Low-Temperature Chlorination-Roasting–Acid-Leaching Uranium Process of Uranium Tailings: Comparison Between Microwave Roasting and Conventional Roasting
Abstract
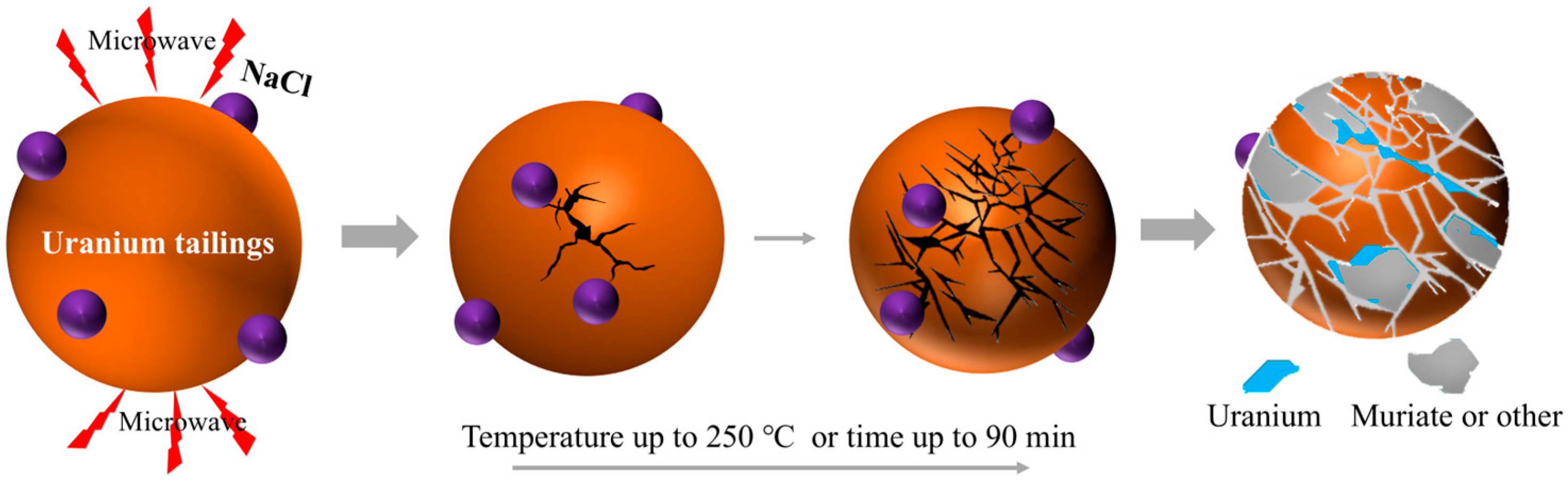
1. Introduction
2. Experimental Process
2.1. Materials
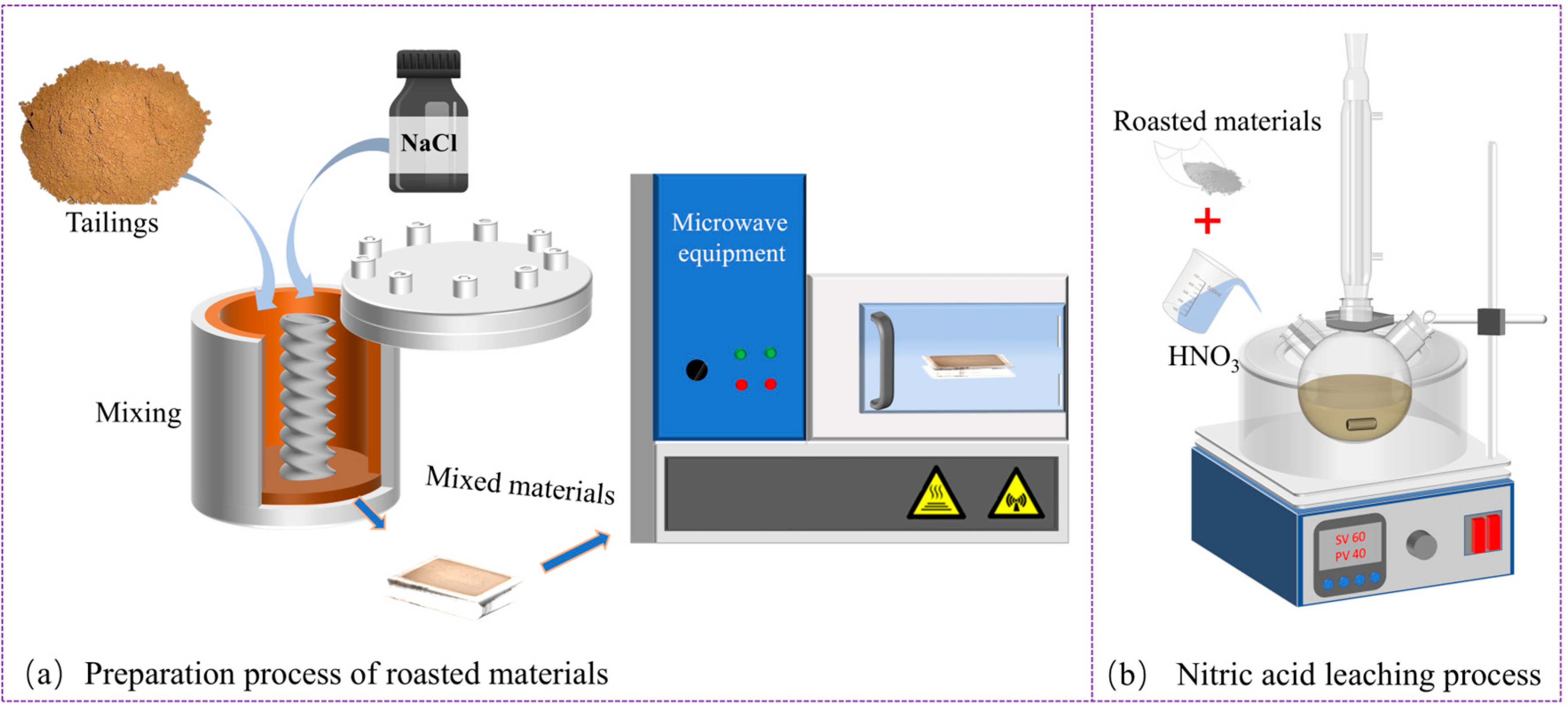
2.2. Preparation and Experimental Process of Roasted Samples
3. Results and Discussion
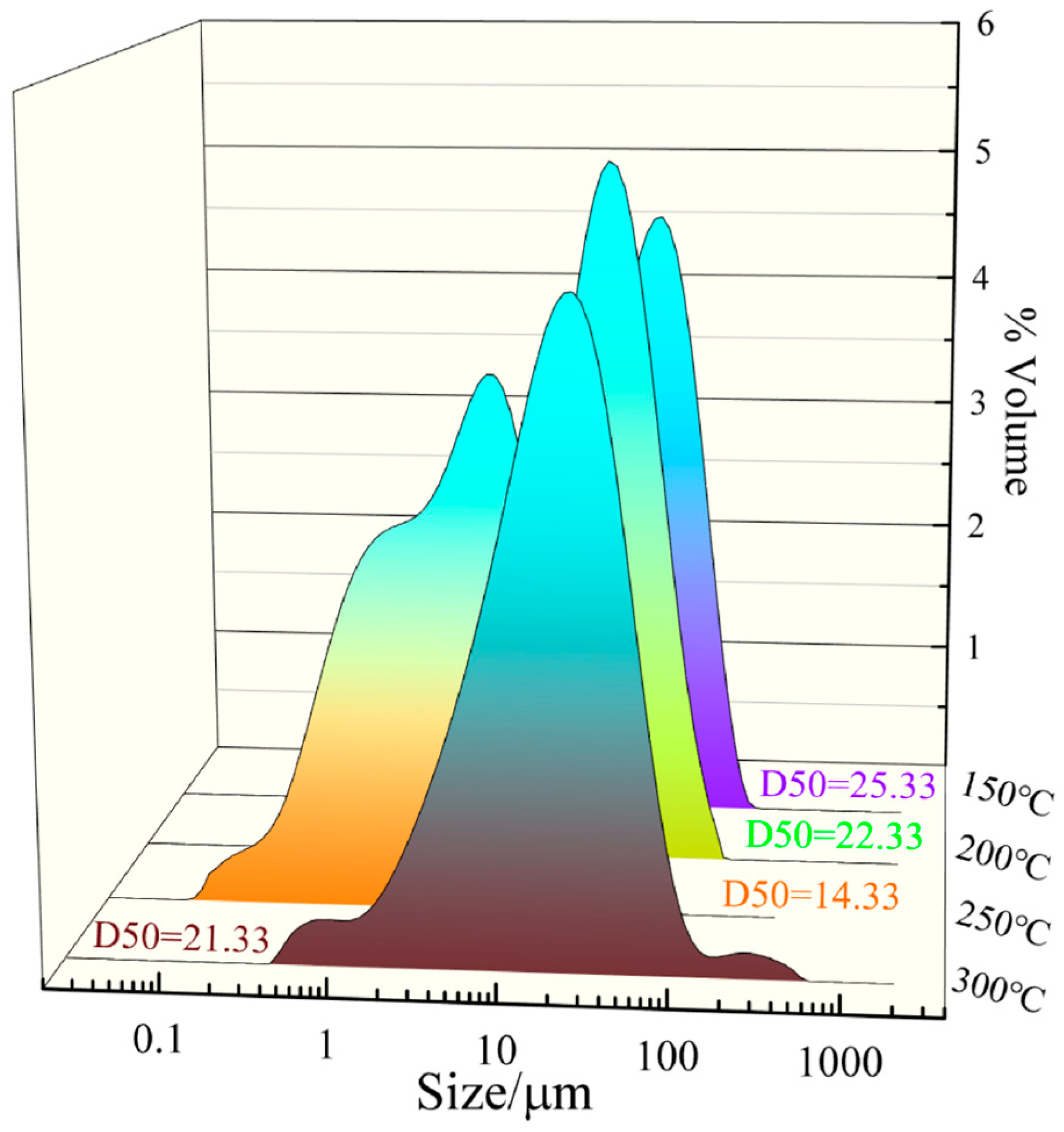
3.1. Effect of Microwave Power on Heating Behavior
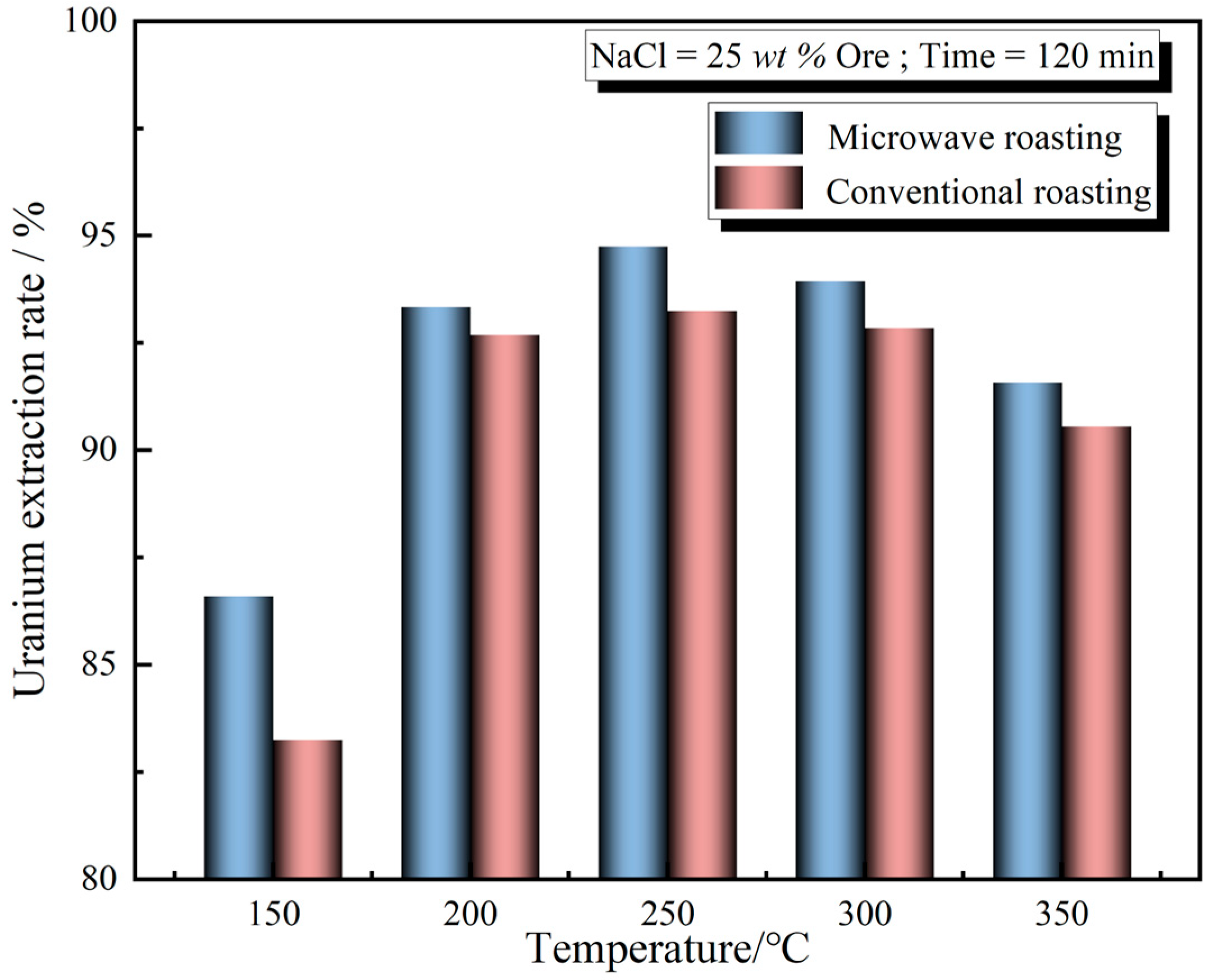
3.2. Effect of Chlorination-Roasting Temperature
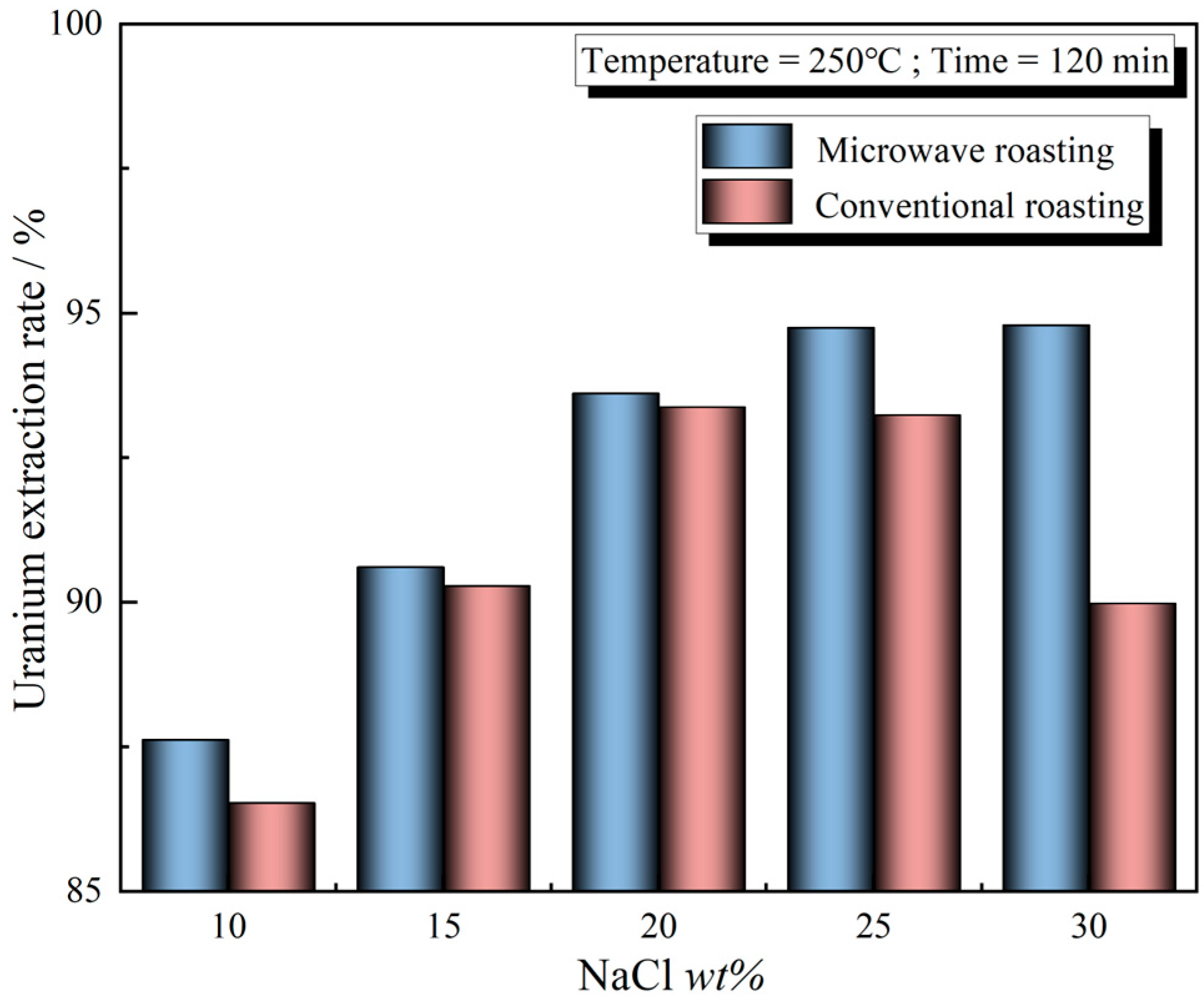
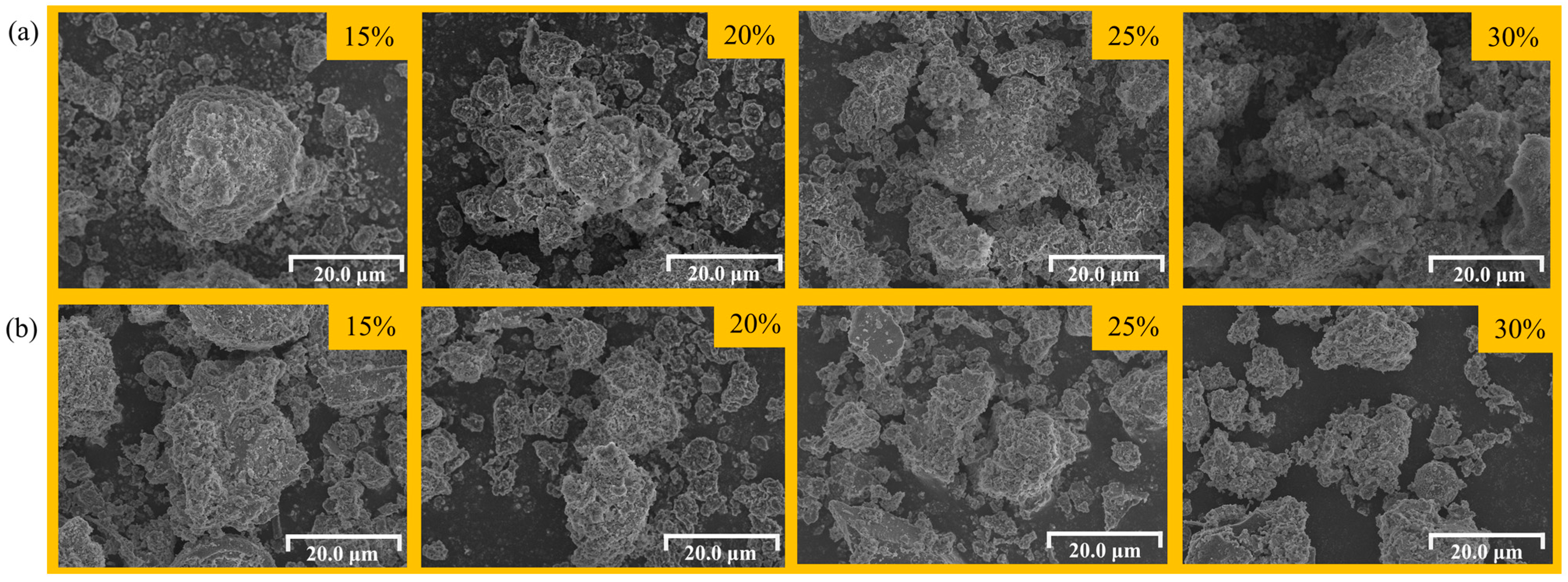
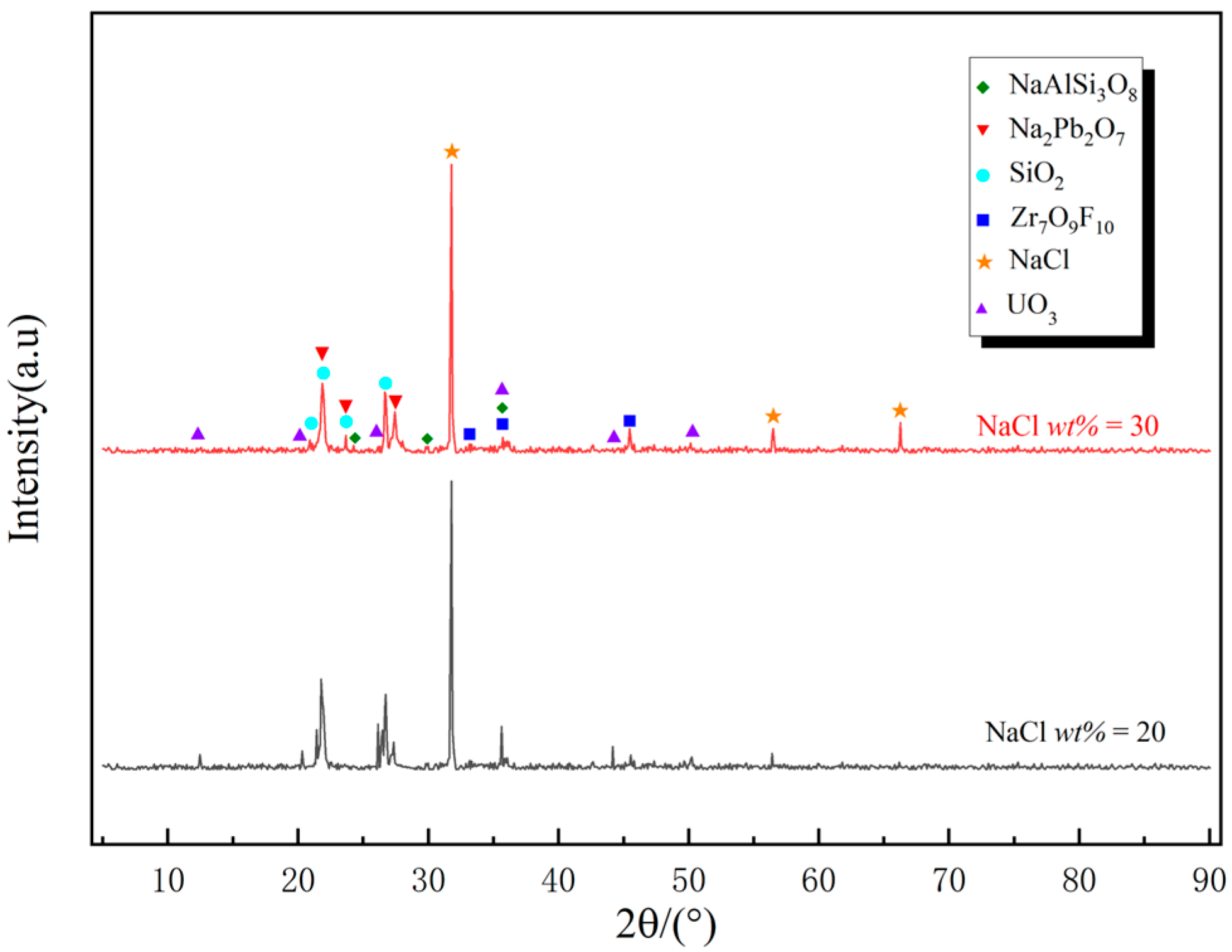
3.3. Effect of NaCl Addition Amount
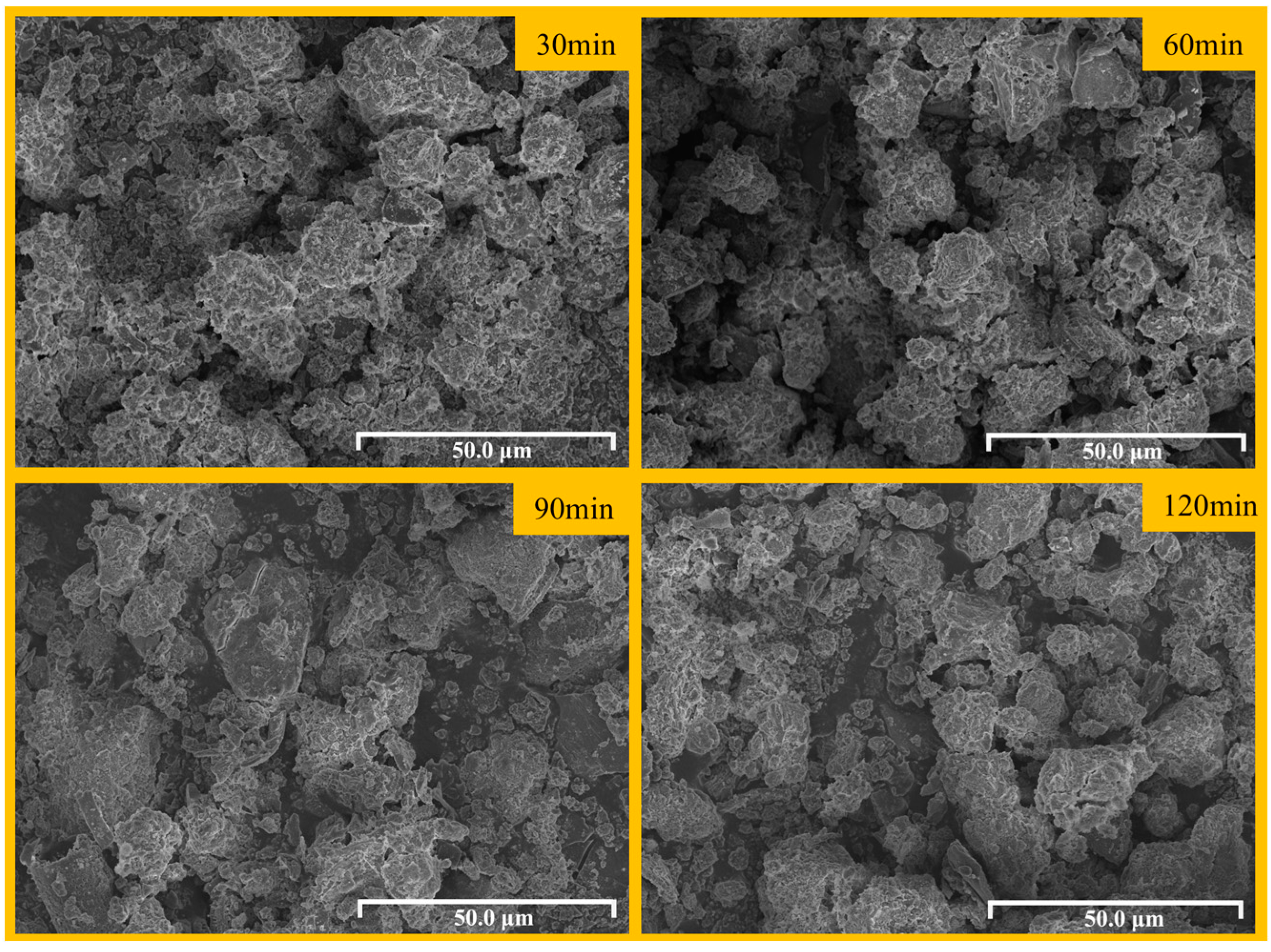
3.4. Effect of Chlorination-Roasting Time
3.5. Analysis of Microwave Chlorination-Roasting Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Xing, W. Study on the Characteristics and Evolution Trends of Global Uranium Resource Trade from the Perspective of a Complex Network. Sustainability 2022, 14, 15295. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Z.; Sun, Z.; Chen, G.; Xu, L.; Liao, Q. Optimization of bioleaching high-fluorine and low-sulfur uranium ore by response surface method. J. Radioanal. Nucl. Chem. 2019, 322, 781–790. [Google Scholar] [CrossRef]
- Shang, D.; Geissler, B.; Mew, M.; Satalkina, L.; Zenk, L.; Tulsidas, H.; Barker, L.; El-Yahyaoui, A.; Hussein, A.; Taha, M.; et al. Unconventional uranium in China’s phosphate rock: Review and outlook. Renew. Sustain. Energy Rev. 2021, 140, 110740. [Google Scholar] [CrossRef]
- Akhtar, S.; Yang, X.; Pirajno, F. Sandstone type uranium deposits in the Ordos Basin, Northwest China: A case study and an overview. J. Asian Earth Sci. 2017, 146, 367–382. [Google Scholar] [CrossRef]
- Schreinemachers, C.; Leinders, G.; Modolo, G.; Verwerft, M.; Binnemans, K.; Cardinaels, T. The conversion of ammonium uranate prepared via solgel synthesis into uranium oxides. Nucl. Eng. Technol. 2020, 52, 1013–1021. [Google Scholar] [CrossRef]
- Du, M.; Hylton, T.D.; Robinson, S.M. Chemical processes for recovery and purification of high-purity uranium-234 from aged plutonium-238. J. Radioanal. Nucl. Chem. 2021, 327, 417–424. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Liu, J.; Sun, Z.; Chen, G.; Yang, M.; Liu, Y.; Wang, D.; Ma, C.; Kong, D. Weak acid leaching of uranium ore from a high carbonate uranium deposit. J. Radioanal. Nucl. Chem. 2022, 331, 2583–2596. [Google Scholar] [CrossRef]
- Van Lien, T.; Dinh, T.T.; Dung, N.T.K. Study on leaching systems and recovery for PALUA–PARONG low grade uranium sandstone ores. Hydrometallurgy 2020, 191, 105164. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Zhang, X.; Huang, J. Kinetics of Uranium Extraction from Uranium Tailings by Oxidative Leaching. JOM 2016, 68, 1990–2001. [Google Scholar] [CrossRef]
- Charalambous, F.A.; Ram, R.; McMaster, S.; Pownceby, M.I.; Tardio, J.; Bhargava, S.K. Leaching behaviour of natural and heat-treated brannerite-containing uranium ores in sulphate solutions with iron (III). Miner. Eng. 2014, 57, 25–35. [Google Scholar] [CrossRef]
- Li, S.; Qin, S.; Kang, L.; Liu, J.; Wang, J.; Li, Y. An Efficient Approach for Lithium and Aluminum Recovery from Coal Fly Ash by Pre-Desilication and Intensified Acid Leaching Processes. Metals 2017, 7, 272. [Google Scholar] [CrossRef]
- Widowati, J.A.; Rosita, W.; Petrus, H.T.B.M.; Anggara, F.; Putra, R.D.D.; Suyanti. Recovery of Rare Earth Elements from Coal Fly Ash with High Calcium and Iron Contents Using Alkaline and Acid Leaching. Min. Metall. Explor. 2024. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Recovery of Gallium from Corundum Flue Dust by Two-Stage Alkali Leaching, Carbonation, Acid Leaching and Solvent Extraction Process. Metals 2018, 8, 545. [Google Scholar] [CrossRef]
- Yubo, G.; Zhongkui, Z.; Jiamin, L.; Guanchao, L.; Chao, L.; Zhanxue, S.; Lili, Z.; Zhihui, Y.; Miaomiao, R. Combined use of CaCl2 roasting and nitric acid leaching for the removal of uranium and radioactivity from uranium tailings. J. Radioanal. Nucl. Chem. 2020, 325, 657–665. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, B.; Wang, Q.; Li, Z.; Tian, Q. Recovery of Zinc and Lead from Copper Smelting Slags by Chlorination Roasting. JOM 2021, 73, 1861–1870. [Google Scholar] [CrossRef]
- Ran, Y.; Qu, G.; Yang, J.; Zhou, S.; Li, B.; Wang, H.; Wei, Y. Efficient separation and extraction of lithium from low-grade claystone by chloride salt-enhanced roasting process. J. Clean. Prod. 2024, 434, 140156. [Google Scholar] [CrossRef]
- Kristianová, E.; Vu, N.H.; Dvořák, P.; Tomaško, T.; Vaculíková, L. Extraction of Li, K, and Rb from zinnwaldite by chlorination roasting with CaCl2. Miner. Eng. 2023, 204, 108420. [Google Scholar] [CrossRef]
- Kumari, A.; Raj, R.; Randhawa, N.S.; Sahu, S.K. Energy efficient process for recovery of rare earths from spent NdFeB magnet by chlorination roasting and water leaching. Hydrometallurgy 2021, 201, 105581. [Google Scholar] [CrossRef]
- Yuan, Y.-Z.; Zhang, Y.-M.; Liu, T.; Chen, T.-J. Microwave roasting with size grading based on the influence of carbon on vanadium extraction from stone coal via microwave roasting-acid leaching. RSC Adv. 2017, 7, 1387–1395. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, L.; Han, Z.; Liu, J.; Zhang, L.; Yang, C.; Xu, Z.; Liu, P. Defluorination study of spent carbon cathode by microwave high-temperature roasting. J. Environ. Manag. 2022, 302, 114028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, F.; Xue, X.; Jiang, T. Effects of microwave and conventional blank roasting on oxidation behavior, microstructure and surface morphology of vanadium slag with high chromium content. J. Alloys Compd. 2016, 686, 356–365. [Google Scholar] [CrossRef]
- Bai, T.; Lei, H.-Y.; Lei, Z.; Li, S. Dearsenification of gold concentrates via microwave roasting. Can. Metall. Q. 2020, 59, 60–66. [Google Scholar] [CrossRef]
- Cardenia, C.; Balomenos, E.; Panias, D. Optimization of Microwave Reductive Roasting Process of Bauxite Residue. Metals 2020, 10, 1083. [Google Scholar] [CrossRef]
- Tang, S.; Fu, C.; Tan, L.; Liu, T.; Mao, J.; Ren, X.; Su, H.; Long, D.; Chai, Q.; Huang, Z.; et al. Imaging-guided synergetic therapy of orthotopic transplantation tumor by superselectively arterial administration of microwave-induced microcapsules. Biomaterials 2017, 133, 144–153. [Google Scholar] [CrossRef]
- Spooren, J.; Abo Atia, T. Combined microwave assisted roasting and leaching to recover platinum group metals from spent automotive catalysts. Miner. Eng. 2020, 146, 106153. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Dash, N.; Dhawan, N.; Rath, S.S. Microwave-assisted reduction roasting—magnetic separation studies of two mineralogically different low-grade iron ores. Int. J. Miner. Metall. Mater. 2020, 27, 1449–1461. [Google Scholar] [CrossRef]
- Li, K.; Jiang, Q.; Gao, L.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. Investigations on the microwave absorption properties and thermal behavior of vanadium slag: Improvement in microwave oxidation roasting for recycling vanadium and chromium. J. Hazard. Mater. 2020, 395, 122698. [Google Scholar] [CrossRef]
- Lin, S.; Gao, L.; Yang, Y.; Chen, J.; Guo, S.; Omran, M.; Chen, G. Efficiency and sustainable leaching process of manganese from pyrolusite-pyrite mixture in sulfuric acid systems enhanced by microwave heating. Hydrometallurgy 2020, 198, 105519. [Google Scholar] [CrossRef]
- Lu, G.-M.; Li, Y.-h.; Hassani, F.; Zhang, X. The influence of microwave irradiation on thermal properties of main rock-forming minerals. Appl. Therm. Eng. 2017, 112, 1523–1532. [Google Scholar] [CrossRef]
- Amankwah, R.K.; Ofori-Sarpong, G. Microwave heating of gold ores for enhanced grindability and cyanide amenability. Miner. Eng. 2011, 24, 541–544. [Google Scholar] [CrossRef]
- Olubambi, P.A. Influence of microwave pretreatment on the bioleaching behaviour of low-grade complex sulphide ores. Hydrometallurgy 2009, 95, 159–165. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Qiao, X.-C.; Yu, J.-G. Aluminum release from microwave-assisted reaction of coal fly ash with calcium carbonate. Fuel Process. Technol. 2015, 134, 303–309. [Google Scholar] [CrossRef]
- Liu, C.; Ju, S.H.; Zhang, L.B.; Srinivasakannan, C.; Peng, J.H.; Le, T.Q.X.; Guo, Z.Y. Recovery of valuable metals from jarosite by sulphuric acid roasting using microwave and water leaching. Can. Metall. Q. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Zhang, M.-p.; Liu, C.-h.; Zhu, X.-j.; Xiong, H.-b.; Zhang, L.-b.; Gao, J.-y.; Liu, M.-h. Preparation of ammonium molybdate by oxidation roasting of molybdenum concentrate: A comparison of microwave roasting and conventional roasting. Chem. Eng. Process. Process Intensif. 2021, 167, 108550. [Google Scholar] [CrossRef]
- Li, H.; Long, H.; Zhang, L.; Yin, S.; Li, S.; Zhu, F.; Xie, H. Effectiveness of microwave-assisted thermal treatment in the extraction of gold in cyanide tailings. J. Hazard. Mater. 2020, 384, 121456. [Google Scholar] [CrossRef]
- Su, G.; Guo, Z.; Guo, P.; Li, F.; Zhang, Q.; Zhou, H.; Chang, J. Study on the effect of microwave roasting pretreatment on nickel extraction from nickel-containing residue using sulfuric acid. Green Process. Synth. 2021, 10, 507–517. [Google Scholar] [CrossRef]
- Zeng, K.; Quan, X.; Jiang, Q.; Jiang, Z.; Qiu, F. An Efficient Dealkalization of Red Mud Through Microwave Roasting and Water Leaching. JOM 2022, 74, 3221–3231. [Google Scholar] [CrossRef]
- Jena, S.K.; Dhawan, N.; Rath, S.S.; Rao, D.S.; Das, B. Studies on Extraction of Potassium from Feldspar by Roast-leach Method Using Phosphogypsum and Sodium Chloride. Sep. Purif. Technol. 2016, 161, 104–111. [Google Scholar] [CrossRef]
- Tian, L.; Gong, A.; Wu, X.; Li, J.; Liu, C.; Yu, X.; Xu, Z. Non-isothermal kinetic studies of rubidium extraction from muscovite using a chlorination roasting-water leaching process. Powder Technol. 2020, 373, 362–368. [Google Scholar] [CrossRef]
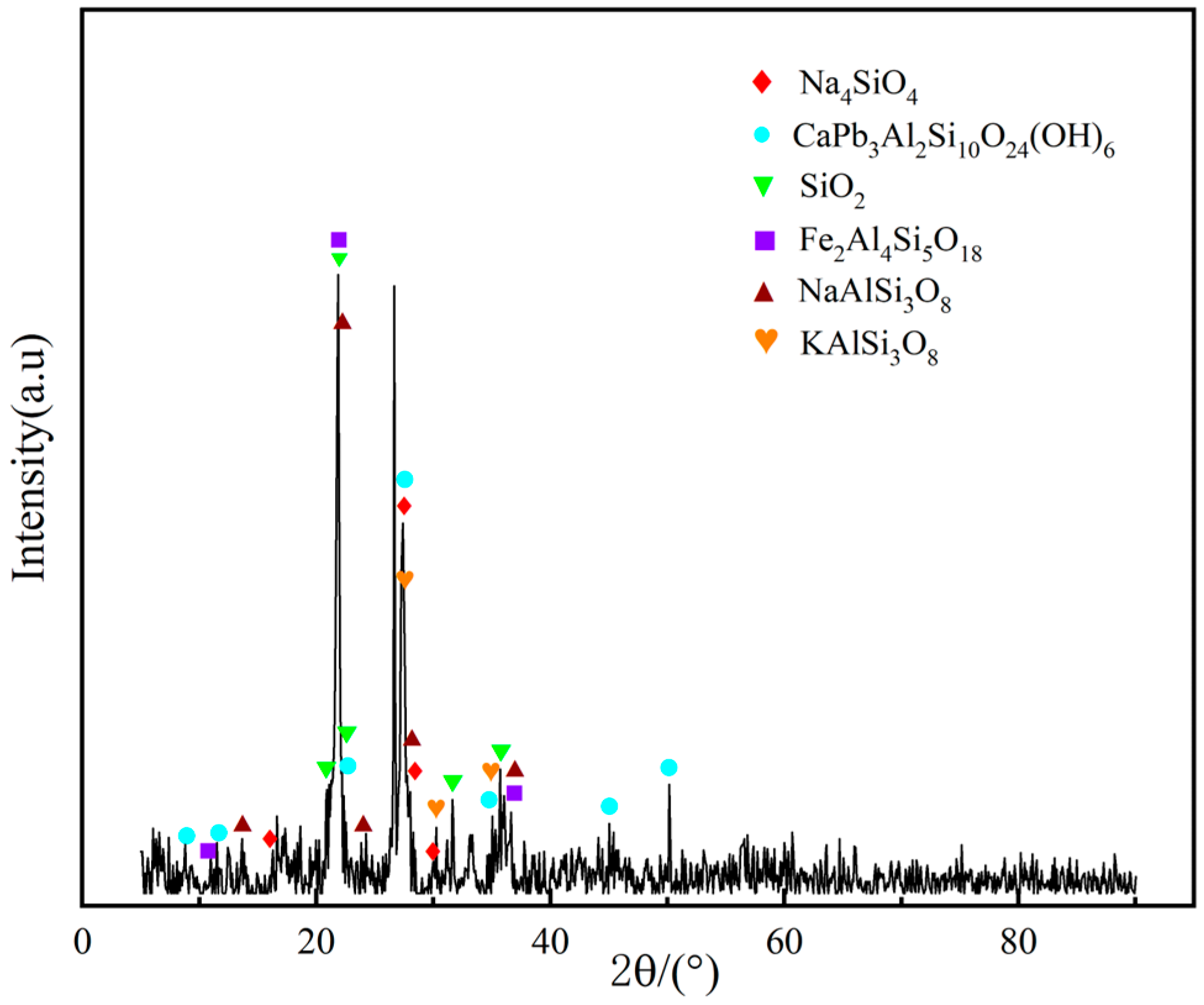



| Chemical Component | SiO2 | P2O5 | Al2O3 | Fe2O3 | ZrO2 | CaO | U |
|---|---|---|---|---|---|---|---|
| Content (%) | 63.36 | 16.04 | 3.72 | 3.83 | 5.03 | 1.16 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Song, J.; Hu, T.; Zhang, L.; Wang, Y.; Zou, F. Low-Temperature Chlorination-Roasting–Acid-Leaching Uranium Process of Uranium Tailings: Comparison Between Microwave Roasting and Conventional Roasting. Processes 2025, 13, 82. https://doi.org/10.3390/pr13010082
Hu J, Song J, Hu T, Zhang L, Wang Y, Zou F. Low-Temperature Chlorination-Roasting–Acid-Leaching Uranium Process of Uranium Tailings: Comparison Between Microwave Roasting and Conventional Roasting. Processes. 2025; 13(1):82. https://doi.org/10.3390/pr13010082
Chicago/Turabian StyleHu, Jinming, Jianwei Song, Tu Hu, Libo Zhang, Yue Wang, and Fa Zou. 2025. "Low-Temperature Chlorination-Roasting–Acid-Leaching Uranium Process of Uranium Tailings: Comparison Between Microwave Roasting and Conventional Roasting" Processes 13, no. 1: 82. https://doi.org/10.3390/pr13010082
APA StyleHu, J., Song, J., Hu, T., Zhang, L., Wang, Y., & Zou, F. (2025). Low-Temperature Chlorination-Roasting–Acid-Leaching Uranium Process of Uranium Tailings: Comparison Between Microwave Roasting and Conventional Roasting. Processes, 13(1), 82. https://doi.org/10.3390/pr13010082






