Study on the Deactivation Mechanism of Ru/C Catalysts
Abstract
1. Introduction
2. Experimental Methods and Materials
2.1. The Synthesis Method of the Catalyst Used in This Paper
2.2. DMHAN and MMH Concentrations Analysis
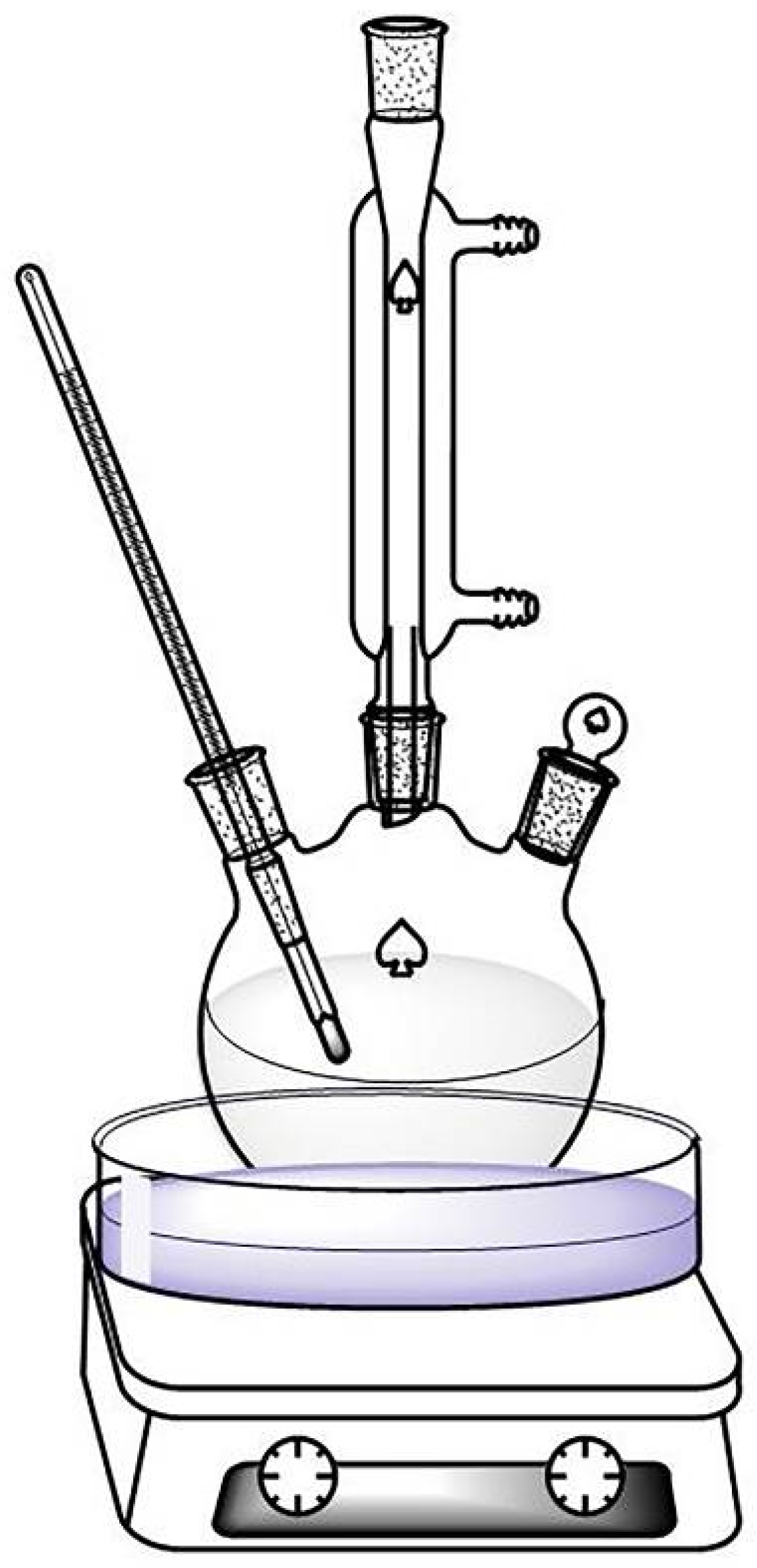
2.3. The Catalyst Treatment Process
2.4. Characterization of Catalyst
2.5. Ru Concentrations Analysis
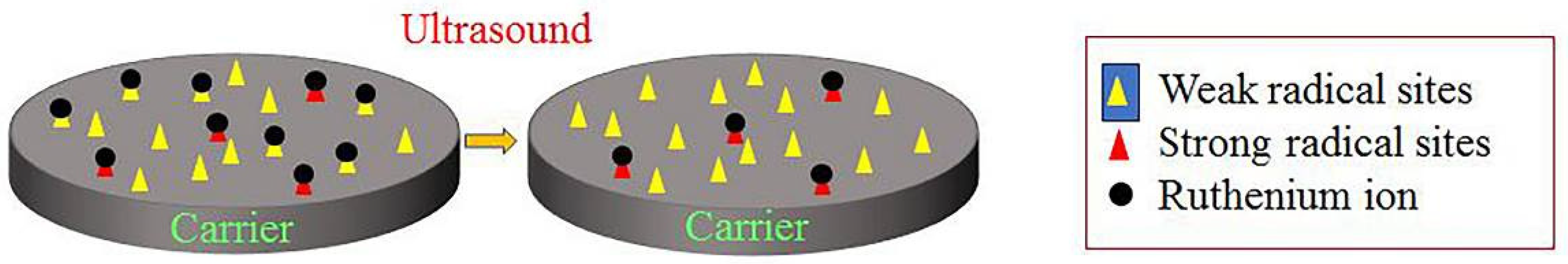
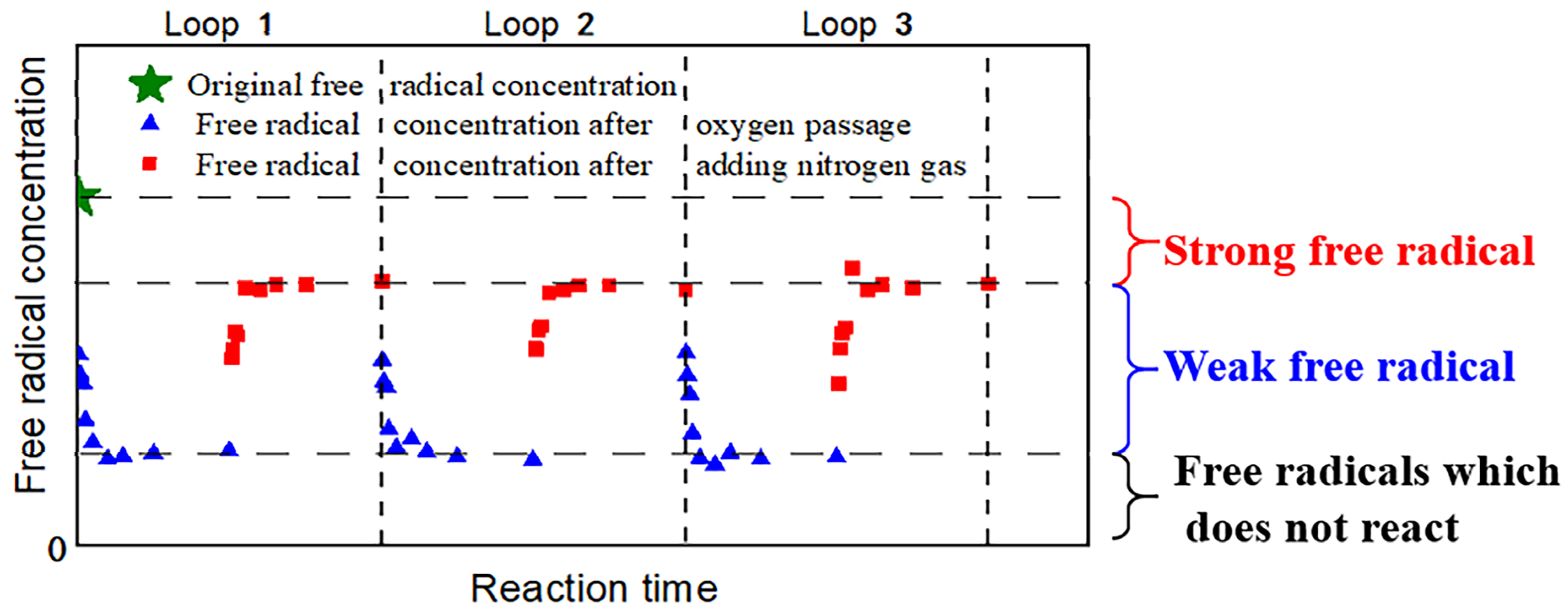
2.6. Experiment to Determine the Types of Free Radical Sites on Carbon-Based Supports
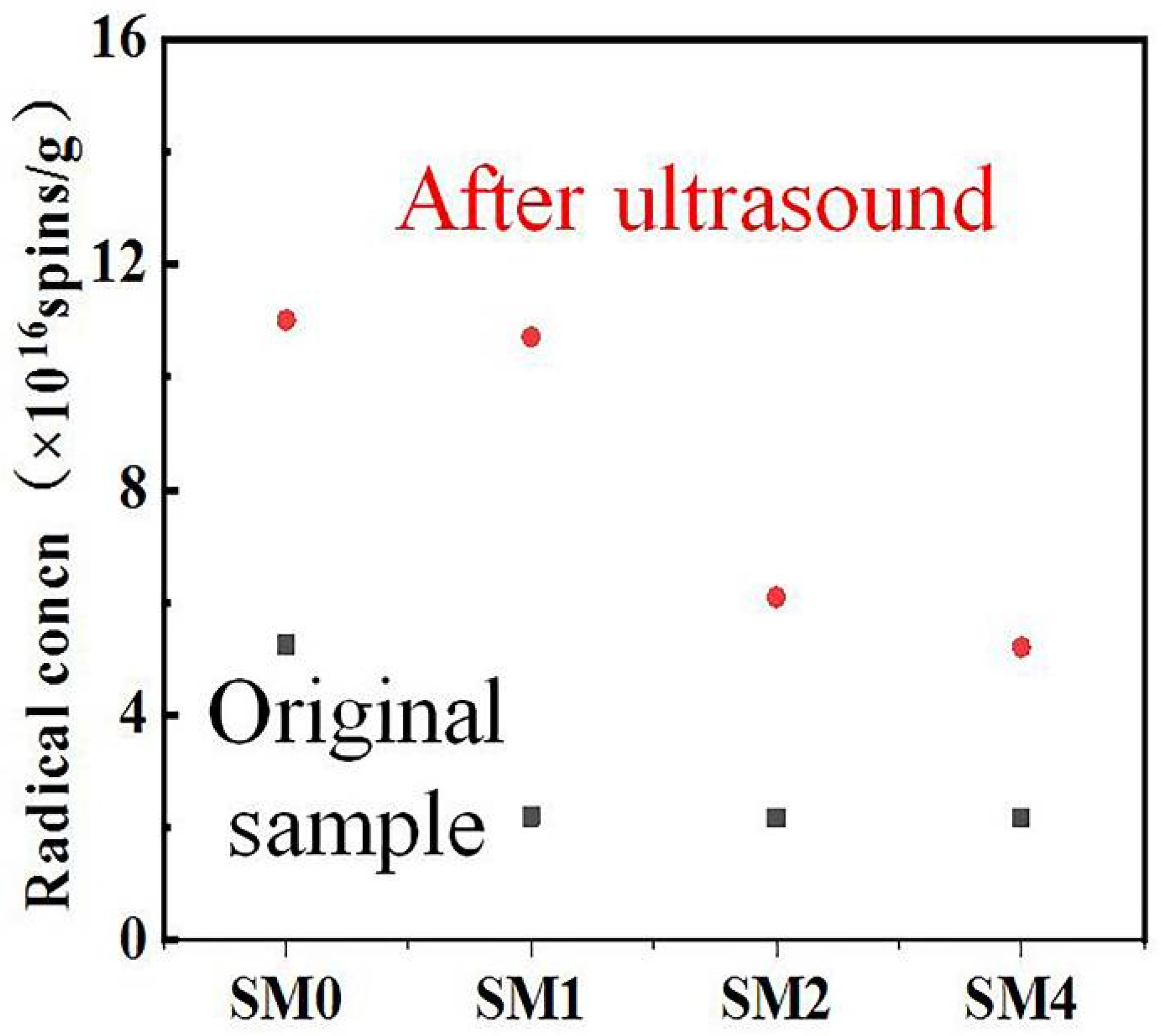
2.7. Ultrasound Experiment
3. Results and Discussion
3.1. Analysis of Free Radical Site Types
3.2. Scanning Electron Microscope Analysis
3.3. Analysis of Activity and Pore Structure
4. Summary
- (1)
- Ru ions on the catalysts adsorb onto the free radical active sites of the carbon-based support. Under ultrasound conditions, some Ru ions desorb from these sites. Those that desorb may re-adsorb onto weak free radical sites, while those that remain adsorbed may occupy strong free radical sites.
- (2)
- The adsorption curves of all four catalysts exhibit Type I adsorption with a H4 type hysteresis loop, characteristic of solids containing narrow slit pores, akin to those found in activated carbon.
- (3)
- After several hundred hours of reaction, SM1 and SM2 experience a slight reduction in specific surface area and pore volume compared to SM0. However, there is no observed change in catalyst activity, and the pore diameter remains largely unchanged. This observation primarily suggests that the micropores on the catalyst’s surface have undergone corrosion and damage, without any discernible alteration in the distribution of internal micropores.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fishwick, T. The development of conventional safety culture in the nuclear industry. Part 2—Manufacture and reprocessing of nuclear fuel. Loss Prevent. Bull. 2022, 288, 7–12. [Google Scholar]
- Boparai, A.S. Gas Chromatographic Analysis of Nuclear Fuel Reprocessing TRUEX-CCI4 Process Solvent. J. Chromatogr. Sci. 1986, 24, 434–437. [Google Scholar] [CrossRef]
- NFCC. Status of Nuclear Fuel Reprocessing, Spent Fuel Storage and High-Level Waste Disposal. 1978. Available online: https://www.osti.gov/biblio/5964412 (accessed on 11 January 1978).
- Soelberg, N.R.; Garn, T.G.; Greenhalgh, M.R.; Law, J.D.; Jubin, R.; Strachan, D.M.; Thallapally, P.K. Radioactive iodine and krypton control for nuclear fuel reprocessing facilities. Sci. Technol. Nucl. Install. 2013, 2013, 702496. [Google Scholar] [CrossRef]
- Li, T.; Liu, F.; Zhou, J.; Zuo, C.; Yan, T.; Zheng, W. Establishment and Verification of the Kinetics Model of Uranium Continuous Dissolution by Using Discrete Element Method. Processes 2023, 11, 2343. [Google Scholar] [CrossRef]
- Vance, R.F. Characterization of the Head End Cells at the West Valley Nuclear Fuel Reprocessing Plant; West Valley Nuclear Services Co.: West Valley, NY, USA, 1986. [Google Scholar] [CrossRef][Green Version]
- Liu, H.J.; Wu, H. Numerical Investigation of Uranium Nitrate Atomization Properties during the Nuclear Fuel Reprocessing. Energy Conserv. Technol. 2016, 34, 3–6,12. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Wang, J.F.; Zhang, T.X. Power Reactor Nuclear Fuel Reprocessing Technology; China Atomic Energy Press: Beijing, China, 2013. [Google Scholar]
- Zhang, H.; Ye, G.A.; Li, L.; Yang, H.; Li, H.R. Preparation of 2AF Solution by Electrochemical Method Ⅱ. Electrooxidation of Pu(Ⅲ) to Prepare 2AF Solution. J. Nucl. Radiochem. 2010, 32, 145–148. [Google Scholar]
- Ren, F.Y.; Zhou, Z.X. Foreign Nuclear Fuel Reprocessing; Atomic Energy Press: Beijing, China, 2006. [Google Scholar]
- Chang, L.; Tian, B.S.; Li, W.M. Oxidation Decomposition of Hydrazine in Nitric Acid. J. Nucl. Radiochem. 2005, 27, 96–99. [Google Scholar] [CrossRef]
- Ren, X.G.; Li, M.H.; Wang, A.Q.; Li, L.; Wang, X.D.; Zhang, T. Catalytic Decomposition of Hydroxyl Ammonium Nitrate at Room Temperature. Chin. J. Catal. 2007, 28, 1–2. [Google Scholar] [CrossRef]
- Wei, C.; Saraf, S.R.; Rogers, W.J.; Mannan, M.S. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim. Acta 2004, 421, 1–9. [Google Scholar] [CrossRef]
- Iwata, Y.; Koseki, H. Risk evaluation of decomposition of hydroxylamine/water solution at various concentrations. Process Saf. Prog. 2002, 21, 136–141. [Google Scholar] [CrossRef]
- Breschet, C.; Miquel, P. Improvement of the procedure used to treat highly irradiated fuels. In Proceedings of the International Solvent Extraction Conference, Lyon, France, 8–14 September 1974; London Society of Chemical Industry: London, UK, 1971; pp. 565–576. [Google Scholar]
- Jiang, S.J.; Ren, F.Y. Nuclear Fuel Reprocessing Technology; Atomic Energy Press: Beijing, China, 1995. [Google Scholar]
- Benedict, M. Nuclear Chemical Engineering, 2nd ed.; McCraw-Hill BooK Company: New York, NY, USA, 1981. [Google Scholar]
- Hou, X.L.; Zhang, S.Q.; Hu, J.; Wei, X.F.; Zhao, H.G. Study of plutonium valence adjustment in 1AF solution in purex process with nitrogen oxide. Nucl. Sci. Eng. 1995, 15, 165–171. [Google Scholar]
- Zhang, S.Q.; Wei, X.F.; Ye, G.A.; Zhang, X.Y.; Zhuang, W.X.; Liu, S.Y.; Fu, L.C. Research on Regulating Plutonium Valence State with N2O4 in Purex Process. Atom. Energy Sci. Technol. 1993, 72, 130–137. [Google Scholar]
- Schmieder, H.; Baumgartner, B.; Goldacker, H.; Hausberger, H.; Warnecke, E. Electrolytic Methods for Ap-plication in the Purex Process. In Proceedings of the International Solvent Extration Conference; Thornton, J.D., Naylor, A., Jeffreys, G.V., Eds.; Society of Chemical Industru: London, UK, 1974; pp. 200–206. [Google Scholar]
- Duan, Y.F. Photochemical reaction in Pu (III)—Fe (II)—N2H5+—HNO3 solution. J. Nucl. Radiochem. 1987, 9, 200–206. [Google Scholar]
- Chang, L. Study on Price Adjustment of Plutonium by Catalytic Oxidation. Master’s Thesis, China Institute of Atomic Energy, Beijing, China, 2008. [Google Scholar]
- Anan’Ev, A.; Boltoeva, M.Y.; Sukhov, N.; Bykov, G.; Ershov, B. Catalytic decomposition of hydrazine in weakly alkaline solutions on platinum nanoparticles. Radiochemistry 2004, 46, 578–582. [Google Scholar] [CrossRef]
- Anan’Ev, A.; Shilov, V. Catalytic Reduction of Pu (IV) with Formic Acid in Nitric Acid Solutions. Radiochemistry 2004, 46, 242–245. [Google Scholar] [CrossRef]
- Ananiev, A.; Shilov, V.; Moisy, P.; Madic, C. Heterogeneous catalytic redox reactions of neptunium ions in the aqueous system HNO3-HCOOH. Radiochim. Acta 2004, 92, 81–88. [Google Scholar] [CrossRef]
- Ananiev, A.; Shilov, V.; Moisy, P.; Madic, C. Heterogeneous catalytic oxidation of neptunium (IV) in nitric acid solutions. Radiochim. Acta 2003, 91, 499–504. [Google Scholar] [CrossRef]
- Ananiev, A.; Shilov, V.; Brossard, P. Kinetics of the platinum catalyzed hydrazoic acid decomposition in acidic media. Appl. Catal. A-Gen. 2004, 257, 151–156. [Google Scholar] [CrossRef]
- Ananiev, A.; Broudic, J.C.; Brossard, P. The platinum catalyzed hydrazine decomposition in non-nitrate acidic media. Appl. Catal. A-Gen. 2003, 242, 1–10. [Google Scholar] [CrossRef]
- Boltoeva, M.Y.; Shilov, V.; Anan’Ev, A. Reactivity of platinum nanoaggregates in catalytic reduction of U (VI) with hydrazine in acid solutions. Radiochemistry 2007, 49, 603–606. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Q.; Guo, J.H.; Liu, L.S.; Wang, C.S.; Chang, S.W.; Li, R.X.; Ou, Y.Y.G.; Tian, B.S. Pt-Catalyzed Oxidation of Pu(Ⅲ) in Nitric Acid. J. Nucl. Radiochem. 2012, 34, 218–222. [Google Scholar]
- Li, B.; He, T.; Zuo, C.; Cao, Z.; Yan, T.; Zheng, W. The reaction kinetics and mechanism of catalytic decomposition of hydrazine nitrate on Ru/C catalyst in nitric acid solutions. New J. Chem. 2023, 47, 7583–7587. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Chen, Q.; Chen, X.; Yan, T.; Zheng, W.; Zuo, C. Catalytic decomposition of hydroxylamine nitrate and hydrazine nitrate using Ru/ZSM-5 catalyst under mild reaction conditions. RSC Adv. 2022, 12, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Anan’Ev, A.; Boltoeva, M.Y.; Sharygin, L.; Grigor’ev, M.; Shilov, V. Reactivity of platinum nanoaggregates on various types of supports in catalytic decomposition of hydrazine in acid solutions. Radiochemistry 2006, 48, 119–124. [Google Scholar] [CrossRef]
- Zhou, S.X. Study on Deactivation Mechanism of Hydrazine Decomposition Catalyst. Master’s Thesis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China, 1989. [Google Scholar]
- Mary, S.; Kappenstein, C.; Balcon, S.; Rossignol, S.; Gengembre, E. Monopropellant decomposition catalysts. I. Ageing of highly loaded Ir/Al2O3 catalysts in oxygen and steam. Influence of chloride content. Appl. Catal. A Gen. 1999, 182, 317–325. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zaffran, J.; Yang, B. Dry reforming of methane over the cobalt catalyst: Theoretical insights into the reaction kinetics and mechanism for catalyst deactivation. Appl. Catal. B Environ. 2020, 270, 118859. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Pan, X.; Ma, H.; Bao, X. Deactivation mechanism and regeneration of carbon nanocomposite catalyst for acetylene hydrochlorination. Appl. Catal. B Environ. 2017, 210, 116–120. [Google Scholar] [CrossRef]
- Bonnin, A.E.L.; Pouilloux, Y.; Coupard, V.; Uzio, D.; Pinard, L. Deactivation mechanism and regeneration study of Zn/HZSM-5 catalyst in ethylene transformation. Appl. Catal. A Gen. 2021, 611, 117976. [Google Scholar] [CrossRef]
- Otor, H.O.; Steiner, J.B.; Garcia-Sancho, C.; Alba-Rubio, A.C. Encapsulation methods for control of catalyst deactivation: A review. ACS Catal. 2020, 10, 7630–7656. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, T.; Han, Y.; Zhang, X.; Wang, M.; Li, C. Deactivation Mechanism and Anti-Deactivation Measures of Metal Catalyst in the Dry Reforming of Methane: A Review. Atmosphere 2023, 14, 770. [Google Scholar] [CrossRef]
- Goodman, E.D.; Johnston-Peck, A.C.; Dietze, E.M.; Wrasman, C.J.; Hoffman, A.S.; Abild-Pedersen, F.; Bare, S.R.; Plessow, P.N.; Cargnello, M. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2019, 2, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, M.; Fu, L.; Wu, C.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. A thorough observation of an ozonation catalyst under long-term practical operation: Deactivation mechanism and regeneration. Sci. Total Environ. 2022, 830, 154803. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, G.; Rogers, K.; Lin, H.; Zheng, Y.; Ng, S. Hydrotreating of waste cooking oil over supported CoMoS catalyst–Catalyst deactivation mechanism study. Mol. Catal. 2017, 443, 228–240. [Google Scholar] [CrossRef]
- Xu, L.; Wu, Q.; Chang, H.; Li, G.; Zou, J.; Wang, S. Chemical deactivation of Selective Catalytic Reduction catalyst: Investigating the influence and mechanism of SeO2 poisoning. Fuel 2020, 269, 117435. [Google Scholar] [CrossRef]
- Li, Y.; Yellezuome, D.; Li, C.; Liu, R. Deactivation mechanism and regeneration effect of bi-metallic Fe-Ni/ZSM-5 catalyst during biomass catalytic pyrolysis. Fuel 2022, 312, 122924. [Google Scholar] [CrossRef]
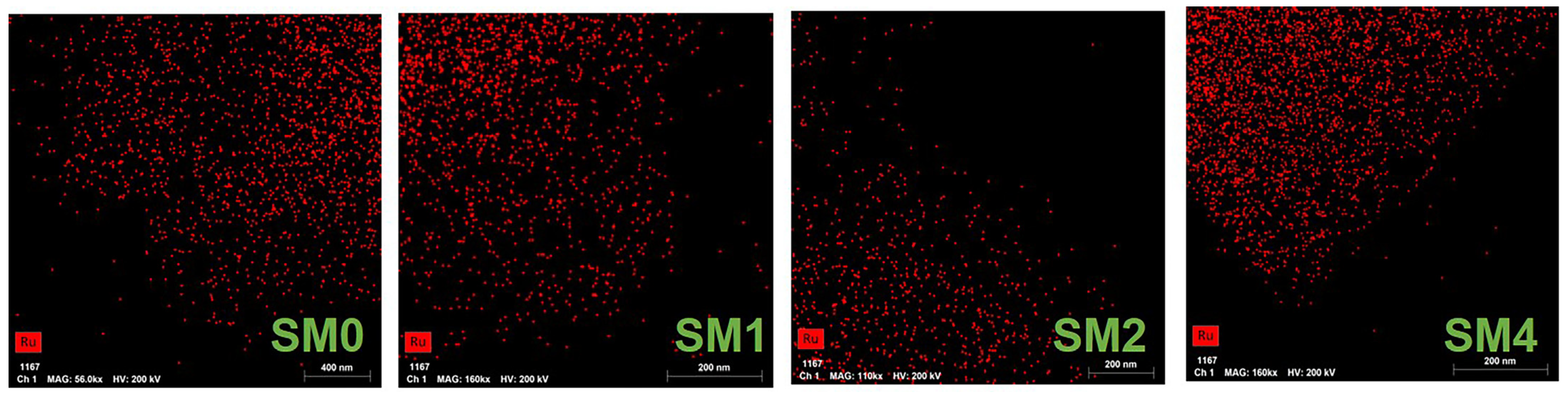
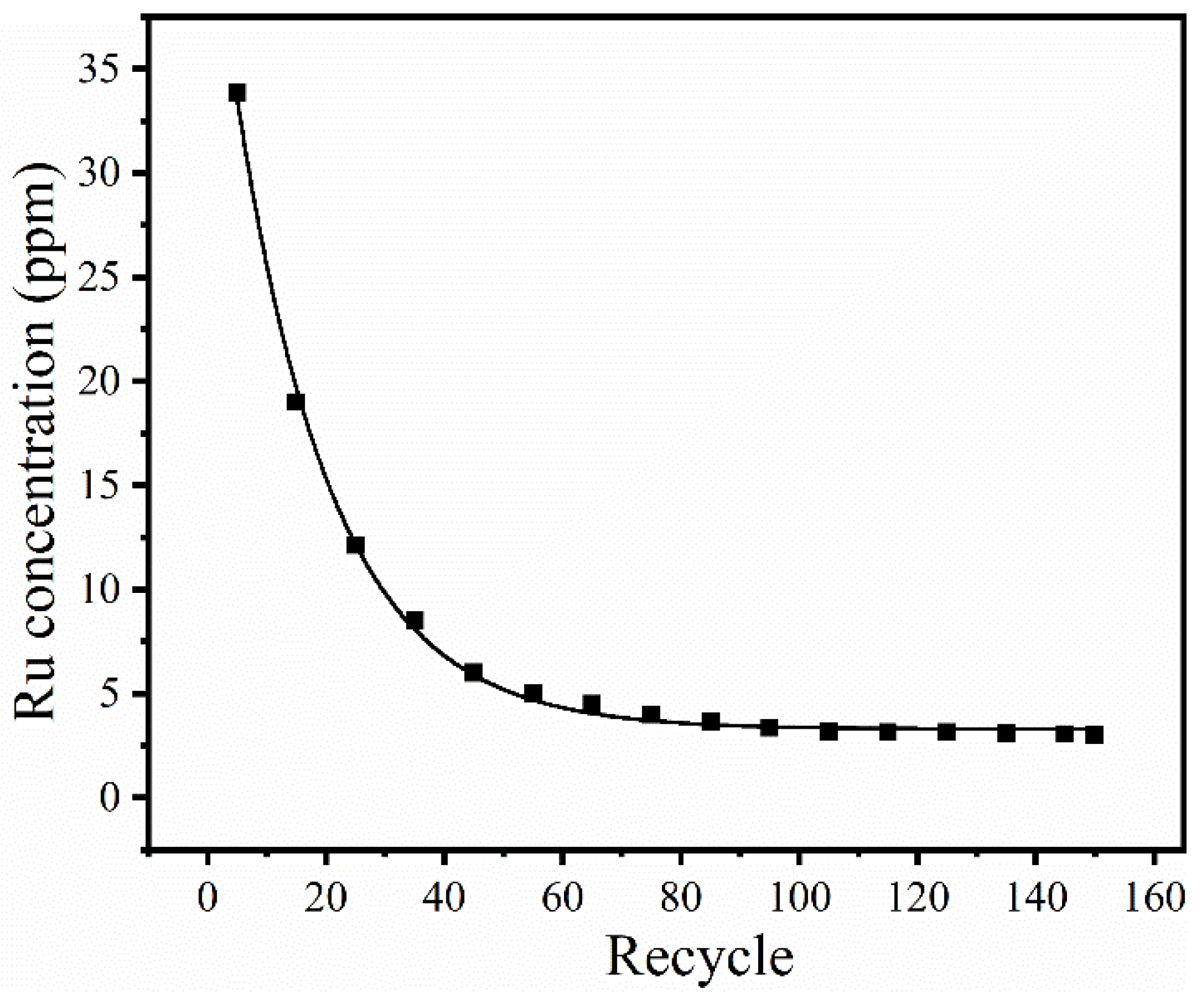
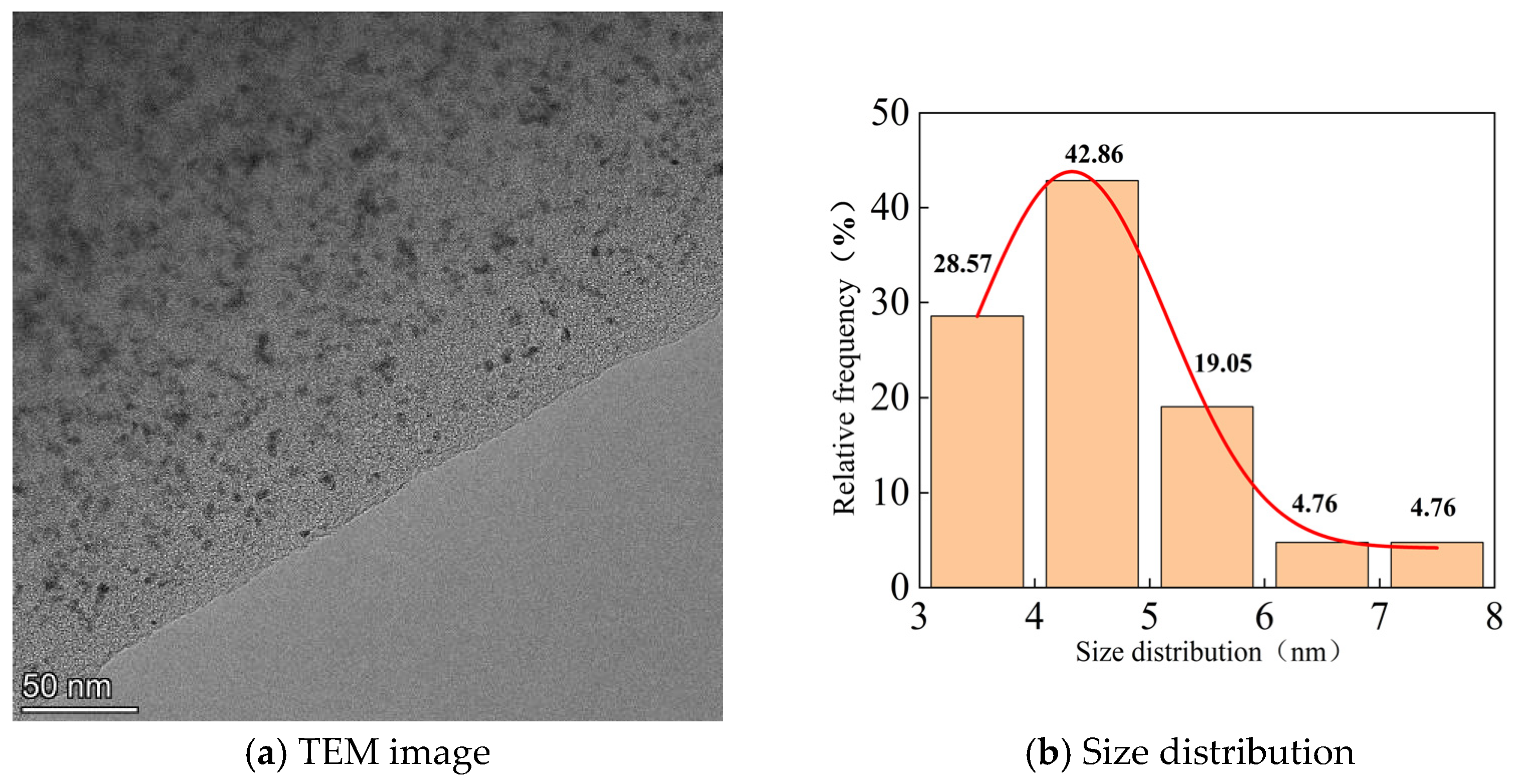
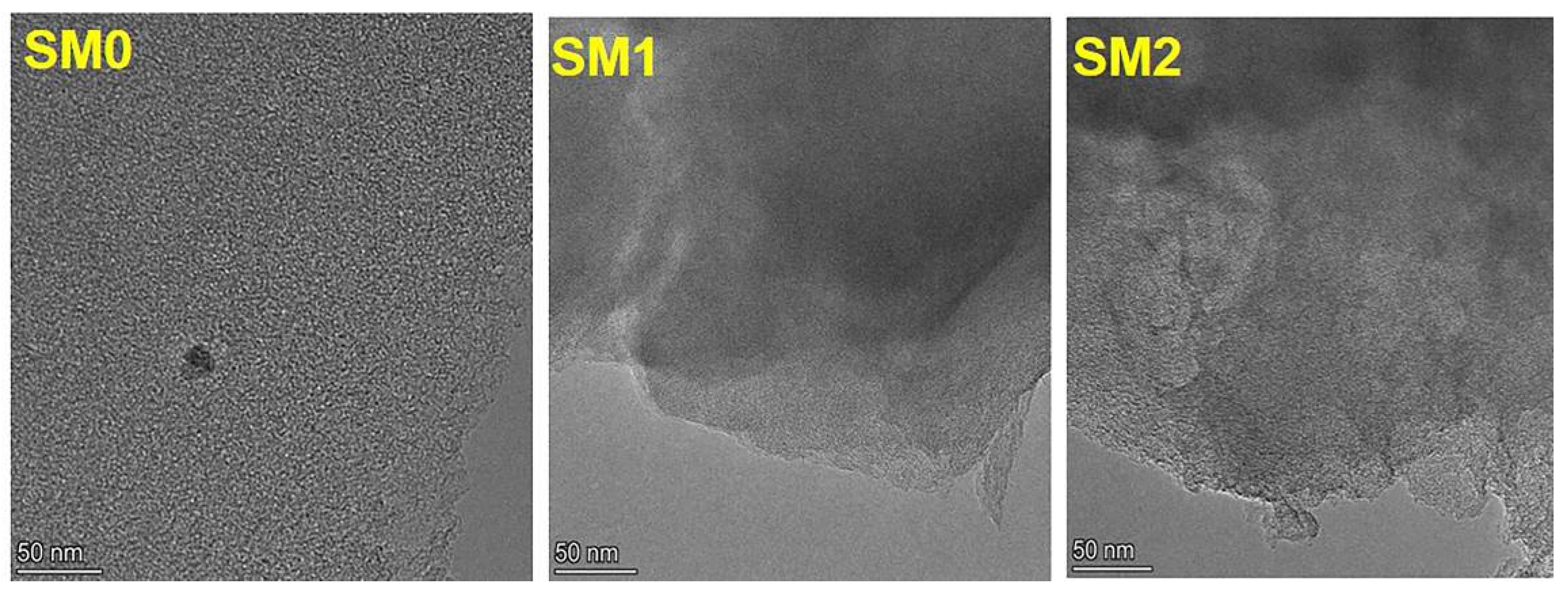

| Number | Sample Description |
|---|---|
| SM0 | 5% Ru/C catalyst-1, a particle size range of 4–8 mesh |
| SM1 | 5% Ru/C catalyst for 100 reactions, a particle size range of 4–8 mesh |
| SM2 | 5% Ru/C catalyst for 150 reactions, a particle size range of 4–8 mesh |
| SM4 | 5% Ru/C-2 catalyst, a particle size range of 10–20 mesh |
| Catalyst Samples | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| SM0 | 1407.4 | 1.2 | 0.5 |
| SM1 | 1058.3 | 1.2 | 0.4 |
| SM2 | 939.3 | 1.2 | 0.4 |
| SM4 | 1165.8 | 1.2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Li, T.; Li, B.; Chen, X.; Zuo, C.; Zheng, W. Study on the Deactivation Mechanism of Ru/C Catalysts. Processes 2024, 12, 1138. https://doi.org/10.3390/pr12061138
Cao Z, Li T, Li B, Chen X, Zuo C, Zheng W. Study on the Deactivation Mechanism of Ru/C Catalysts. Processes. 2024; 12(6):1138. https://doi.org/10.3390/pr12061138
Chicago/Turabian StyleCao, Zhi, Tianchi Li, Baole Li, Xiwen Chen, Chen Zuo, and Weifang Zheng. 2024. "Study on the Deactivation Mechanism of Ru/C Catalysts" Processes 12, no. 6: 1138. https://doi.org/10.3390/pr12061138
APA StyleCao, Z., Li, T., Li, B., Chen, X., Zuo, C., & Zheng, W. (2024). Study on the Deactivation Mechanism of Ru/C Catalysts. Processes, 12(6), 1138. https://doi.org/10.3390/pr12061138






