Numerical Investigation of Heat Transfer Characteristics of Trapezoidal Fin Phase Change Thermal Energy Storage Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Model
2.2. Mathematical Model
2.3. Boundary Conditions and Computational Settings
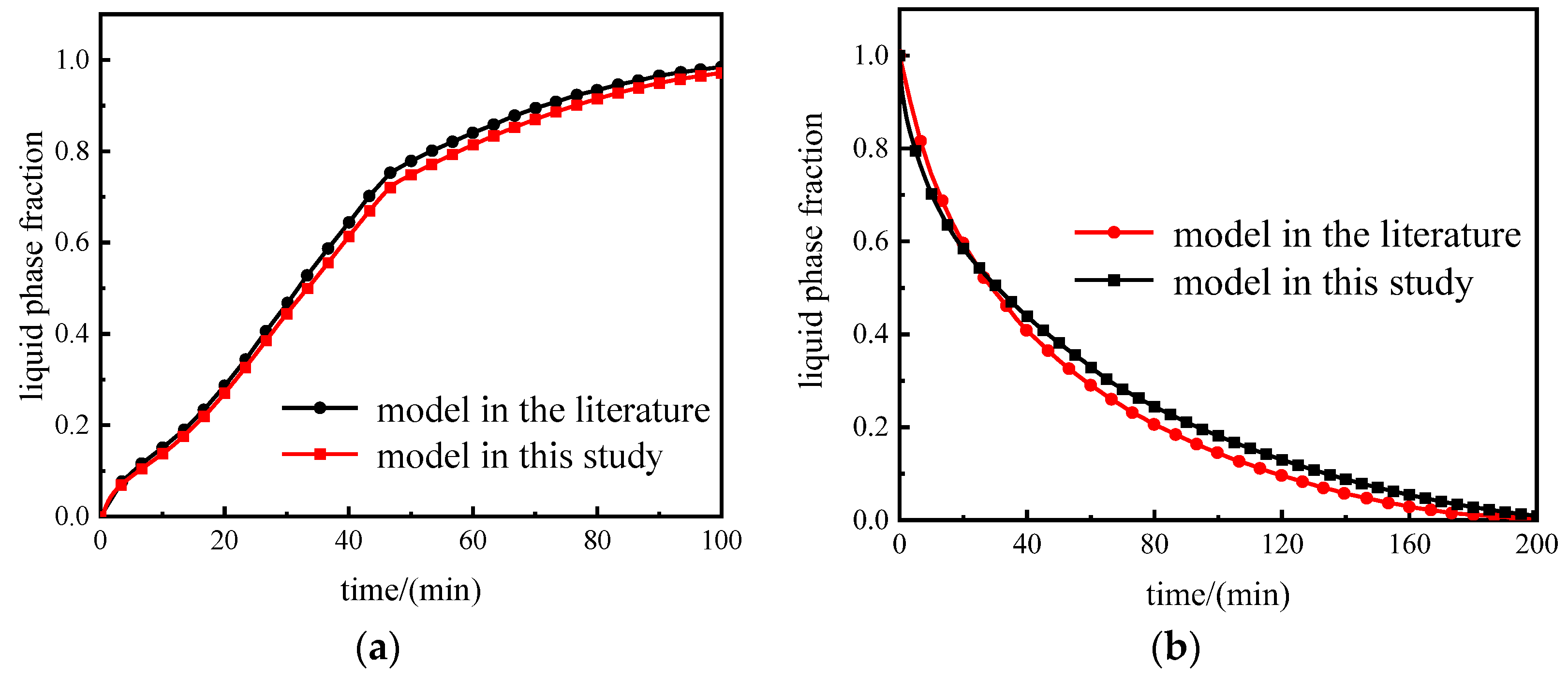
2.4. Mesh Independence and Model Validation
3. Results and Discussion
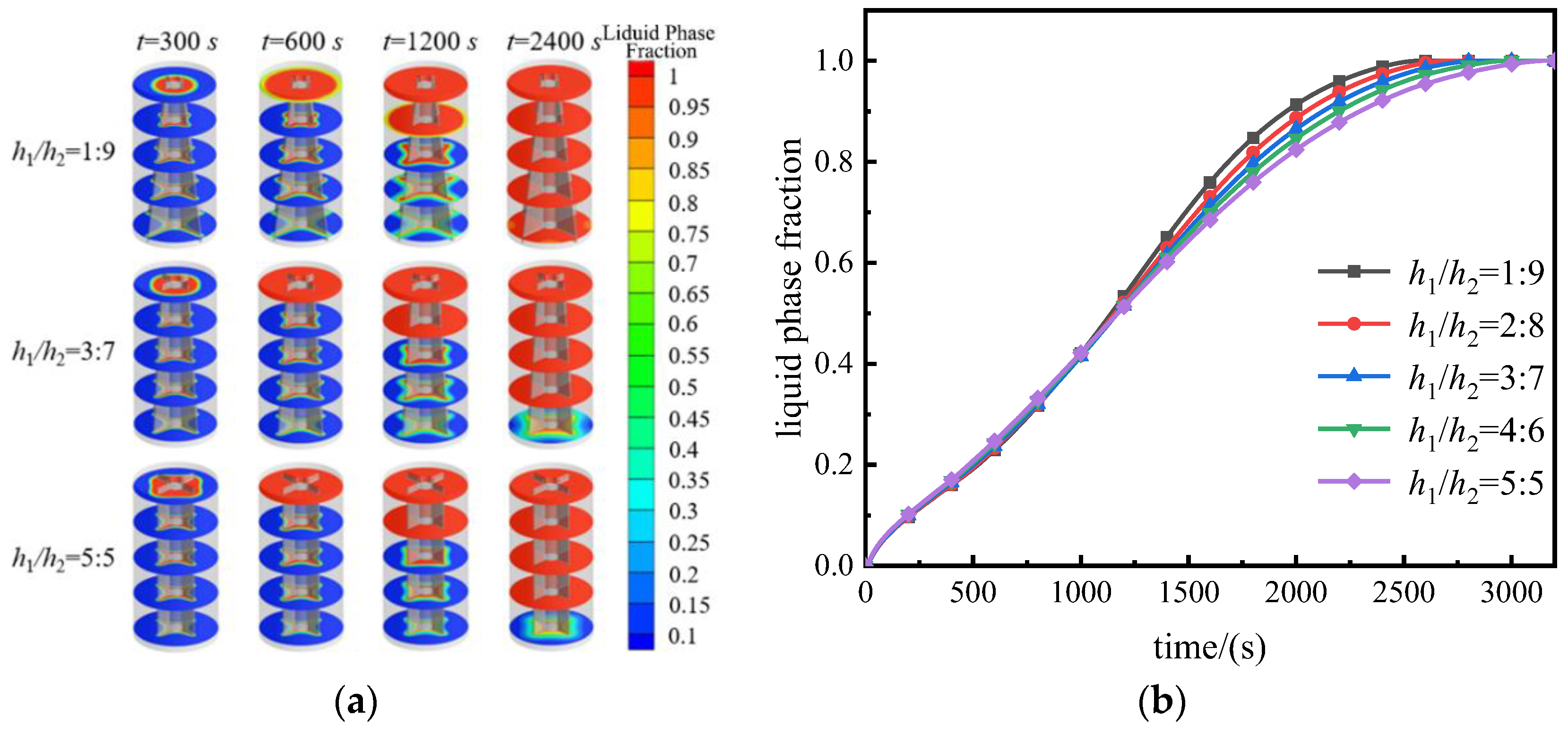
3.1. Melting Process
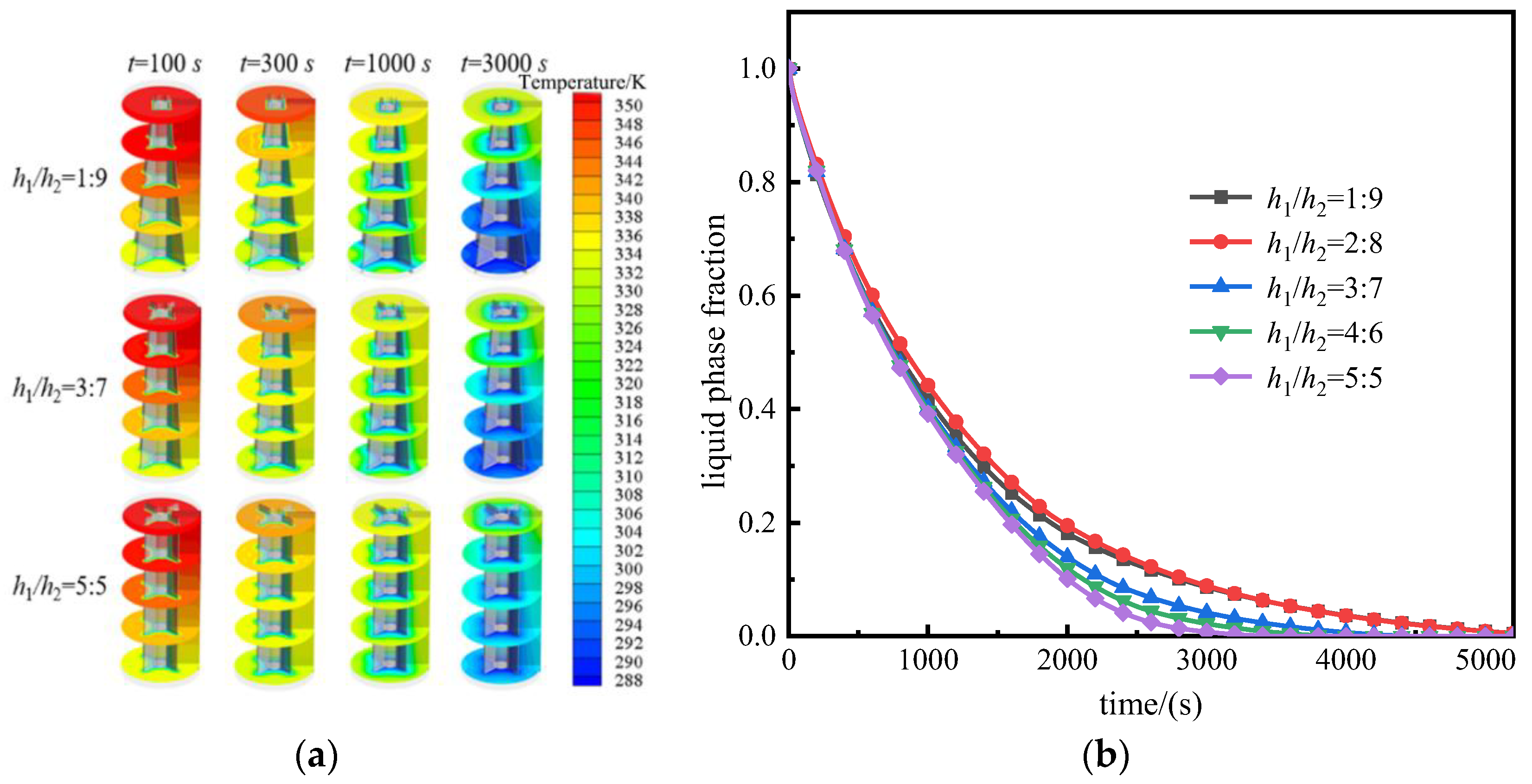
3.2. Solidification Process
3.2.1. Upright Placement of TESU
3.2.2. Upside-Down Placement of TESU
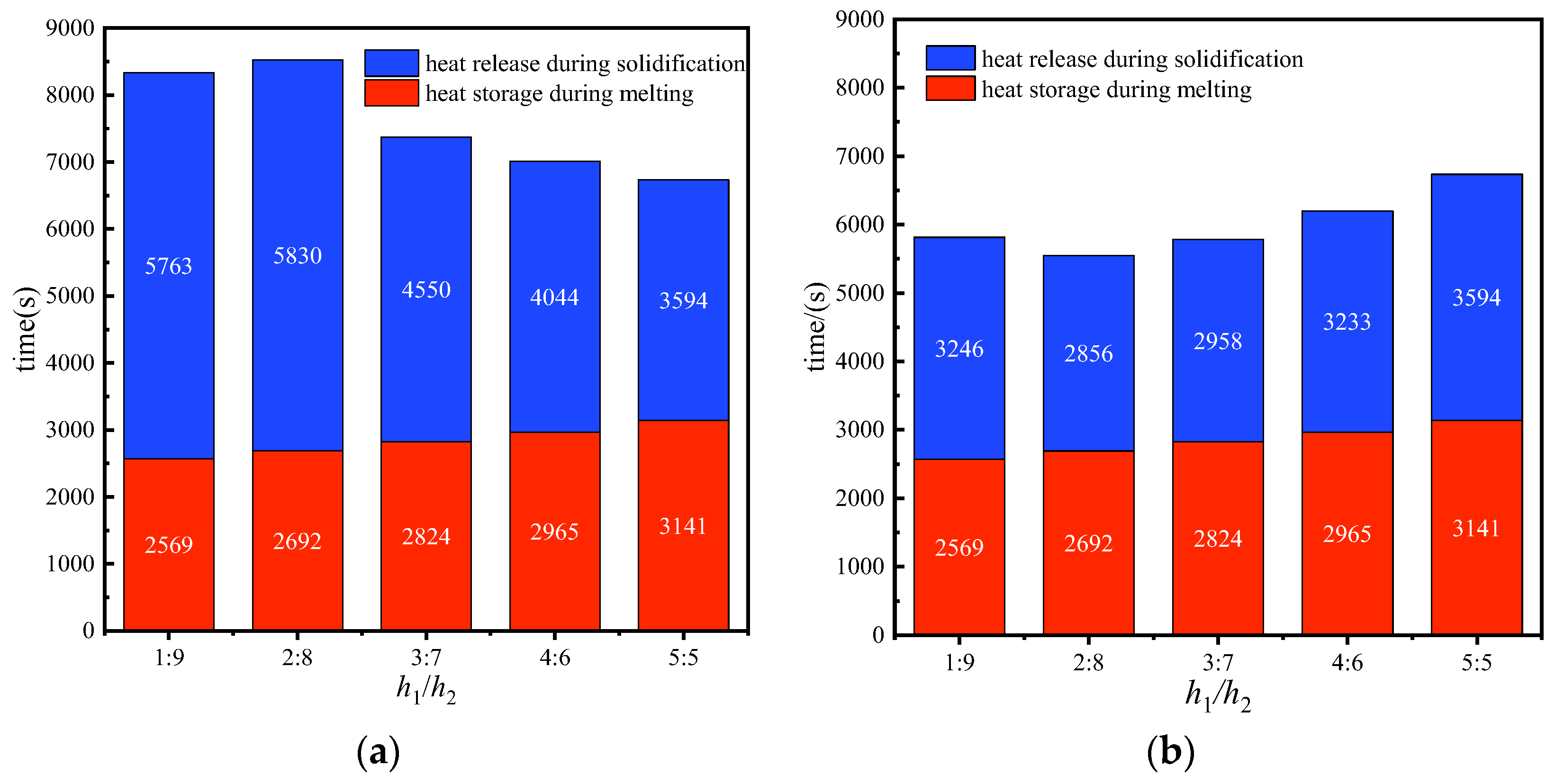
3.3. Comprehensive Thermal Energy Storage and Release Performance
4. Conclusions
- During the melting process, the fin structure with h1/h2 = 1:9 exhibited the most optimal enhancement in heat transfer for the storage unit, resulting in a reduction of the melting time by 18.24% compared to the structure with h1/h2 = 5:5;
- During the solidification process, when the storage unit was upright, the structure with h1/h2 = 5:5 enhanced heat transfer best, resulting in a decrease in the heat storage time by 37.64% compared to the unit with h1/h2 = 1:9. Conversely, when the storage unit was placed upside-down, the unit with h1/h2 = 2:8 demonstrated the best enhancement in heat transfer, resulting in a reduction of the heat storage time by 20.6% compared to the unit with h1/h2 = 5:5;
- When heat storage and release are considered together, the unit with h1/h2 = 5:5 shows the shortest overall energy storage and release time, with the energy storage unit placed in the upright position during both melting and solidification processes. The energy storage unit with h1/h2 = 2:8 takes the shortest time to melt in the upright placement and then to solidify in the upside-down placement.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, H.T.; Zhang, G.; Jiang, J.Z. Numerical simulation on thermal performance of multiple-pipe heat storage. Fluid Mach. 2014, 42, 56–61. [Google Scholar]
- Hei, Y.X.; Qu, M.L.; Huang, P.; Zhang, T.Y.; Li, Z. Study on multi-temperature heat release characteristics of phase change heat storage. Fluid Mach. 2022, 50, 21–26. [Google Scholar]
- Lin, D.G.; Mao, Y.N.; Li, X.H. Simulation study on the heat storage characteristics of the internal fin type casing phase change regenerator. Fluid Mach. 2021, 49, 84–89. [Google Scholar]
- Huang, K.L.; Chang, Q.P.; Song, J.S.; Feng, G.; Liu, K. Analysis of influence factors to heat transfer of concentric tube air type phase change heat accumulator. Fluid Mach. 2018, 46, 74–78. [Google Scholar]
- Qu ML Zhang, T.Y.; Zhang, R.; Fan, Y. Experimental study on charge and discharge thermal efficiency of cascade air source heat pump phase change regenerator. Fluid Mach. 2019, 47, 66–71. [Google Scholar]
- Sun, J.C.; Du, P.; Li, P.S.; Zhang, Y.; Li, W. The numerical simulation about energy storage process of spiral tube phase-change energy storage box. Fluid Mach. 2017, 45, 76–81. [Google Scholar]
- Meng, F.; Che, C.; Wu, Y.; Wei, J.; Rong, J.; Yang, X.; Li, D.; Yang, R.; Wang, Z. Thermal Storage Performance of a Shell and Tube Phase Change Heat Storage Unit with Different Thermophysical Parameters of the Phase Change Material. Processes 2024, 12, 123. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Liu, Y.; Lai, B.; Ke, Z.; Wu, W. Investigations of Lithium-Ion Battery Thermal Management System with Hybrid PCM/Liquid Cooling Plate. Processes 2023, 11, 57. [Google Scholar] [CrossRef]
- Ashok Kumar, J.; Muthuvel, S.; Issac Selvaraj, R.V.; Ramoni, M.; Shanmugam, R.; Pandian, R.S. Mechanical Property Comparison of Geopolymer Brick Dried by Electrical and Passive Solar Devices with Phase Change Material (Paraffin Wax). Processes 2024, 12, 28. [Google Scholar] [CrossRef]
- Yang, T.Y.; King, W.P.; Mil Jkovic, N. Phase change material-based thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Yuan, Y.; Cao, X.; Xiang, B.; Du, Y. Effect of installation angle of fins on melting characteristics of annular unit for latent heat thermal energy storage. Sol. Energy 2016, 136, 365–378. [Google Scholar] [CrossRef]
- Wang, W.W.; Wang, L.B.; He, Y.L. Parameter effect of a phase change thermal energy storage unit with one shell and one finned tube on its energy efficiency ratio and heat storage rate. Appl. Therm. Eng. 2016, 93, 50–60. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Bai, Q.; Zhang, Q.; Jin, L.; Yan, J. Thermal performance of a shell-and-tube latent heat thermal energy storage unit: Role of annular fins. Appl. Energy 2017, 202, 558–570. [Google Scholar] [CrossRef]
- Ren, Z.; Huang, H.; Gao, J.; Zhao, M. Analysis of heat transfer characteristics of three-dimensional tubular ribbed chase change heat storage unit. J. Chin. Soc. Power Eng. 2022, 42, 437–443. [Google Scholar]
- Zhang, S.; Pu, L.; Xu, L.; Dai, M. Study on dominant heat transfer mechanism in vertical smooth/finned-tube thermal energy storage during charging process. Appl. Therm. Eng. 2022, 204, 117935. [Google Scholar] [CrossRef]
- Zonouzi, S.A.; Dadvar, A. Numerical investigation of using helical fins for the enhancement of the charging process of a latent heat thermal energy storage system. J. Energy Storage 2022, 49, 104157. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Yang, B.; Yang, X.; Li, M.J. Thermal assessment on solid-liquid energy storage tube packed with non-uniform angled fins. Sol. Energy Mater. Sol. Cells 2022, 236, 111526. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Yang, B.; Yang, X.; Yan, J. Melting assessment on the angled fin design for a novel latent heat thermal energy storage tube. Renew. Energy 2022, 183, 406–422. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, Z.; Luo, X.; Yan, J. Effect of fin number on the melting phase change in a horizontal finned shell-and-tube thermal energy storage unit. Sol. Energy Mater. Sol. Cells 2022, 236, 111527. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Fabrykiewicz, M. Thermal Energy Storage with PCMs in Shell-and-Tube Units: A Review. Energies 2023, 16, 936. [Google Scholar] [CrossRef]
- Najim, F.T.; Mohammed, H.I.; Al-Najjar, H.M.T.; Thangavelu, L.; Mahmoud, M.Z.; Mahdi, J.M.; Tiji, M.E.; Yaïci, W.; Talebizadehsardari, P. Improved Melting of Latent Heat Storage Using Fin Arrays with Non-Uniform Dimensions and Distinct Patterns. Nanomaterials 2022, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Vivekananthan, M.; Amirtham, V.A. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Duan, J.; Xiong, Y.L.; Yang, D. Study on the effect of multiple spiral fins for improved phase change process. Appl. Therm. Eng. 2020, 169, 114966. [Google Scholar] [CrossRef]



| Parameters | Paraffin | Copper |
|---|---|---|
| solidus temperature/K | 322.36 | |
| liquidus temperature/K | 334.14 | |
| latent heat of fusion/(J/kg) | 142,716.3 | |
| density/(kg/m3) | 902.2 | 8430 |
| specific heat capacity/(J/(kg·K) | 2300 | 375 |
| thermal conductivity of the solid phase/(W/(m·K)) | 0.2437 | 108.86 |
| thermal conductivity of the liquid phase/(W/(m·K)) | 0.177 | |
| dynamic viscosity/(kg/(m·s)) | 0.00324 | |
| thermal expansion coefficient/(K−1) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Yang, C.; Xu, M.; Zhang, Y. Numerical Investigation of Heat Transfer Characteristics of Trapezoidal Fin Phase Change Thermal Energy Storage Unit. Processes 2024, 12, 1080. https://doi.org/10.3390/pr12061080
Luo H, Yang C, Xu M, Zhang Y. Numerical Investigation of Heat Transfer Characteristics of Trapezoidal Fin Phase Change Thermal Energy Storage Unit. Processes. 2024; 12(6):1080. https://doi.org/10.3390/pr12061080
Chicago/Turabian StyleLuo, Haobing, Changchuan Yang, Meng Xu, and Ying Zhang. 2024. "Numerical Investigation of Heat Transfer Characteristics of Trapezoidal Fin Phase Change Thermal Energy Storage Unit" Processes 12, no. 6: 1080. https://doi.org/10.3390/pr12061080
APA StyleLuo, H., Yang, C., Xu, M., & Zhang, Y. (2024). Numerical Investigation of Heat Transfer Characteristics of Trapezoidal Fin Phase Change Thermal Energy Storage Unit. Processes, 12(6), 1080. https://doi.org/10.3390/pr12061080





