Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases and Study of Their Aggregative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of Study
2.2. Methods
3. Results and Discussion
3.1. Effect of Quinoline as a Component of a Dispersion Medium on the Composition, Structure, and Aggregation Stability of Asphaltenes
3.2. Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases at Various Temperatures
3.3. Composition, Structure, and Aggregation Stability of Asphaltene-like Nitrogenous Bases
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shatalova, N.V.; Apasov, T.K.; Shatalov, A.V.; Grigoriev, B.V. Renovation way to restore well productivity using wave fields. J. Min. Inst. 2022, 258, 986–997. [Google Scholar] [CrossRef]
- Chengzhi, Q.; Guzev, M.A.; Poplygin, V.V.; Kunitskikh, A.A. Predicting the permeability of the near-bottomhole zone during wave impact. J. Min. Inst. 2022, 258, 998–1007. [Google Scholar] [CrossRef]
- Podoprigora, D.; Byazrov, R.; Sytnik, J. The comprehensive overview of large-volume surfactant slugs injection for enhancing oil recovery: Status and the outlook. Energies 2022, 15, 8300. [Google Scholar] [CrossRef]
- Krivoshchekov, S.N.; Kochnev, A.A.; Ravelev, K.A. Development of an algorithm for determining the technological parameters of acid composition injection during treatment of the near-bottomhole zone, taking into account economic efficiency. J. Min. Inst. 2021, 250, 587–595. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2006; pp. 1–16. [Google Scholar]
- Abbas, H.A.; Manasrah, A.D.; Carbognani, L.; Sebakhy, K.O.; El Nokab, M.E.H.; Hacini, M.; Nassar, N.N. A study on the characteristics of Algerian Hassi-Messaoud asphaltenes: Solubility and precipitation. Pet. Sci. Technol. 2022, 40, 1279–1301. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Salenzadeh, M.; Husein, M.M.; Ghotbi, C.; Dabir, B.; Taghikhani, V. In-depth characterization of light, medium and heavy oil asphaltenes as well as asphaltenes subfractions. Fuel 2022, 324, 124525. [Google Scholar] [CrossRef]
- Groenzin, H.; Mullins, O.C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14, 677–684. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Shuler, B.; Meyer, G.; Pena, D.; Mullins, O.C.; Gross, L. Unraveling the Molecular Structures of Asphaltenes by Atomic Force Microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef]
- Acevedo, S.; Castro, A.; Negrin, J.G.; Fernandez, A.; Escobar, G.; Piscitelli, V.; Delolme, F.; Dessalces, G. Relations between asphaltene structures and their physical and chemical properties: The rosary-type structure. Energy Fuels 2007, 21, 2165–2175. [Google Scholar] [CrossRef]
- Sergun, V.P.; Cheshkova, T.V.; Sagachenko, T.A.; Min, R.S. Structural units with sulfur and ether/ester bonds in molecules of high- and low-molecular-weight asphaltenes of USA heavy oil. Pet. Chem. 2016, 56, 10–15. [Google Scholar] [CrossRef]
- McKenna, A.M.; Donald, L.J.; Fitzsimmons, J.E.; Juyal, P.; Spicer, V.; Standing, K.G.; Marshall, A.G.; Rodgers, R.P. Heavy petroleum composition. 3. Asphaltene aggregation. Energy Fuels 2013, 27, 1246–1256. [Google Scholar] [CrossRef]
- Akbarzadeh, K.; Hammami, A.; Kharrat, A.; Zhang, D.; Allenson, S.; Creek, J.; Kabir, S.; Jamaluddin, A.; Marshall, A.G.; Rodgers, R.P.; et al. Asphaltenes—Problematic but Rich in Potential. Oilfield Rev. 2007, 19, 22–43. [Google Scholar]
- Evdokimov, I.N.; Fesan, A.A.; Losev, A.P. New answers to the optical interrogation of asphaltenes: Monomers and primary aggregates from steady-state fluorescence studies. Energy Fuels 2016, 30, 4494–4503. [Google Scholar] [CrossRef]
- Li, X.; Chi, P.; Sun, Q. Effects of asphaltene concentration and asphaltene agglomeration on viscosity. Fuel 2019, 255, 115825. [Google Scholar] [CrossRef]
- Golubev, I.A.; Golubev, A.V.; Laptev, A.B. Practice of using the magnetic treatment devices to intensify the processes of primary oil treating. J. Min. Inst. 2020, 245, 554–560. [Google Scholar] [CrossRef]
- Javanbakht, G.; Sedghi, M.; Welch, W.; Goual, L.; Hoepfner, M.P. Molecular polydispersity improves prediction of asphaltene aggregation. J. Mol. Liq. 2018, 256, 382–394. [Google Scholar] [CrossRef]
- Korobov, G.Y.; Parfenov, D.V.; Van, T.N. Mechanism of the formation of asphalt-resin and paraffin deposits and factors influencing their intensity. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2023, 334, 103–116. [Google Scholar] [CrossRef]
- Nurgalieva, K.S.; Saychenko, L.A.; Riazi, M. Improving the efficiency of oil and gas wells complicated by the formation of asphalt-resin-paraffin deposits. Energies 2021, 14, 6673. [Google Scholar] [CrossRef]
- Rogachev, M.K.; Nguyen Van, T.; Aleksandrov, A.N. Technology for preventing the wax deposit formation in gas-lift wells at offshore oil and gas fields in Vietnam. Energies 2021, 14, 5016. [Google Scholar] [CrossRef]
- Martyanov, O.N.; Larichev, Y.V.; Morozov, E.V.; Trukhan, S.N.; Kazarian, S.G. The stability and evolution of oil systems studied via advanced methods in situ. Russ. Chem. Rev. 2017, 86, 999–1023. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in asphaltene science and the Yen-Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Rogel, E. Studies on asphaltene aggregation via computational chemistry. Colloids Surf. A 1995, 104, 85–93. [Google Scholar] [CrossRef]
- Sheremata, J.M.; Gray, M.R.; Dettman, H.D.; McCaffrey, W.C. Quantitative molecular representation and sequential optimization of Athabasca asphaltenes. Energy Fuels 2004, 18, 1377–1384. [Google Scholar] [CrossRef]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular assembly model for aggregation of petroleum asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Lai, C.H.; Kazarian, S.G. In situ chemical imaging of asphaltene precipitation from crude oil induced by n-heptane. Energy Fuels 2014, 28, 964–971. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Morozov, E.V.; Subramani, V.; Martyanov, O.N.; Kazarian, S.G. Chemical Visualization of Asphaltenes Aggregation Processes Studied in Situ with ATR-FTIR Spectroscopic Imaging and NMR Imaging. J. Phys. Chem. C 2015, 119, 2646–2660. [Google Scholar] [CrossRef]
- Ganeeva, Y.M.; Yusupova, T.N.; Romanov, G.V. Asphaltene nano-aggregates: Structure, phase transitions and effect on petroleum systems. Russ. Chem. Rev. 2011, 80, 993. [Google Scholar] [CrossRef]
- Efimov, I.; Smyshlyaeva, K.I.; Povarov, V.G.; Buzyreva, E.D.; Zhitkov, N.V.; Vovk, M.A.; Rudko, V.A. UNIFAC residual marine fuels stability prediction from NMR and elemental analysis of SARA components. Fuel 2023, 352, 129014. [Google Scholar] [CrossRef]
- Guzmán, R.; Ancheyta, J.; Trejo, F.; Rodríguez, S. Methods for determining asphaltene stability in crude oils. Fuel 2017, 188, 530–543. [Google Scholar] [CrossRef]
- Smyshlyaeva, K.I.; Rudko, V.A.; Kuzmin, K.A.; Povarov, V.G. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel 2022, 328, 125291. [Google Scholar] [CrossRef]
- Silva, H.S.; Sodero, A.C.; Bouyssiere, B.; Carrier, H.; Korb, J.; Alfarra, A.; Vallverdu, G.; Bégué, D.; Baraille, I. Molecular dynamics study of nanoaggregation in asphaltene mixtures: Effects of the N, O, and S heteroatoms. Energy Fuels 2016, 30, 5656–5664. [Google Scholar] [CrossRef]
- Sodero, A.C.; Silva, H.S.; Level, P.G.; Bouyssiere, B.; Korb, J.; Carrier, H.; Alfarra, A.; Bégué, D.; Baraille, I. Investigation of the effect of sulfur heteroatom on asphaltene aggregation. Energy Fuels 2016, 30, 4758–4766. [Google Scholar] [CrossRef]
- Mizuhara, J.; Liang, Y.; Masuda, Y.; Kobayashi, K.; Iwama, H.; Yonebayashi, H. Evaluation of Asphaltene Adsorption Free Energy at the Oil–Water Interface: Role of Heteroatoms. Energy Fuels 2020, 34, 5267–5280. [Google Scholar] [CrossRef]
- Lyulin, S.V.; Glova, A.D.; Falkovich, S.G.; Ivanov, V.A.; Nazarychev, V.M.; Lyulin, A.V.; Larin, S.V.; Antonov, S.V.; Ganan, P.; Kenny, J.M. Computer Simulation of Asphaltenes. Pet. Chem. 2018, 58, 983–1004. [Google Scholar] [CrossRef]
- Ekramipooya, A.; Valadi, F.M.; Farisabadi, A.; Gholami, M.R. Effect of the heteroatom presence in different positions of the model asphaltene structure on the self-aggregation: MD and DFT study. J. Mol. Liq. 2021, 334, 116109. [Google Scholar] [CrossRef]
- Takanohashi, T.; Sato, S.; Tanaka, R. Structural relaxation behaviors of three different asphaltenes using MD calculations. Pet. Sci. Technol. 2004, 22, 901–914. [Google Scholar] [CrossRef]
- Ramírez, L.; Moncayo-Riascos, I.; Cortés, F.B.; Franco, C.A.; Ribadeneira, R. Molecular dynamics study of the aggregation behavior of polycyclic aromatic hydrocarbon molecules in n-heptane–toluene mixtures: Assessing the heteroatom content effect. Energy Fuels 2021, 35, 3119–3129. [Google Scholar] [CrossRef]
- Sedghi, M.; Goual, L.; Welch, W.; Kubelka, J. Effect of asphaltene structure on association and aggregation using molecular dynamics. J. Phys. Chem. B 2013, 117, 5765–5776. [Google Scholar] [CrossRef]
- Yaseen, S.; Mansoori, G.A. Asphaltene aggregation due to waterflooding (A molecular dynamics study). J. Petrol. Sci. Eng. 2018, 170, 177–183. [Google Scholar] [CrossRef]
- Cheshkova, T.V.; Sergun, V.P.; Kovalenko, E.Y.; Gerasimova, N.N.; Sagachenko, T.A.; Min, R.S. Resins and Asphaltenes of Light and Heavy Oils: Their Composition and Structure. Energy Fuels 2019, 33, 7971–7982. [Google Scholar] [CrossRef]
- Ayurova, A.M.; Gerasimova, N.N.; Sagachenko, T.A. High- and low-molecular nitrogenous bases in highly paraffinic oils. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2017, 328, 28–35. [Google Scholar]
- Kovalenko, E.Y.; Sagachenko, T.A.; Min, R.S. Effect of nitrogen compounds in oil on formation of asphaltene aggregates. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2016, 327, 119–126. [Google Scholar]
- Larichev, Y.V.; Kovalenko, E.Y.; Martyanov, O.N. Effect of Nitrogen Bases on the Structure of Primary Asphaltene Clusters and Dynamics of Aggregation of Heavy Oil Fractions. Pet. Chem. 2019, 59, 1195–1200. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N.; Iovik, Y.A.; Kolobova, E.N.; Grabchenko, M.V.; Kurzina, I.A.; Mukhamatdinov, I.I. Effect of hydrogen-donor of heavy crude oil catalytic aquathermolysis in the presence of a nickel-based catalyst. Catalysts 2022, 12, 1154. [Google Scholar] [CrossRef]
- Yan, Y.; de Klerk, A.; Prado, G.H.C. Visbreaking of Vacuum Residue Deasphalted Oil: New Asphaltenes Formation. Energy Fuels 2020, 34, 5135–5147. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N. NiO based catalysts obtained “in-situ” for heavy crude oil upgrading: Effect of NiO precursor on the catalytic cracking products composition. J. Taiwan Inst. Chem. Eng. 2021, 127, 151–156. [Google Scholar] [CrossRef]
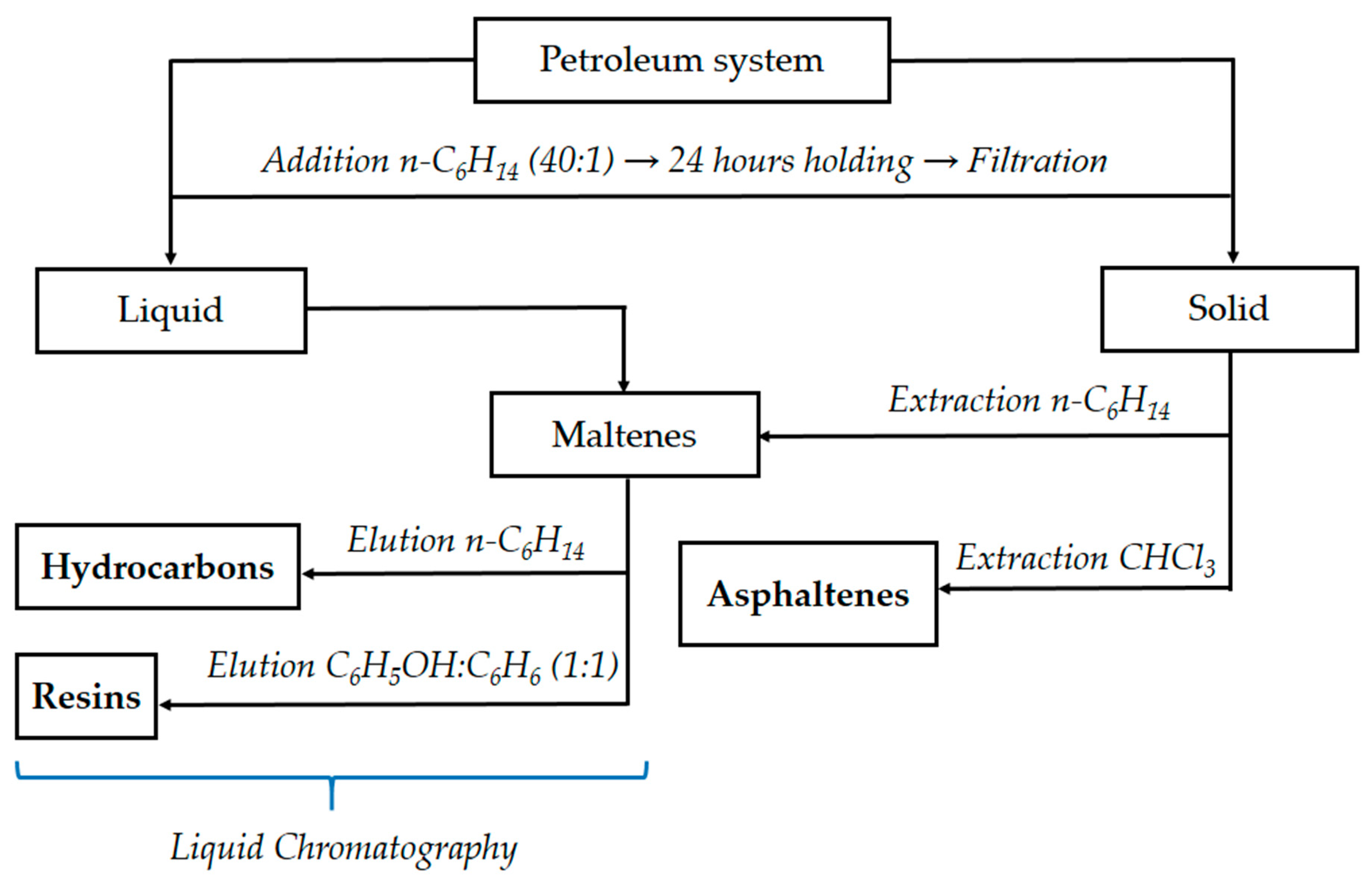
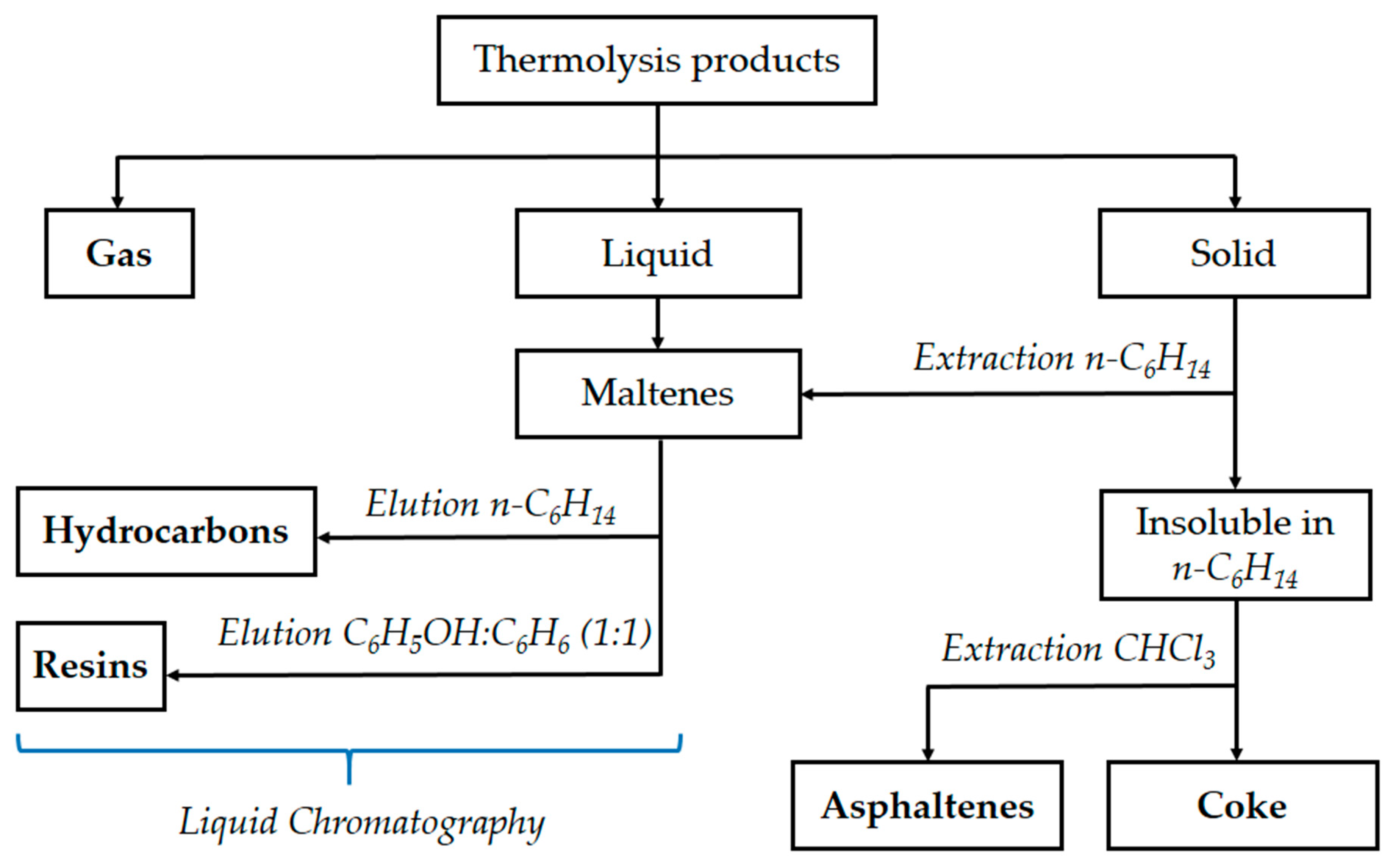


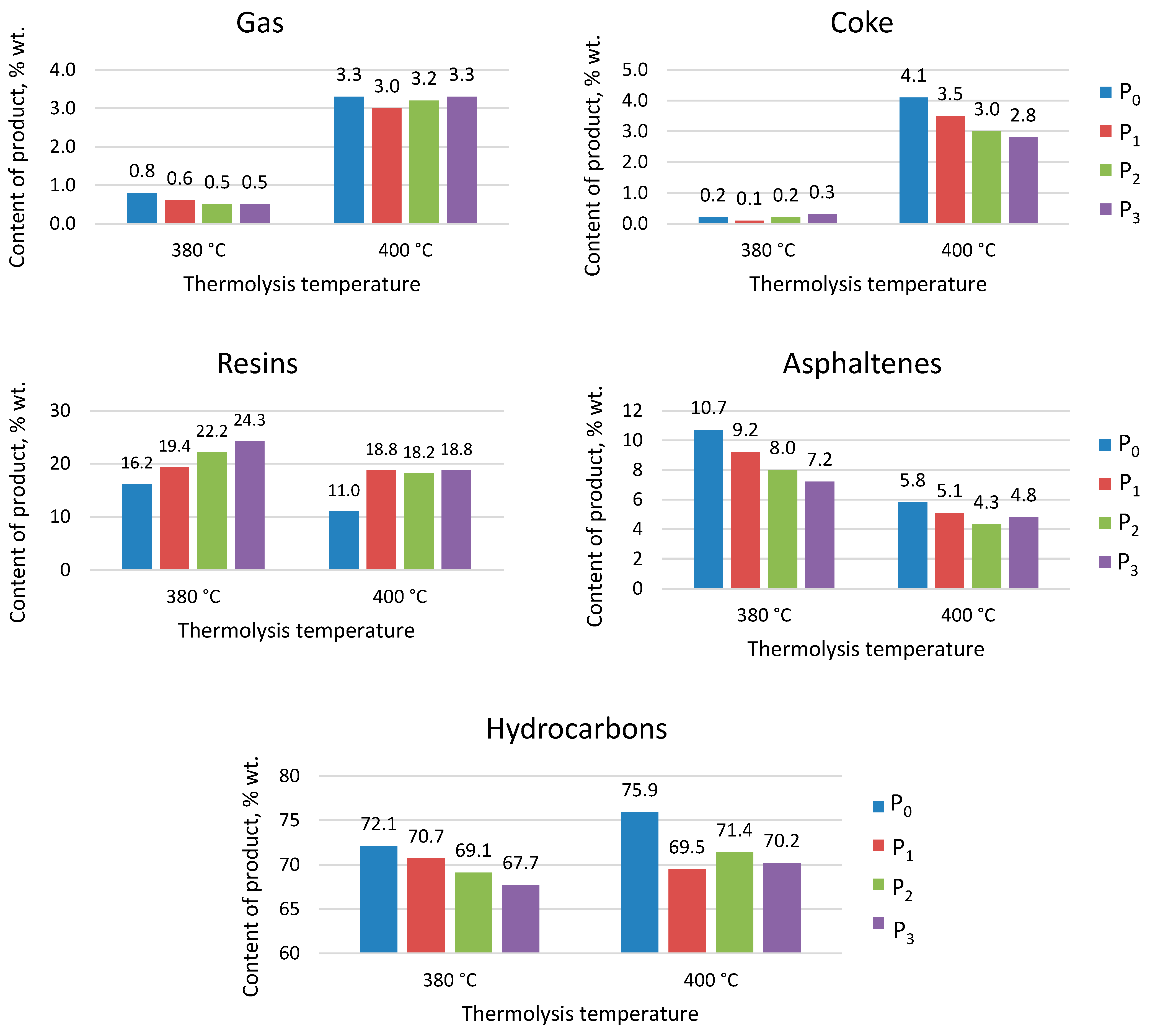
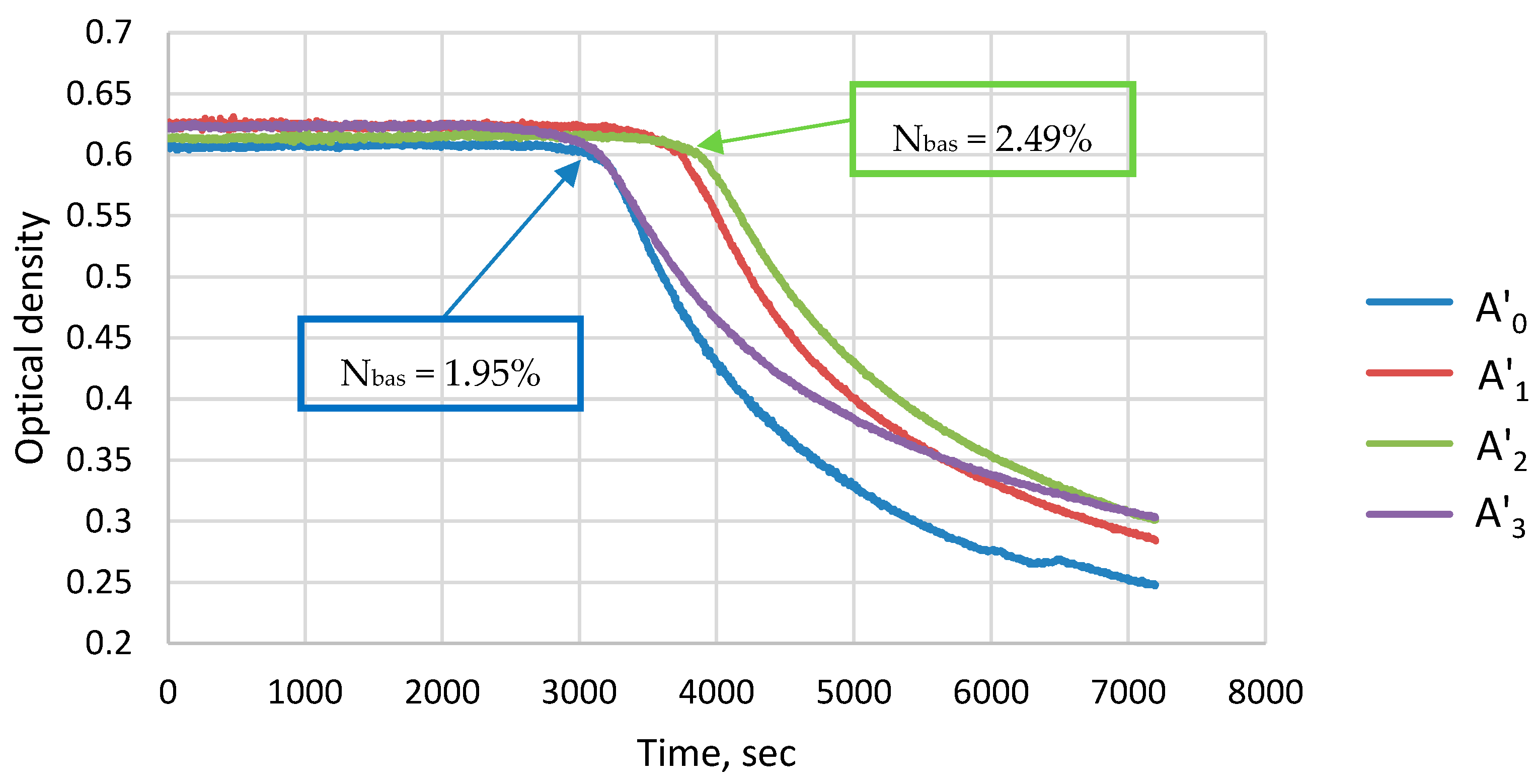
| Object of Study | Abbreviation | Quinoline Content, % wt. | Nitrogen Content, % wt. |
|---|---|---|---|
| Initial crude oil | P0 | - | 0.4 |
| Model petroleum system 1 | P1 | 5.5 | 1.0 |
| Model petroleum system 2 | P2 | 14.6 | 2.0 |
| Model petroleum system 3 | P3 | 23.8 | 3.0 |
| Elemental Composition, % wt. | Group Composition, % wt. | ||||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Hydrocarbons | Resins | Asphaltenes |
| 81.67 | 12.38 | 0.41 | 4.01 | 1.53 | 64.69 | 24.06 | 11.25 |
| Parameters | Object | |||
|---|---|---|---|---|
| A0 | A1 | A2 | A3 | |
| MW, amu | 1368 | 1640 | 1725 | 2018 |
| Elemental composition, % wt. | ||||
| C | 82.17 | 82.89 | 82.82 | 82.91 |
| H | 7.96 | 8.04 | 7.88 | 7.91 |
| Nbas | 1.39 | 1.72 | 2.14 | 2.69 |
| S | 4.18 | 3.98 | 3.89 | 3.80 |
| O | 4.30 | 3.37 | 3.27 | 2.69 |
| Distribution of carbon among structural fragments of asphaltenes, % | ||||
| fa | 47.3 | 47.6 | 48.6 | 49.2 |
| fn | 19.4 | 20.7 | 21.6 | 21.3 |
| fp | 33.3 | 31.7 | 29.8 | 29.5 |
| Parameters | Object | |||
|---|---|---|---|---|
| A’0 | A’1 | A’2 | A’3 | |
| MW, amu | 615 | 771 | 715 | 690 |
| Nbas, % wt. | 1.95 | 2.35 | 2.49 | 2.04 |
| Distribution of carbon among structural fragments of asphaltenes, % | ||||
| fa | 65.2 | 67.1 | 67.4 | 67.8 |
| fn | 21.5 | 21.2 | 21.4 | 21.2 |
| fp | 13.3 | 11.7 | 11.2 | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korneev, D.; Fialkovsky, I. Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases and Study of Their Aggregative Stability. Processes 2024, 12, 1448. https://doi.org/10.3390/pr12071448
Korneev D, Fialkovsky I. Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases and Study of Their Aggregative Stability. Processes. 2024; 12(7):1448. https://doi.org/10.3390/pr12071448
Chicago/Turabian StyleKorneev, Dmitry, and Igor Fialkovsky. 2024. "Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases and Study of Their Aggregative Stability" Processes 12, no. 7: 1448. https://doi.org/10.3390/pr12071448
APA StyleKorneev, D., & Fialkovsky, I. (2024). Thermolytic Synthesis of Asphaltene-like Nitrogenous Bases and Study of Their Aggregative Stability. Processes, 12(7), 1448. https://doi.org/10.3390/pr12071448






