Abstract
The purpose of this work is to obtain the optimum conditions for leaching the zinc contained in an industrial lead smelting slag. In this type of slag, zinc oxide, sulfide, and ferrite are contained. Zinc extraction from these compounds consists of using a single aqueous medium, where oxidant alkaline leaching with NaOH and NaClO was used. The parameters evaluated during the experiment were as follows: percentage of solids, NaOH/NaClO ratio, and temperature. The maximum amount of recovered Zn obtained during the leaching was 58%. This percentage was achieved by using the following optimal conditions: temperature of 60 °C, 0.22 of NaOH/NaClO ratio, 10% of solids, and a reaction time of 40 min. The calculated values of activation energy confirmed that the rate-limiting step of the reaction using the decreasing particle model is diffusion. The maximum percentage of zinc obtained could only have been achieved if the zinc oxide and part of the zinc sulfide (both present in almost equal proportions in the sample) were leached into the alkaline aqueous medium in the presence of NaClO.
1. Introduction
Lead slag is a byproduct that can be generated in two ways: through lead smelting and through lead recovery from lead-acid batteries. From these processes, primary and secondary slag are obtained, respectively, which have similar physical properties, have a black color, and are glassy in appearance. The mineral phases present in primary lead slag include wustite (FeO), fayalite (ZnFe2O4), willemite (Zn2SiO4), and magnetite (Fe3O4). Lead is usually embedded as droplets in the glassy matrix of the slag [1].
According to Yin et al. (2016), in the 1980s, it was considered that metallurgical slags were chemically inert because the metals are associated with glass and silicate [2], which is why the slags were dumped in large areas of terrain or used in pavements [3].
However, recent studies have proven that leaching from slag landfills leads to the contamination of soils, sediments, and streams. Ref. [2] mentioned that lead slag contains zinc (ZnO, ZnS), Sb, As, and Cd, among other metals, which are toxic and even carcinogenic [3]. These metals are quickly mobilized in the soil, causing significant oral bioaccessibility, which is why an improper handling of this type of material can cause serious problems to ecosystems [3,4]. Some researchers have found that the leaching of heavy metals from slag occurs in a greater proportion at acidic pH, except for lead, which is easily released at alkaline pH, and cadmium, which is the most mobile metal [5].
According to Xia et al. (2019), there are various methods for treating hazardous waste, such as incineration, biological treatment, pyrometallurgical and hydrometallurgical treatment, and solidification/stabilization [4]. Some researchers have evaluated the use of Pb/Zn slags in construction materials, finding that it can be used in mortar and concrete mixtures as a replacement for part of the material without losing the mechanical properties of the material, keeping heavy metals immobilized [6,7,8,9,10,11].
Additionally, slag can be processed by using various methods to reduce its impact on the environment and recover the metallic values it contains through pyrometallurgical and hydrometallurgical routes. According to Pan et al. (2019), the valuable metals that can be recovered from slag are lead, zinc, and iron [1]. Due to the zinc content present in slags (≥1%), they can be considered as a secondary supply source. On the other hand, zinc has an important commercial value, so the recovery values can represent a considerable economic benefit, in addition to the fact that there is currently a shortage of zinc ores [12].
In the case of zinc recovery, different hydrometallurgical and pyrometallurgical procedures have been used for their recovery from industrial waste. In the case of materials composed of a greater proportion of ZnO, hydrometallurgical procedures are preferred, while pyrometallurgical processes are optimal for raw materials with sulfides and carbonates [13].
In the case of hydrometallurgical procedures for zinc recovery, leaching in acidic or basic media has been applied. Pang et al. (2022) carried out a leaching study on a lead–zinc slag at different pH scales with the objective of knowing the distribution and leaching of heavy metals. They found that zinc, when present as ZnS, was not leached if the pH value was less than four because zinc was stable in the glassy phase, in the solid solution phase, and in the ZnS compound. They also found that in an alkaline environment, zinc precipitated as Zn(OH)2 and Zn(CO)3 [14].
Some of the leaching media that have been used during acid leaching are sulfuric acid (H2SO4), nitric acid (HNO3), hydrochloric acid (HCl), and citric acid (C6H8O7), with high recovery rates [15,16,17]. In the case of the alkaline leaching of zinc-rich waste, the leaching medium that has frequently been used is NaOH [18,19,20]. Table 1 shows the leaching conditions of zinc from different types of metallurgical waste.

Table 1.
Leaching conditions of zinc from different types of metallurgical waste.
The fact that research continues exploring how to recover metals from slags, in this case, zinc from lead slags, is because zinc is present as an oxide and as a sulfide. The processes mentioned and referred to in the literature are generally aimed at preferably leaching one of the two compounds, zinc oxide or zinc sulfide. The first can dissolve in acidic or alkaline medium. But the second is generally dissolved in an oxidizing acid medium. In this context, the present work addresses the leaching of zinc in an alkaline oxidizing medium to determine if, with this, the dissolution of both zinc compounds can be obtained.
2. Materials and Methods
Samples of lead smelting slag were ground and meshed to obtain a 100% −75 μm particle size. Table 2 shows the approximate composition of the lead slag. Table 2 shows that the sample has 11.42% of zinc, of which 82% of zinc is distributed as ZnS (39.8%) and ZnO (42.2%). The rest of the zinc (18%) is distributed as ferrite, ZnFe2O4.

Table 2.
Approximate chemical composition of the lead slag grounded at −75 µm.
Leaching experiments were conducted in a 4 L batch reactor. Temperature was maintained using a heating grate. A constant agitation speed of 700 rpm was kept using a mechanical stirrer. Temperature values of 20 to 80 °C were used. The percentage of solids used was 5, 10, and 15%. The ratios of NaOH (IQUISA, 98%)(IQUISA, Mexico City, Mexico) and NaClO (HYCEL, 13–15%)(HYCEL, Mexico City, Mexico) were 0.07, 0.19, 0.22, and 0.41 (NaOH/NaClO, w/w%). The maximum molarity of NaOH and NaClO used was 1.4 and 0.5 M, respectively. Residual solids were analyzed by scanning electron microscopy (FEI Quanta 600)(FEI Company, Hillsbury, USA), a X-ray fluorescence spectrophotometer (RIGAKU Primus II)(Rigaku, Tokio, Japan), and X-ray diffractometer (PANALYTICAL, Empyrean) (Malvern Panalytical, Worcestershire, UK); 25 cm3 aliquots were taken at different intervals for chemical analysis using a Coupled Plasma Emission Spectrophotometer (PERKIN ELMER Optima 8300) (PerkinElmer, Waltham, MA, USA).
3. Results and Discussion
This section provides a description of the experimental results and their interpretation and analysis, as well as the experimental conclusions.
3.1. Effect of the Main Parameters
The effect of the experimental parameters on zinc extraction was determined using an analysis of variance (ANOVA), with the objective of evaluating the effect of each of the parameters involved in zinc extraction.
Table 3 shows the main effects, as determined by ANOVA, of the process of extracting zinc through leaching tests. The amount of Zn extracted during the tests was determined according to Fisher’s ratio (F). The data were provided by the ANOVA according to the following: degrees of freedom (DF), sum of squares (SS), and mean of squares (MS). These data indicate that under the conditions studied, the percentage of solids, including a higher ratio of F and a lower probability of its effect being zero, is the most significant parameter during the zinc extraction of the slag from the blow furnace, followed by temperature and, lastly, the NaClO/NaOH ratio.

Table 3.
The analysis of variance of the experimental parameters of zinc extraction.
The results of the ANOVA indicate the factor that has the most variability in the range of levels tested. To specify which level provides the greatest response, the main effects graphs are presented in the form of bar graphs (Figure 1).
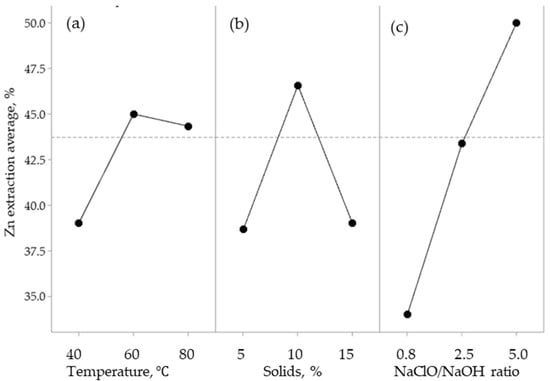
Figure 1.
Effect of the main parameters in the zinc extraction from lead smelting slag: (a) % solids; (b) NaOH/HClO ratio; and (c) temperature.
Figure 1a shows the effect of the percentage of solids in the percentage of zinc extraction; 40% of zinc dissolution was obtained at 5% of solids. Increasing the percentage of solids to 10%, 55% of zinc dissolved was reached. However, at 15% of solids, there was a decrease of the extraction of zinc, to 40%. Since the stirring conditions are the same for the three percentages of solids, the higher the percentage, the greater the stirring necessary to maintain the diffusion of the reagents. If this does not happen, there is a maximum limit condition, which, in this case, was 10%.
Figure 1b shows that the Zn extraction increased from 34% to 58% by increasing the NaOH/NaClO ratio from 0.07 to 0.22. However, after 0.22, the effect of increasing the NaOH and NaClO ratios decreases the extraction significantly.
In Figure 1c, it can be seen that increasing the temperature from 40 to 80 °C increases the percentage of zinc extraction; the same behavior was seen by Stefanova and collaborators (2012) when they performed the alkaline lixiviation of zinc dust of stainless steel production, where it was found that increasing the temperature from 25 to 95 °C improves the extraction of zinc in a strong NaOH solution [28].
Therefore, it can be determined that the optimal conditions needed to maximize zinc extraction are 10% solids, 80 °C, and a 0.22 NaClO/NaOH ratio.
As mentioned, the maximum zinc extraction obtained was 58%. This result indicates that possibly mainly the zinc oxide was dissolved, and part of the zinc sulfide. This is because both species contain almost the same amount of zinc in the sample, 40% in each of them, and because franklinite is the most stable species. Therefore, it can be assumed that 40% of the zinc in the oxide was extracted, and the rest, 18%, comes from the partial dissolution of the sulfide. Next, a thermodynamic analysis of the system was carried out to support this statement.
3.2. Thermodynamics
The experimental results can be explained from the perspective of thermodynamics. In this section, the Gibbs free energy of the possible reactions are calculated, and the Eh-pH is analyzed to find the Zn stability areas during alkaline leaching in the Zn-Cl-Na-S-H2O system. According to the Eh-pH diagram shown in Figure 2, the sphalerite, ZnS, in the presence of an oxidant, such as sodium hypochlorite, and under alkaline conditions, i.e., in a pH range of 9 to 12, and the HZn ion can be obtained. The combination of an oxidant and an alkali metal hydroxide is effective for selectively dissolving the ZnS [29].
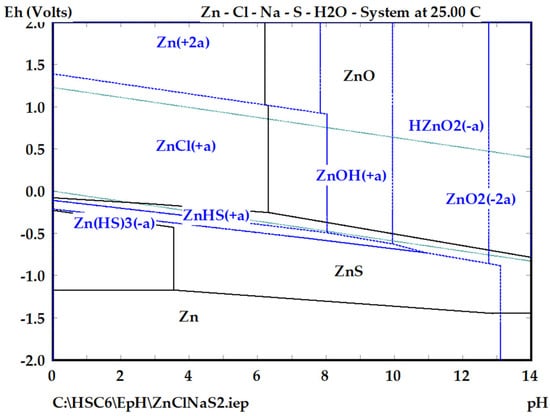
Figure 2.
Eh-pH diagram for the Zn-Cl-Na-S system at 333 K using HSC Chemistry 6 [30].
Considering the thermodynamic data obtained using the software HSC Chemistry 6 version 6.12 [30], HZn is the species predominate under alkaline and oxidizing conditions, according to Equation (1):
ZnS(s) + NaClO(ia) + OH− = HZnO2− + NaCl + S° (ΔG° 333 K =−189.98 kJ)
Sodium hypochlorite reacts with zinc sulfide in the presence of hydroxide to produce a soluble zincate, sodium chloride, and elemental sulfur. According to Figure 2, the ZnS can react and carry out the formation of the anionic compound HZn, the ZnOH+ ion, and the ZnCl+. This may occur according to the following reactions, of which their Gibbs free energies were obtained using the HSC Chemistry 6 software [30]:
ZnS + 2NaClO + O2(g) = ZnCl2+ Na2SO4 (ΔG°333 K = −879.89 kJ)
As can be observed in reactions 2 and 3, which do not include the presence of hypochlorite, it appears to be favorable for only the leaching and even oxidation of sulfur to sulfate, only if under strongly alkaline conditions and in the presence of oxygen. By making a comparison between both reactions, the formation of the HZn ion turns out to be a little more favorable than the formation of the ZnOH+ ion. However, reaction 2 produces acidity, which is the reason why good control of the alkalinity must be obtained. According to the diagram of Figure 2, the formation of one species or another depends on the pH.
According to reaction 4, the dissolution of ZnS using hypochlorite and without the presence of NaOH shows the oxidation of sulfur to sulfate.
On the other hand, Figure 2 indicates that the zinc oxide (ZnO) under alkaline conditions will not dissolve; however, under oxidizing conditions, it can be dissolved. As reported by the HSC program, the following reactions can be carried out:
ZnO + 2NaClO+ H2O = ZnCl2+ 2NaOH + O2(g) (ΔG°333 K = −21.92 kJ)
ZnO + OH− = ZnOH+ + 0.5O2(g) + 2e− (ΔG°333 K = 128.42 kJ)
The ZnO in the presence of hypochlorite and hydroxide (reaction (5)) reacts to form the HZn ion and is observed to be thermodynamically favorable.
Thermodynamic analysis reveals that ZnO is stable under alkaline conditions if there is no other agent, such as hypochlorite, as shown in Equations (7) and (8). These reactions have a positive free energy, indicating that the reactions are not possible. Therefore, the dissolution of ZnO is favorable only in the presence of an oxidant agent, in this case, sodium hypochlorite. It is important to mention that the presence of oxygen in open reactors, where there is contact of the solution with the ambient oxygen, as well as conditions of high concentration of NaOH, allow for oxidizing conditions that cause the oxide to dissolve in alkaline conditions, without adding an additional oxidant [27].
3.3. Kinetic Analysis
Identification of the Reaction Mechanism
For the identification of the reaction mechanism, the experimental data obtained during the zinc leaching were analyzed with the shrinking particle model (SPM). The model was analyzed for spherical particles of decreasing size because there was no ash formation, and the reactant particle decreases in size during the reaction, as shown in Figure 3.
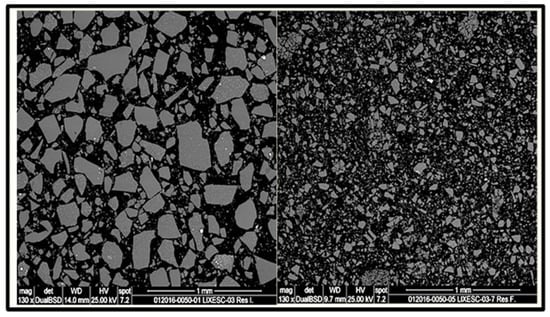
Figure 3.
SEM images of slag sample before (left) and after (right) leaching.
As can be seen in Figure 3, the images provided by SEM show the particle size of the slag before and after being subjected to the alkaline leaching process and, it is observed how the particle size of the slag has decreased significantly after treatment with NaOH and NaClO.
In this model, the leaching reaction may be governed or controlled by chemical reaction and diffusion through the liquid film [31].
The fraction of zinc reacted at any time (t), when controlled by a chemical reaction, can be predicted with Equation (9). However, when the regime is diffusion-controlled, the fraction of zinc reacted at any time (t) can be predicted with Equation (10):
where Kr and Kd are the apparent rate constant for chemical reaction and diffusion control, respectively, and x is the reactant of the Zn fraction and can be calculated from the ratio of the zinc concentration at different time intervals (c), with respect to the initial concentration (C0), according to the following relation:
Krt = 1 − (1 − x)1/3
Kdt = 1 − (1 − x)2/3
It was also analyzed by the model called the stochastic control model [32], where the rate constant Ks of the stochastic model is transformed to a variable that changes with the time according to Equation (12):
Kst =(1 − x)−2/3 − 1
The following figures show the behavior of zinc extraction with respect to time for the variables evaluated, as well as its effect on the rate constant using the three kinetic models.
Figure 4a shows that in the first 15 min there is no difference in the percentage of zinc extraction at the three percentages of solids. After 15 min, the extraction starts to be higher for the tests with 10% solids. From 5 to 10% solids, the effect is on mass: the higher the percentage, the greater the amount extracted. However, at 15%, the extraction decreases. If diffusion is the limiting step, the higher the concentration of solids, the larger the layer required to diffuse reagents and products, making the rate slower.
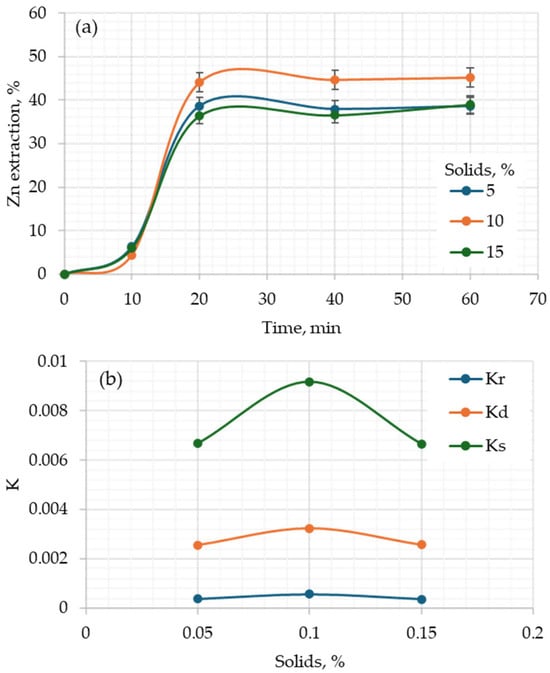
Figure 4.
Effect of percentage of solids on (a) the zinc extraction vs. time and (b) rate constant of the kinetics models. Conditions: 20 °C, NaClO/NaOH = 2.5.
Regarding Figure 4b, the behavior of the apparent kinetic constants of the evaluated models is shown. The effect of the percentage of solids can be observed, regardless of the model (since the K values are relative or apparent). It is observed that the value of the rate constant increases when going from 5 to 10% and then decreases to 15%. This is more noticeable in the stochastic model than in the diffusion model, and much less in the chemical reaction model. As mentioned, the stochastic model considers the change in particle size as the reaction occurs. The fact that the reaction constant decreases is indicative that the size has stopped changing, precisely because the reaction has no longer continued or has stopped. In the case of diffusion, the layer of product formed (solid or liquid) has hindered the reaction, thereby decreasing the value of the apparent kinetic constant.
Figure 5a shows the effect of the NaClO/NaOH ratio, observing that by increasing said ratio, the amount of the maximum extracted zinc, obtained after 20 min, increases. After this time, the extraction percentage is no longer significantly higher, remaining practically the same as at 20 min. However, the maximum amount of zinc extracted was obtained at 40 min. This increase is because, initially, there is a greater slope, given by the apparent velocity constant, as seen in Figure 5b. This figure clearly shows this increase, being more noticeable in the constants obtained by the stochastic models and the shrinking particle model with a diffusion-limited stage.
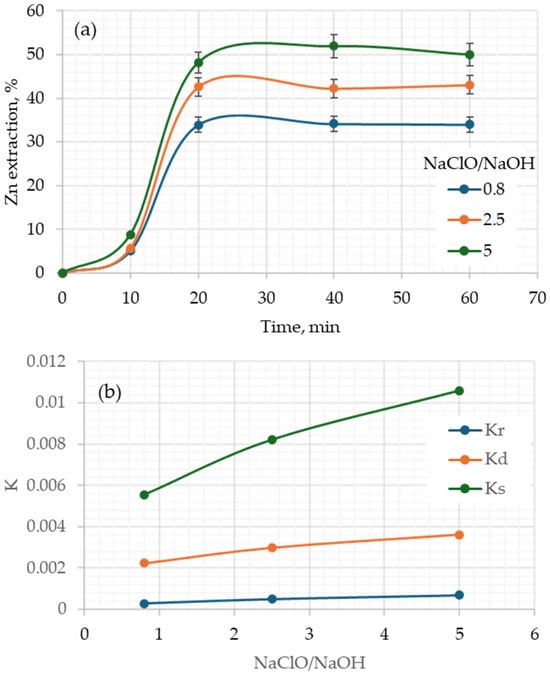
Figure 5.
Effect of NaClO/NaOH ratio on (a) the zinc extraction vs. time and (b) rate constant of the kinetics models. Conditions: 60 °C, 10 % solids.
However, although these two models visually show the trend better, this is due to relative value. Calculating the percentages of increase, based on the lowest ratio of 0.08, it is observed that the value of the apparent rate constant kr (chemical control) increases by 78 and 150%, for the values of 2.5 and 5, respectively. For ks (stochastic model), the increase is 47 and 90%, and only 32 and 62% for kd (diffusion control). On the other hand, it is observed that by increasing the NaClO/NaOH ratio from 0.8 to 2.5 and 5, that is, by 3.125 and 6.25 times, the apparent rate constant that shows the least impact on its increase is kd. That is to say, the apparent velocity constant values have a lower percentage increase in the diffusion model, which is giving an indication that this may be the limiting stage.
The effect of temperature is shown in Figure 6a,b. An increase in the amount of zinc extraction is observed in the first 20 min, for all temperatures. After said time, there is no significant increase. In these first 20 min, it is observed that the slope is greater as it goes from 20 to 40 to 60 °C. And it then decreases to 80 °C. This is clearly observed with the values of the apparent velocity constants obtained for the three kinetic models. In the three cases, the apparent constant increases up to 60 °C and then obtains a lower value at 80 °C. When comparing the relative values in each model, smaller proportional increases in the constant kd are obtained, due to diffusion. Even the value of kd at 80 °C is the highest, indicating that at this temperature, there is no effect on diffusion. In the other models, the proportional values are higher between 20 and 60 °C and lower at 80 °C, indicating that temperature may have a greater effect.
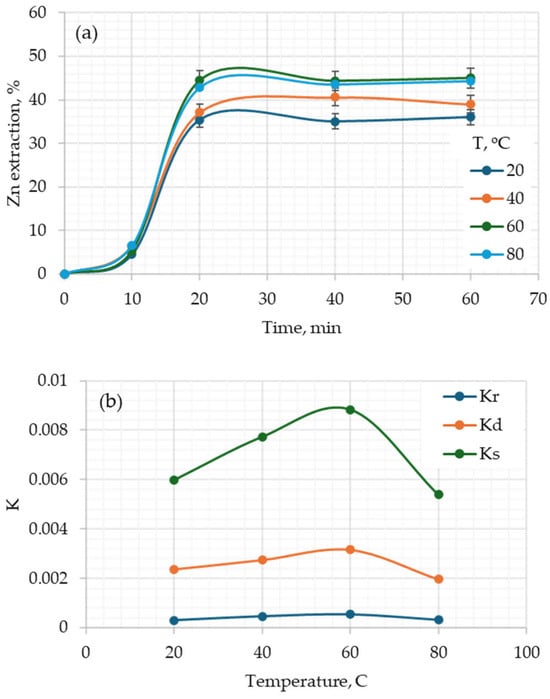
Figure 6.
Effect of temperature on (a) the zinc extraction vs. time and (b) rate constant of the kinetics models. Conditions: 5% solids, NaClO/NaOH = 2.5.
The above coincides with the fact that when leaching is controlled by diffusion, the impact of temperature is less than when the control is by chemical reaction.
To determine which model governs the leaching of zinc and which stage governs the reaction, the activation energy was calculated according to the shrinking particle model and the stochastic model Equations (9), (10) and (12).
To calculate the activation energy (Ea), apparent rate constants calculated from the slopes of the kinetic models at different temperatures were used to determine the limiting step of the zinc leaching process.
The graph of ln k versus 1/T (Figure 7) provides a line with a slope of −Ea/R and the intersection of ln K, according to the linearized Arrhenius equation [33]:
where K is the constant apart from velocity, A is an exponential factor, R is the universal gas constant, and T is the temperature in K.
ln K = ln A − (Ea/RT)
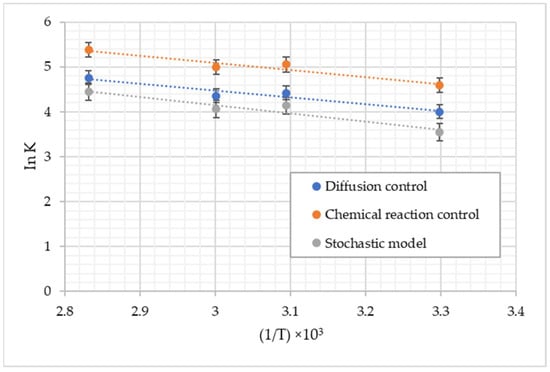
Figure 7.
Arrhenius graph for the Zn leaching of the blow-oven slag.
The estimated values for the activation energy were 13.27, 12.69, and 15.26 kJ/mol, for control by chemical reaction, diffusion control, and stochastic model, respectively, as seen in Table 4.

Table 4.
Calculated value of the activation energy for each model.
Some researchers point out that the activation energy for diffusion control is between 8.37 and 20.92 kJ in the leaching process under atmospheric pressure conditions and 0 to 100 °C [32,33], so it can be confirmed that the zinc leaching contained in lead smelting slags is controlled by diffusion through the liquid film.
4. Conclusions
The leaching of the blast furnace lead smelting slag generated during the processing of Pb concentrate in the metallurgical industry was analyzed on a laboratory scale to obtain optimum conditions for zinc recovery. The experimental results of alkaline leaching show that the percentage of solids, NaClO/NaOH ratio, and temperature are factors that have a substantial effect on zinc extraction. This amount, under such conditions, could only have been achieved if the zinc oxide and part of the zinc sulfide were leached. According to the results of the ANOVA, the percentage of solids has the most significant effect during the leaching, followed by the NaOH and NaClO ratio and, lastly, by the temperature. The maximum amount of recovered zinc was 58%, using 10% solids, a NaOH (0.45 M) and NaClO (0.1 M) ratio of 0.22, and a temperature of 60 °C, in 40 min. Three kinetic models were used: particle decreasing by chemical control, particle decreasing by diffusion control, and the stochastic model. Activation energies were calculated for these models. According to activation energy values obtained, from 12 to 15 kJ (in the temperature range of 20 to 80 °C), the model that controls the zinc leaching process of lead smelting slag is the model of particle decreasing controlled by diffusion.
Author Contributions
Conceptualization, I.A.-G. and F.R.C.-P.; methodology, M.d.J.S.-A.; software, J.M.N.I.; validation, A.M.-L., M.d.J.S.-A. and J.C.-F.; formal analysis, I.A.-G.; investigation, J.M.N.I.; resources, I.A.-G.; writing—original draft preparation, J.M.N.I.; writing—review and editing, N.G.P.-R. and F.R.C.-P.; visualization, A.M.-L.; supervision, J.C.-F.; project administration, I.A.-G.; funding acquisition, I.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The author Najera-Ibarra thanks CONAHCyT for their scholarship. The authors thank Met Mex Peñoles for materials and chemical analysis support.
Conflicts of Interest
Authors Isaías Almaguer-Guzman, Josue Chaidez-Felix were employed by the company Met Mex Peñoles. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. In Resources, Conservation and Recycling; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 146, pp. 140–155. [Google Scholar] [CrossRef]
- Yin, N.H.; Sivry, Y.; Guyot, F.; Lens, P.N.L.; van Hullebusch, E.D. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.R.; da Silva, F.B.V.; Araújo, P.R.M.; do Nascimento, C.W.A. Assessing human health risks and strategies for phytoremediation in soils contaminated with As, Cd, Pb, and Zn by slag disposal. Ecotoxicol. Environ. Saf. 2017, 144, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Prasad, A. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Alwaeli, M. Application of granulated lead-zinc slag in concrete as an opportunity to save natural resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Kanneboina, Y.Y.; Jothi Saravanan, T.; Kabeer, K.I.S.A.; Bisht, K. Valorization of lead and zinc slags for the production of construction materials—A review for future research direction. In Construction and Building Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 367. [Google Scholar] [CrossRef]
- Nath, S.K. Fly ash and zinc slag blended geopolymer: Immobilization of hazardous materials and development of paving blocks. J. Hazard. Mater. 2020, 387, 121673. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Misra, A.; Chaudhary, S. Strength and Abrasion Characteristics of ISF Slag Concrete. J. Mater. Civ. Eng. 2013, 25, 1611–1618. [Google Scholar] [CrossRef]
- Xia, M.; Muhammad, F.; Zeng, L.; Li, S.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.C.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Peng, P.; Xie, H.; Lu, L. Leaching of a sphalerite concentrate with H2SO4-HNO3 solutions in the presence of C2Cl4. Hydrometallurgy 2005, 80, 265–271. [Google Scholar] [CrossRef]
- Erdem, M.; Özverdi, A. Environmental risk assessment and stabilization/solidification of zinc extraction residue: II. Stabilization/solidification. Hydrometallurgy 2011, 105, 270–276. [Google Scholar] [CrossRef]
- Pang, L.; Wang, D.; Wang, H.; An, M.; Wang, Q. Occurrence and leaching behaviors of heavy-metal elements in metallurgical slags. Constr. Build. Mater. 2022, 330, 127268. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Kinetic model for direct leaching of zinc sulfide concentrates at high slurry and solute concentration. Hydrometallurgy 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Yan, X.; Wang, X.; Zhang, J.; Huang, J.; Zhao, J. Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. J. Ind. Eng. Chem. 2015, 27, 368–372. [Google Scholar] [CrossRef]
- Ashtari, P.; Pourghahramani, P. Selective mechanochemical alkaline leaching of zinc from zinc plant residue. Hydrometallurgy 2015, 156, 165–172. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Jiang, J.; Zhang, C. Optimized Hydrometallurgical Route to Produce Ultrafine Zinc Powder from Industrial Wastes in Alkaline Medium. Procedia Environ. Sci. 2012, 16, 674–682. [Google Scholar] [CrossRef][Green Version]
- Stefanova, A.; Aromaa, J.; Forsen, O. Alkaline leaching of zinc from argon oxygen decolonization dust from stainless steel production. Physicochem. Probl. Miner. Process. 2013, 49, 37–46. [Google Scholar] [CrossRef]
- Song, S.; Sun, W.; Wang, L.; Liu, R.; Han, H.; Hu, Y.; Yang, Y. Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J. Environ. Chem. Eng. 2019, 7, 102777. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, H.; Zhang, C.; Liu, B.; Wang, L.; Peng, W.; Cao, Y.; Song, X.; Zhu, X. A novel method for the separation of zinc and cobalt from hazardous zinc–cobalt slag via an alkaline glycine solution. Sep. Purif. Technol. 2021, 273, 119009. [Google Scholar] [CrossRef]
- Xin, C.; Xia, H.; Jiang, G.; Zhang, Q.; Zhang, L.; Xu, Y. Studies on Recovery of Valuable Metals by Leaching Lead–Zinc Smelting Waste with Sulfuric Acid. Minerals 2022, 12, 1200. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Li, S.; Wang, Y.; Zhu, S. Zinc recovery from metallurgical slag and dust by coordination leaching in NH3–CH3COONH4–H2O system. R. Soc. Open Sci. 2018, 5, 180660. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.S.; Rüşen, A.; Topçu, M.A. Recovery of Lead and Zinc from Complex Industrial Waste of Zinc Process with Ammonium Acetate. JOM 2023, 75, 1158–1168. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Song, S.; Xue, N.; Zhang, J.; Yang, S.; Liu, C. Experimental and kinetic study of zinc leaching from metallurgical slag by 5-sulfosalicylic acid. Physicochem. Probl. Miner. Process. 2021, 57, 8–20. [Google Scholar] [CrossRef]
- Varga, T.; Bokányi, L.; Török, T. On the Aqueous Recovery of Zinc from Dust and Slags of the Iron and Steel Production Technologies. Int. J. Metall. Mater. Eng. 2016, 212, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, A.; Aromaa, J.; Forsen, O. Alkaline leaching of zinc from stainless steel electric arc furnace dusts. Physicochem. Probl. Miner. Process. 2015, 51, 293–302. [Google Scholar]
- Dahal, M. Selective Leach Recovery of Zinc from a Composite Sulphide Ore Deposit, Tailings, Crushed Ore or Mine Sludge. U.S. Patent No. US 8,961,911 B2, 24 February 2015. [Google Scholar]
- Roine, A.; Lamberg, P.; Mansikk-Aho, J.; Björklund, P.; Kentala, J.; Talonet, T.; Vartiainen, A. HSC Chemistry 6. version 6.12. Outokumpu Research Oy: Helsinki, Finland, 2007. [Google Scholar]
- Levenspiel, O.; Conesa, J. Ingeniería de las Reacciones Químicas; Limusa: Ciudad de México, Mexico, 2004. [Google Scholar]
- Ciminelli, V.; Osseo, K. Kinetics of pyrite oxidation in sodium carbonate solutions. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1995, 26, 677–685. [Google Scholar] [CrossRef]
- Habashi, F. Kinetics of Metallurgical Process; Métallurgie Extractive: Québec City, QC, Canada, 1999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).