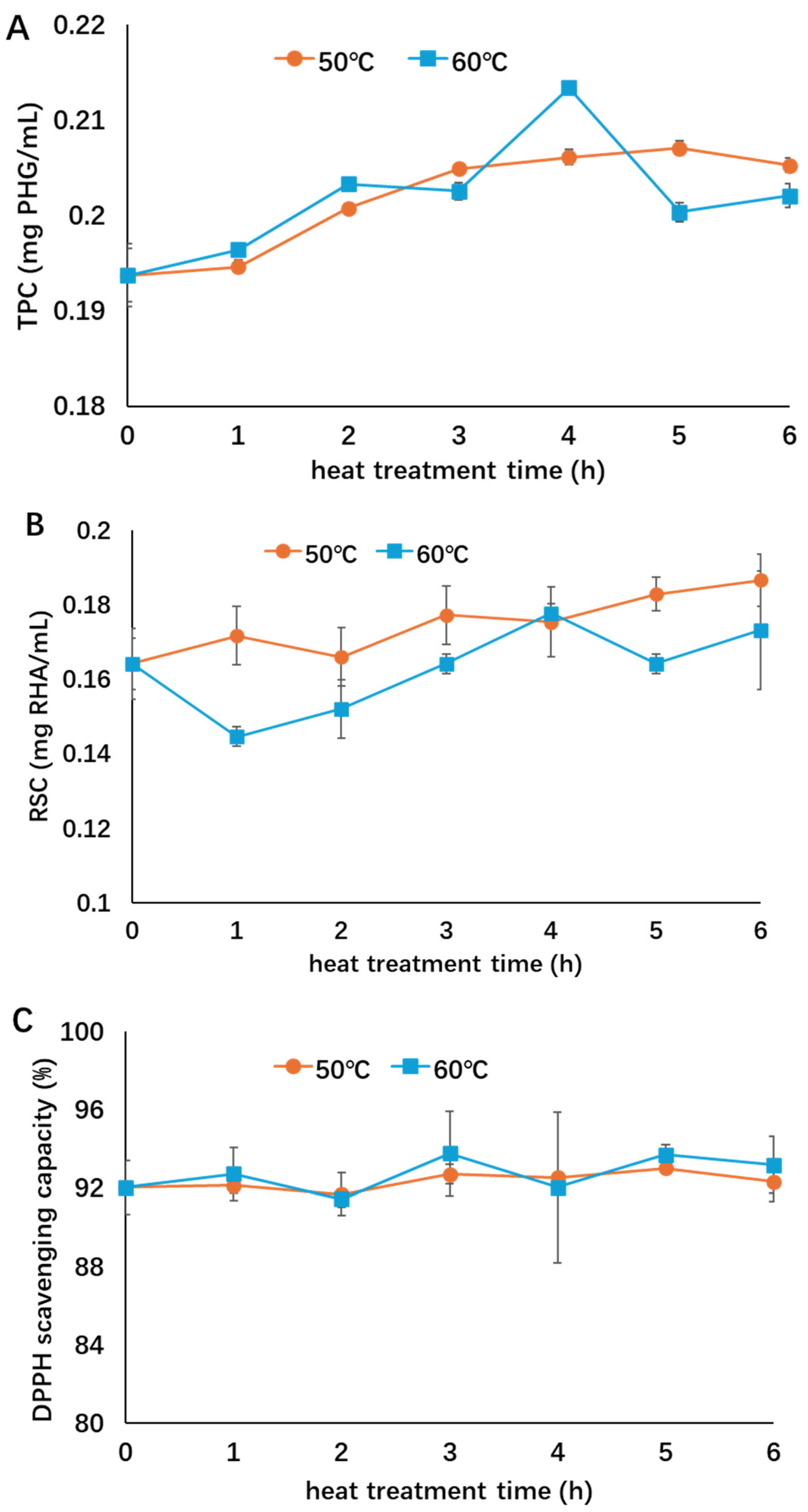
The stability of supernatants obtained without rotary evaporation was evaluated to investigate the influence of thermal treatment on the composition and thermal stability of the non-concentrated extracts to evaluate the influence of the solvents used for dissolving the extract. Similar to the concentrated extracts, the RSC of supernatants treated at 50 and 60 °C both increased (Figure 3), suggesting degradation of polysaccharides under thermal treatment. No significant change in antioxidant activity was observed. However, the TPC of non-concentrated extract increased during the treatment, probably because polyphenols were released from their bindings with other components such as polysaccharides during thermal treatment [11,18]. When the influence of different treatment temperatures was considered, no significant difference was observed in the TPC, RSC, or antioxidant activity between the two temperatures. Compared with the concentrated extract in Figure 2, the non-concentrated extract showed a better thermal stability at both 50 and 60 °C, suggesting that the solvent could help to preserve the bioactivity of the extract at relatively lower heating temperatures, probably by reducing dissolved oxygen and thereby slowing the oxidative process of phenolic compounds.
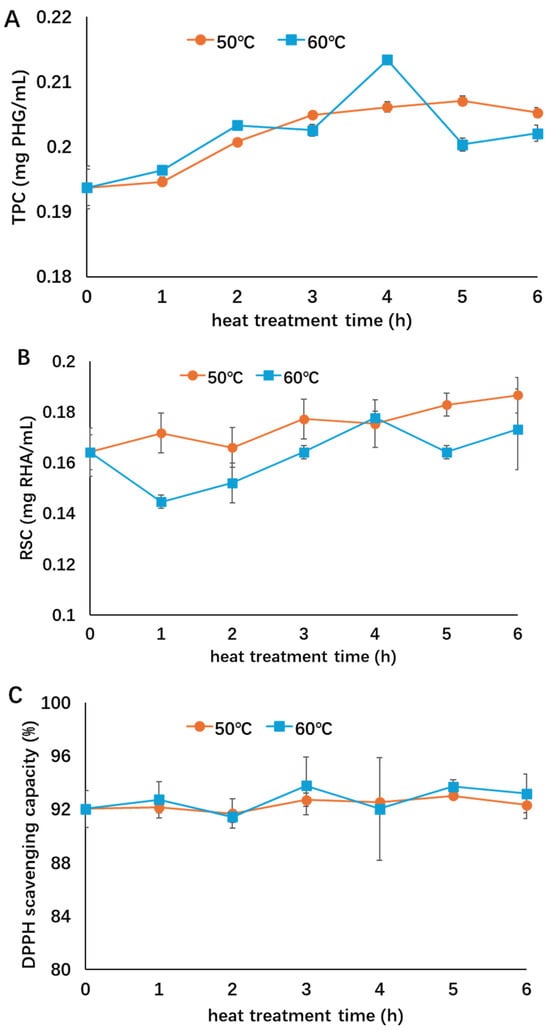
Figure 3.
Influence of thermal treatment on the bioactivities of non-concentrated extract ((A): TPC; (B): RSC; (C): antioxidant activity). Different symbols indicate heat treatment at 50 °C (○, orange) and 60 °C (□, blue). This figure was adopted from the lead author’s thesis [28].
The phytochemical content and thermal stability of the non-concentrated extract depend mainly on their chemical composition, extraction procedure, and treatment temperature and period. For example, the polyphenolic compounds in red grape skin extract were very stable during heating at 40 °C but were found to decrease at 60 and 80 °C [35]. Degradation of phenolic compounds and antioxidants was reported in sea buckthorn extract when heated at 60 °C for 25 min, as a significant decrease in TPC and DPPH radical scavenging activity was observed [15]. However, the phenol content in grape marc extract obtained after filtration did not decrease at 80 °C and an increase in TPC was observed when the temperature reached 100 and 150 °C, while the antioxidant activities of the extract treated at 100 and 150 °C were both higher than that at 80 °C, as some polyphenols associated with polysaccharides could be released due to the thermal degradation of polysaccharides [18], which might explain the increasing TPC in the present study. No significant change in antioxidant activity of extract treated at 80 °C has been reported; however, an increment was observed at 100 °C and degradation was found at 150 °C, indicating that heat treatment might contribute to antioxidant activity by releasing more polyphenols, but higher temperature could also induce the degradation of these polyphenols [18].
3.3. Influence of Storage Conditions
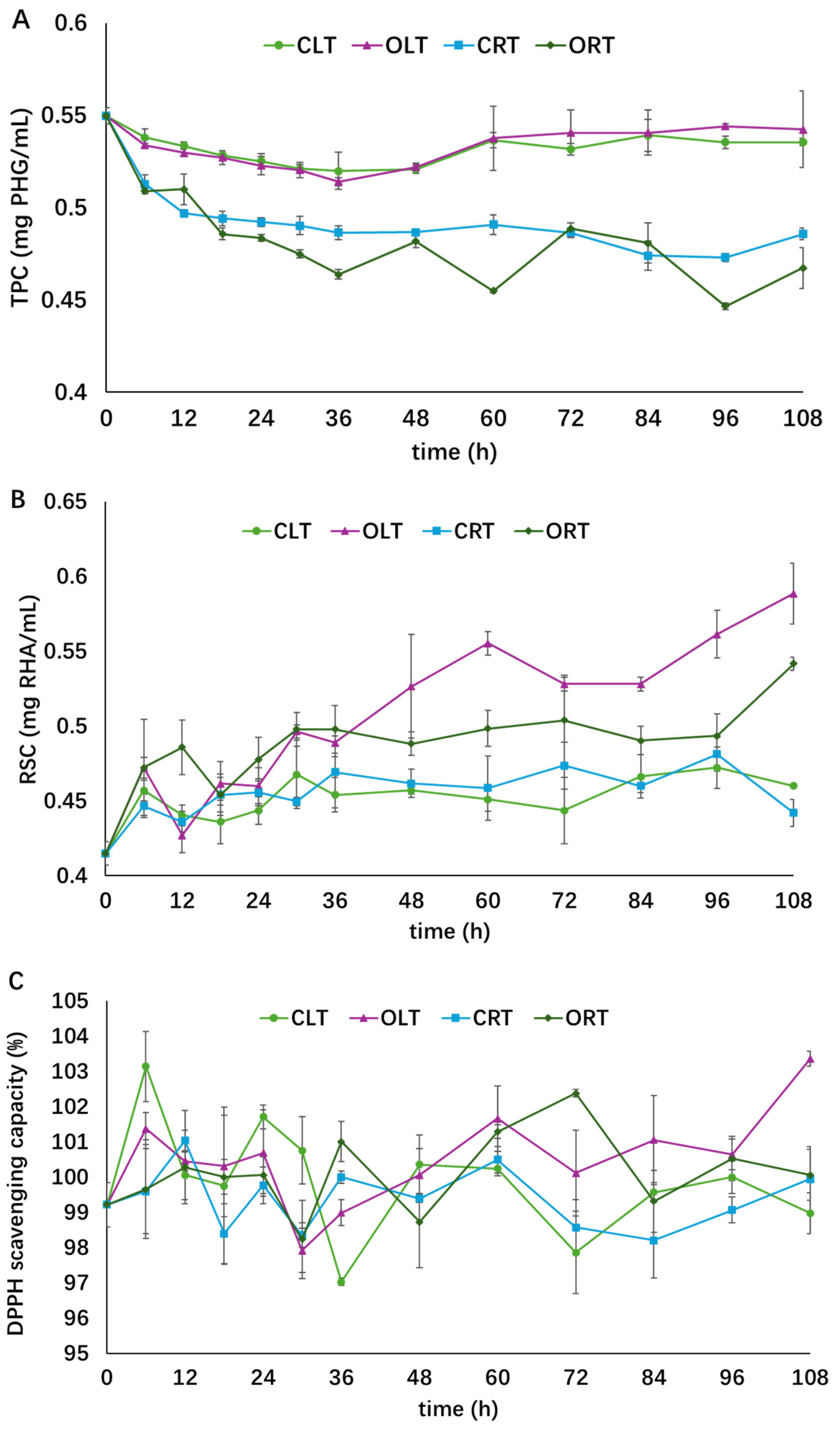
The stability of the concentrated extract under different storage conditions is shown in Figure 4. As time went on, a decrease in TPC and an increase in RSC was observed, while the antioxidant activity remained relatively stable in all four groups. The order of the TPC of the four groups at the end of storage was OLT ≈ CLT > CRT > ORT, and the order of RSC was OLT > ORT > CLT ≈ CRT. The “≈” indicates no significant difference between treatments. At the end of storage, the TPC of extract stored at 4 °C remained stable while those stored at 25 °C in closed or open tubes decreased by 12 and 15%, respectively. RSC in the OLT and ORT groups increased by 42 and 31%, respectively, while the increases in CLT and CRT were only 9 and 7%, respectively, towards the end of storage. TPC was more sensitive to storage temperature than to air exposure, suggesting that low storage temperature might inhibit the degradation of phenolic compounds. Exposure to oxygen might accelerate polysaccharide degradation in A. nodosum extract, as the RSC was higher in the groups exposed to air. RSC is a typical label reflecting polysaccharide degradation, which is usually induced by thermal, oxidative, hydrolytic, and enzymatic processes [31]. Similar findings have been reported in thermal and freezing treatment of garlic [31,36]. No significant change was observed in the DPPH radical scavenging activity in all four groups, suggesting that the antioxidants in the algae extract were stable under the storage conditions applied in the present research.
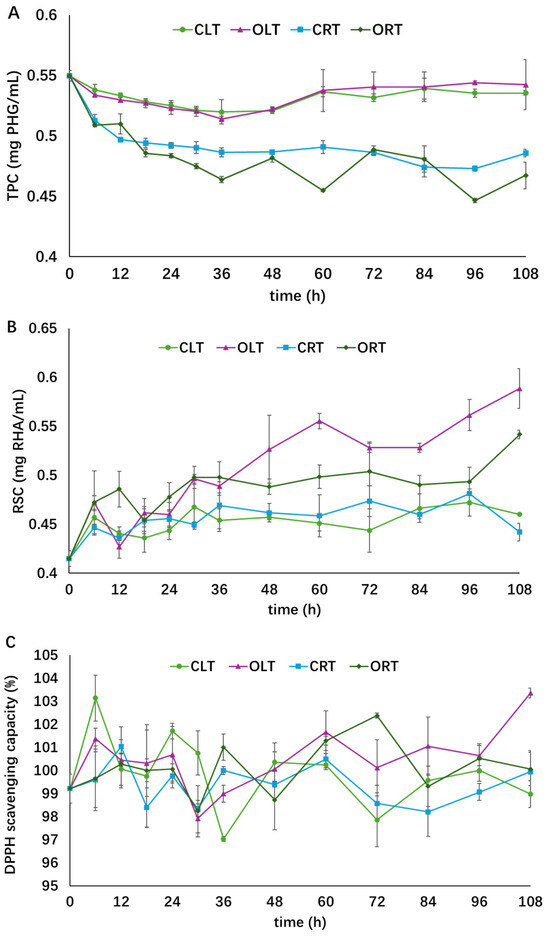
Figure 4.
Influence of various storage conditions on the total phenol content (graph (A)), reducing sugar content (graph (B)), and antioxidant activity (graph (C)). Different symbols indicate different treatment groups (○: closed low temperature (CLT), light green; □: closed room temperature (CRT), blue; Δ: open low temperature (OLT), purple; ◇: open room temperature (ORT), dark green). This figure was adopted from the lead author’s thesis [28].
Polyphenols are usually less protected once they are extracted from their source material [37]. Their degradation could be attributed to heat, light, oxygen, pH, etc. Phenolic compounds in plant extracts showed good stability during long-period storage at low or room temperatures in previous studies. As reported by Tao et al. [38], after 30-day storage, the TPC of wine lees extract stored at 4 and 20 °C decreased by 12.5 and 12.1%, respectively. A storage experiment of 400 days was performed on grape marc phenolics in darkness at 4 and 25 °C, and the TPC remained stable during this long-period storage [39]. Two major anthocyanins in roselle extract, delphinidin 3-O-sambubioside and cyaniding 3-O-sambubioside, decreased by 11% and 17% after storage at 4 °C for 60 days, respectively, and the degradation of the two compounds was more rapid at higher temperatures of 20, 30, and 37 °C [40], which is in agreement with the findings of the present study that the TPC of algae extract stored at room temperature decreased more rapidly than those stored at 4 °C. The form of extract being stored, e.g., powder or liquid, also had an influence on the antioxidant activity. For instance, powdered olive leaf extract stored at 4 °C had a stronger antioxidant capacity than those stored at 25 and 35 °C, while the liquid extract stored at 25 °C had the highest antioxidant capacity [37]. This observation is different from the present study because the powdered extract has been re-diluted and analyzed as liquid extract, since all extracts used in the present study were concentrated to reduce the solvent without re-dilution. It has been stated by Ahmad-Qasem et al. [37] that neither the extract form nor the storage temperature had a significant impact on the antioxidant capacity, though the TPC was influenced by the dehydration technique. They found that the TPC of powdered extract dehydrated at 55 °C under vacuum was more sensitive to high storage temperature; however, storage conditions barely affected the TPC of extract dehydrated at 120 °C. Although the TPC and antioxidant activity of algae extract remained relatively stable in the present research, further investigation on the stability of A. nodosum extract over longer storage periods is needed.
4. Conclusions
The phenolic extract obtained from the edible brown algae A. nodosum exhibited good stability under thermal treatment and various storage conditions. An increase in RSC was observed in all treated extracts especially at higher heating temperatures (80 and 90 °C) and during storage with exposure to air, indicating that the degradation of polysaccharides during thermal treatment and storage might be affected by heating temperature and oxygen. The TPC and antioxidant activity of the concentrated extracts decreased by 1~5% and 0.5~10% after thermal treatment (40–90 °C), respectively. The antioxidant activity of the non-concentrated extract remained stable at a medium heating temperature, 50 and 60 °C, while the TPC and RSC both increased. The ethanol in the solvent helped to preserve the TPC, RSC, and antioxidant activity in the non-concentrated extract at 50 and 60 °C, and further investigation might be needed at temperatures higher than 60 °C. After storage at 4 and 25 °C with/without exposure to oxygen for 108 h, the antioxidant activity of the algae extract remained stable. The decrease in TPC and antioxidant activity was the lowest in the extract stored in sealed tubes at 4 °C, indicating that storage of the extracts in sealed containers at a low temperature is a good choice to preserve their bioactivities. Further research into identifying the specific compounds in the extract and conducting kinetic studies on its degradation would enhance our understanding of how thermal treatment and storage affect the bioactivities of A. nodosum extract.
Author Contributions
Conceptualization, X.L. and W.Y.; methodology, X.L.; formal analysis, X.L.; data curation, X.L.; writing—original draft preparation, X.L.; writing—review and editing, W.Y.; supervision, W.Y.; project administration, W.Y.; funding acquisition, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US Department of Agriculture National Institute of Food and Agriculture, Hatch Project NC02866 and Multi-state Project NC00425, the Guangxi Natural Science Foundation (2021GXNSFBA220050), and Guangxi Scientific Project (GUIKEAD22035010).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TPC | Total phenol content |
| RSC | Reducing sugar content |
| BHT | Butylated hydroxytoluene |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| CLT | Closed low temperature |
| CRT | Closed room temperature |
| OLT | Open low temperature |
| ORT | Open room temperature |
| PHG | Phloroglucinol |
| DNS | 3,5-dinitrosalicylic acid |
| RHA | Rhamnose |
References
- Gisbert, M.; Franco, D.; Sineiro, J.; Moreira, R. Antioxidant and antidiabetic properties of phlorotannins from Ascophyllum nodosum seaweed extracts. Molecules 2023, 28, 4937. [Google Scholar] [CrossRef] [PubMed]
- Agregan, R.; Munekata, P.E.; Dominguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcate and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Wang, Y.; Yang, H.; Li, H.; Xu, W.; Chen, G.J.; Zhu, H.J. Physicochemical characterization, antioxidant and immunostimulatory activities of sulfated polysaccharides extracted from Ascophyllum nodosum. Molecules 2018, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, G.H.; Wang, L.J.; Yuan, W.Q. Optimization of antioxidant extraction from edible brown algae Ascophyllum nodosum using response surface methodology. Food Bioprod. Process. 2019, 114, 205–215. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-rich extracts from the edible seaweed, Ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. J. Funct. Foods 2014, 10, 201–209. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef]
- Cassani, L.; Silva, A.; Carpena, M.; Pellegrini, M.C.; García-Pérez, P.; Grosso, C.; Barroso, M.F.; Simal-Gandara, J.; Gómez-Zavaglia, A.; Prieto, M.A. Phytochemical compounds with promising biological activities from Ascophyllum nodosum extracts using microwave-assisted extraction. Food Chem. 2024, 438, 138037. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.Q.; Liu, Y. Antibacterial effects of brown algae extract against tilapia spoilage bacteria Pseudomonas fluorescens and Shewanella putrefaciens. BioResources 2023, 18, 2897–2912. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.L. Editorial: Chemical and biological changes of polyphenols caused by food thermal processing. Front. Nutr. 2022, 9, 948894. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Onofrei, C.; Turturica, M.; Bahrim, G.; Rapeanu, G.; Stanciuc, N. The kinetics of thermal degradation of polyphenolic compounds from elderberry (Sambucus nigra L.) extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Turturica, M.; Stanciuc, N.; Bahrim, G.; Rapeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts—A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Ursache, F.M.; Ghinea, I.O.; Turturica, M.; Aprodu, I.; Rapeanu, G.; Stanciuc, N. Phytochemical content and antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) as affected by heat treatment—Quantitative spectroscopic and kinetic approaches. Food Chem. 2017, 233, 442–449. [Google Scholar] [CrossRef]
- Maskat, M.Y.; Tan, S.M. Effect of heat treatment on the physico-chemical properties of Mengkudu (Morinda citrifolia) extract. Int. Food Res. J. 2011, 18, 1007–1011. [Google Scholar]
- Chang, X.L.; Wang, C.H.; Feng, Y.M.; Liu, Z.P. Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from Aloe vera Miller. J. Food Eng. 2006, 75, 245–251. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
- Bremenkamp, I.; Sousa-Gallagher, M.J. Design and development of an edible coating for a ready-to-eat fish product. Polymers 2024, 16, 346. [Google Scholar] [CrossRef]
- Khorami, F.; Babaei, S.; Valizadeh, S.; Nseri, M.; Golmakani, M.T. Bilayer coatings for extension of the shelf life of fish fillets: Incorporating seaweed sulfated polysaccharides in chitosan-alginate LbL structures. Food Sci. Nutr. 2023, 12, 2511–2522. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food Bioprocess Technol. 2018, 12, 199–210. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Zheng, T.; Ho, C.T.; Huang, Q.R.; Wu, Q.L.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Yu, L.M.; Wu, Y.X.; Liu, D.J.; Sheng, Z.L.; Liu, J.M.; Chen, H.G.; Feng, W.H. The kinetic behavior of antioxidant activity and the stability of aqueous and organic polyphenol extracts from navel orange peel. Food Sci. Technol. 2022, 42, e90621. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.Q.; Zhao, R.Y. Extraction of antioxidants from brown algae Ascophyllum nodosum using a binary solvent extraction system. ACS Food Sci. Technol. 2021, 1, 1041–1049. [Google Scholar] [CrossRef]
- Garriga, M.; Almaraz, M.; Marchiaro, A. Determination of reducing sugars in extracts of Undaria pinnatifida (harvey) algae by UV-visible spectrophotometry (DNS method). Actas Ing. 2017, 3, 173–179. [Google Scholar]
- Zeng, Z.C.; Hu, X.T.; McClements, D.J.; Luo, S.J.; Liu, C.M.; Gong, E.S.; Huang, K.C. Hydrothermal stability of phenolic extracts of brown rice. Food Chem. 2019, 271, 114–121. [Google Scholar] [CrossRef]
- Liu, X. Extraction and Anti-Bacterial Effects of Edible Brown Algae Extracts. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2020. [Google Scholar]
- Zhang, Y.; Hawboldt, K.; MacQuarrie, S. Extraction of bioactive compounds from beachcast brown algae: A review on accelerated solvent extraction and subcritical water extraction. RSC Sustain. 2024, 2, 2069. [Google Scholar] [CrossRef]
- Bradley, T.D.; Mitchell, J.R. The determination of the kinetics of polysaccharide thermal degradation using high temperature viscosity measurements. Carbohydr. Polym. 1988, 9, 257–267. [Google Scholar] [CrossRef]
- Lu, X.M.; Li, N.Y.; Qiao, X.G.; Qiu, Z.C.; Liu, P.L. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT-Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Makris, D.P.; Yannakopoulou, K.; Kalogeropoulos, N.; Michali, I.; Karathanos, V.T. Thermal stability of anthocyanin extract of Hibiscus sabdariffa L. in the presence of β–cyclodextrin. J. Agric. Food Chem. 2008, 56, 10303–10310. [Google Scholar] [CrossRef]
- Turturica, M.; Stanciuc, N.; Bahrim, G.; Rapeanu, G. Investigations on sweet cherry phenolic degradation during thermal treatment based on fluorescence spectroscopy and inactivation kinetics. Food Bioprocess Technol. 2016, 9, 1706–1715. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.Y.; Meng, Q.F.; Li, D.H.; Garg, S.; Teng, L.R.; Wen, J.Y. Forced degradation of flavonol glycosides extracted from Ginkgo biloba. Chem. Res. Chin. Univ. 2013, 29, 667–670. [Google Scholar] [CrossRef]
- Tomaz, I.; Sikuten, I.; Preiner, D.; Andabaka, Z.; Huzanic, N.; Leskovic, M.; Kontic, J.K.; Asperger, D. Stability of polyphenolic extracts from red grape skins after thermal treatments. Chem. Pap. 2019, 73, 195–203. [Google Scholar] [CrossRef]
- Li, N.Y.; Lu, X.M.; Pei, H.B.; Qiao, X.G. Effect of freezing pretreatment on the processing time and quality of black garlic. J. Food Process Eng. 2014, 38, 329–335. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Ahmad-Qasem, B.H.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Drying and storage of olive leaf extracts. Influence on polyphenols stability. Ind. Crops Prod. 2015, 79, 232–239. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Spigno, G. Grape marc phenolics: Extraction kinetics, quality and stability of extracts. J. Food Process Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).