Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Microgreens Samples
2.2. Supramolecular Solvent-Based Extraction
2.3. Bioactive Compound Contents and Antioxidant Activity
2.4. Taguchi Experimental Design
2.5. Mathematical Analysis
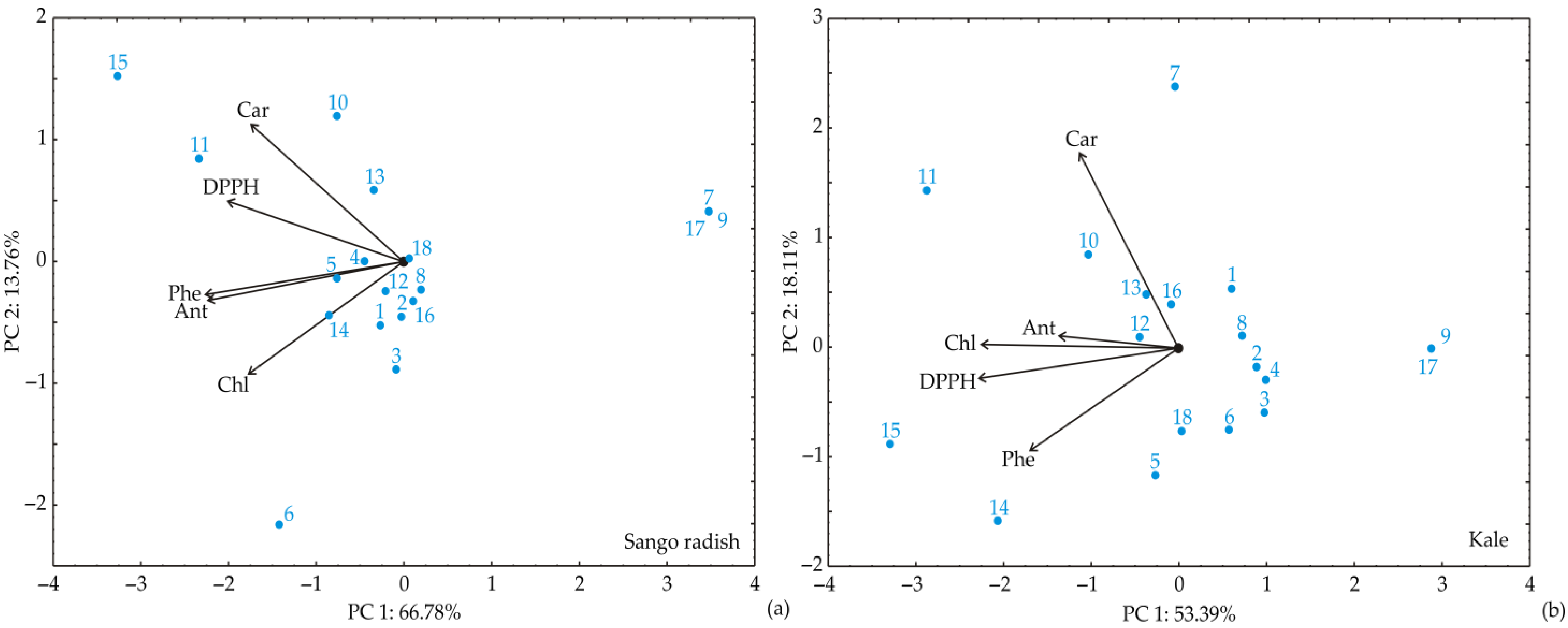
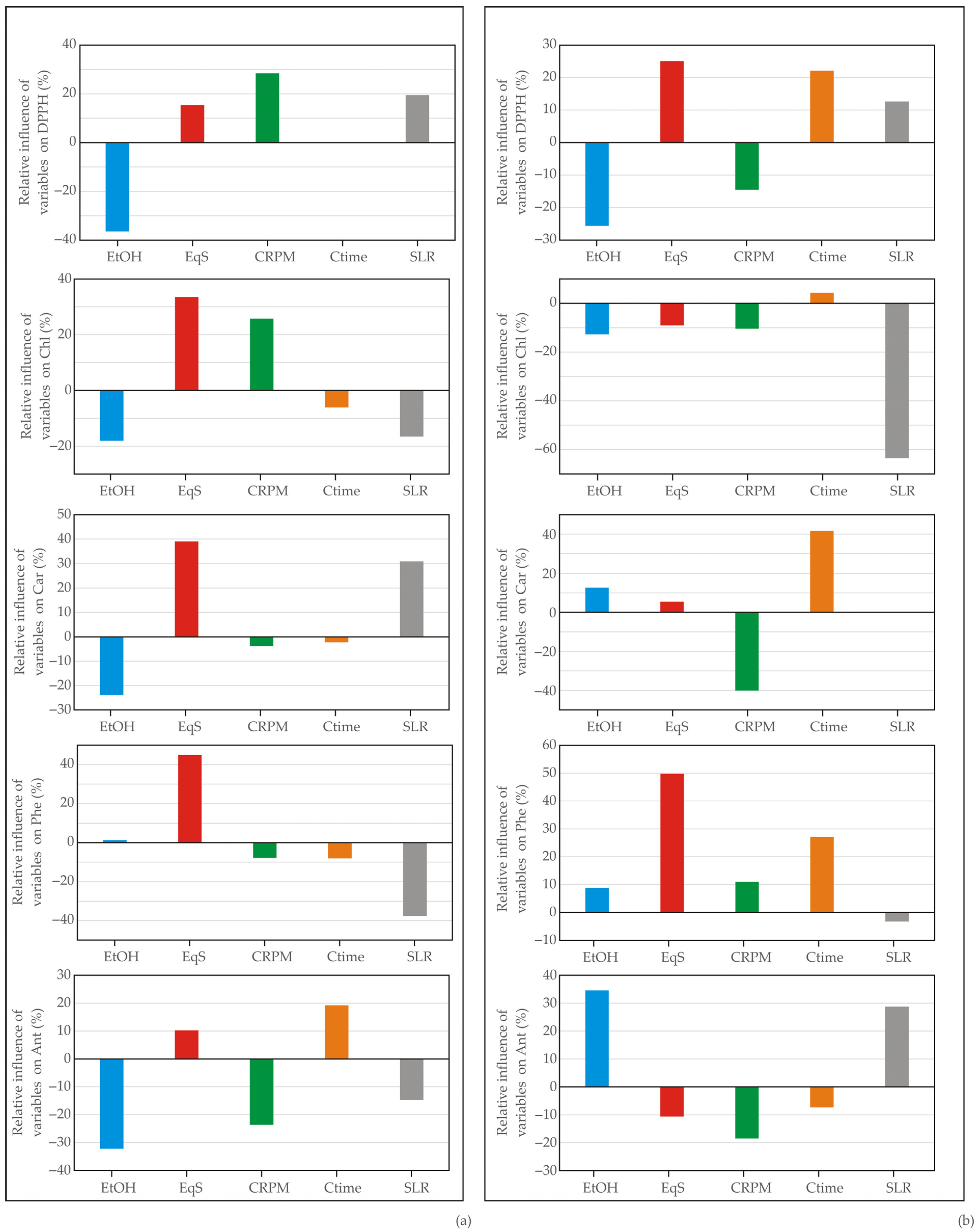
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mir, S.; Shah, M.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Santamaria, P.; Caponio, F. Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens. Foods 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Park, B.-J.; Kang, H.M.; Lee, Y.-T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2019, 309, 125763. [Google Scholar] [CrossRef] [PubMed]
- Komeroski, M.; Rios, A.; Flôres, S.H.; Klug, T. Overview on bioactive compounds’ profile of Brassicaceae microgreens: An approach on different production systems and the use of elicitors. Acta Bot. Bras. 2023, 37, e20230113. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Xie, Z.; Yu, L.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular solvent extraction of bioactives from coffee cherry pulp. J. Food Eng. 2020, 278, 109933. [Google Scholar] [CrossRef]
- Moral, A.; Caballo, C.; Sicilia, M.D. Highly efficient microextraction of chlorophenoxy acid herbicides in natural waters using a decanoic acid-based nanostructured solvent prior to their quantitation by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2012, 709, 59–65. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Sicilia, M.D.; Rubio, S. Supramolecular solvents in the extraction of organic compounds. A review. Anal. Chim. Acta 2010, 677, 108–130. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamadon, A.M.; Niakousari, M. Optimization of ultrasound-assisted extraction of anthocyanins from red cabbage using Taguchi design method. J. Food Sci. Technol. 2015, 52, 8140–8147. [Google Scholar] [CrossRef] [PubMed]
- Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency. Polymers 2022, 14, 3282. [Google Scholar] [CrossRef] [PubMed]
- Jagirani, M.S.; Soylak, M. Supramolecular solvents: A review of a modern innovation in liquid-phase microextraction technique. Turk. J. Chem. 2021, 45, 1651–1677. [Google Scholar] [PubMed]
- Cardeñosa, V.; Lunar, M.L.; Rubio, S. Generalized and rapid supramolecular solvent-based sample treatment for the determination of annatto in food. J. Chromatogr. A 2011, 1218, 8996–9002. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šeregelj, V.; Šovljanski, O.; Tumbas Šaponjac, V.; Švarc Gajić, J.; Brezo-Borjan, T.M.; Pezo, L. In vitro antioxidant, antihyperglycemic, anti-inflammatory, and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crops Prod. 2021, 169, 113672. [Google Scholar] [CrossRef]
- Šovljanski, O.; Lončar, B.; Pezo, L.; Saveljić, A.; Tomić, A.; Brunet, S.; Filipović, V.; Filipović, J.; Čanadanović-Brunet, J.; Ćetković, G.; et al. Unlocking the Potential of the ANN Optimization in Sweet Potato Varieties Drying Processes. Foods 2023, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Šovljanski, O.; Šeregelj, V.; Pezo, L.; Tumbas Šaponjac, V.; Vulić, J.; Cvanić, T.; Markov, S.; Ćetković, G.; Čanadanović-Brunet, J. Horned Melon Pulp, Peel, and Seed: New Insight into Phytochemical and Biological Properties. Antioxidants 2022, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Krunić, M. Anthocyanin profiles and biological properties of caneberry (Rubus spp.) press residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Brlek, T.; Pezo, L.; Voća, N.; Krička, T.; Vukmirović, Đ.; Čolović, R.; Bodroža-Solarov, M. Chemometric approach for assessing the quality of olive cake pellets. Fuel Proc. Technol. 2013, 116, 250–256. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Grahovac, J.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of relative influence of operational and environmental factors. J. Biotechnol. 2022, 350, 31–41. [Google Scholar] [CrossRef]
- Šovljanski, O.; Saveljić, A.; Tomić, A.; Šeregelj, V.; Lončar, B.; Cvetković, D.; Ranitović, A.; Pezo, L.; Ćetković, G.; Markov, S.; et al. Carotenoid-Producing Yeasts: Selection of the Best-Performing Strain and the Total Carotenoid Extraction Procedure. Processes 2022, 10, 1699. [Google Scholar] [CrossRef]
- Goldberg, D.E. Genetic Algorithms in Search, Optimization and Machine Learning, 1st ed.; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1989. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energies 2019, 159, 1075–1087. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Shamsuddin, A.H.; Ong, H.C.; Milano, J.; Kusumo, F.; Sebayang, A.H.; Dharma, S.; Ibrahim, H.; Husin, H.; et al. Optimization of Cerbera manghas biodiesel production using artificial neural networks integrated with ant colony optimization. Energies 2019, 12, 3811. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from polana raspberry (Rubus idaeus L.) fruit and juice—In Vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, A.; Gaspar, P.; Neves, A.R.; Forster, J. Engineering of microbial cell factories for the production of plant polyphenols with health-beneficial properties. Curr. Pharm. Des. 2018, 24, 2208–2225. [Google Scholar] [CrossRef] [PubMed]
- Gunjal, M.; Singh, J.; Kaur, J.; Kaur, S.; Nanda, V.; Mehta, C.M.; Bhadariya, V.; Rasane, P. Comparative analysis of morphological, nutritional, and bioactive properties of selected microgreens in alternative growing medium. S. Afr. J. Bot. 2024, 165, 188–201. [Google Scholar] [CrossRef]
- Toro, M.T.; Fustos-Toribio, R.; Ortiz, J.; Becerra, J.; Zapata, N.; López-Belchí, M.D. Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High-Temperature Stress. Antioxidants 2024, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Makhal, P.; Jaryal, S.; More, N.; Kaki, V.R. Anthocyanins: Plant-based flavonoid pigments with diverse biological activities. Int. J. Plant Based Pharm. 2020, 2, 118–127. [Google Scholar] [CrossRef]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens: A novel and promissory source of sustainable bioactive compounds. Cur. Res. Food Sci. 2023, 100480. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, B.; Shi, J.; Chu, J.; Zhang, X.; Shi, Q. Efficient preparation of caffeoylquinic acids from the flowers of Artemisia anomala by supramolecular solvent/equilibrium solution extraction followed by re-extraction. Sep. Purif. Technol. 2021, 265, 118478. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Osabutey, J.; Mintah, B.K.; Tano-Debrah, K.; Ma, Y. Cleavage of macromolecule (protein/polysaccharide)-phenolic bond in soybean cell wall through Lactobacillus casei and Lactobacillus helviticus mixed culture solid-state fermentation for chlorogenic acid extraction. Food Biosci. 2023, 55, 102903. [Google Scholar] [CrossRef]
- Boateng, I.D.; Kumar, R.; Daubert, C.R.; Flint-Garcia, S.; Mustapha, A.; Kuehnel, L.; Agliata, J.; Li, Q.; Wan, C.; Somavat, P. Sonoprocessing improves phenolics profile, antioxidant capacity, structure, and product qualities of purple corn pericarp extract. Ultrason. Sonochem. 2023, 95, 106418. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Aboagarib, E.A.A.; Yan, D.; Li, M.; Wahia, H.; Mustapha, A.T.; Zhou, C.; Ma, H. Novel two-pot approach ultrasonication and deep eutectic solvent pretreatments for watermelon rind delignification: Parametric screening and optimization via response surface methodology. Energy 2020, 203, 117872. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Ferreira, S.L.C.; Novaes, C.G.; Dos Santos, A.M.P.; Valasques, G.S.; da Mata Cerqueira, U.M.F.; dos Santos Alves, J.P. Simultaneous optimization of multiple responses and its application in Analytical Chemistry–A review. Talanta 2019, 194, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D. Application of graphical optimization, desirability, and multiple response functions in the extraction of food bioactive compounds. Food Eng. Rev. 2023, 15, 309–328. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N. Novel solid-state fermentation extraction of 5-O-caffeoylquinic acid from heilong48 soybean using Lactobacillus helviticus: Parametric screening and optimization. LWT-Food Sci. Technol. 2021, 149, 111809. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, J.; Tang, Y.; Wang, X.; Zhang, J.; Yao, X.; Jiang, W.; Duan, J.A. Optimization of Ultrasound-Assisted Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Functional Components and Removal of Toxic Components from Ginkgo biloba Leaves. BioMed Res. Int. 2018, 2018, 4598067. [Google Scholar] [CrossRef]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria × ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 2023, 29, 1–13. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella thermophila: Optimization of process parameters and modelling by artificial neural network. Proc. Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Silva, E.M.; Rogez, J. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and Isolation of Phenolic Compounds. Nat. Prod. Isol. 2012, 427–464. [Google Scholar]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.L.G.; Kudo, M.; Lopes, C.T.; Tarley, C. Development and multivariate optimization of nanostructured supramolecular liquid-liquid microextraction validated method for highly sensitive determination of methyl parathion in water samples. J. Molec. Liq. 2020, 308, 113026. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A. A green and efficient vortex-assisted liquid-phase microextraction based on supramolecular solvent for UV-VIS determination of nitrite in processed meat and chicken products. Food Chem. 2020, 332, 127395. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Elik, A.; Altunay, N. Ultrasound-assisted supramolecular solvent dispersive liquid-liquid microextraction for preconcentration and determination of Cr(VI) in waters and total chromium in beverages and vegetables. J. Mol. Liq. 2021, 329, 115556. [Google Scholar] [CrossRef]
- Kashanaki, R.; Ebrahimzadeh, H.; Moradi, M. Ultrasound-assisted supramolecular solvent microextraction coupled with graphite furnace atomic absorption spectrometry for speciation analysis of inorganic arsenic. Anal. Met. 2017, 9, 3121–3127. [Google Scholar] [CrossRef]



| Independent Variables | Coded Symbol | Levels of Variation in the Coded and Examined Form | |||
|---|---|---|---|---|---|
| 1 (Low) | 2 (Middle) | 3 (High) | |||
| Ethanol (%) | X1 | 16 | / | 36 | |
| SUPRAS: Equilibrium ratio (mL/mL) | X2 | 0.5 | 1 | 2 | |
| Centrifugation rate (rpm) | X3 | 3000 | 3500 | 4000 | |
| Centrifugation time (min) | X4 | 10 | 20 | 30 | |
| Solid-liquid ratio (mg/mL) | X5 | 10 | 30 | 50 | |
| Dependent variables | Coded Symbol | Constraints | |||
| DPPH (µM TE/100 g) | Y1 | “Larger is better” (Maximize value) | |||
| Chlorophylls (mg/100 g) | Y2 | ||||
| Carotenoids (mg/100 g) | Y3 | ||||
| Phenolics (mg/100 g) | Y4 | ||||
| Anthocyanidins (mg/100 g) | Y5 | ||||
| Experimental design | |||||
| Run | X1 | X2 | X3 | X4 | X5 |
| 1 | 16 | 0.5 | 3000 | 10 | 10 |
| 2 | 16 | 0.5 | 3500 | 20 | 30 |
| 3 | 16 | 0.5 | 4000 | 30 | 50 |
| 4 | 16 | 1 | 3000 | 10 | 30 |
| 5 | 16 | 1 | 3500 | 20 | 50 |
| 6 | 16 | 1 | 4000 | 30 | 10 |
| 7 | 16 | 2 | 3000 | 20 | 50 |
| 8 | 16 | 2 | 3500 | 30 | 10 |
| 9 | 16 | 2 | 4000 | 10 | 30 |
| 10 | 36 | 0.5 | 3000 | 20 | 10 |
| 11 | 36 | 0.5 | 3500 | 30 | 30 |
| 12 | 36 | 0.5 | 4000 | 10 | 50 |
| 13 | 36 | 1 | 3000 | 30 | 50 |
| 14 | 36 | 1 | 3500 | 10 | 10 |
| 15 | 36 | 1 | 4000 | 20 | 30 |
| 16 | 36 | 2 | 3000 | 20 | 30 |
| 17 | 36 | 2 | 3500 | 10 | 50 |
| 18 | 36 | 2 | 4000 | 20 | 10 |
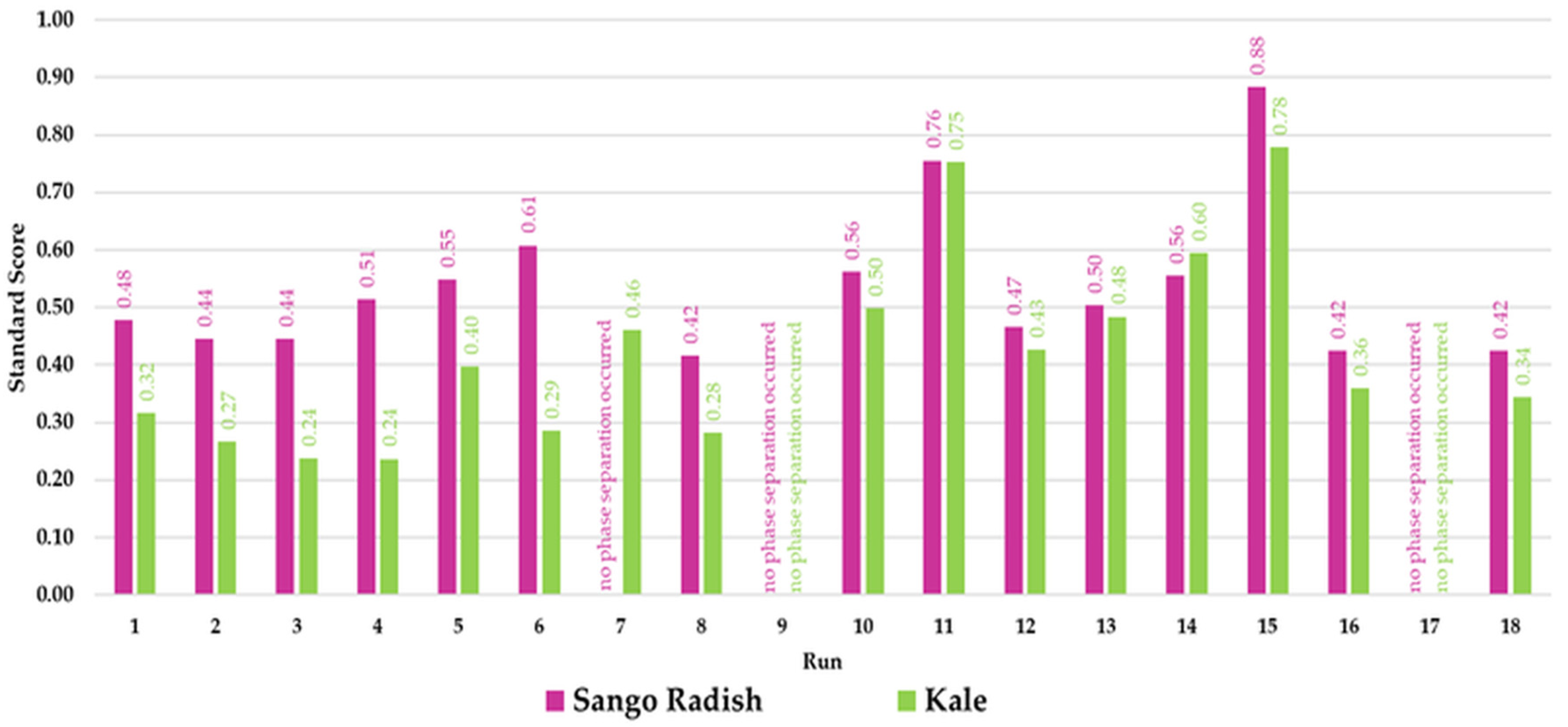
| Run | Sango Radish Microgreen Sample | ||||
|---|---|---|---|---|---|
| DPPH (µM TE/100 g) | Chlorophylls (mg/100 g) | Carotenoids (mg/100 g) | Phenolics (mg/100 g) | Anthocyanidins (mg/100 g) | |
| 1 | 1771.51 ± 15.03 d | 956.47 ± 7.97 f | 3.57 ± 0.07 e | 4728.83 ± 5.59 de | 466.14 ± 21.84 ab |
| 2 | 1386.13 ± 42.74 c | 625.30 ± 8.97 d | 2.68 ± 0.44 de | 4615.69 ± 129.52 cd | 591.00 ± 4.08 ef |
| 3 | 1147.01 ± 40.42 b | 417.08 ± 2.31 a | 0.33 ± 0.03 a | 6027.29 ± 48.34 h | 693.54 ± 1.67 hi |
| 4 | 1011.41 ± 35.22 b | 1017.31 ± 43.79 fg | 7.52 ± 0.16 h | 3318.34 ± 165.86 a | 599.64 ± 7.21 f |
| 5 | 2368.71 ± 19.63 f | 1051.44 ± 31.78 g | 5.66 ± 0.07 g | 4386.59 ± 136.30 c | 522.52 ± 6.14 d |
| 6 | 2688.31 ± 29.63 g | 1996.87 ± 48.85 h | 1.16 ± 0.01 ab | 4989.46 ± 61.40 efg | 564.37 ± 3.65 e |
| 7 | No phase separation occurred | ||||
| 8 | 1802.21 ± 13.86 d | 471.46 ± 5.20 abc | 2.19 ± 0.07 cd | 3303.62 ± 94.36 a | 672.40 ± 14.77 gh |
| 9 | No phase separation occurred | ||||
| 10 | 2841.43 ± 33.85 h | 681.33 ± 20.63 d | 9.43 ± 0.29 i | 3845.89 ± 98.44 b | 477.19 ± 7.37 bc |
| 11 | 4589.02 ± 94.08 j | 859.81 ± 13.26 e | 8.79 ± 0.55 i | 6566.06 ± 130.77 i | 640.29 ± 15.45 g |
| 12 | 2156.02 ± 16.96 e | 479.59 ± 2.67 abc | 2.25 ± 0.34 cd | 5209.94 ± 18.66 fg | 581.74 ± 13.70 ef |
| 13 | 1148.13 ± 8.73 b | 501.39 ± 2.86 bc | 7.58 ± 0.45 h | 5260.59 ± 63.43 fg | 507.63 ± 11.48 cd |
| 14 | 1503.62 ± 51.63 c | 821.00 ± 6.53 e | 4.72 ± 0.02 f | 5276.66 ± 14.18 g | 722.42 ± 1.33 i |
| 15 | 646.51 ± 46.37 a | 876.63 ± 17.12 e | 12.00 ± 0.62 j | 6435.87 ± 212.98 i | 798.48 ± 13.78 j |
| 16 | 2070.31 ± 85.01 e | 520.17 ± 5.44 c | 1.62 ± 0.03 bc | 4952.91 ± 7.72 ef | 485.27 ± 7.44 bc |
| 17 | No phase separation occurred | ||||
| 18 | 4158.21 ± 53.96 i | 416.48 ± 6.90 ab | nd | 4203.46 ± 15.90 bc | 427.71 ± 13.86 a |
| Run | Kale Microgreen Sample | ||||
|---|---|---|---|---|---|
| DPPH (µM TE/100 g) | Chlorophylls (mg/100 g) | Carotenoids (mg/100 g) | Phenolics (mg/100 g) | Anthocyanidins (mg/100 g) | |
| 1 | 916.32 ± 24.09 b | 1542.96 ± 77.76 e | 13.54 ± 0.41 fg | 3329.57 ± 68.37 e | nd |
| 2 | 1647.12 ± 47.96 ef | 472.75 ± 23.19 a | 5.94 ± 0.19 c | 2481.27 ± 79.96 bc | 41.41 ± 1.40 c |
| 3 | 1918.31 ± 61.57 h | 370.66 ± 20.21 a | 3.53 ± 0.21 ab | 2695.28 ± 23.97 cd | 21.22 ± 1.02 b |
| 4 | 1869.18 ± 32.75 gh | 621.37 ± 29.84 b | 5.48 ± 0.28 c | 2500.84 ± 43.30 bc | 8.4 ± 0.86 a |
| 5 | 2417.03 ± 19.57 j | 811.23 ± 17.40 c | 6.53 ± 0.08 c | 5503.6 ± 62.05 i | 21.12 ± 1.02 b |
| 6 | 1249.52 ± 11.38 c | 1905.27 ± 65.93 g | 4.83 ± 0.04 bc | 3867.91 ± 93.84 fg | nd |
| 7 | 1735.22 ± 46.22 fg | 954.06 ± 13.79 d | 23.64 ± 1.64 h | 1594.79 ± 39.29 a | 89.99 ± 4.63 e |
| 8 | 1344.74 ± 60.72 cd | 1660.28 ± 11.93 f | 9.19 ± 0.77 d | 2869.26 ± 61.99 d | nd |
| 9 | No phase separation occurred | ||||
| 10 | 3121.22 ± 72.34 k | 4760.08 ± 37.68 k | 15.71 ± 0.26 g | 2643.03 ± 110.73 cd | 7.03 ± 0.87 a |
| 11 | 4338.04 ± 44.83 m | 6554.13 ± 46.26 l | 23.74 ± 0.21 h | 3759.08 ± 55.38 f | 43.28 ± 2.17 c |
| 12 | 2166.11 ± 24.14 i | 3332.03 ± 20.78 j | 12.06 ± 0.52 ef | 3811.05 ± 138.03 fg | 3.04 ± 0.41 a |
| 13 | 1543.31 ± 35.06 de | 1956.51 ± 35.51 g | 9.64 ± 0.49 d | 2355.59 ± 109.76 b | 165.4 ± 4.31 h |
| 14 | 4398.01 ± 102.53 m | 4708.27 ± 51.63 k | 2.3 ± 0.14 a | 4824.13 ± 119.84 h | 75.2 ± 2.08 d |
| 15 | 5214.96 ± 94.46 n | 6645.59 ± 18.71 l | 5.7 ± 0.24 c | 4015.22 ± 74.45 g | 153.2 ± 4.17 g |
| 16 | 3645.44 ± 46.33 l | 2515.11 ± 14.99 h | 10.09 ± 0.74 de | 1637.42 ± 53.03 a | nd |
| 17 | No phase separation occurred | ||||
| 18 | 222.53 ± 8.42 a | 2893.33 ± 37.28 i | 3.78 ± 0.09 ab | 3260.83 ± 21.38 e | 19.2 ± 1.06 b |
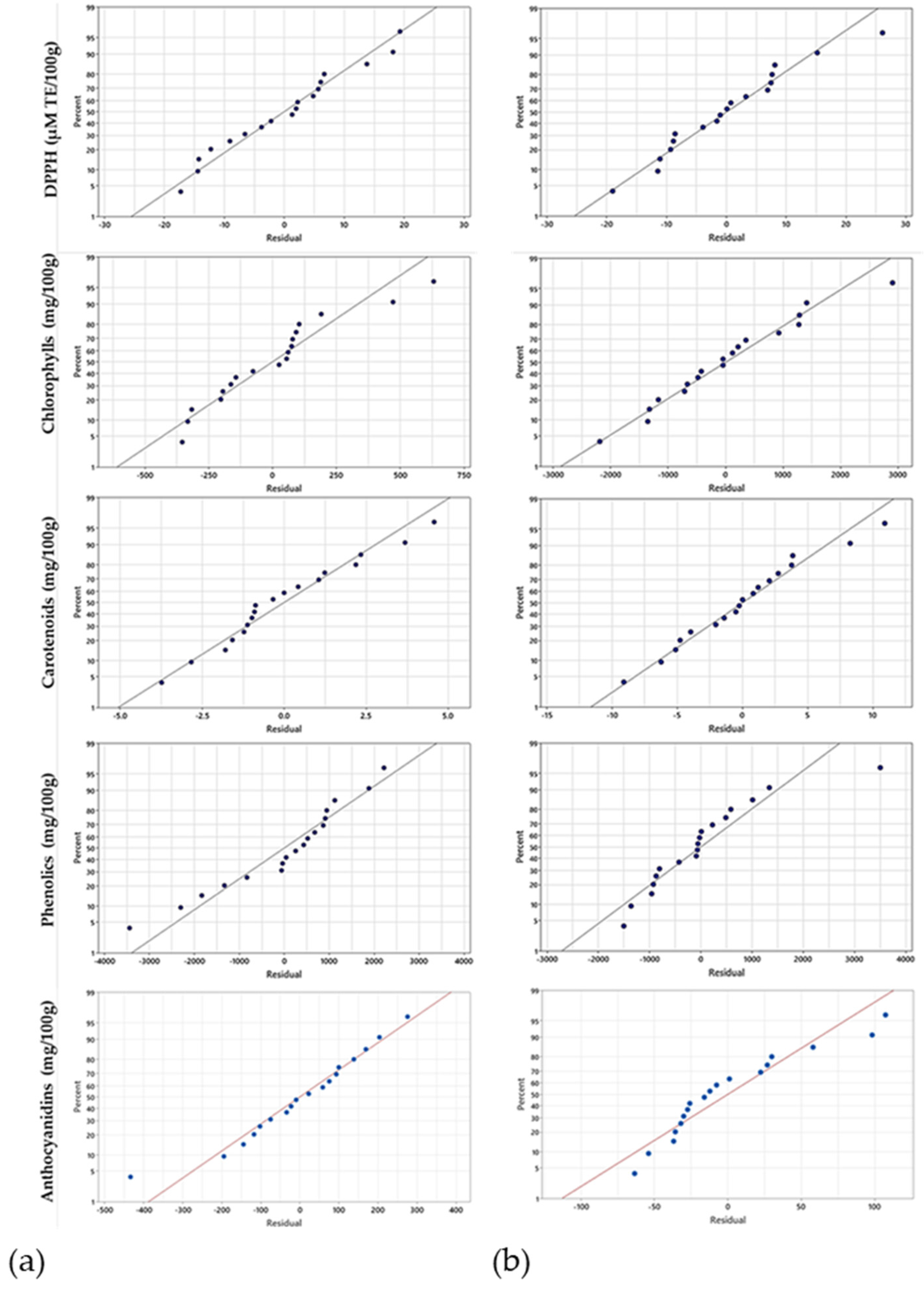
| ANN Characteristics | Network Name | Training Performance | Training Error | Training Algorithm | Error Function | Hidden Activation | Output Activation |
|---|---|---|---|---|---|---|---|
| Sango radish | |||||||
| DPPH | MLP 5-8-1 | 1.00 | 0.00 | BFGS 870 | SOS | Logistic | Tanh |
| Chlorophylls | MLP 5-9-1 | 1.00 | 0.00 | BFGS 86 | SOS | Tanh | Identity |
| Carotenoids | MLP 5-9-1 | 1.00 | 0.00 | BFGS 75 | SOS | Tanh | Identity |
| Phenolics | MLP 5-9-1 | 1.00 | 0.00 | BFGS 82 | SOS | Exponential | Identity |
| Anthocyanidins | MLP 5-6-1 | 1.00 | 0.00 | BFGS 93 | SOS | Exponential | Identity |
| Kale | |||||||
| DPPH | MLP 5-8-1 | 1.00 | 0.00 | BFGS 4989 | SOS | Exponential | Logistic |
| Chlorophylls | MLP 5-11-1 | 1.00 | 0.00 | BFGS 75 | SOS | Logistic | Identity |
| Carotenoids | MLP 5-4-1 | 1.00 | 0.00 | BFGS 106 | SOS | Tanh | Identity |
| Phenolics | MLP 5-8-1 | 1.00 | 0.00 | BFGS 76 | SOS | Logistic | Identity |
| Anthocyanidins | MLP 5-5-1 | 1.00 | 0.00 | BFGS 56 | SOS | Tanh | Identity |
| SUPRAS Outputs for Optimized Extract | Predicted Values | Experimentally Obtained Values | Divergence Degree (%) |
|---|---|---|---|
| Sango radish microgreen | |||
| DPPH (µM TE/100 g) | 5734 | 5557 | −3.09 |
| Chlorophylls (mg/100 g) | 876.44 | 897.13 | +2.36 |
| Carotenoids (mg/100 g) | 12.21 | 12.11 | −0.82 |
| Phenolics (mg/100 g) | 6378.84 | 6494.15 | +1.81 |
| Anthocyanidins (mg/100 g) | 803.27 | 799.85 | −0.43 |
| Kale microgreen | |||
| DPPH (µM TE/100 g) | 5242 | 5107 | −2.57 |
| Chlorophylls (mg/100 g) | 6624.31 | 6861.47 | +3.58 |
| Carotenoids (mg/100 g) | 152.66 | 152.64 | −0.01 |
| Phenolics (mg/100 g) | 4013.08 | 4061.34 | +1.2 |
| Anthocyanidins (mg/100 g) | 152.66 | 151.1 | −1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučetić, A.; Pezo, L.; Šovljanski, O.; Vulić, J.; Travičić, V.; Ćetković, G.; Čanadanović-Brunet, J. Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization. Processes 2024, 12, 1451. https://doi.org/10.3390/pr12071451
Vučetić A, Pezo L, Šovljanski O, Vulić J, Travičić V, Ćetković G, Čanadanović-Brunet J. Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization. Processes. 2024; 12(7):1451. https://doi.org/10.3390/pr12071451
Chicago/Turabian StyleVučetić, Anja, Lato Pezo, Olja Šovljanski, Jelena Vulić, Vanja Travičić, Gordana Ćetković, and Jasna Čanadanović-Brunet. 2024. "Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization" Processes 12, no. 7: 1451. https://doi.org/10.3390/pr12071451
APA StyleVučetić, A., Pezo, L., Šovljanski, O., Vulić, J., Travičić, V., Ćetković, G., & Čanadanović-Brunet, J. (2024). Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization. Processes, 12(7), 1451. https://doi.org/10.3390/pr12071451











