Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents
Abstract
1. Introduction
2. Modelling Approach and Reaction Mechanism
3. Model Validation
4. Result and Discussion
5. Estimation of Diffusion Coefficient and Effect of Co-Solvent on CO2 Diffusion in Various Aqueous and Water-Lean Solvent Systems
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Qian, J.; He, Y. Water-lean triethylenetetramine/N, N-diethylethanolamine/n-propanol biphasic solvents: Phase-separation performance and mechanism for CO2 capture. Sep. Purif. Technol. 2022, 289, 120740. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Luo, J. Life cycle cost analysis of power generation from underground coal gasification with carbon capture and storage (CCS) to measure the economic feasibility. Resour. Policy 2024, 92, 104996. [Google Scholar] [CrossRef]
- Desai, B.H. United Nations Environment Programme (UNEP). Yearb. Int. Environ. Law 2020, 31, 319–325. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. Process Intensif. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Rubin, E.S.; Davison, J.E.; Herzog, H. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents–A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-lean solvents for post-combustion CO2 capture: Fundamentals, uncertainties, opportunities, and outlook. Chem. Rev. 2017, 117, 9594–9624. [Google Scholar] [CrossRef]
- Ping, T.; Dong, Y.; Shen, S. Energy-efficient CO2 capture using nonaqueous absorbents of secondary alkanolamines with a 2-butoxyethanol cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 18071–18082. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. Investigating opportunities for water-lean solvents in CO2 capture: VLE and heat of absorption in water-lean solvents containing MEA. Sep. Purif. Technol. 2020, 231, 115883. [Google Scholar] [CrossRef]
- Wang, N.; Peng, Z.; Gao, H.; Sema, T.; Shi, J.; Liang, Z. New insight and evaluation of secondary Amine/N-butanol biphasic solutions for CO2 Capture: Equilibrium Solubility, phase separation Behavior, absorption Rate, desorption Rate, energy consumption and ion species. Chem. Eng. J. 2022, 431, 133912. [Google Scholar] [CrossRef]
- Shi, X.; Li, C.; Guo, H.; Shen, S. Density, viscosity, and excess properties of binary mixtures of 2-(methylamino) ethanol with 2-methoxyethanol, 2-ethoxyethanol, and 2-butoxyethanol from 293.15 to 353.15 K. J. Chem. Eng. Data 2019, 64, 3960–3970. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Pereira, L.M.; AlHajaj, A.; Vega, L.F. Performance of non-aqueous amine hybrid solvents mixtures for CO2 capture: A study using a molecular-based model. J. CO2 Util. 2020, 35, 126–144. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, P.; He, X.; Yu, Q. Preparation of intercalated MXene by TPAOH and its adsorption characteristics towards U (VI). J. Radioanal. Nucl. Chem. 2024, 333, 1999–2014. [Google Scholar] [CrossRef]
- Garcia, M.; Knuutila, H.K.; Aronu, U.E.; Gu, S. Influence of substitution of water by organic solvents in amine solutions on absorption of CO2. Int. J. Greenh. Gas Control 2018, 78, 286–305. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Hu, L.; Liu, J.; Zhou, J.; Cen, K. Phase-changing solution PZ/DMF for efficient CO2 capture and low corrosiveness to carbon steel. Fuel 2018, 216, 418–426. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, C.; Chen, H.; Shen, Y.; Zhang, S.; Wang, L.; Chen, J. Novel biphasic solvent with tunable phase separation for CO2 capture: Role of water content in mechanism, kinetics, and energy penalty. Environ. Sci. Technol. 2019, 53, 4470–4479. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Pan, T.-H.; Wong, D.S.-H.; Jang, S.-S.; Chi, Y.-W.; Yeh, C.-H. Plantwide control of CO2 capture by absorption and stripping using monoethanolamine solution. Ind. Eng. Chem. Res. 2011, 50, 1338–1345. [Google Scholar] [CrossRef]
- Park, Y.; Lin, K.-Y.A.; Park, A.-H.A.; Petit, C. Recent advances in anhydrous solvents for CO2 capture: Ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front. Energy Res. 2015, 3, 42. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Wang, L.; Li, Q.; Zhang, S.; Chen, B.; Jiang, L.; Zhang, Y. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas. Appl. Energy 2019, 242, 302–310. [Google Scholar] [CrossRef]
- Li, Q.; Gao, G.; Wang, R.; Zhang, S.; An, S.; Wang, L. Role of 1-methylimidazole in regulating the CO2 capture performance of triethylenetetramine-based biphasic solvents. Int. J. Greenh. Gas Control 2021, 108, 103330. [Google Scholar] [CrossRef]
- Hu, H.; Fang, M.; Liu, F.; Wang, T.; Xia, Z.; Zhang, W.; Ge, C.; Yuan, J. Novel alkanolamine-based biphasic solvent for CO2 capture with low energy consumption and phase change mechanism analysis. Appl. Energy 2022, 324, 119570. [Google Scholar] [CrossRef]
- Chronopoulos, T.; Fernandez-Diez, Y.; Maroto-Valer, M.M.; Ocone, R.; Reay, D.A. CO2 desorption via microwave heating for post-combustion carbon capture. Microporous Mesoporous Mater. 2014, 197, 288–290. [Google Scholar] [CrossRef]
- Qian, J.; Sun, R.; Sun, S.; Gao, J. Computer-Free Group-Addition Method for p K a Prediction of 73 Amines for CO2 Capture. J. Chem. Eng. Data 2017, 62, 111–122. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.; Zahra, M.R.A. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. A comparative study of the CO2 absorption in some solvent-free alkanolamines and in aqueous monoethanolamine (MEA). Environ. Sci. Technol. 2016, 50, 7239–7246. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.; Gao, H.; Idem, R.; Tontiwachwuthikul, P.; Olson, W.; Liang, Z. Reaction kinetics of the absorption of carbon dioxide (CO2) in aqueous solutions of sterically hindered secondary alkanolamines using the stopped-flow technique. Chem. Eng. Sci. 2017, 170, 16–25. [Google Scholar] [CrossRef]
- Bihong, L.; Kexuan, Y.; Xiaobin, Z.; Zuoming, Z.; Guohua, J. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture. Appl. Energy 2020, 264, 114703. [Google Scholar] [CrossRef]
- Tan, L.; Shariff, A.; Lau, K.; Bustam, M. Impact of high pressure on high concentration carbon dioxide capture from natural gas by monoethanolamine/N-methyl-2-pyrrolidone solvent in absorption packed column. Int. J. Greenh. Gas Control 2015, 34, 25–30. [Google Scholar] [CrossRef]
- Henni, A.; Mather, A.E. Solubility of carbon dioxide in methyldiethanolamine+ methanol+ water. J. Chem. Eng. Data 1995, 40, 493–495. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Evjen, S.; Pinto, D.D.D.; Knuutila, H.K. The salting-out effect in some physical absorbents for CO2 capture. Chem. Eng. Trans. 2018, 69, 97–102. [Google Scholar]
- Schlecht, M.F. Molecular Modeling on the PC; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Li, Z.; Gan, B.; Li, Z.; Zhang, H.; Wang, D.; Zhang, Y.; Wang, Y. Kinetic mechanisms of methane hydrate replacement and carbon dioxide hydrate reorganization. Chem. Eng. J. 2023, 477, 146973. [Google Scholar] [CrossRef]
- Narimani, M.; Amjad-Iranagh, S.; Modarress, H. Performance of tertiary amines as the absorbents for CO2 capture: Quantum mechanics and molecular dynamics studies. J. Nat. Gas Sci. Eng. 2017, 47, 154–166. [Google Scholar] [CrossRef]
- Song, Z.; Han, D.; Yang, M.; Huang, J.; Shao, X.; Li, H. Formic acid formation via direct hydration reaction (CO+ H2O→ HCOOH) on magnesia-silver composite. Appl. Surf. Sci. 2023, 607, 155067. [Google Scholar] [CrossRef]
- Sharif, M.; Fan, H.; Sultan, S.; Yu, Y.; Zhang, T.; Wu, X.; Zhang, Z. Evaluation of CO2 absorption and stripping process for primary and secondary amines. Mol. Simul. 2023, 49, 565–575. [Google Scholar] [CrossRef]
- Biovia. Material Studio, Biovia: 2019 Version; Available online: https://www.3ds.com/products/biovia/materials-studio (accessed on 24 July 2024).
- Meunier, M. Diffusion coefficients of small gas molecules in amorphous cis-1, 4-polybutadiene estimated by molecular dynamics simulations. J. Chem. Phys. 2005, 123, 134906. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Zhang, G.; Liu, J.; Qian, J.; Zhang, X.; Liu, Z. Review of Research Progress and Stability Studies of Amine-based Biphasic Absorbents for CO2 Capture. J. Ind. Eng. Chem. 2024, 134, 28–50. [Google Scholar] [CrossRef]
- Shamiri, A.; Shafeeyan, M.; Tee, H.; Leo, C.; Aroua, M.; Aghamohammadi, N. Absorption of CO2 into aqueous mixtures of glycerol and monoethanolamine. J. Nat. Gas Sci. Eng. 2016, 35, 605–613. [Google Scholar] [CrossRef]
- Olyaei, E.; Hafizi, A.; Rahimpour, M. Low energy phase change CO2 absorption using water-lean mixtures of glycine amino acid: Effect of co-solvent. J. Mol. Liq. 2021, 336, 116286. [Google Scholar] [CrossRef]
- Versteeg, G.; van Swaaij, W.P.M. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions—II. Tertiary amines. Chem. Eng. Sci. 1988, 43, 587–591. [Google Scholar] [CrossRef]
- Laddha, S.; Danckwerts, P. Reaction of CO2 with ethanolamines: Kinetics from gas-absorption. Chem. Eng. Sci. 1981, 36, 479–482. [Google Scholar] [CrossRef]
- Matsubara, H.; Pichierri, F.; Kurihara, K. Mechanism of diffusion slowdown in confined liquids. Phys. Rev. Lett. 2012, 109, 197801. [Google Scholar] [CrossRef]
- Chemspider. Royal Society of Chemistry Database. 2020. Available online: https://www.chemspider.com/ (accessed on 24 July 2024).
- Yaws, C.L. Yaws’ Thermophysical Properties of Chemicals and Hydrocarbons; Knovel: New York, NY, USA, 2009. [Google Scholar]
- Harun, N.; Masiren, E. Molecular dynamic simulation of amine-CO2 absorption process. Indian J. Sci. Technol. 2017, 10. [Google Scholar] [CrossRef][Green Version]
- Masiren, E.; Harun, N.; Ibrahim, W.; Adam, F. Intermolecular Interaction of Monoethanolamine, Diethanolamine, Methyl diethanolamine, 2-Amino-2-methyl-1-propanol and Piperazine Amines in Absorption Process to Capture CO2 using Molecular Dynamic Simulation Approach. Universiti Malasiya Pahang. The National Conference for Postgraduate Researc. 2016. Available online: http://umpir.ump.edu.my/id/eprint/15835/1/P053%20pg385-397.pdf (accessed on 24 July 2024).
- Yuan, Y.; Rochelle, G.T. CO2 absorption rate in semi-aqueous monoethanolamine. Chem. Eng. Sci. 2018, 182, 56–66. [Google Scholar] [CrossRef]
- Sharif, M.; Han, T.; Wang, T.; Shi, X.; Fang, M.; Shuming, D.; Meng, R.; Gao, X. Investigation of Rational Design of Amine Solvents for CO2 Capture: A Computational Approach. Chem. Eng. Res. Des. 2024, 204, 524–535. [Google Scholar] [CrossRef]
- Sharif, M.; Wang, T.; Xu, Y.; Fang, M.; Wu, H.; Gao, X. Evaluating solvent efficiency for carbon capture: Comparative analysis of temperature, concentration and diffusivity effects. Geoenergy Sci. Eng. 2024, 237, 212833. [Google Scholar] [CrossRef]
- Al-Ghawas, H.A.; Hagewiesche, D.P.; Ruiz-Ibanez, G.; Sandall, O.C. Physicochemical properties important for carbon dioxide absorption in aqueous methyldiethanolamine. J. Chem. Eng. Data 1989, 34, 385–391. [Google Scholar] [CrossRef]
- Varady, M.J.; Knox, C.K.; Cabalo, J.B.; Bringuier, S.A.; Pearl, T.P.; Lambeth, R.H.; Mantooth, B.A. Molecular dynamics study of competing hydrogen bonding interactions in multicomponent diffusion in polyurethanes. Polymer 2018, 140, 140–149. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.; Puxty, G.; Yu, H.; Conway, W.; Fang, M.; Wang, T.; Mulder, R.J. Diffusivity in Novel Diamine-Based Water-Lean Absorbent Systems for CO2 Capture Applications. Ind. Eng. Chem. Res. 2022, 61, 12493–12503. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-W.; Choi, B.-S.; Lee, J.-W. Absorption of carbon dioxide into non-aqueous solutions of N-methyldiethanolamine. Korean J. Chem. Eng. 2006, 23, 806–811. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhang, C.-F.; Zheng, Z.-S. Solubility of hydrogen sulfide and carbon dioxide in a solution of methyldiethanolamine mixed with ethylene glycol. Ind. Eng. Chem. Res. 2002, 41, 6175–6180. [Google Scholar] [CrossRef]


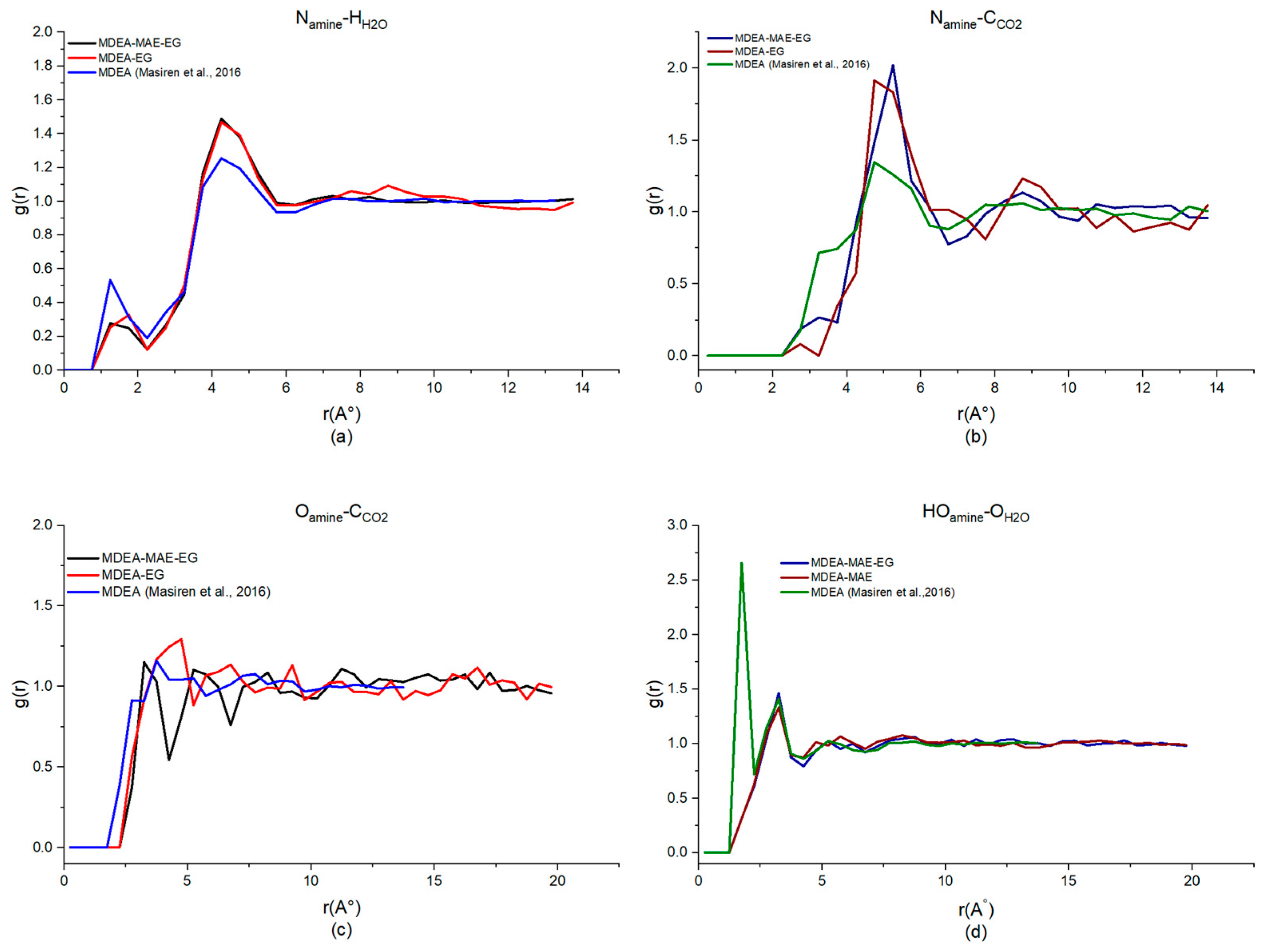
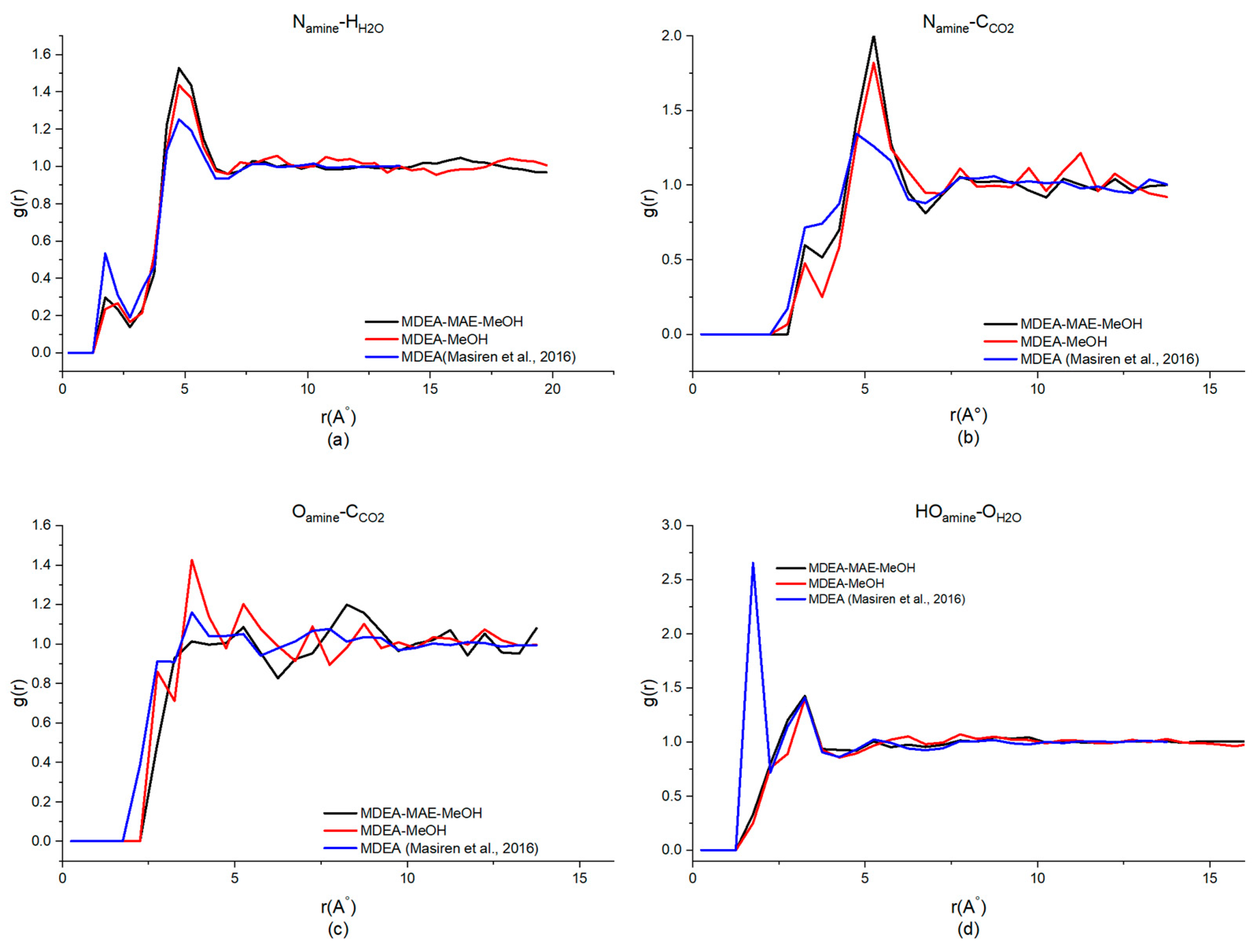
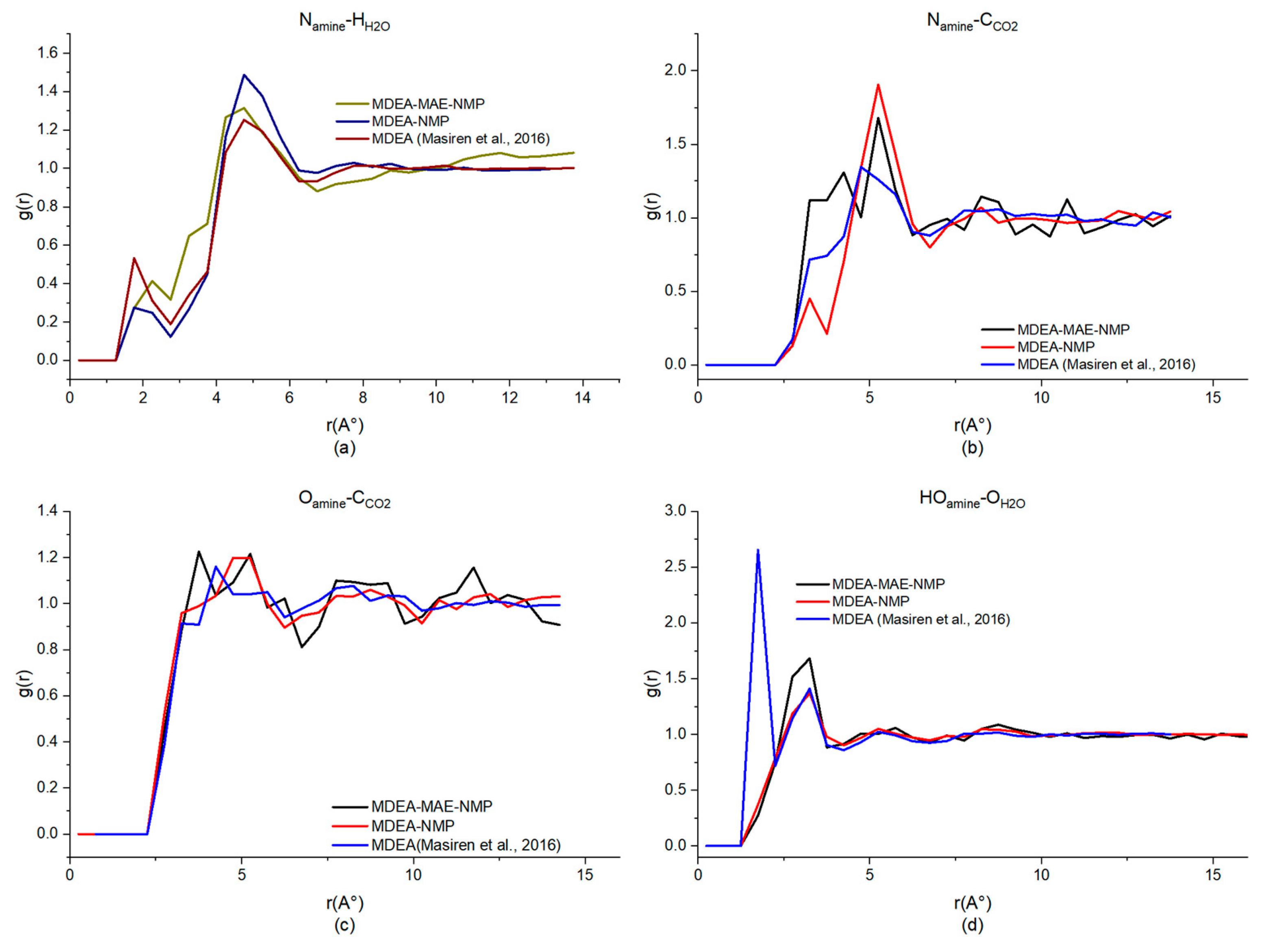
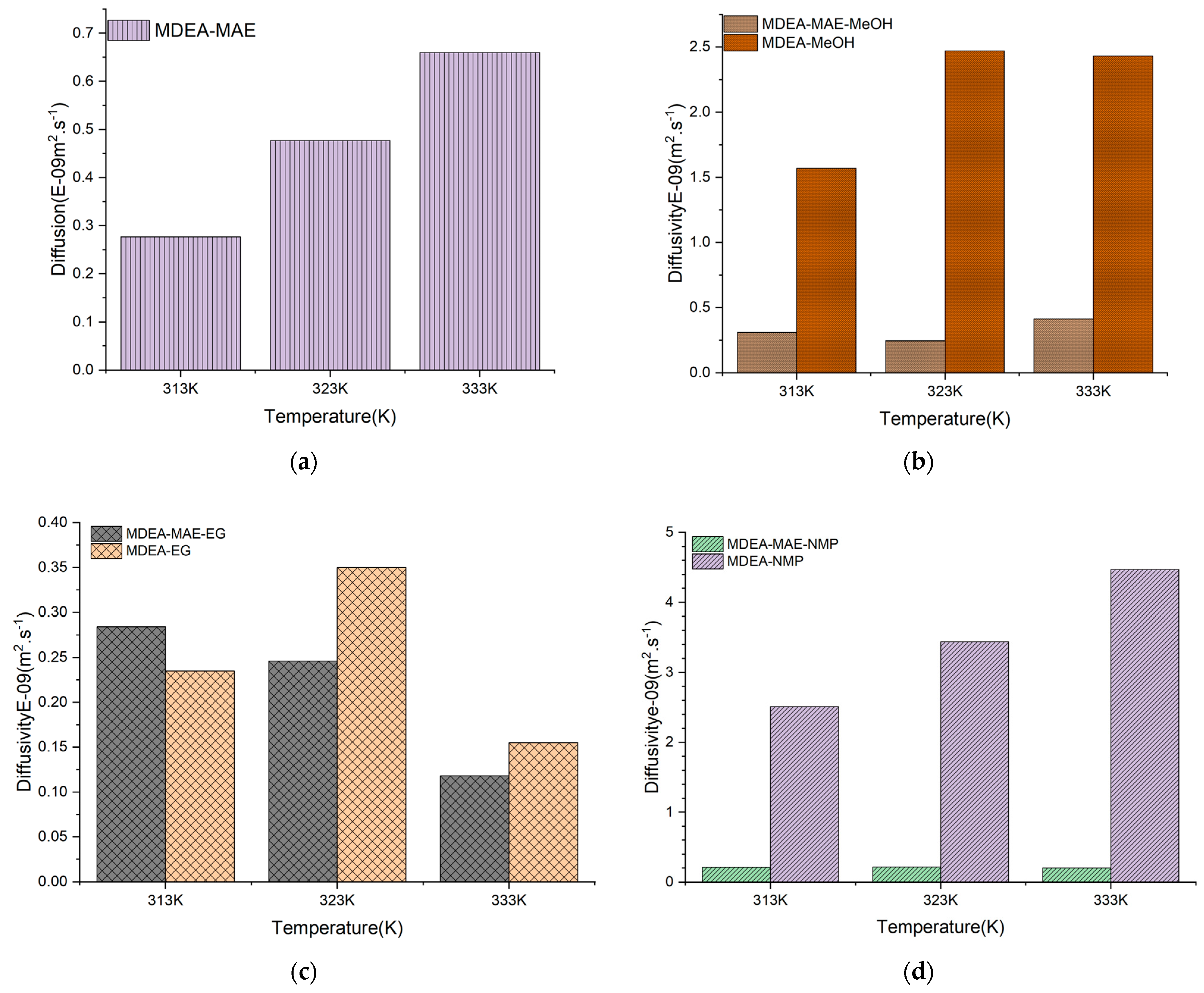

| Generation | Type of Solvents | Energy Consumption (GJ/tCO2) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| 1st Generation 1930s | Amine Solvents 30 wt% MEA PZ aqueous solvents | 3.7–4.0 | Fast reaction rate High capture capacity | High energy penalty solvent regeneration | [21] |
| 2nd Generation 1990s | Blended amine solvent | 2.5–3.2 | Improved capture efficiency Low energy compared to single amine | Possible increased volatility and corrosiveness depending on the blend | [22] |
| 3rd Generation 2000s | Water-lean solvent/Phase-change Absorbent | 1.8–2.4 | Lower overall energy consumption compared to aqueous amine systems Lower water content reduce energy consumption for regeneration Enhanced selectivity and capacity for CO2 capture | Higher viscosity can lead to mass transfer limitations Limited long term operation data available | [23] |
| Name | Molecular Structure | Molecular Weight (g.mol−1) | Density (g.mL−1) | CAS No. |
|---|---|---|---|---|
| MDEA | 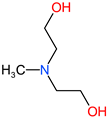 | 119 | 1.1 | 105-59-9 |
| MeOH | 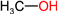 | 32 | 0.80 | 67-56-1 |
| MAE | 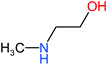 | 75 | 0.94 | 109-83-1 |
| EG | 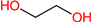 | 64 | 1.1 | 107-21-1 |
| NMP |  | 99 | 1.03 | 872-50-4 |
| System | Descriptions | MDEA | NMP | CO2 | H2O | |
|---|---|---|---|---|---|---|
| MDEA-NMP (40Wt%H2O) | Density of mixture (g.mL−1) | 1.053 | ||||
| No. of molecules | 12 | 10 | 11 | 111 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| MDEA-MeOH (40Wt%H2O) | Descriptions | MDEA | MeOH | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.007 | |||||
| No. of molecules | 120 | 310 | 220 | 2220 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| MDEA-EG (40Wt%H2O) | Descriptions | MDEA | EG | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.067 | |||||
| No. of molecules | 44 | 54 | 40 | 80 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| System | Descriptions | MDEA | MAE | NMP | CO2 | H2O |
| MDEA-MAE-NMP (30%H2O) | Density of mixture (g.mL−1) | 1.044 | ||||
| No. of molecules | 25 | 13 | 20 | 22 | 166 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-MEG (30%H2O) | Descriptions | MDEA | MAE | MEG | CO2 | H2O |
| Density of mixture (g.mL−1) | 1.058 | |||||
| No. of molecules | 252 | 133 | 312 | 222 | 1666 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-MeOH (30%H2O) | Descriptions | MDEA | MAE | MeOH | CO2 | H2O |
| Density of mixture (g.mL−1) | 1.002 | |||||
| No. of molecules | 12 | 6 | 30 | 11 | 83 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-H2O (50%H2O) | Descriptions | MDEA | MAE | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.04 | |||||
| No. of molecules | 25 | 13 | 22 | 277 | ||
| Weight% | 30% | 10% | 10% | 50% |
| Name of Solvent | Experimental Density (X1) [47] | Simulation Density (X2) Present Work | Std. Deviation |
|---|---|---|---|
| NMP | 1.03 | 0.99 | (−) 0.02 |
| MEG | 1.11 | 1.07 | (−) 0.02 |
| MDEA | 1.10 | 1.05 | (−) 0.025 |
| MAE | 0.940 | 0.945 | (+) 0.0025 |
| MeOH | 0.80 | 0.79 | (−) 0.005 |
| Solvent System | Initial Density (X1) | Final Density(X2) | Std. Deviation |
| MDEA-MAE-EG | 1.058 | 0.99 | (−) 0.034 |
| MDEA-EG | 1.067 | 0.99 | (−) 0.0385 |
| MDEA-MAE-MeOH | 1.002 | 0.90 | (−) 0.051 |
| MDEA-MeOH | 1.007 | 0.89 | (−) 0.0585 |
| MDEA-MAE-NMP | 1.044 | 0.92 | (−) 0.062 |
| MDEA-NMP | 1.053 | 0.90 | (−) 0.0765 |
| MDEA-MAE | 1.040 | 0.90 | (−) 0.070 |
| System | Observed Interactions | |||
|---|---|---|---|---|
| Namine-HH2O | Namine-CCO2 | Oamine-CCO2 | HOamine-OH2O | |
| MDEA-NMP (40%H2O) | 4.75, 1.49 | 5.25, 1.90 | 5.25, 1.20 | 3.25, 1.37 |
| MDEA-MAE-NMP (30%H2O) | 4.75, 1.31 | 5.25, 1.67 | 5.25, 1.21 | 3.25, 1.68 |
| MDEA-EG (40%H2O) | 4.75, 1.47 | 4.75, 1.91 | 4.75, 1.29 | 3.25, 1.33 |
| MDEA-MAE-EG (30%H2O) | 4.75, 1.22 | 5.25, 2.02 | 5.25, 1.10 | 3.25, 1.45 |
| MDEA-MeOH (40%H2O) | 4.75, 1.44 | 5.25, 1.82 | 3.75, 1.43 | 3.25, 1.40 |
| MDEA-MAE-MeOH (30%H2O) | 4.75, 1.53 | 5.25, 2.01 | 3.25, 0.92 | 3.25, 1.43 |
| MDEA-MAE (50%H2O) | 4.75, 1.29 | 5.25, 1.67 | 5.25, 1.18 | 3.25, 1.35 |
| MDEA [49,50] | 4.25, 1.25 | 4.75, 1.34 | 3.75, 1.14 | 1.75, 2.65 |
| Solvent System * | Interactions Results | Advantages | Challenges |
|---|---|---|---|
| Methanol | Strong CO2 interaction, slightly weaker solubility | Low molecular weight High solubility Effective interactions with amine and CO2 Enhances Hydrogen bonding | High Volatility Evaporation loss Handling issue [7,8] |
| N-Methyl-2-Pyrolidone | Improved stability and strong hydrogen bonds | High boiling point Strong solvation properties Improved stability for CO2 capture Higher interaction with CO2 Significantly enhances CO2 capture efficiency | Higher viscosity Affects flow properties Increased energy requirement for solvent circulation [48] |
| Ethylene Glycol | Strongest CO2 interaction, enhanced stability | Hygroscopic Nature Interaction shows High affinity for water Stable interactions Balances strong hydrogen bonding with effective CO2 capture | Higher molecular weight Potential viscosity issues [41] |
| MDEA-MAE-Aqueous Solvent | Moderate CO2 capture and stability | Increased hydrogen bonding with water Enhanced CO2 capture efficiency Stable interactions due to higher water content | Possible handling and processing challenges due to higher water content Higher energy requirement for solvent regeneration [49,51] |
| Co-Solvent | Water Concentration | Generalized Impact |
|---|---|---|
| NMP | 30%H2O and 40%H2O | Exhibits stable CO2 interaction across different water concentrations, making it a versatile co-solvent. |
| EG | 30%H2O and 40%H2O | Shows higher CO2 interaction at 40%H2O, indicating better performance at higher water content but is temperature-sensitive. |
| MeOH | 30%H2O and 40%H2O | Demonstrates higher CO2 interaction at 40%H2O, but has handling challenges that must be managed. |
| MDEA-MAE (No Co-Solvent) | 50%H2O | Provides effective CO2 interactions with a good balance at 50%H2O, but lower than with Co-solvent blends |
| Solvent System | CO2 Diffusivity (m2.s−1)/Temperature | ||
|---|---|---|---|
| 313 K | 323 K | 333 K | |
| MDEA-NMP (40%H2O) | 2.51 × 10−9 | 3.44 × 10−9 | 4.47 × 10−9 |
| MDEA-MAE-NMP (30%H2O) | 0.210 × 10−9 | 0.213 × 10−9 | 0.202 × 10−9 |
| MDEA-MeOH (40%H2O) | 1.57 × 10−9 | 2.47 × 10−9 | 3.06 × 10−9 |
| MDEA-MAE-MeOH (30%H2O) | 0.308 × 10−9 | 0.246 × 10−9 | 0.413 × 10−9 |
| MDEA-EG (40%H2O) | 0.235 × 10−9 | 0.350 × 10−9 | 0.155 × 10−9 |
| MDEA-MAE-EG (30%H2O) | 0.284 × 10−9 | 0.244 × 10−9 | 0.1180 × 10−9 |
| MDEA-MAE (50%H2O) | 0.276 × 10−9 | 0.477 × 10−9 | 0.660 × 10−9 |
| 50wt%MDEA | 0.62 × 10−9 (298 K) | 1.20 × 10−9 (313 K) | 1.80 × 10−9 (323 K) |
| CO2 in pure H2O | 1.61 × 10−9 (298 K) | 2.66 × 10−9 (313 K) | 3.78 × 10−9 (323 K) |
| System | MDEA-50wt% | Reference | ||
|---|---|---|---|---|
| Temperature (K) | 298 | 313 | 323 | |
| CO2 Diffusivity in MDEA-50wt% (m2.s−1) | 0.622 × 10−9 | 1.214 × 10−9 | 1.68 × 10−9 1.70 × 10−9 | [54] |
| 0.624 × 10−9 | 1.204 × 10−9 | 1.80 × 10−9 | This work | |
| Density (g.mL−1) of MDEA mixture | 1.0427 | 1.0331 | 1.0269 | [54] |
| 1.047 | 1.047 | 1.047 | This work | |
| System | Pure H2O | Reference | ||
| CO2 Diffusivity (m2.s−1) in H2O | 1.93 × 10−9 | 2.71 × 10−9 | 3.34 × 10−9 | [54] |
| 1.61 × 10−9 | 2.66 × 10−9 | 3.78 × 10−9 | This work | |
| Density of mixture (g.mL−1) | 0.9970 | 0.9922 | 0.9880 | [54] |
| 1.00 | 1.00 | 1.00 | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, M.; Ge, C.; Wang, T.; Zhang, W.; Fang, M.; Gao, X. Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents. Processes 2024, 12, 1588. https://doi.org/10.3390/pr12081588
Sharif M, Ge C, Wang T, Zhang W, Fang M, Gao X. Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents. Processes. 2024; 12(8):1588. https://doi.org/10.3390/pr12081588
Chicago/Turabian StyleSharif, Maimoona, Chunliang Ge, Tao Wang, Wei Zhang, Mengxiang Fang, and Xiang Gao. 2024. "Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents" Processes 12, no. 8: 1588. https://doi.org/10.3390/pr12081588
APA StyleSharif, M., Ge, C., Wang, T., Zhang, W., Fang, M., & Gao, X. (2024). Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents. Processes, 12(8), 1588. https://doi.org/10.3390/pr12081588








