Optimization of Hydrochemical Leaching Process of Kaolinite Fraction of Bauxite with Response Surface Methodology
Abstract
1. Introduction
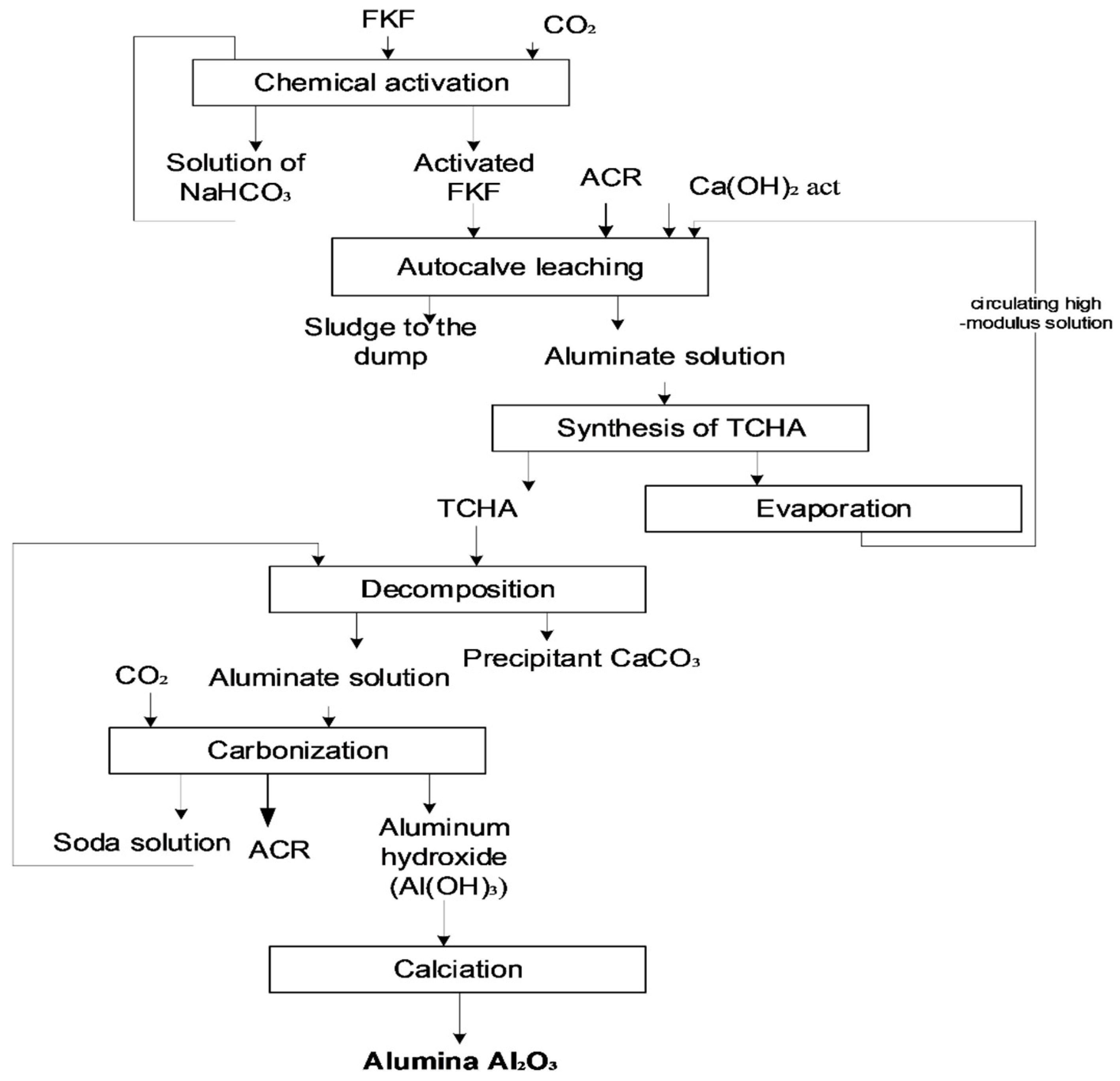
2. Materials and Methods
Experimental Design
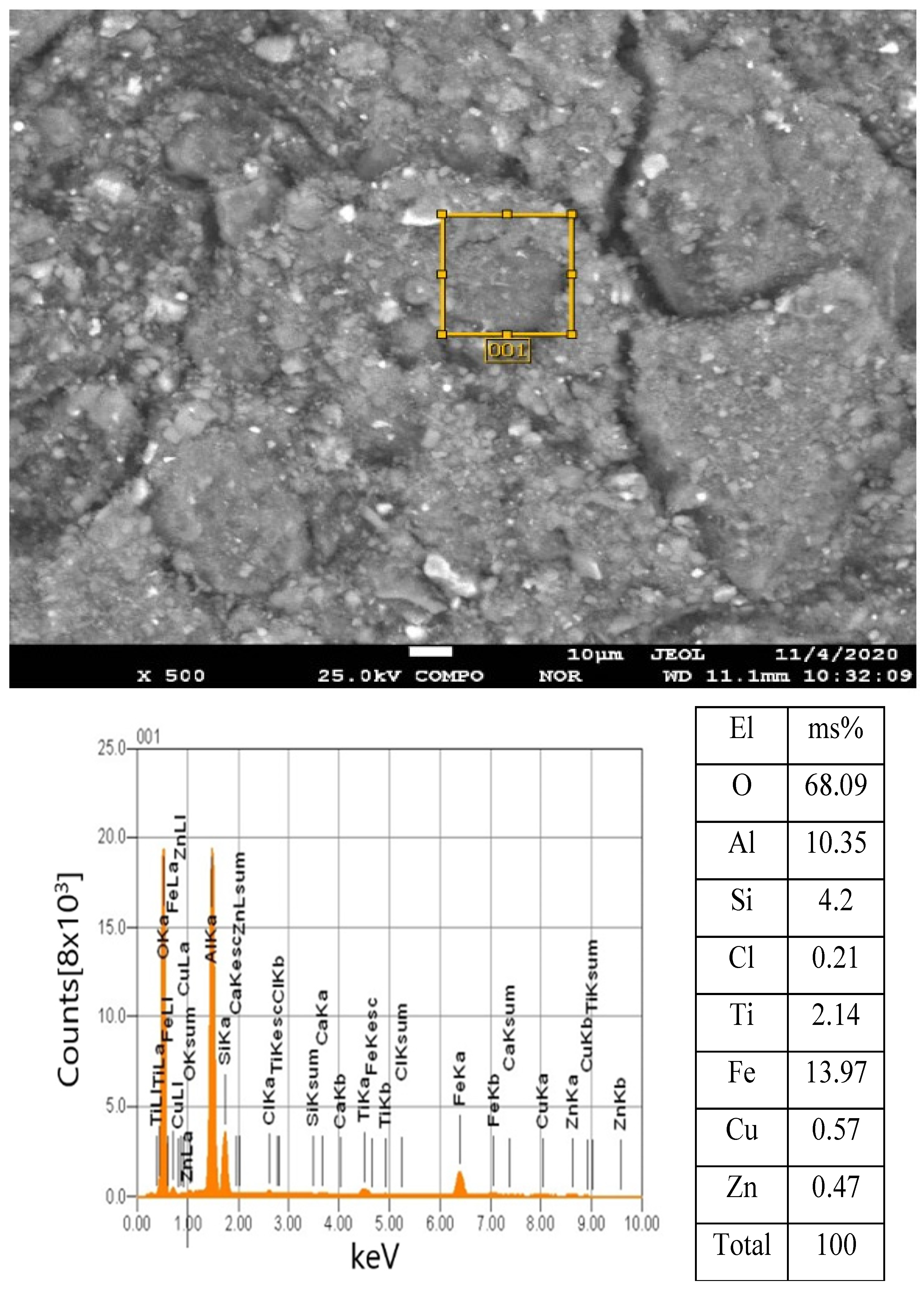
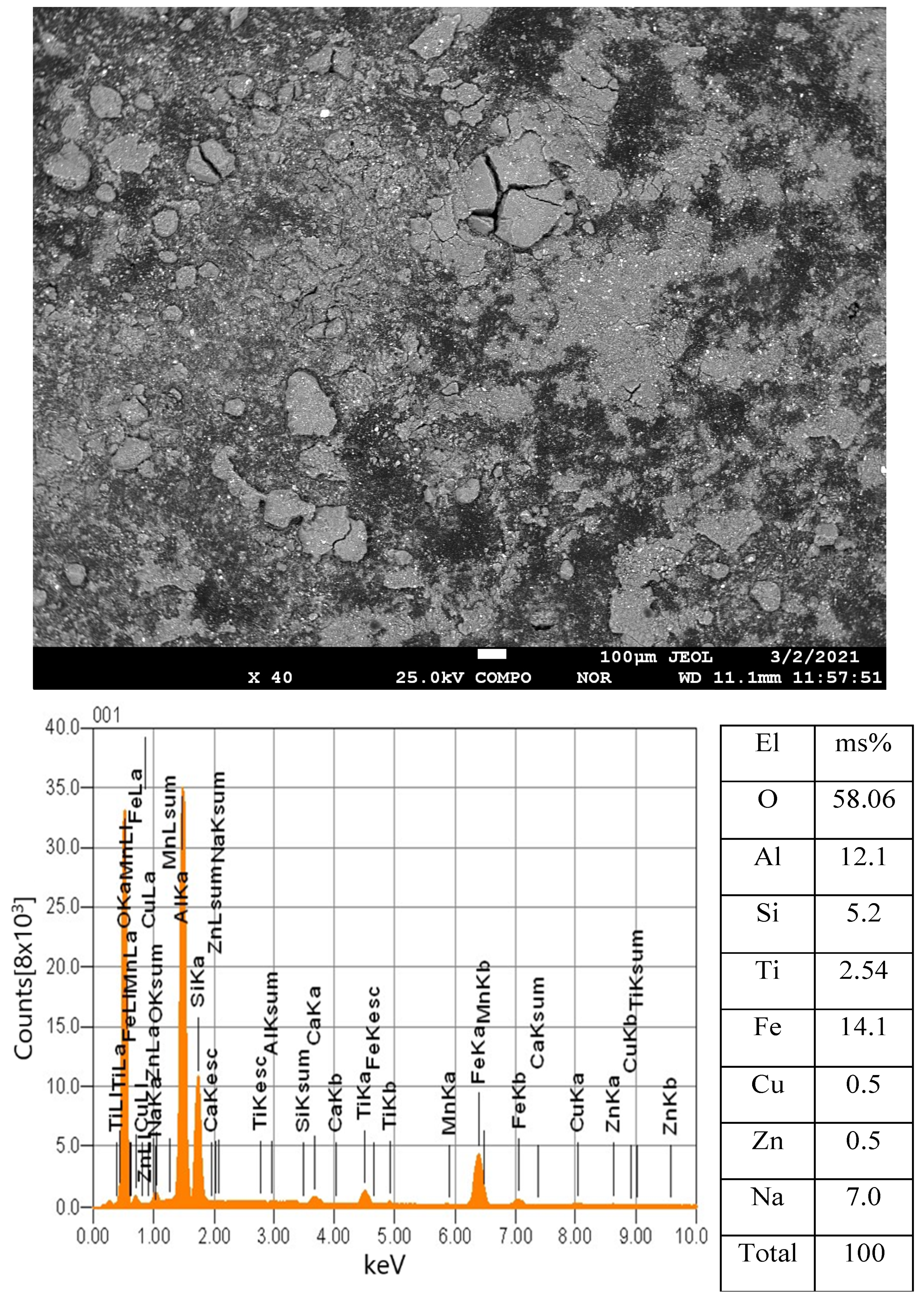
3. Results
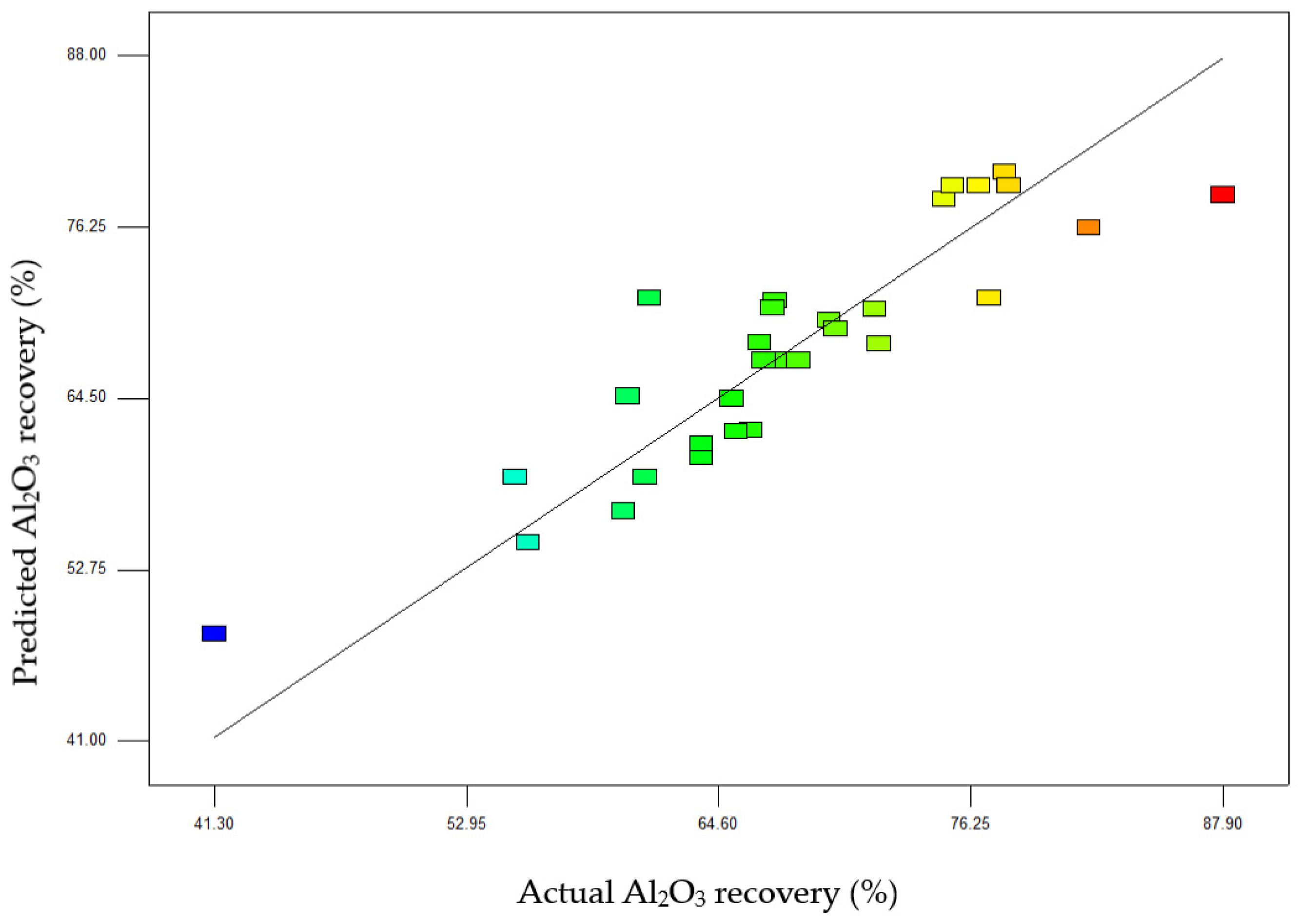
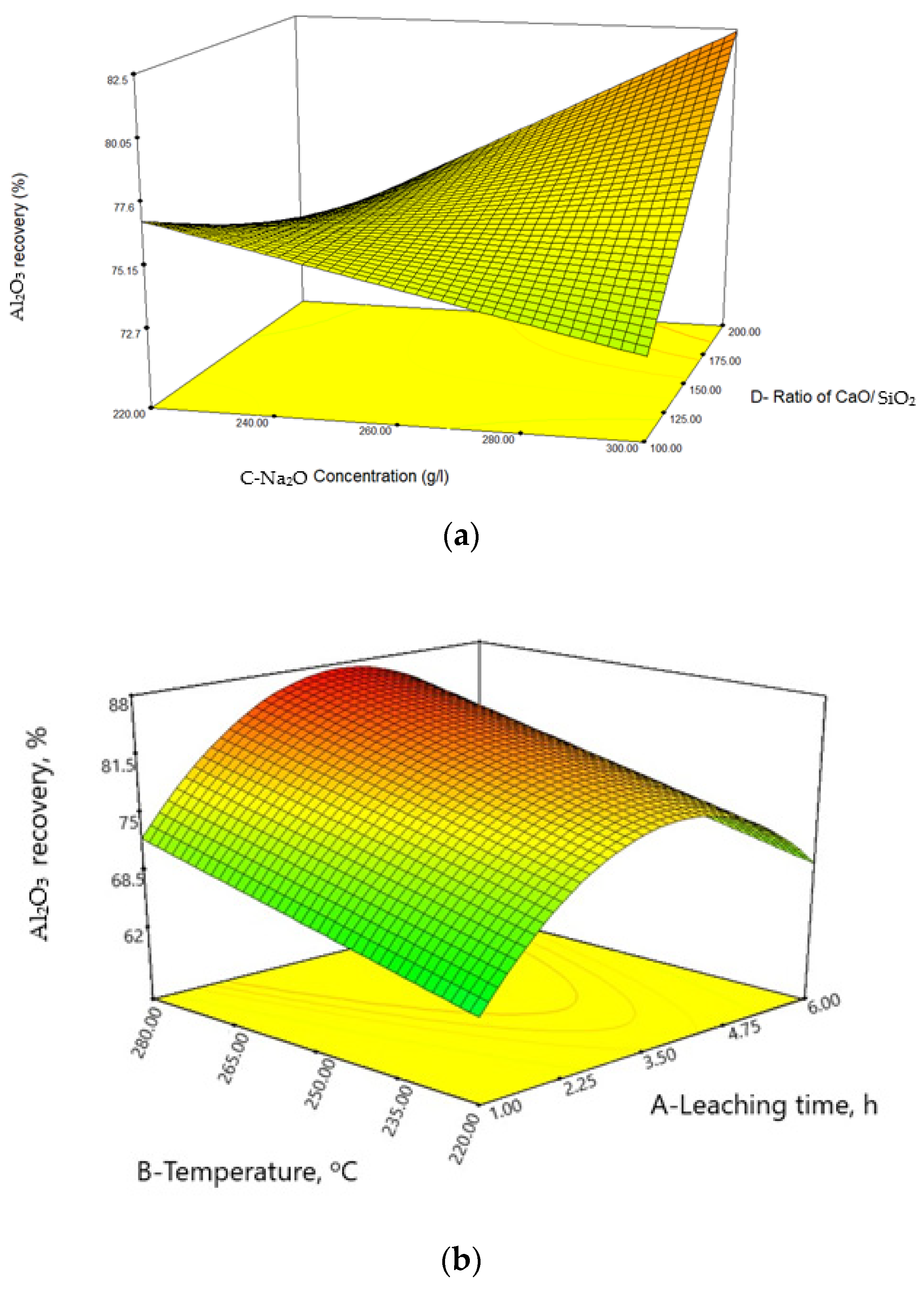
Statistical Analysis and Interpretation of Responses
- –
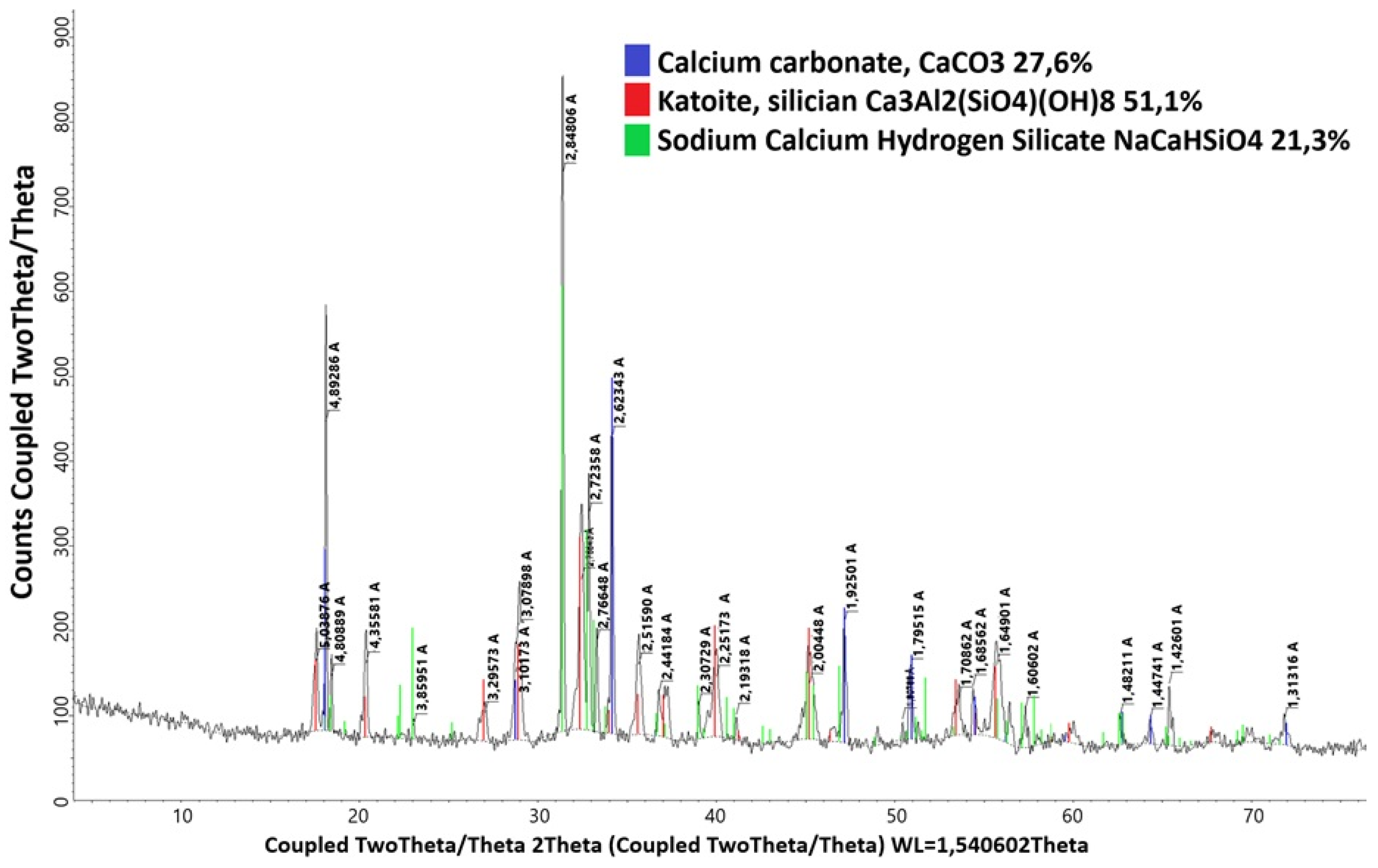
- As a result of the interaction of dawsonite with the active form of calcium oxide, calcium carbonate precipitate and sodium aluminate are formed, which passes into solution:
- –
- As a result of the interaction of sodium aluminum silicate, amorphous silica (obtained as a result of chemical activation) and the active form of calcium oxide, a precipitate consisting of katoite and sodium–calcium–hydrogen silicate is formed, and sodium aluminate passes into the solution:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, P. The Processing of High Silica Bauxites-Review of Existing and Potential Processes. Hydrometallurgy 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in depressants for flotation separation of Cu-Fe sulfide minerals at low alkalinity: A critical review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar]
- Al-Zahrani, A.A.; Abdul-Majid, M.H. Extraction of alumina from local clays by hydrochloric acid process. JKAU Eng. Sci. 2009, 20, 29–41. [Google Scholar] [CrossRef]
- Suss, A.G.; Damaskin, A.A.; Senyuta, A.S.; Panov, A.V.; Smirnov, A.A. The influence of the mineral composition of low-grade aluminum ores on aluminum extraction by acid leaching. Light Met. 2016, 2014, 105–109. [Google Scholar]
- Balmaev, B.G.; Kirov, S.S.; Pak, V.I.; Ivanov, M.A. Kinetics of high-temperature hydrochloric acid leaching of kaolin clays from East Siberian deposits in laboratory and enlarged conditions. Non-Ferr. Met. 2018, 3. [Google Scholar] [CrossRef]
- Allegretta, I.; Pinto, D.; Eramo, G. Effects of grain size on the reactivity of limestone temper in kaolinite clay. Appl. Clay Sci. 2016, 126, 223–234. [Google Scholar] [CrossRef]
- Dubovikov, O.A.; Brichkin, V.N.; Ris, A.D.; Sundurov, A.V. Thermochemical activation of hydrated aluminosilicates and its importance for aluminum production. Non-Ferr. Met. 2018, 2, 3–15. [Google Scholar]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Lou HH Effect of Na2CO3 additive on the activation of coal gangue for aluminum extraction. Int. J. Miner. Process. 2014, 131, 51–57. [Google Scholar] [CrossRef]
- Tang, A.; Su, L.; Li, C.; Wei, W. Effect of mechanical activation on acid-leaching of kaolin residue. Appl. Clay Sci. 2010, 48, 296–299. [Google Scholar] [CrossRef]
- Kuang, J.; Yuan, W.; Li, L.; Hu, J.; Xu, L. Effects of Er(NO3)3, Nd(NO3)3 and Y(NO3)3 on kinetics of dehydroxylation of kaolinite. Powder Technol. 2016, 301, 581–589. [Google Scholar] [CrossRef]
- Souri, A.; Golestani-Fard, F.; Naghizadeh, R.; Veiseh, S. An investigation on pozzolanic activity of Iranian kaolins obtained by thermal treatment. Appl. Clay Sci. 2015, 103, 34–39. [Google Scholar] [CrossRef]
- ElDeeb, A.B.; Brichkin, V.N.; Kurtenkov, R.V.; Bormotov, I.S. Extraction of Alumina from Kaolin: A Comparative Study of Pyrometallurgical and Hydrometallurgical Processes. Appl. Clay Sci. 2019, 172, 146. [Google Scholar] [CrossRef]
- Abdulvaliev, R.A.; Gladyshev, S.V.; Pozmogov, V.A.; Imangalieva, L.M. Method for Preparing Aluminosilicate Raw Materials before Leaching. RK Patent 32333, 31 August 2017. [Google Scholar]
- Gladyshev, S.V.; Kenzhaliev, B.K.; Abikak, Y.B.; Akhmadieva, N.K.; Dyusenova, S.B.; Manapova, A.I.; Bakhshyan, A.I. Method for Processing High-Siliceous Aluminum Ore. Utility Patent 8905, 9 March 2023. [Google Scholar]
- Akhmadiyeva, N.K.; Gladyshev, S.V.; Abdulvaliyev, R.A.; Kenzhaliev, B.K.; Dyusenova, S.B.; Imangaliyeva, L.M.; Bakhshyan, A.I. Air-Slaked Lime Production Method. Utility Patent 8906, 8 February 2024. [Google Scholar]
- Nesterov, A.V.; Oskorbin, A.A. Lime slaking technology. Sci. Tech. Prod. Mag. Silic. Build. Mater. 2019, 8. [Google Scholar] [CrossRef]
- Khomchenko, Y.V.; Barbanyagre, V.D. The influence of fractional composition and water-lime ratio on lime slaking processes. Bull. BSTU Named V.G. Shukhova. 2010, 3, 120–123. [Google Scholar]
- Gavrilenko, V.N.; Petrachkov, F.A. Method for Producing Lime—Fluff. SU Patent 1811541, 24 April 1993. [Google Scholar]
- Nhlanhla, O.; Edison, M. Predictive Models of Leaching Processes: A Critical Review. In Proceedings of the 7th International Conference on Latest Trends in Engineering & Technology (ICLTET’2015), Irene, Pretoria, 26–27 November 2015. [Google Scholar]
- Moradi, M.; Ghanbari, F.; Tabrizi, E.M. Removal of acid yellow 36 using Box–Behnken designed photoelectro-Fenton: A study on removal mechanisms. Toxicol. Environ. Chem. 2015, 97, 700–709. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.-M.; Jiang, B.-H.; Li, L. Optimization of the Operational Parameters for Desalination with Response Surface Methodology during a Capacitive Deionization Process. Desalination 2014, 336, 64–71. [Google Scholar] [CrossRef]
- Dadrasi, A.; Fooladpanjeh, S.; Gharahbagh, A.A. Interactions between HA/GO/epoxy resin nanocomposites: Optimization, modeling and mechanical performance using central composite design and genetic algorithm. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 63. [Google Scholar] [CrossRef]
- Moutiy, E.H.; Tran, L.H.; Mueller, K.K.; Coudert, L.; Blais, J.F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Waste Manag. 2020, 114, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, N.M.; Birloaga, I.; Ferella, F.; Centofanti, M.; Vegliò, F. Preliminary Study on Gold Recovery from High Grade E-waste by Thiourea Leaching and Electrowinning. Minerals 2021, 11, 235. [Google Scholar] [CrossRef]
- Zakrzewska-Koltuniewicz, G.; Herdzik-Koniecko, I.; Cojocaru, C.; Chajduk, E. Experimental design and optimization of leaching process for recovery of valuable chemical elements (U, La, V, Mo, Yb and Th) from low-grade uranium ore. J. Hazard. Mater. 2014, 275, 136–145. [Google Scholar] [CrossRef] [PubMed]

| ID | Parameters | Range | |
|---|---|---|---|
| Variable Parameters | 1 | Leaching Time | 1–6 h |
| 2 | Temperature | 220–280 °C | |
| 3 | Na2O Concentration | 220–300 g/L | |
| 4 | CaO/SiO2 | 100–200% | |
| Fixed Parameters | 5 | Pulp Density | 20% |
| 7 | Stirring Rate | 300 RPM |
| Process Parameters | Units | Symbols | Low Level | High Level |
|---|---|---|---|---|
| Leaching Time | H | A | 1 | 6 |
| Temperature | °C | B | 220 | 280 |
| Na2O Concentration | g/L | C | 220 | 300 |
| XRF Analysis Results (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | MgO | Al2O3 | CaO | TiO2 | Na2O | K2O | Other |
| 16.9 | 19.6 | 0.26 | 39.8 | 1.45 | 3.4 | 0.18 | 0.06 | 18.35 |
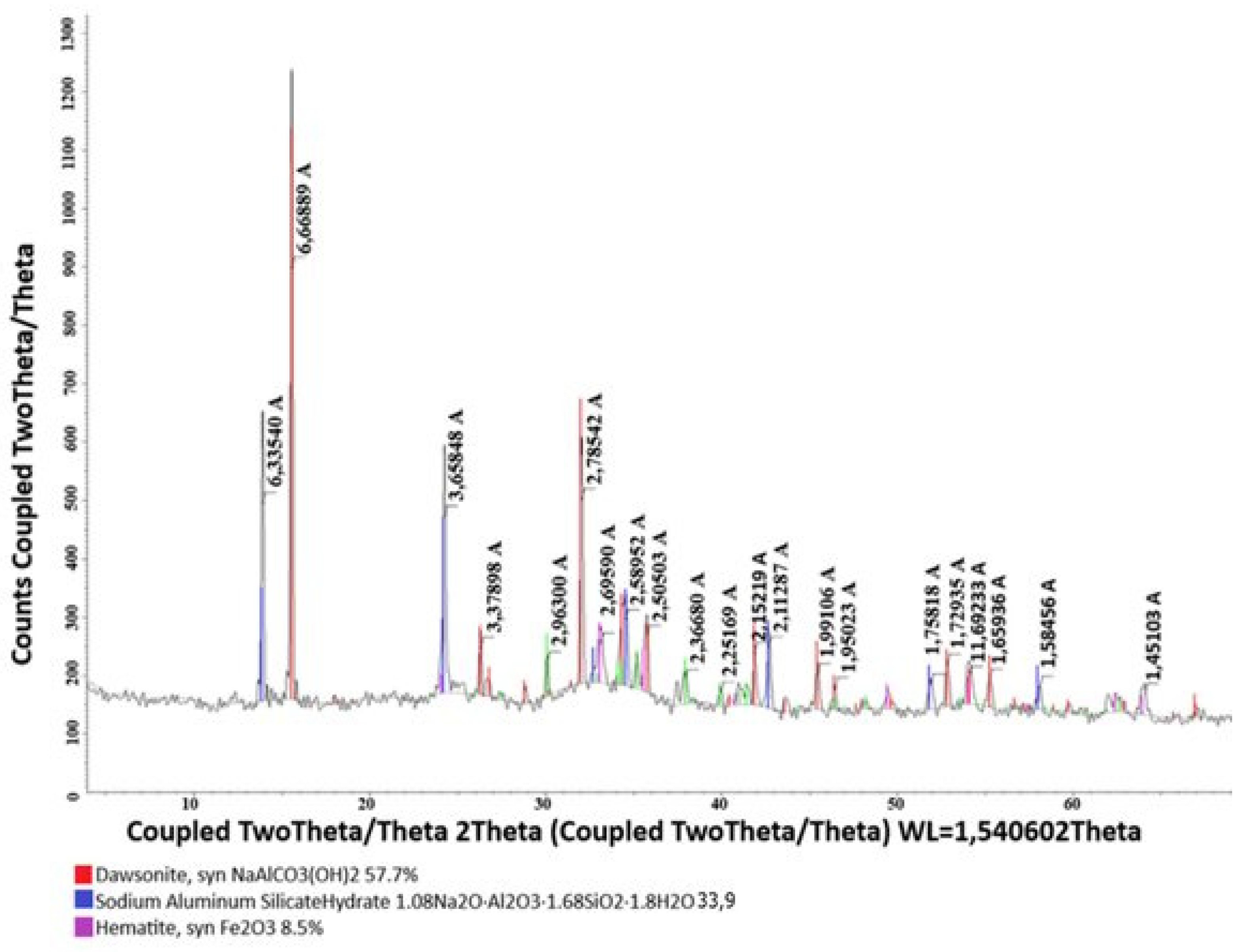
| Name of Phases | Content, % | |||
|---|---|---|---|---|
| Duration, min | ||||
| Initial | 30 | 60 | 90 | |
| Gibbsite | 47.5 | 40.8 | 31.2 | - |
| Kaolinite-1A | 13.6 | 34.9 | 45.1 | - |
| Hematite | 18.2 | 18.2 | 18.2 | 8.5 |
| Anataz | 7.7 | 6.1 | 5.5 | - |
| Quartz | 13.0 | - | - | - |
| Dawsonite | - | - | - | 57.7 |
| Sodium hydroaluminosilicate | - | - | - | 33.9 |
| XRF Analysis Results (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | MgO | Al2O3 | CaO | TiO2 | Na2O | K2O | Other |
| 16.9 | 19.6 | 0.26 | 39.8 | 1.45 | 3.4 | 6.7 | 0.06 | 11.8 |
| Runs | Leaching Time (H) | Temperature (°C) | Na2O Concentration (g/L) | CaO/SiO2 | Al2O3 (%) |
|---|---|---|---|---|---|
| 1 | 3.50 | 250 | 260 | 150 | 78 |
| 2 | 1.00 | 230 | 260 | 150 | 66.1 |
| 3 | 1.00 | 230 | 220 | 100 | 55.8 |
| 4 | 3.50 | 250 | 260 | 150 | 76.6 |
| 5 | 6.00 | 280 | 300 | 100 | 67.5 |
| 6 | 6.00 | 220 | 220 | 100 | 65.4 |
| 7 | 6.00 | 220 | 260 | 150 | 60.4 |
| 8 | 1.00 | 250 | 260 | 150. | 66.5 |
| 9 | 6.00 | 240 | 220 | 200 | 63.8 |
| 10 | 3.50 | 230 | 220 | 150 | 77.1 |
| 11 | 3.00 | 220 | 260 | 150 | 72 |
| 12 | 1.00 | 280 | 220 | 200 | 63.8 |
| 13 | 1.00 | 240 | 220 | 100 | 61.2 |
| 14 | 1.00 | 240 | 220 | 200 | 55.2 |
| 15 | 3.50 | 250 | 260 | 150 | 75.4 |
| 16 | 6.00 | 240 | 300 | 100 | 68.3 |
| 17 | 3.50 | 230 | 220 | 150 | 61.4 |
| 18 | 1.00 | 280 | 300 | 200 | 69.7 |
| 19 | 3.50 | 280 | 260 | 150 | 75 |
| 20 | 6.00 | 240 | 300 | 200 | 67.1 |
| 21 | 1.00 | 220 | 300 | 200 | 65.2 |
| 22 | 1.00 | 240 | 300 | 100 | 60.2 |
| 23 | 3.50 | 260 | 260 | 150 | 77.8 |
| 24 | 3.50 | 220 | 260 | 150 | 70 |
| 25 | 6.00 | 240 | 220 | 100 | 67.2 |
| 26 | 3.50 | 230 | 260 | 200 | 71.8 |
| 27 | 5.00 | 260 | 260 | 150 | 87.9 |
| 28 | 3.50 | 230 | 300 | 150 | 81.7 |
| 29 | 6.00 | 280 | 300 | 100 | 66.7 |
| 30 | 1.00 | 220 | 300 | 100 | 41.3 |
| Std. Dev. | 5.67 | R-Squared | 0.6886 |
| Mean | 67.87 | Adj R-Squared | 0.6074 |
| CV% | 8.35 | Pred R-Squared | 0.4805 |
| PRESS | 1231.37 | Adeq Precision | 10.202 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1632.15 | 6 | 272.02 | 8.48 | <0.0001 | Significant |
| Leaching Time | 189.17 | 1 | 189.17 | 5.89 | 0.0234 | |
| Temperature | 266.07 | 1 | 266.07 | 8.29 | 0.0085 | |
| Na2O Concentration | 30.75 | 1 | 30.75 | 0.96 | 0.3378 | |
| CaO/SiO2 | 28.62 | 1 | 28.62 | 0.89 | 0.3548 | |
| CD | 159.18 | 1 | 159.18 | 4.96 | 0.0360 | |
| A2 | 890.19 | 1 | 890.19 | 27.74 | <0.0001 | |
| Residual | 738.13 | 23 | 32.09 | |||
| Lack of Fit | 611.18 | 19 | 32.17 | 1.01 | 0.5615 | Not significant |
| Pure Error | 126.95 | 4 | 272.02 | 8.48 | <0.0001 | |
| Leaching Time | Temperature °C | Na2O Concentration | CaO/SiO2 Ratio | Provision from the Optimization | Experimental Results | |
|---|---|---|---|---|---|---|
| Al2O3 | Desirability | Al2O3 | ||||
| 5 | 260 | 260 | 1.5 | 87.9 | 0.916 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abikak, Y.; Bakhshyan, A.; Dyussenova, S.; Gladyshev, S.; Kassymzhanova, A. Optimization of Hydrochemical Leaching Process of Kaolinite Fraction of Bauxite with Response Surface Methodology. Processes 2024, 12, 1440. https://doi.org/10.3390/pr12071440
Abikak Y, Bakhshyan A, Dyussenova S, Gladyshev S, Kassymzhanova A. Optimization of Hydrochemical Leaching Process of Kaolinite Fraction of Bauxite with Response Surface Methodology. Processes. 2024; 12(7):1440. https://doi.org/10.3390/pr12071440
Chicago/Turabian StyleAbikak, Yerkezhan, Arina Bakhshyan, Symbat Dyussenova, Sergey Gladyshev, and Asiya Kassymzhanova. 2024. "Optimization of Hydrochemical Leaching Process of Kaolinite Fraction of Bauxite with Response Surface Methodology" Processes 12, no. 7: 1440. https://doi.org/10.3390/pr12071440
APA StyleAbikak, Y., Bakhshyan, A., Dyussenova, S., Gladyshev, S., & Kassymzhanova, A. (2024). Optimization of Hydrochemical Leaching Process of Kaolinite Fraction of Bauxite with Response Surface Methodology. Processes, 12(7), 1440. https://doi.org/10.3390/pr12071440









