A Review of Fracturing and Enhanced Recovery Integration Working Fluids in Tight Reservoirs
Abstract
1. Introduction
2. The Function of Integrated Fracturing and Enhanced Recovery and the Characteristics of Working Fluids
2.1. The Function of Integrated Fracturing and Enhanced Recovery
2.2. Characteristics of Integrated Fracturing and Enhanced Recovery Fluid
3. Types and Characteristics of Integrated Fracturing and Enhanced Recovery Fluids
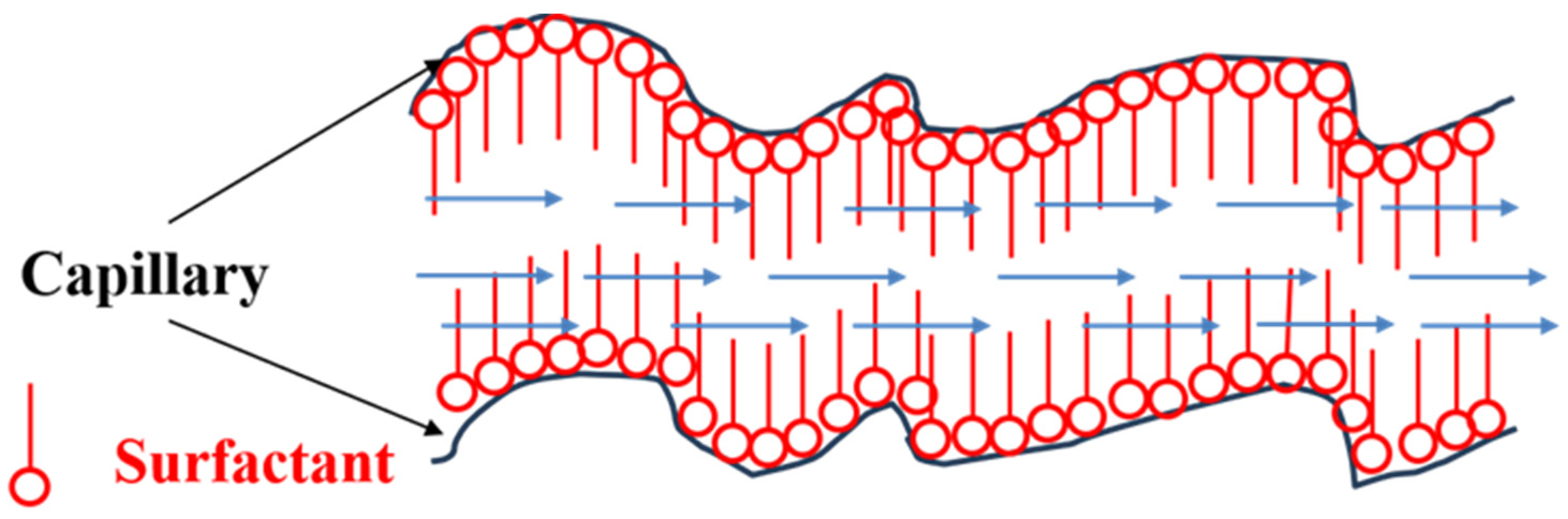
3.1. Polymer-Based Working Fluids
3.2. Surfactant-Based Fracturing Fluids
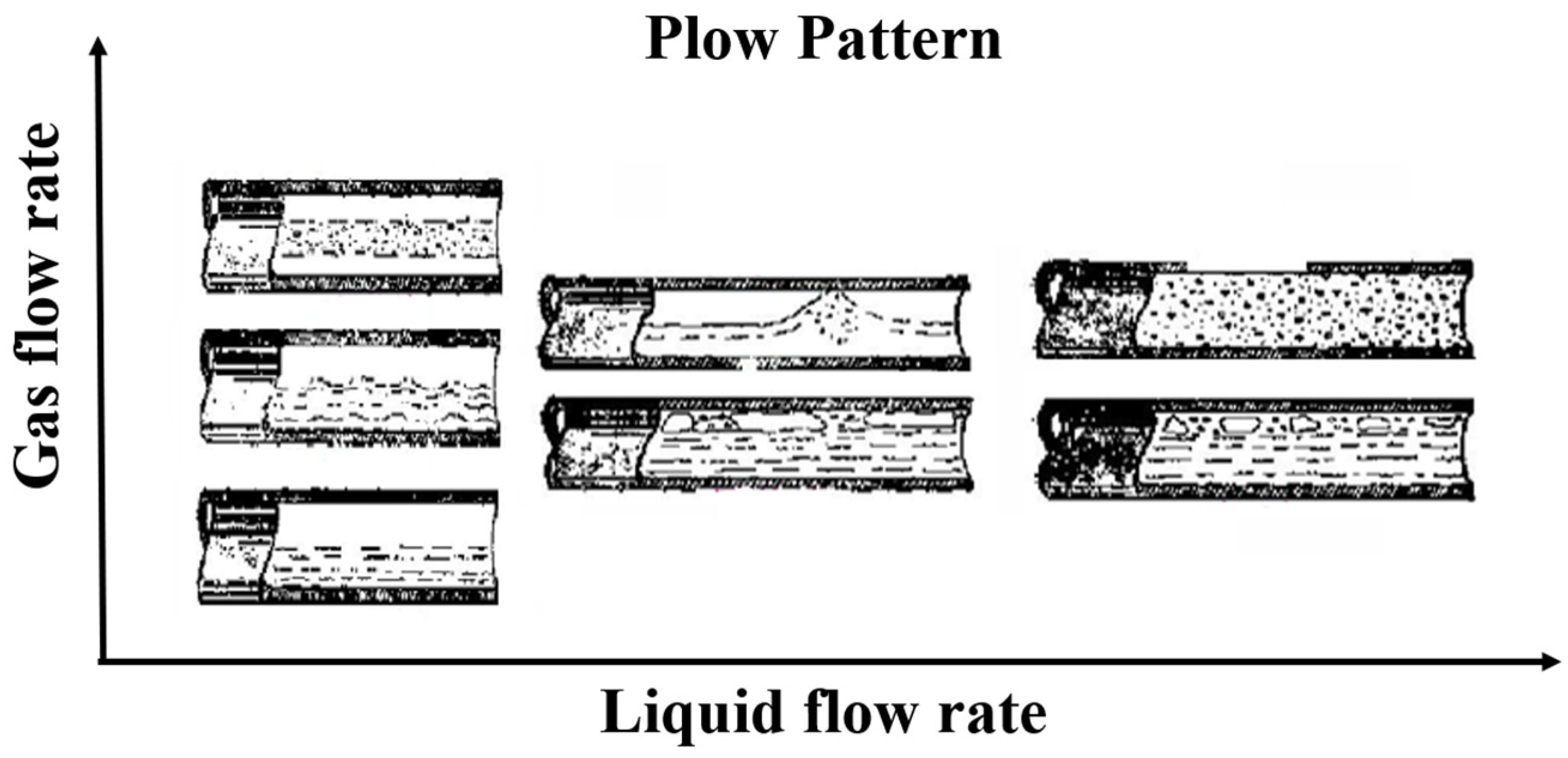
3.3. Foam-Based Working Fluid
3.4. Gas-Based Working Fluid
4. Fracturing and Enhanced Recovery Integration Fluid: Challenges and Prospects
4.1. Fracturing and Enhanced Recovery Coordination Effect
- (1)
- The development of working fluids with oil displacement, such as cationic surfactant and carboxymethyl hydroxyethyl cellulose composite working fluid systems. Combining the advantages of surfactants and polymers, these have lower surface tension and interfacial tension, altering rock wettability and improving displacement efficiency.
- (2)
- Microbially enhanced oil recovery is one of the most effective techniques recognized for recovering residual oil and heavy oil reservoirs. Researching endogenous microbial activators and biopolymer emulsifiers compounded with oil displacement agents for specific reservoirs and injecting them into the formation during fracturing operations can activate reservoir microorganisms through processes such as shut-in wells, leading to the degradation and dispersion emulsification of crude oil.
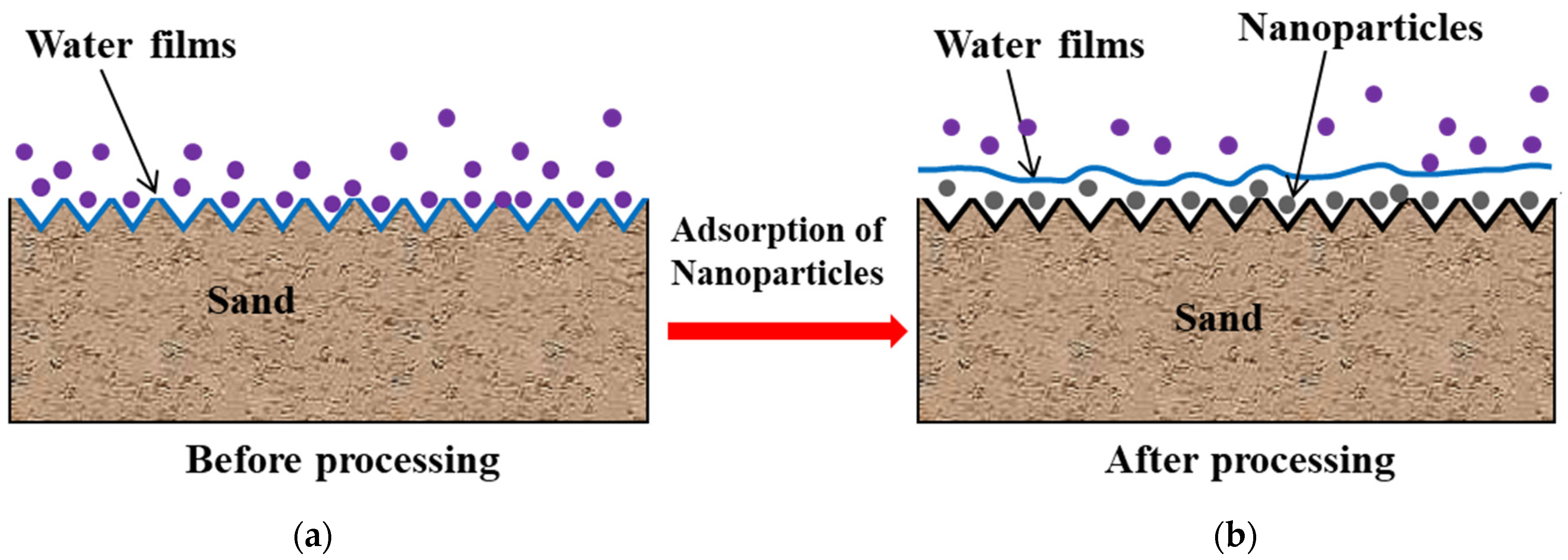
4.2. Nanomaterial Pressure Reduction and Enhanced Injection
- (1)
- Enlarging the Effective Pore Radius
- (2)
- Superhydrophobic Effect
- (3)
- Anti-Swelling Performance
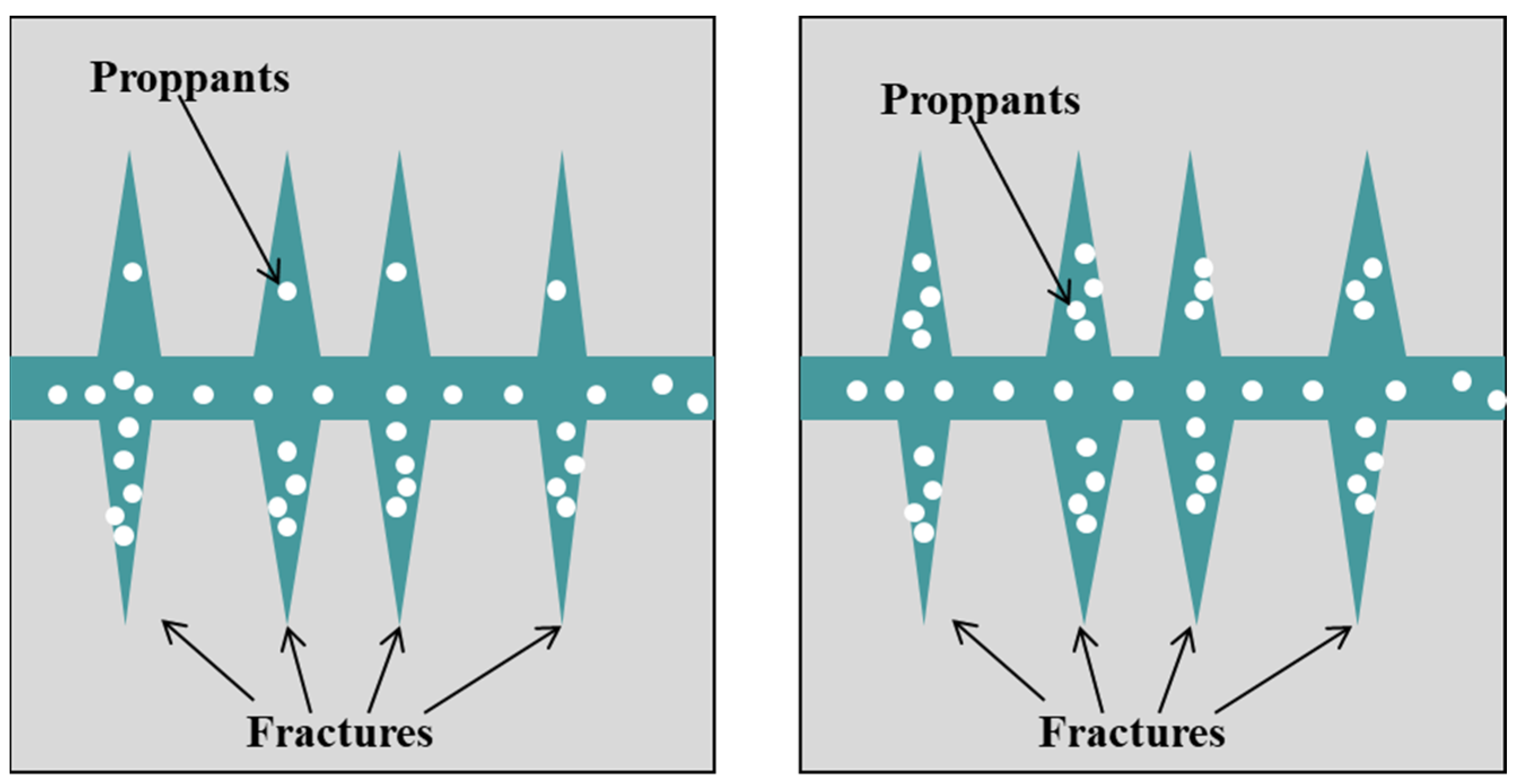
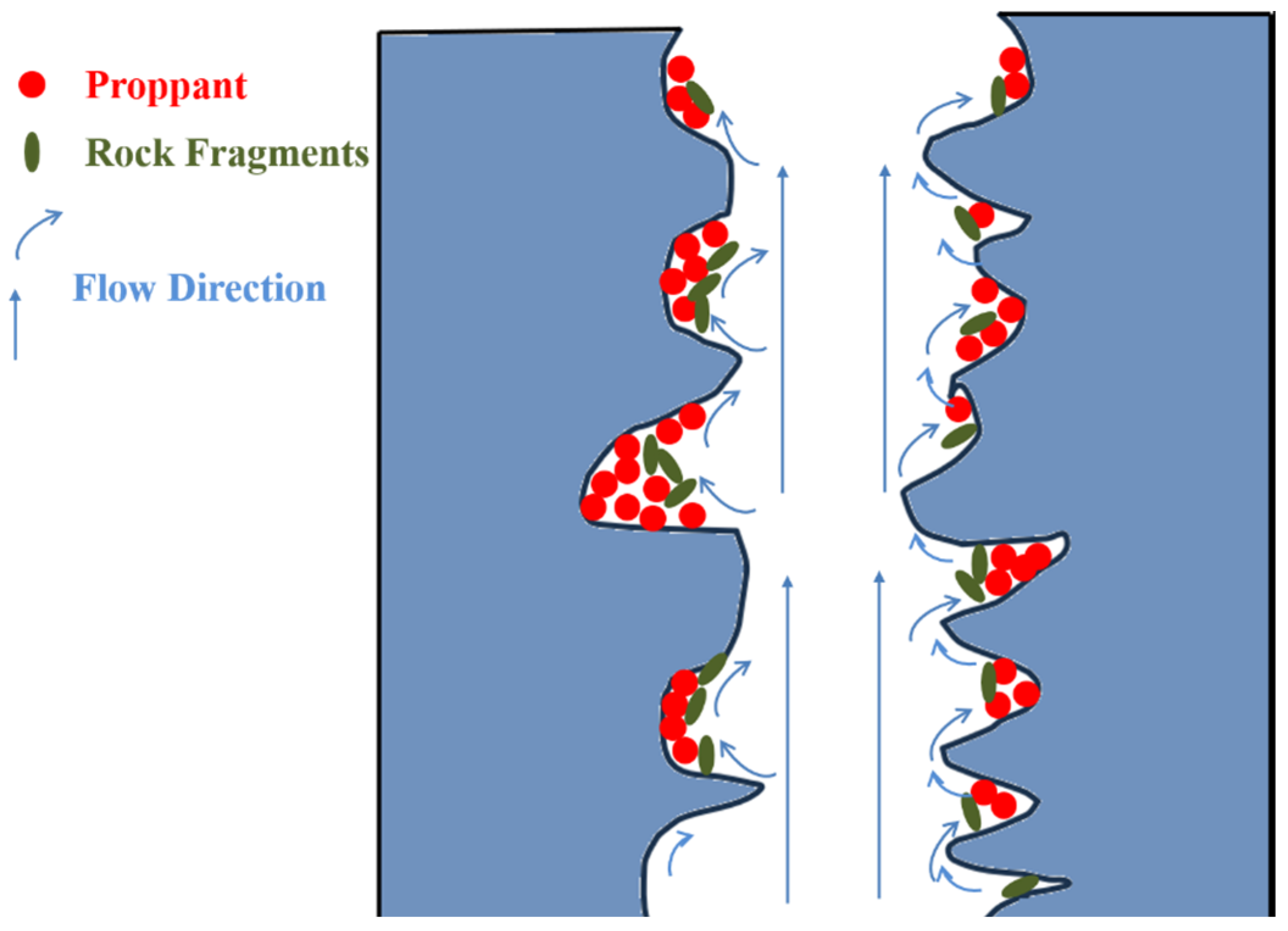
4.3. Fracture–Pore Coupling Frictional Resistance Evolution
4.4. Green and Environmentally Friendly Integrated Fluid
- (1)
- Fracturing can produce thousands to tens of thousands of cubic meters of fluid, leading to rising costs and imposing a heavy burden on the ecological environment. Therefore, it is necessary to strengthen the recycling of fracturing fluids. The recycling of fracturing wastewater simplifies the treatment process and reduces fracturing costs, and given the special environment of offshore fracturing operations, the urgent need for multiple recycling of fracturing fluids is emphasized.
- (2)
- “CO2+”-enhanced fracturing and stimulation (such as CO2 + solvent, CO2 + expansion agent, CO2 + thickening agent, etc.) will play an important role, and the promotion of supercritical CO2 fracturing will provide an opportunity for the wider use of CO2. CO2-responsive microemulsion is a promising solvent.
- (3)
- Developing high-temperature controllable gelling/breaking CO2-responsive clean fracturing fluids can overcome the poor temperature resistance of traditional clean fracturing fluids and have advantages such as low reservoir damage and environmental friendliness. The biodegradable, environmentally friendly, and outstanding properties of green biosurfactants, crosslinkers, and amino-acid-based green surfactants will unleash their application potential.
5. Summary
- (1)
- Fracturing and subsequent production enhancement are related. Fracturing fluids need to meet the performance requirements for stages such as creating fractures, suspending proppants, supporting fracture networks, and flowback. Additionally, they must consider production enhancement requirements. Polymer additives exhibit strong thickening abilities but may have difficulties in complete breaking and may leave large residues. Clean fracturing fluids have low surface–interface tension but weaker mechanical properties and higher costs. Foam-based fluids have low filtration loss and minimal reservoir damage but have poor sand-carrying capabilities. Integrated fracturing fluids should be tailored to specific conditions, integrating the advantages of various additives to complete integrated operations.
- (2)
- Nano-scale depressurization and injection technology involves carrying specific nanoparticles into a reservoir through a dispersing medium, adsorbing them onto fracture surfaces, and altering surface wettability and microstructures to achieve depressurization and injection enhancement. The main mechanisms include (a) expanding the effective pore radius; (b) the superhydrophobic effect; and (c) anti-swelling performance.
- (3)
- Injecting fluids with fracturing and oil displacement properties during the fracturing process can expand the fluid filtration range in the reservoir, reduce rock fracturing pressure, increase reservoir elastic energy, and displace crude oil, thereby increasing production. Surfactants with polymerization; biodegradability and the properties of bio-based surface-active agents, crosslinkers, and amino-acid-based green surfactants demonstrate application potential. Polymer-layered silicate nanocomposites with excellent thermal stability and mechanical properties will provide important support for the effectiveness of integrated fracturing and enhanced recovery enhancement fluids.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soeder, D.J. The successful development of gas and oil resources from shales in North America. J. Pet. Sci. Eng. 2018, 163, 399–420. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.; Mao, J.; Tan, H.; Zhang, Y.; Song, Z. Review of friction reducers used in slickwater fracturing fluids for shale gas reservoirs. J. Nat. Gas Sci. Eng. 2018, 62, 302–313. [Google Scholar] [CrossRef]
- Zeng, B.; Cheng, L.; Li, C. Low velocity non-linear flow in ultra-low permeability reservoir. J. Pet. Sci. Eng. 2011, 80, 1–6. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Z.; Song, X.; Wu, W.; Sun, Y.; Cheng, Y.; Wang, X.; Yan, X.; Dai, C. Study on the main factors and mechanism of functional silica nanofluid spontaneous imbibition for enhanced oil recovery. J. Mol. Liq. 2024, 394, 123699. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Chen, Q.; Weifeng, S.; Du, S. Research on the Enhanced Oil Recovery Technique of Horizontal Well Volume Fracturing and CO2 Huff-n-Puff in Tight Oil Reservoirs. ACS Omega 2021, 6, 28485–28495. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wei, X.; Chen, Z.; Rahman, S.S.; Pu, C.; Li, X.; Zhang, Y. Numerical simulation and parametric analysis for designing High Energy Gas Fracturing. J. Nat. Gas Sci. Eng. 2018, 53, 218–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, G.; Liu, J. Modeling Method to Characterize the Pore Structure of Fractured Tight Reservoirs. Appl. Sci. 2022, 12, 2078. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Zhao, W.; Li, W.; Zhang, J. Experimental of hydraulic fracture propagation using fixed-point multistage fracturing in a vertical well in tight sandstone reservoir. J. Pet. Sci. Eng. 2018, 171, 704–713. [Google Scholar] [CrossRef]
- Pană, I.; Gheţiu, I.V.; Stan, I.G.; Dinu, F.; Brănoiu, G.; Suditu, S. The Use of Hydraulic Fracturing in Stimulation of the Oil and Gas Wells in Romania. Sustainability 2022, 14, 5614. [Google Scholar] [CrossRef]
- Mayerhofer, M.J.; Lolon, E.P.; Youngblood, J.E.; Heinze, J.R. Integration of Microseismic Fracture Mapping Results with Numerical Fracture Network Production Modeling in the Barnett Shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006. [Google Scholar]
- Barbati, A.C.; Desroches, J.; Robisson, A.; McKinley, G.H. Complex Fluids and Hydraulic Fracturing. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 415–453. [Google Scholar] [CrossRef]
- Lashari, N.; Hussain, T.; Ganat, T.; Kalam, S.; Hussain, K.; Aslam, S.; Ahmed, S. Synergistic effect of graphene oxide and partially hydrolyzed polyacrylamide for enhanced oil recovery: Merging coreflood experimental and CFD modeling approaches. J. Mol. Liq. 2024, 394, 123733. [Google Scholar] [CrossRef]
- Goldstein, B.D.; Brooks, B.W.; Cohen, S.D.; Gates, A.E.; Honeycutt, M.E.; Morris, J.B.; Orme-Zavaleta, J.; Penning, T.M.; Snawder, J. The Role of Toxicological Science in Meeting the Challenges and Opportunities of Hydraulic Fracturing. Toxicol. Sci. 2014, 139, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Legemah, M.; Guerin, M.; Sun, H.; Qu, Q. Novel High-Efficiency Boron Crosslinkers for Low-Polymer-Loading Fracturing Fluids. SPE J. 2014, 19, 737–743. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, B.; Chen, Z.; Nie, D. The status quo review and suggested policies for shale gas development in China. Renew. Sustain. Energy Rev. 2016, 59, 420–428. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, S.; Ge, H.; Wang, X.; Lei, X.; Xiao, B. A new method for evaluation of fracture network formation capacity of rock. Fuel 2014, 140, 778–787. [Google Scholar] [CrossRef]
- Liang, T.; Wen, Y.; Qu, M.; Yang, C.; Wu, W.; Ma, T.; Raj, I.; Hou, J. Constructing a viscoelastic film for enhanced oil recovery via self-adsorption of amphiphilic nanosheets at oil-water interface. J. Mol. Liq. 2024, 394, 123679. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Zhang, Z.; Yang, B.; Zhang, W.; Zhang, H. Preparation of a novel ultra-high temperature low-damage fracturing fluid system using dynamic crosslinking strategy. Chem. Eng. J. 2018, 354, 913–921. [Google Scholar] [CrossRef]
- Cao, B.; Lu, X.; Xie, K.; Ding, H.; Xiao, Z.; Cao, W.; Zhou, Y.; He, X.; Li, Y.; Li, H. The pore-scale mechanisms of surfactant-assisted spontaneous and forced imbibition in water-wet tight oil reservoirs. J. Pet. Sci. Eng. 2022, 213, 110371. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Li, Y.; Wang, R.; Chen, J.; Wu, Y.; Dai, C. Interface properties evolution and imbibition mechanism of gel breaking fluid of clean fracturing fluid. J. Mol. Liq. 2022, 359, 118952. [Google Scholar] [CrossRef]
- Shun, L.; Jun, N.; Xianli, W.; Xiong, L.; Zhaoqin, H.; Desheng, Z.; Pengju, R. A dual-porous and dual-permeable media model for imbibition in tight sandstone reservoirs. J. Pet. Sci. Eng. 2020, 194, 107477. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Yan, L.; Liu, S.; Liu, Y. On the Imbibition Model for Oil-Water Replacement of Tight Sandstone Oil Reservoirs. Geofluids 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Qiu, R.; Gu, C.; Xue, P.; Xu, D.; Gu, M. Imbibition characteristics of sandstone cores with different permeabilities in nanofluids. Pet. Explor. Dev. 2022, 49, 374–381. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Gao, Q.; Liu, Y.; Huang, H.; Liu, S. Effect of Salinity on the Imbibition Recovery Process of Tight Sandstone Reservoirs. Processes 2022, 10, 228. [Google Scholar] [CrossRef]
- Barbucci, R.; Pasqui, D.; Favaloro, R.; Panariello, G. A thixotropic hydrogel from chemically cross-linked guar gum: Synthesis, characterization and rheological behaviour. Carbohydr. Res. 2008, 343, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Hashmet, M.R.; Onur, M.; Tan, I.M. Empirical Correlations for Viscosity of Polyacrylamide Solutions with the Effects of Temperature and Shear Rate. II. J. Dispers. Sci. Technol. 2014, 35, 1685–1690. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Liao, Y.; Xu, T.; Zhang, H.; Du, A.; Yang, X.; Lin, C.; Mao, J. Evaluation of temperature resistance of non chemical crosslinked double-tailed hydrophobically associating polymer fracturing fluid. React. Funct. Polym. 2022, 175, 105268. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Shen, Y.; Lai, X.; Guo, X. Study on properties of hydrophobic associating polymer as drag reduction agent for fracturing fluid. J. Polym. Res. 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Q.; Zhou, Z.; Mu, Y. Synthesis and evaluation of water-soluble fracturing fluid thickener based on hydrophobic association. Mater. Lett. 2022, 325, 132857. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, L.; Ma, C. Synthesis and properties of fluorinated hydrophobic association polyacrylamide as thickener for hydraulic fracturing fluid. Iran. Polym. J. 2017, 26, 589–595. [Google Scholar] [CrossRef]
- Ma, X.; Mu, H.; Hu, Y.; Yang, S. Synthesis and properties of hydrophobically associating polymer fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127013. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Li, W.; Zeng, P.; Zheng, Z.; Feng, Y.; Yin, H. Comparative Studies on Hydraulic Fracturing Fluids for High-Temperature and High-Salt Oil Reservoirs: Synthetic Polymer versus Guar Gum. ACS Omega 2021, 6, 25421–25429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mao, J.; Zhao, J.; Zhang, H.; Xue, J.; Zhang, Q.; Zhang, Y.; Yang, X.; Lin, C.; Mao, J.; et al. Synthesis of a salt-responsive hydrophobically associating polymer for fracturing fluid based on self-assembling transition. J. Mol. Liq. 2022, 366, 120201. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Zeng, L.; Miao, W.; Wu, Y. Study of emulsion polymerization stabilized by amphiphilic polymer nanoparticles. Colloid Polym. Sci. 2011, 289, 1543–1551. [Google Scholar] [CrossRef]
- Miao, G.; Zhang, H.; Yang, Y.; Qu, J.; Ma, X.; Zheng, J.; Liu, X. Synthesis and performance evaluation of crosslinker for seawater-based fracturing fluid. J. Appl. Polym. Sci. 2022, 140, 53372. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, M.; Bi, W.; Cheng, Y.; Ma, Z.; Liu, K.; Li, Y.; Dai, C. Synthesis and analysis of drag reduction performance of the temperature-resistant polymer slickwater for high temperature reservoirs. J. Mol. Liq. 2024, 397, 124137. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Zhang, S.; Feng, Y. Smart thermoviscosifying polymer for improving drag reduction in slick-water hydrofracking. Fuel 2020, 278, 118408. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Z.; Xiong, Y.; Chen, N.; Lan, J.; He, Z.; Dai, S.; Huang, D. Molecularly designed nonionic hydrophobic association polymers with anti-salt capacity: Enhancing drag reduction at high mineralization levels. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132827. [Google Scholar] [CrossRef]
- Gallegos, T.J.; Varela, B.A.; Haines, S.S.; Engle, M.A. Hydraulic fracturing water use variability in theUnitedStates and potential environmental implications. Water Resour. Res. 2015, 51, 5839–5845. [Google Scholar] [CrossRef]
- Gong, L.X.; Zhang, X.F. A new approach to the synthesis of hydrophobically associating polyacrylamide via the inverse miniemulsion polymerization in the presence of template. Express Polym. Lett. 2009, 3, 778–787. [Google Scholar] [CrossRef]
- Ji, K.; Jia, W.; He, G.; Chen, G.; Yu, L. Rheological behavior of hydrophobic association polyacrylamides with high salt resistance and corrosion inhibition: Cross-linking modification based on trace dopamine hydrochloride derivatives. J. Mol. Liq. 2024, 400, 124520. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Bai, H.; Wei, D.; Ma, J.; Yang, P.; Zhang, F.; Yuan, L. A new approach to study the friction-reduction characteristics of viscous/conventional slickwater in simulated pipelines and fractures. J. Nat. Gas Sci. Eng. 2020, 83, 103620. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, X.; Huang, Y.; Zhao, M.; Zhang, L.; Dai, C. Ultra-deep reservoirs gel fracturing fluid with stepwise reinforcement network from supramolecular force to chemical crosslinking. Energy 2024, 293, 130632. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X. The Shear Mechanisms of Natural Fractures during the Hydraulic Stimulation of Shale Gas Reservoirs. Materials 2016, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mao, J.; Yang, X.; Zhao, J.; Smith, G.S. Advances in waterless fracturing technologies for unconventional reservoirs. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 41, 237–251. [Google Scholar] [CrossRef]
- Barré, L.L.; Vaughan, A.S.; Sutton, S.J. On the solid-state ageing behaviour of the polysaccharide, guar gum. J. Mater. Sci. 2007, 42, 5497–5507. [Google Scholar] [CrossRef]
- Bahamdan, A.; Daly, W.H. Hydrophobic guar gum derivatives prepared by controlled grafting processes. Polym. Adv. Technol. 2007, 18, 652–659. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Yang, B.; Yang, X.; Zhang, W.; Zhang, C.; Smith, G.S. Experimental evaluation of a novel modification of anionic guar gum with maleic anhydride for fracturing fluid. Rheol. Acta 2019, 58, 173–181. [Google Scholar] [CrossRef]
- Song, J.; Fan, W.; Long, X.; Zhou, L.; Wang, C.; Li, G. Rheological behaviors of fluorinated hydrophobically associating cationic guar gum fracturing gel. J. Pet. Sci. Eng. 2016, 146, 999–1005. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Du, B.; Li, P.; Li, H. Associating and rheological behaviors of fluorinated cationic guar gum in aqueous solutions. Carbohydr. Polym. 2013, 95, 637–643. [Google Scholar] [CrossRef]
- Abdel-Halim, E.; Al-Deyab, S.S. Hydrogel from crosslinked polyacrylamide/guar gum graft copolymer for sorption of hexavalent chromium ion. Carbohydr. Polym. 2011, 86, 1306–1312. [Google Scholar] [CrossRef]
- Yusof NS, M.; Khan, M.N. Quantitative correlation between counterion-affinity to cationic micelles and counterion-induced micellar growth. Adv. Colloid Interface Sci. 2013, 193–194, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, Z.; Song, Z.; Bai, J.; Zhang, Y.; Luo, J.; Du, Y.; Jiang, Q. Self-assembly properties of ultra-long-chain gemini surfactant with high performance in a fracturing fluid application. J. Appl. Polym. Sci. 2016, 134, 44602. [Google Scholar] [CrossRef]
- Ma, L.; Guo, Y.; Feng, R.; Xiang, P.; Li, C. Synthesis and Micellar Behaviors of an Anionic Polymerizable Surfactant. J. Chin. Chem. Soc. 2014, 61, 583–588. [Google Scholar] [CrossRef]
- Baruah, A.; Shekhawat, D.S.; Pathak, A.K.; Ojha, K. Experimental investigation of rheological properties in zwitterionic-anionic mixed-surfactant based fracturing fluids. J. Pet. Sci. Eng. 2016, 146, 340–349. [Google Scholar] [CrossRef]
- Al-Sadat, W.; Nasser, M.; Chang, F.; Nasr-El-Din, H.; Hussein, I. Laboratory evaluation of the effects of additives and pH on the thermorheological behavior of a viscoelastic zwitterionic surfactant used in acid stimulation. J. Pet. Sci. Eng. 2014, 122, 458–467. [Google Scholar] [CrossRef]
- Baruah, A.; Pathak, A.K.; Ojha, K. Study on the Thermal Stability of Viscoelastic Surfactant-Based Fluids Bearing Lamellar Structures. Ind. Eng. Chem. Res. 2015, 54, 7640–7649. [Google Scholar] [CrossRef]
- Du, A.; Yang, J.; Jiang, M.; Zeng, L.; Mao, J. A green alternative to replace anionic viscoelastic surfactant based fracturing fluid with enhanced properties. J. Mol. Liq. 2024, 393, 123653. [Google Scholar] [CrossRef]
- da Silva, M.A.; Weinzaepfel, E.; Afifi, H.; Eriksson, J.; Grillo, I.; Valero, M.; Dreiss, C.A. Tuning the Viscoelasticity of Nonionic Wormlike Micelles with β-Cyclodextrin Derivatives: A Highly Discriminative Process. Langmuir 2013, 29, 7697–7708. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, A. Synthesis and physiochemical characterization of zwitterionic surfactant for application in enhanced oil recovery. J. Mol. Liq. 2017, 243, 61–71. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, N.; Mandal, A. Synthesis and evaluation of physicochemical properties of anionic polymeric surfactant derived from Jatropha oil for application in enhanced oil recovery. J. Ind. Eng. Chem. 2016, 43, 106–116. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, M.; Fang, B.; Wang, J.; Guo, Y. Wormlike Micelles in Mixed Amino Acid-Based Anionic Surfactant and Zwitterionic Surfactant Systems. J. Surfactants Deterg. 2015, 18, 589–596. [Google Scholar] [CrossRef]
- Thampi, N.V.; Ojha, K.; Nair, U.G. Effect of Branched Alcohols on Phase Behavior and Physicochemical Properties of Winsor IV Microemulsions. J. Surfactants Deterg. 2013, 17, 371–381. [Google Scholar] [CrossRef]
- Gu, F.; An, J.; Fu, L. Simulation and experimental study on amphiphilic modified graphene oxide for EOR. Mater. Today Commun. 2024, 39, 109188. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, Y.; Yang, W.; Wang, J.; Fan, Y.; Lu, J. Experimental Study of Sulfonate Gemini Surfactants as Thickeners for Clean Fracturing Fluids. Energies 2018, 11, 3182. [Google Scholar] [CrossRef]
- Mao, J.; Yang, X.; Chen, Y.; Zhang, Z.; Zhang, C.; Yang, B.; Zhao, J. Viscosity reduction mechanism in high temperature of a Gemini viscoelastic surfactant (VES) fracturing fluid and effect of counter-ion salt (KCl) on its heat resistance. J. Pet. Sci. Eng. 2018, 164, 189–195. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef]
- Zarchi, M.B.; Divsalar, A.; Abrari, K.; Rezaei, A. Multiple spectroscopic studies of the interaction between a quaternary ammonium-based cationic Gemini surfactant (as a carrier) and human erythropoietin. J. Biomol. Struct. Dyn. 2017, 36, 3479–3486. [Google Scholar] [CrossRef]
- Wanniarachchi WA, M.; Ranjith, P.G.; Perera MS, A. Shale gas fracturing using foam-based fracturing fluid: A review. Environ. Earth Sci. 2017, 76, 91. [Google Scholar] [CrossRef]
- Wanniarachchi, W.A.M.; Ranjith, P.G.; Perera, M.S.A.; Lashin, A.; Al Arifi, N.; Li, J.C. Current opinions on foam-based hydro-fracturing in deep geological reservoirs. Geomech. Geophys. Geo-Energy Geo-Resour. 2015, 1, 121–134. [Google Scholar] [CrossRef]
- Gandossi, L.; Von Estorff UGandossi, L.; Von Estorff, U. An Overview of Hydraulic Fracturing and Other Formation Stimulation Technologies for Shale Gas Production; Publications Office of the European Union: Luxembourg, 2015; pp. 15–16. [Google Scholar]
- Rognmo, A.; Horjen, H.; Fernø, M. Nanotechnology for improved CO 2 utilization in CCS: Laboratory study of CO 2 -foam flow and silica nanoparticle retention in porous media. Int. J. Greenh. Gas Control. 2017, 64, 113–118. [Google Scholar] [CrossRef]
- Tang, M.; He, S.; Xiong, J.; Zheng, F. A research into recyclable foam fluid for petroleum exploration and development. J. Nat. Gas Sci. Eng. 2014, 21, 241–247. [Google Scholar] [CrossRef]
- Wanniarachchi, W.A.M.; Ranjith, P.G.; Perera, M.S.A.; Rathnaweera, T.D.; Zhang, D.C.; Zhang, C. Investigation of effects of fracturing fluid on hydraulic fracturing and fracture permeability of reservoir rocks: An experimental study using water and foam fracturing. Eng. Fract. Mech. 2018, 194, 117–135. [Google Scholar] [CrossRef]
- Asharfizadeh, S.N.; Ganjiade, A. Liquid foams: Properties, structures, prevailing phenomena and their applications in chemical/biochemical processes. Adv. Colloid Interface Sci. 2024, 325, 103109. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Sun, X.; Gao, R.; Zeng, F.; Tontiwachwuthikul, P.; Liang, Z. Rheological properties study of foam fracturing fluid using CO 2 and surfactant. Chem. Eng. Sci. 2017, 170, 720–730. [Google Scholar] [CrossRef]
- Edrisi, A.R.; Kam, S.I. A New Foam Rheology Model for Shale-Gas Foam Fracturing Applications. In Proceedings of the SPE Canadian Unconventional Resources Conference, Calgary, AB, Canada, 30 October–1 November 2012. [Google Scholar]
- Gu, M.; Mohanty, K. Effect of foam quality on effectiveness of hydraulic fracturing in shales. Int. J. Rock Mech. Min. Sci. Géoméch. Abstr. 2014, 70, 273–285. [Google Scholar] [CrossRef]
- Tian, M.; Li, Q.; Ahmad, N.; Zhang, M.; Gong, J.; Li, C.; Zhao, C. Reversible phase separation of CO2 responsive microemulsion: Judgment on whether and how regulated by ratio of ion to responsive surfactant concentration. Sep. Purif. Technol. 2024, 336, 126326. [Google Scholar] [CrossRef]
- Gajbhiye; Kam, S. Characterization of foam flow in horizontal pipes by using two-flow-regime concept. Chem. Eng. Sci. 2011, 66, 1536–1549. [Google Scholar] [CrossRef]
- Cabeza, L.F.; de Gracia, A.; Fernández, A.I.; Farid, M.M. Supercritical CO2 as heat transfer fluid: A review. Appl. Therm. Eng. 2017, 125, 799–810. [Google Scholar] [CrossRef]
- Wright, M.C.; Court, R.W.; Kafantaris, F.-C.A.; Spathopoulos, F.; Sephton, M.A. A new rapid method for shale oil and shale gas assessment. Fuel 2015, 153, 231–239. [Google Scholar] [CrossRef]
- He, Z.; Tian, S.; Li, G.; Wang, H.; Shen, Z.; Xu, Z. The pressurization effect of jet fracturing using supercritical carbon dioxide. J. Nat. Gas Sci. Eng. 2015, 27, 842–851. [Google Scholar] [CrossRef]
- Abedini, A.; Torabi, F. On the CO2 storage potential of cyclic CO2 injection process for enhanced oil recovery. Fuel 2014, 124, 14–27. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Xiao, M.; Ren, Y.; Yang, J.; Hu, Z. Turbulent Rayleigh–Bénard convection in a supercritical CO2-based binary mixture with cross-diffusion effects. Int. J. Heat Mass Tran. 2024, 228, 125648. [Google Scholar] [CrossRef]
- Ishida, T.; Aoyagi, K.; Niwa, T.; Chen, Y.; Murata, S.; Chen, Q.; Nakayama, Y. Acoustic emission monitoring of hydraulic fracturing laboratory experiment with supercritical and liquid CO2. Geophys. Res. Lett. 2012, 39, L052788. [Google Scholar] [CrossRef]
- Zhou, M.; Ni, R.; Zhao, Y.; Huang, J.; Deng, X. Research progress on supercritical CO2 thickeners. Soft Matter 2021, 17, 5107–5115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Ma, Z.; Ranjith, P. Combined micro-proppant and supercritical carbon dioxide (SC-CO2) fracturing in shale gas reservoirs: A review. Fuel 2021, 305, 121431. [Google Scholar] [CrossRef]
- Memon, S.; Feng, R.; Ali, M.; Bhatti, M.A.; Giwelli, A.; Keshavarz, A.; Xie, Q.; Sarmadivaleh, M. Supercritical CO2-Shale interaction induced natural fracture closure: Implications for scCO2 hydraulic fracturing in shales. Fuel 2021, 313, 122682. [Google Scholar] [CrossRef]
- Di, Q.F.; Shuai, H.U.A.; Ding, W.P.; Wei, G.O.N.G.; Cheng, Y.C.; Feng, Y. Application of support vector machine in drag reduction effect prediction of nano-particles adsorption method on oil reservoir’s micro-channels. J. Hydrodyn. Ser. B 2015, 27, 99–104. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Li, L.; Yang, Z.; Liu, Y.; Fang, J.; Dai, C.; You, Q. Experimental study on pressure-decreasing performance and mechanism of nanoparticles in low permeability reservoir. J. Pet. Sci. Eng. 2018, 166, 693–703. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Di, Q.-F.; Wang, X.-L.; Ding, W.-P.; Gong, W. Numerical Study of the Relationship between Apparent Slip Length and Contact Angle by Lattice Boltzmann Method. J. Hydrodyn. 2012, 24, 535–540. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Luo, J. Biopolymer/montmorillonite nanocomposite: Preparation, drug-controlled release property and cytotoxicity. Nanotechnology 2008, 19, 065707. [Google Scholar] [CrossRef]
- Yu, X.; Ji, R.; Li, Q.; Yong, S.; Yong, H.; Su, G. Core-shell polymer microsphere PSN@SiO2 based on “dry water microreactor” for enhanced oil recovery and its properties. Geoenergy Sci. Eng. 2024, 239, 212972. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, H.; Zheng, L.; Yang, J.; Zhu, J.; Nan, Y.; Su, G.; Yu, X. Preparation of amphiphilic Janus nanosheets based on thermally expandable microspheres and evaluation of their physical and chemical properties. Fuel 2024, 358, 130253. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Van Meerbeek, A. Chapter 10.3 Clay Mineral– and Organoclay–Polymer Nanocomposite. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 583–621. [Google Scholar]
- Mansa, R.; Detellier, C. Preparation and Characterization of Guar-Montmorillonite Nanocomposites. Materials 2013, 6, 5199–5216. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Ng, P.K. Natural Biopolymer-Based Nanocomposite Films for Packaging Applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]



| Operation Process | Characteristics of Working Fluid |
|---|---|
| Fracturing | High-temperature resistance, shear resistance, salt resistance, sand-carrying capacity, anti-swelling property, friction reduction, and low fluid loss |
| Production | Low interfacial tension, anti-swelling property, low residue, moderate return permeability, easy breakage of gel, and low damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, J.; Dong, Z.; Tan, W.; Zhang, Y.; Liang, T.; Xing, L.; Wang, Z. A Review of Fracturing and Enhanced Recovery Integration Working Fluids in Tight Reservoirs. Processes 2024, 12, 1241. https://doi.org/10.3390/pr12061241
Shang J, Dong Z, Tan W, Zhang Y, Liang T, Xing L, Wang Z. A Review of Fracturing and Enhanced Recovery Integration Working Fluids in Tight Reservoirs. Processes. 2024; 12(6):1241. https://doi.org/10.3390/pr12061241
Chicago/Turabian StyleShang, Jianping, Zhengliang Dong, Wenyuan Tan, Yanjun Zhang, Tuo Liang, Liang Xing, and Zhaohuan Wang. 2024. "A Review of Fracturing and Enhanced Recovery Integration Working Fluids in Tight Reservoirs" Processes 12, no. 6: 1241. https://doi.org/10.3390/pr12061241
APA StyleShang, J., Dong, Z., Tan, W., Zhang, Y., Liang, T., Xing, L., & Wang, Z. (2024). A Review of Fracturing and Enhanced Recovery Integration Working Fluids in Tight Reservoirs. Processes, 12(6), 1241. https://doi.org/10.3390/pr12061241







