Synthesis of Hureaulite Mn5(H2O)4(PO3OH)2(PO4)2 with an Open 3D Network Structure as Electrode Material for Electrochemical Capacitors
Abstract
1. Introduction
2. Methodology
2.1. Synthesis of Mn5(H2O)4(PO3OH)2(PO4)2
2.2. Material Characterization
2.3. Electrochemical Characterization
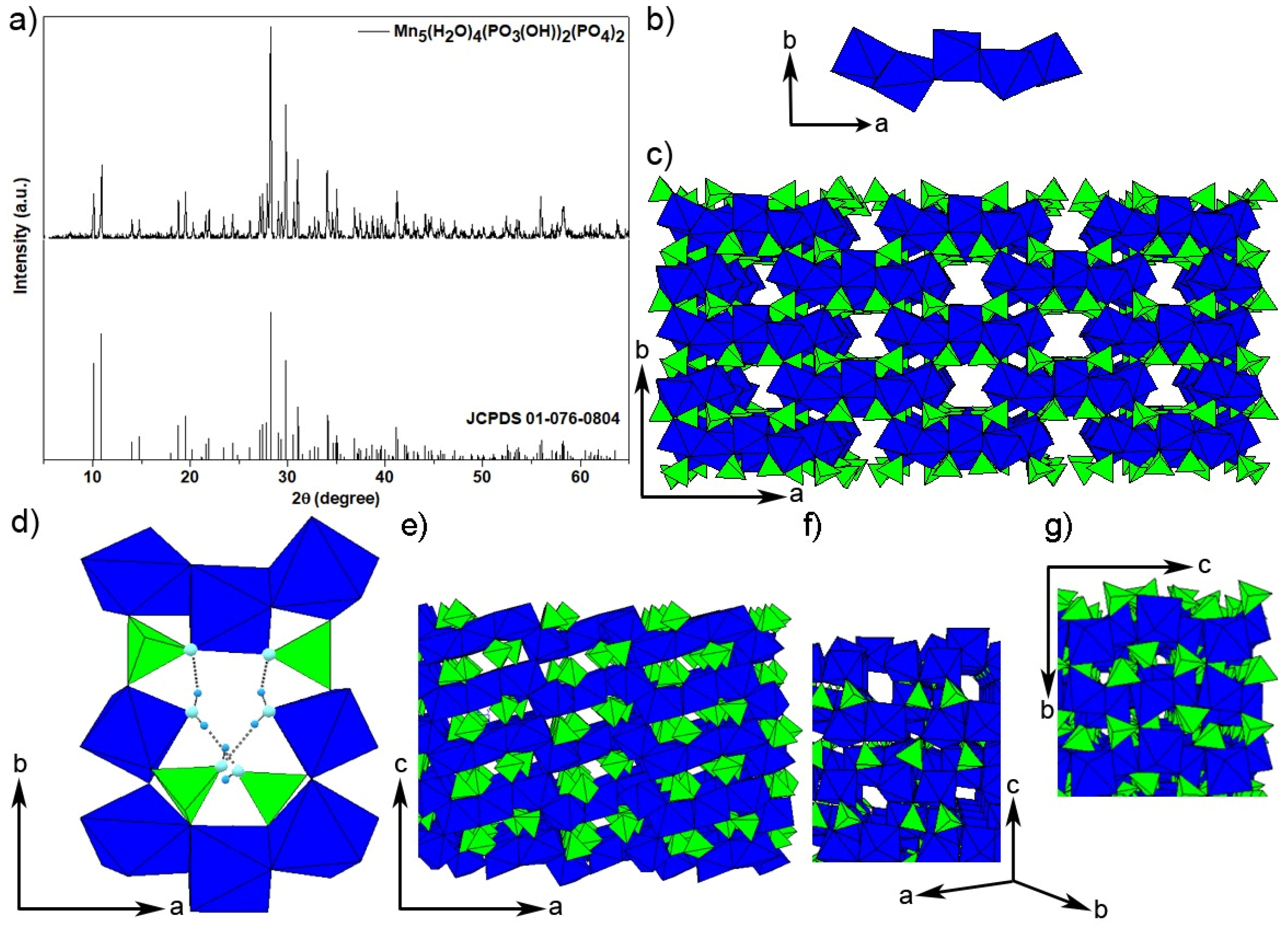
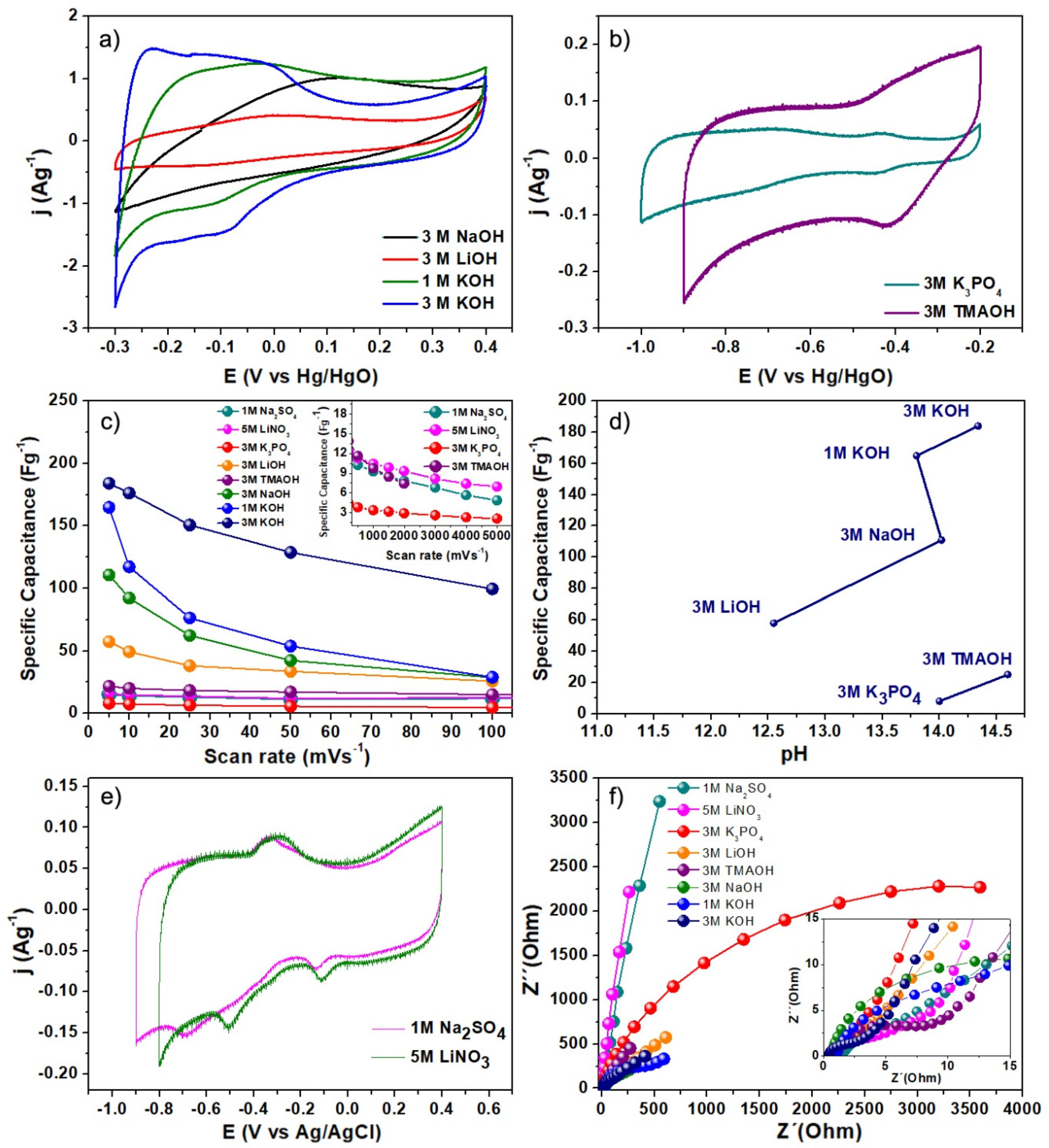
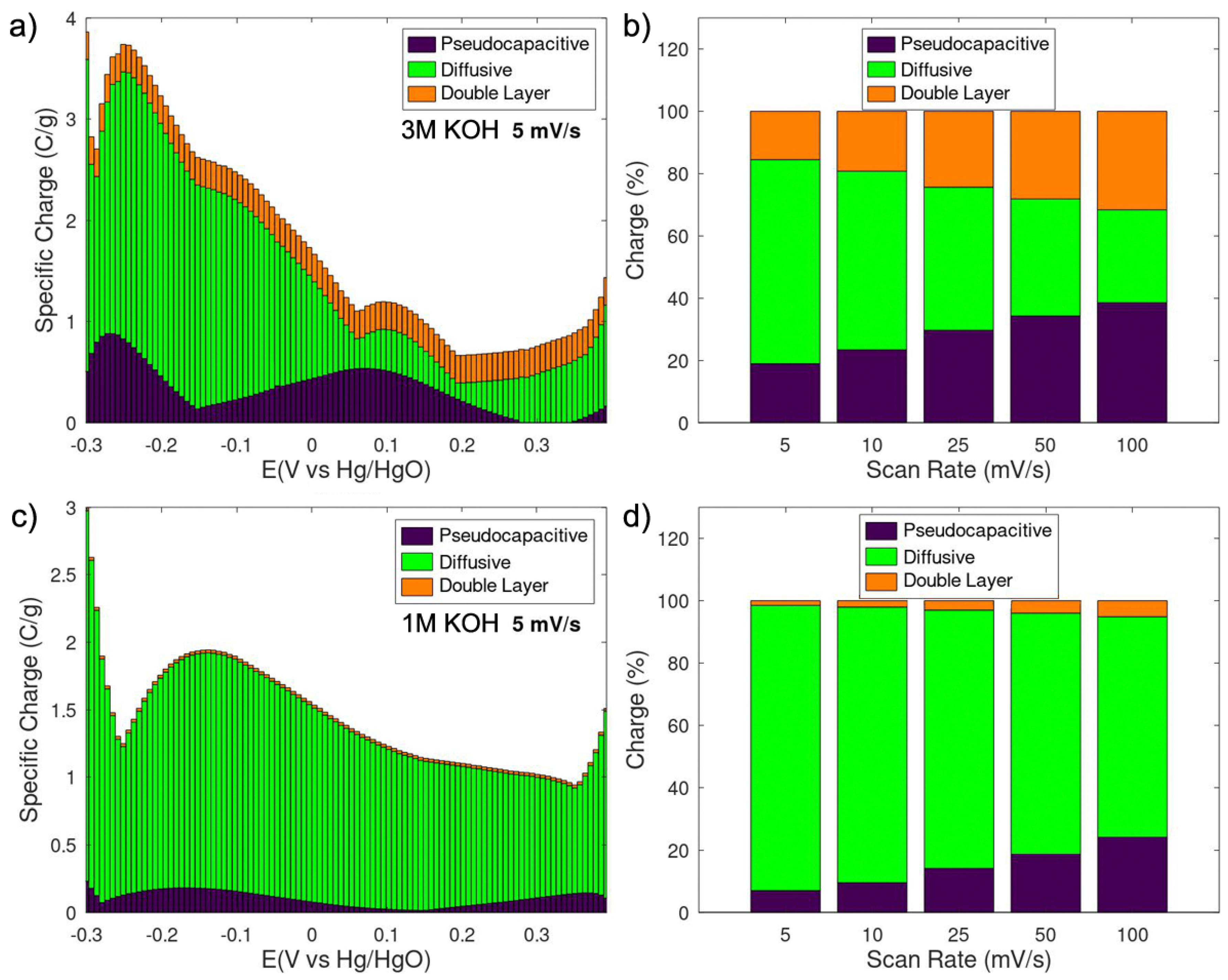
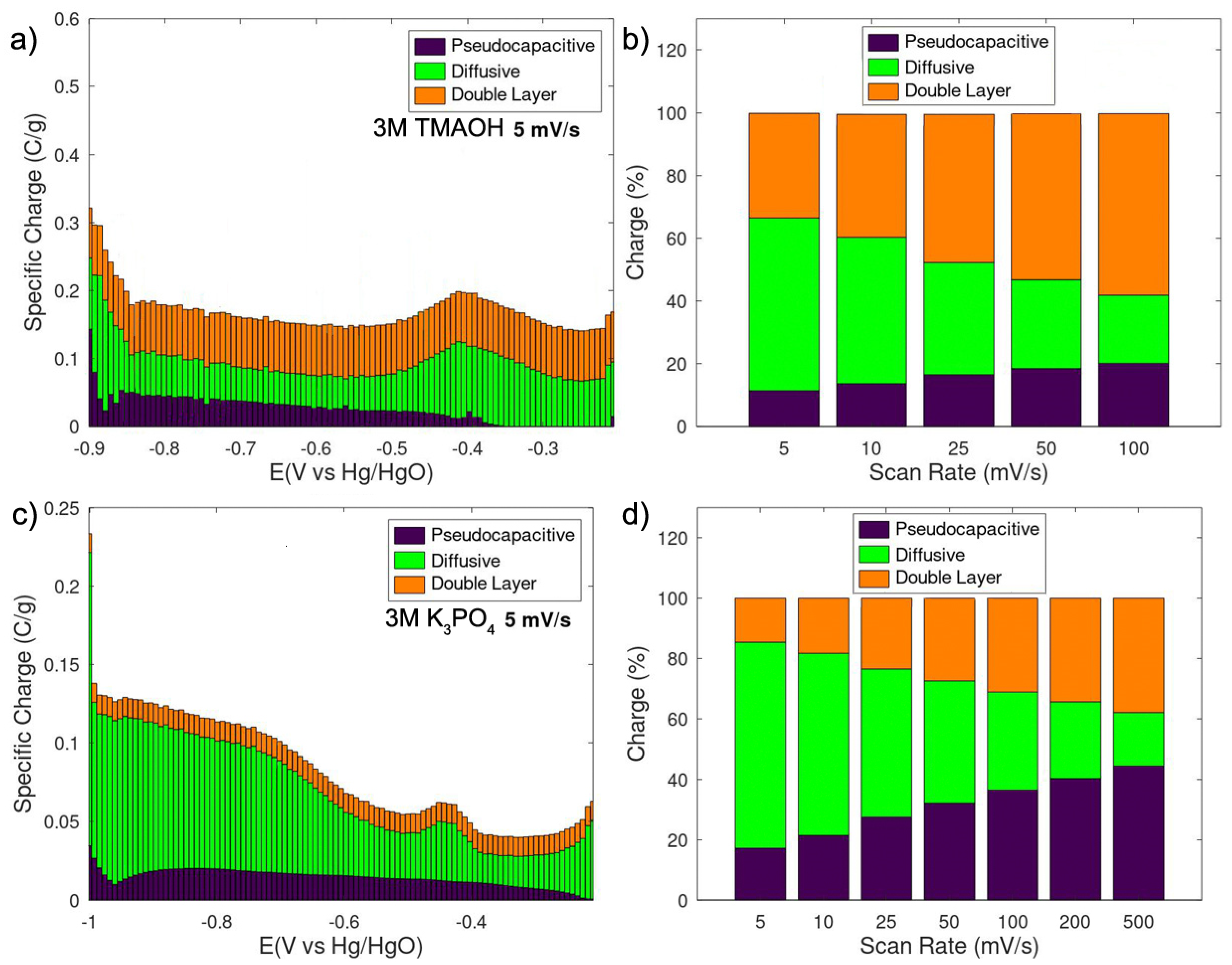
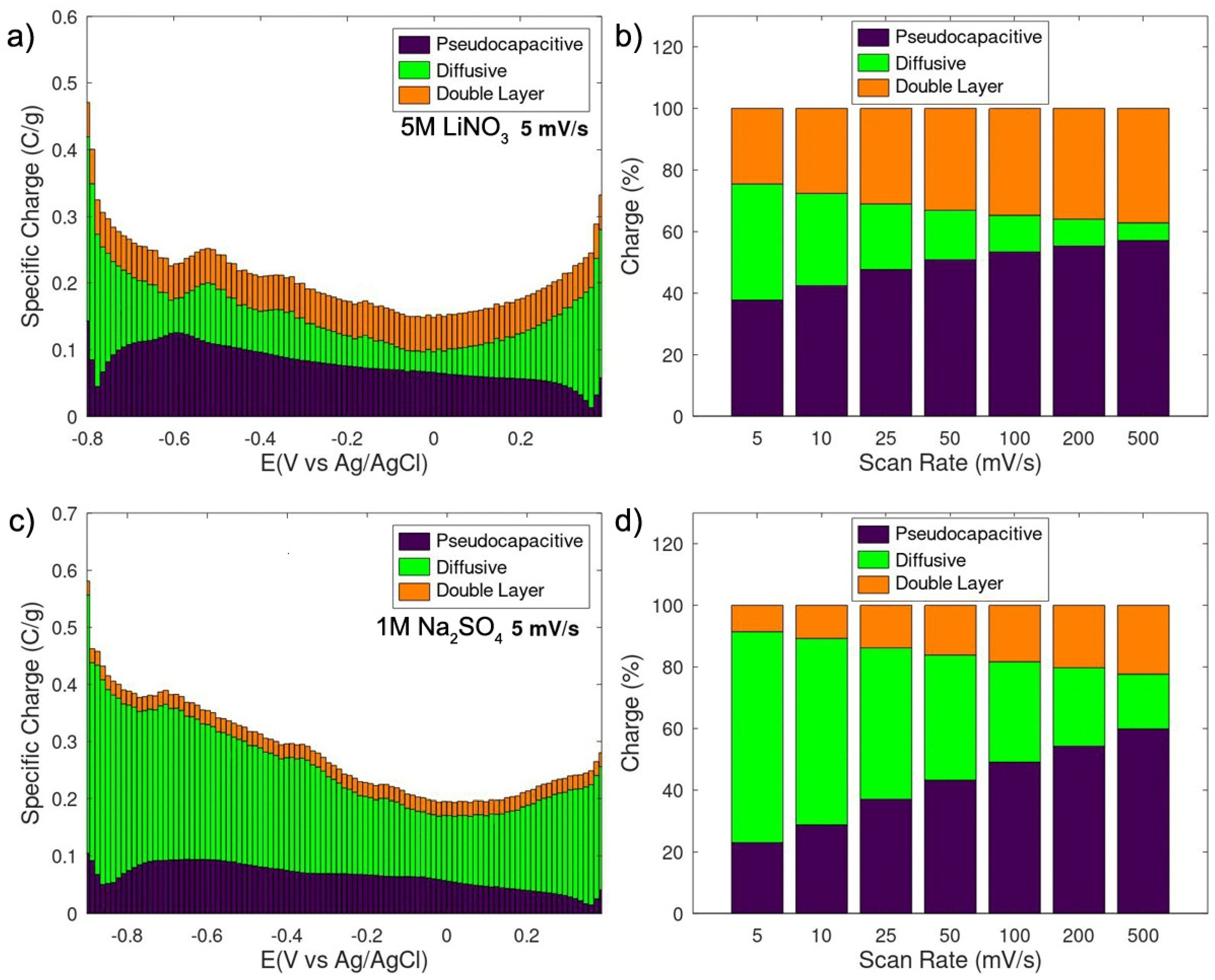
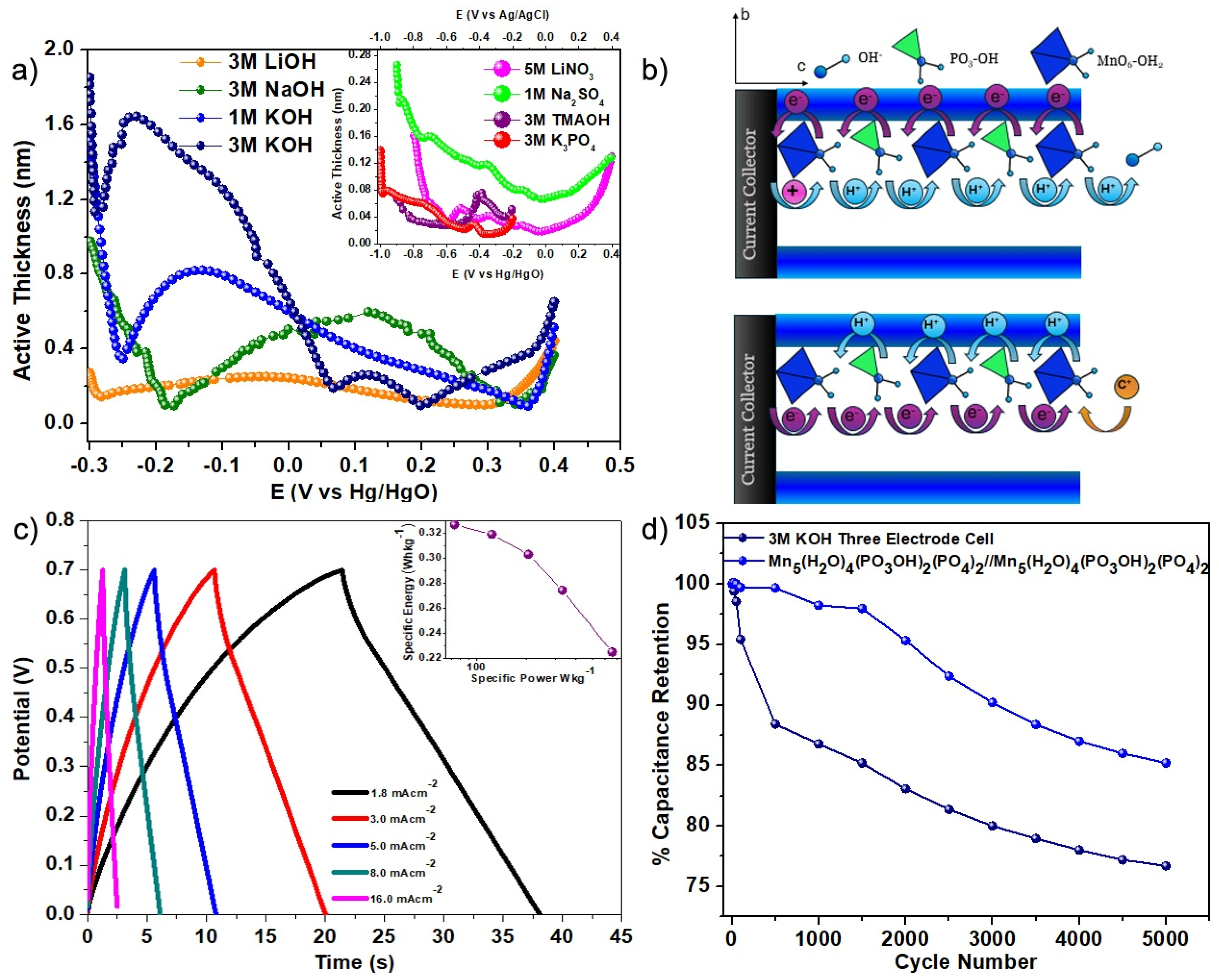
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, W.; Turcheniuk, K.; Naumov, O.; Mysyk, R.; Wang, F.; Liu, M.; Kim, D.; Ren, X.; Magasinski, A.; Yu, M.; et al. Materials and technologies for multifunctional, flexible or integrated supercapacitors and batteries. Mater. Today 2021, 48, 176–197. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Momen, R.; Tao, S.; Xiong, D.; Song, Z.; Xiao, X.; Deng, W.; Hou, H.; Yasar, S.; Altin, S.; et al. Metal–Organic Framework Materials for Electrochemical Supercapacitors. Nano-Micro Lett. 2022, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Porto, R.; Bouhtiyya, S.; Pierson, J.; Morel, A.; Capon, F.; Boulet, P.; Brousse, T. VN thin films as electrode materials for electrochemical capacitors. Electrochim. Acta 2014, 141, 203–211. [Google Scholar] [CrossRef]
- Brian Evans Conway. Electrochemical Supercapacitors. In Scientific Fundamentals and Technological Applications, 1st ed.; Kluwer Academic/Plenum Publisher: New York, NY, USA, 1999. [Google Scholar]
- Boyd, S.; Ganeshan, K.; Tsai, W.-Y.; Wu, T.; Saeed, S.; Jiang, D.-E.; Balke, N.; van Duin, A.C.T.; Augustyn, V. Effects of interlayer confinement and hydration on capacitive charge storage in birnessite. Nat. Mater. 2021, 20, 1689–1694. [Google Scholar] [CrossRef]
- Panigrahi, K.; Howli, P.; Chattopadhyay, K.K. 3D network of V2O5 for flexible symmetric supercapacitor. Electrochim. Acta 2020, 337, 135701. [Google Scholar] [CrossRef]
- Majumdar, D.; Mandal, M.; Bhattacharya, S.K. V2O5 and its Carbon-Based Nanocomposites for Supercapacitor Applications. ChemElectroChem 2018, 6, 1623–1648. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Lo, W.C.; Genc, A.; LeBeau, J.; Augustyn, V. Transition from Battery to Pseudocapacitor Behavior via Structural Water in Tungsten Oxide. Chem. Mater. 2017, 29, 3928–3937. [Google Scholar] [CrossRef]
- Porto, R.L.; Cortez, I.E.M.; Brousse, T.; Martínez, J.A.A.; Pavón, L.A.L. MnPO4·H2O as Electrode Material for Electrochemical Capacitors. J. Electrochem. Soc. 2018, 165, A2349–A2356. [Google Scholar] [CrossRef]
- Martínez, J.A.Z.; Náñez, S.E.G.; Le Calvez, E.; Porto, R.L.; Cortez, I.E.M.; Brousse, T.; Pavón, L.A.L. Layered Vanadium Phosphates as Electrodes for Electrochemical Capacitors Part I: The case of VOPO4·2H2O. J. Electrochem. Soc. 2021, 168, 070531. [Google Scholar] [CrossRef]
- Martínez, J.A.Z.; Náñez, S.E.G.; Le Calvez, E.; Porto, R.L.; Cortez, I.E.M.; Brousse, T.; Pavón, L.A.L. Layered Vanadium Phosphates as Electrodes for Electrochemical Capacitors Part II: The case of VOPO4·CTAB and K0.5VOPO4·1.5H2O. J. Electrochem. Soc. 2021, 168, 090520. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, W.-B.; Zhang, L.; Guo, Y.-W.; Zhou, X.; Zhang, X.-L.; Han, X.-W.; Long, J. Alkali cation intercalation manganese phosphate hydrate boosting electrochemical kinetics for pseudocapacitive energy storage. J. Mater. 2022, 8, 833–842. [Google Scholar] [CrossRef]
- Pan, M.-Y.; Lu, S.-T.; Li, Y.-Y.; Fan, Y. Synthetic hureaulite as anode material for lithium-ion batteries. J. Appl. Electrochem. 2023, 53, 1015–1022. [Google Scholar] [CrossRef]
- Moore, P.B.; Araki, T. Hureaulite, Mn52+(H2O)4[PO3(OH)]2[PO4]2: Its atomic arrangement. Am. Mineral. 1973, 58, 302–307. Available online: http://www.minsocam.org/ammin/AM58/AM58_302.pdf (accessed on 7 April 2024).
- Menchetti, S.; Sabelli, C. The crystal structure of hureaulite, Mn5(HOPO3)2(PO4)2(H2O)4. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 2541–2548. [Google Scholar] [CrossRef]
- De Amorim, H.S.; Amaral, M.R.D.; Moreira, L.F.; Mattievich, E. Structure refinement of synthetic hureaulite: Mn5(H2O)4[PO3(OH)]2[PO4]2. J. Mater. Sci. Lett. 1996, 15, 1895–1897. [Google Scholar] [CrossRef]
- Gatta, G.D.; Redhammer, G.J.; Vignola, P.; Meven, M.; McIntyre, G.J. Single-crystal neutron diffraction and Mössbauer spectroscopic study of hureaulite, (MnFe)5(PO4)2(HPO4)2(H2O)4. Eur. J. Mineral. 2016, 28, 93–103. [Google Scholar] [CrossRef]
- Hartl, A.; Park, S.-H.; Hoelzel, M.; Paul, N.; Gilles, R. Proton conductivity in a hureaulite-type compound, Mn5[(PO4)2(PO3(OH))2](HOH)4. J. Solid State Chem. 2019, 277, 290–302. [Google Scholar] [CrossRef]
- Qiu, G.; Gao, Z.; Yin, H.; Feng, X.; Tan, W.; Liu, F. Synthesis of MnPO4·H2O by refluxing process at atmospheric pressure. Solid State Sci. 2010, 12, 808–813. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Chen, X.; Feng, X.; Tan, W.; Qiu, G. Synthesis of hureaulite by a reflux process at ambient temperature and pressure. Microporous Mesoporous Mater. 2012, 153, 115–123. [Google Scholar] [CrossRef]
- Sronsri, C.; U-Yen, K.; Sittipol, W. Application of synthetic hureaulite as a new precursor for the synthesis of lithiophilite nanoparticles. Solid State Sci. 2020, 110, 106469. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; Scholz, R.; López, A.; Belotti, F.M. Vibrational spectroscopic characterization of the phosphate mineral hureaulite—(Mn, Fe)5(PO4)2(HPO4)2·4(H2O). Vib. Spectrosc. 2013, 66, 69–75. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; Bruque, S. Characterization of manganese(III) orthophosphate hydrate. Inorg. Chem. 1990, 29, 1334–1337. [Google Scholar] [CrossRef]
- Boonchom, B.; Youngme, S.; Maensiri, S.; Danvirutai, C. Nanocrystalline serrabrancaite (MnPO4·H2O) prepared by a simple precipitation route at low temperature. J. Alloys Compd. 2008, 454, 78–82. [Google Scholar] [CrossRef]
- Šoptrajanov, B.; Stefov, V.; Kuzmanovski, I.; Jovanovski, G. Fourier transform infrared and Raman spectra of manganese hydrogenphosphate trihydrate. J. Mol. Struct. 1999, 482–483, 103–107. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Dong, L.; Chen, Z.; Lu, H. High-Performance All-Solid-State Supercapacitor Based on the Assembly of Graphene and Manganese(II) Phosphate Nanosheets. J. Phys. Chem. C 2014, 118, 18884–18891. [Google Scholar] [CrossRef]
- Ma, X.-J.; Zhang, W.-B.; Kong, L.-B.; Luo, Y.-C.; Kang, L. Electrochemical performance in alkaline and neutral electrolytes of a manganese phosphate material possessing a broad potential window. RSC Adv. 2016, 6, 40077–40085. [Google Scholar] [CrossRef]
- Mirghni, A.A.; Madito, M.J.; Masikhwa, T.M.; Oyedotun, K.O.; Bello, A.; Manyala, N. Hydrothermal synthesis of manganese phosphate/graphene foam composite for electrochemical supercapacitor applications. J. Colloid Interface Sci. 2017, 494, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.-C.; Xu, M.-W.; Bao, S.-J.; Cai, C.-J.; Wang, R.-Y.; Jia, D.-Z. Effect of alkaline and alkaline–earth cations on the supercapacitor performance of MnO2 with various crystallographic structures. J. Solid State Electrochem. 2013, 17, 1357–1368. [Google Scholar] [CrossRef]
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and Reversible Surface Redox Reaction in Nanocrystalline Vanadium Nitride Supercapacitors. Adv. Mater. 2006, 18, 1178–1182. [Google Scholar] [CrossRef]
- Zhou, M.; Vassallo, A.; Wu, J. Data-Driven Approach to Understanding the In-Operando Performance of Heteroatom-Doped Carbon Electrodes. ACS Appl. Energy Mater. 2020, 3, 5993–6000. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Porto, R.; Gómez, I. Synthesis of Manganese Oxide Nanocompounds for Electrodes in Electrochemical Capacitors. Synth. React. Inorg. Met. Nano-Metal Chem. 2012, 42, 833–838. [Google Scholar] [CrossRef]
- Porto, R.L.; Frappier, R.; Ducros, J.; Aucher, C.; Mosqueda, H.; Chenu, S.; Chavillon, B.; Tessier, F.; Cheviré, F.; Brousse, T. Titanium and vanadium oxynitride powders as pseudo-capacitive materials for electrochemical capacitors. Electrochim. Acta 2012, 82, 257–262. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, Y.; Ju, Z.; Ji, Y.; Yan, Y.; Liu, Y.; Yu, G. Understanding Charge Storage in Hydrated Layered Solids MOPO4 (M = V, Nb) with Tunable Interlayer Chemistry. ACS Nano 2020, 14, 13824–13833. [Google Scholar] [CrossRef]
- Trasatti, S. Effect of the nature of the metal on the dielectric properties of polar liquids at the interface with electrodes. A phenomenological approach. J. Electroanal. Chem. Interfacial Electrochem. 1981, 123, 121–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Zhuo, K.; Cherevko, S.; Kim, W.-J.; Chung, C.-H. Facile preparation of three-dimensional porous hydrous ruthenium oxide electrode for supercapacitors. J. Power Sources 2013, 244, 806–811. [Google Scholar] [CrossRef]
- Brousse, T.; Toupin, M.; Dugas, R.; Athouel, L.; Crosnier, O.; Bélanger, D. Crystalline MnO2 as Possible Alternatives to Amorphous Compounds in Electrochemical Supercapacitors. J. Electrochem. Soc. 2006, 153, A2171. [Google Scholar] [CrossRef]
- De Grotthuss, C.J.T. Mémoire sur la décomposition de l’eau: Et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Ann. Chim. 1806, 58, 54–75. [Google Scholar]
- Marx, D. Proton Transfer 200 Years after von Grotthuss: Insights from Ab Initio Simulations. Chemphyschem 2006, 7, 1848–1870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the Energy Density of Aqueous Batteries via Facile Grotthuss Proton Transport. Angew. Chem. 2020, 133, 4215–4220. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Hao, L.; Cheng, P.; Chen, Y.; Zhang, Z. Grotthuss Proton-Conductive Covalent Organic Frameworks for Efficient Proton Pseudocapacitors. Angew. Chem. Int. Ed. 2021, 60, 21838–21845. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Fregonara, G.; Trasatti, S. Inner and Outer active surface of RuO2 electrodes. Electrochem. Acta 1990, 35, 263. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Polleux, J.; Dunn, B.; Tolbert, S.H. Templated Nanocrystal-Based Porous TiO2 Films for Next-Generation Electrochemical Capacitors. J. Am. Chem. Soc. 2009, 131, 1802–1809. [Google Scholar] [CrossRef]


| Electrode | Areal Capacitance (µFcm−2) | Ref |
|---|---|---|
| Double-Layer Capacitance | 50 | [40,41] |
| Ruthenium Oxides | 390 | [42] |
| Metal Nitrides | 50–300 | [38] |
| Manganese Oxides | 110–123 | [43] |
| MnPO4 3M KOH | 1350 | [13] |
| VOPO4•2H2O in 3M LiOH | 500 | [14] |
| VOPO4•2H2O in 3M KOH | 430 | [14] |
| VOPO4•2H2O in 3M LiNO3 | 240 | [14] |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 3M KOH | 4600 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 1M KOH | 4100 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 3M NaOH | 2870 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 3M LiOH | 1450 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 3M TMAOH | 550 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 1M K3PO4 | 200 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 5M LiNO3 | 400 | This Work |
| Mn5(H2O)4(PO3OH)2(PO4)2 in 1M Na2SO4 | 375 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Guajardo, C.I.; Zúñiga Martínez, J.A.; Berlanga Pérez, R.; López Pavón, L.A.; Lucio Porto, R. Synthesis of Hureaulite Mn5(H2O)4(PO3OH)2(PO4)2 with an Open 3D Network Structure as Electrode Material for Electrochemical Capacitors. Processes 2024, 12, 1622. https://doi.org/10.3390/pr12081622
García Guajardo CI, Zúñiga Martínez JA, Berlanga Pérez R, López Pavón LA, Lucio Porto R. Synthesis of Hureaulite Mn5(H2O)4(PO3OH)2(PO4)2 with an Open 3D Network Structure as Electrode Material for Electrochemical Capacitors. Processes. 2024; 12(8):1622. https://doi.org/10.3390/pr12081622
Chicago/Turabian StyleGarcía Guajardo, Cesar Iván, Jorge Alexis Zúñiga Martínez, Roxana Berlanga Pérez, Luis Alberto López Pavón, and Raúl Lucio Porto. 2024. "Synthesis of Hureaulite Mn5(H2O)4(PO3OH)2(PO4)2 with an Open 3D Network Structure as Electrode Material for Electrochemical Capacitors" Processes 12, no. 8: 1622. https://doi.org/10.3390/pr12081622
APA StyleGarcía Guajardo, C. I., Zúñiga Martínez, J. A., Berlanga Pérez, R., López Pavón, L. A., & Lucio Porto, R. (2024). Synthesis of Hureaulite Mn5(H2O)4(PO3OH)2(PO4)2 with an Open 3D Network Structure as Electrode Material for Electrochemical Capacitors. Processes, 12(8), 1622. https://doi.org/10.3390/pr12081622








