The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor
Abstract
1. Introduction
- Search and development of new homogeneous and heterogeneous catalysts;
- Modification of the synthesis reactor;
- Change in technological synthesis conditions.
2. Experimental Section
2.1. Materials and Reagents
2.2. Alcohol Drying
2.3. Synthesis of Fatty Acid Esters in Perfect Mixing Reactor
2.4. Synthesis of Fatty Acid Esters in Plug Flow Reactor
2.5. Synthesis of Ethyl Oleate via Acid Etherification
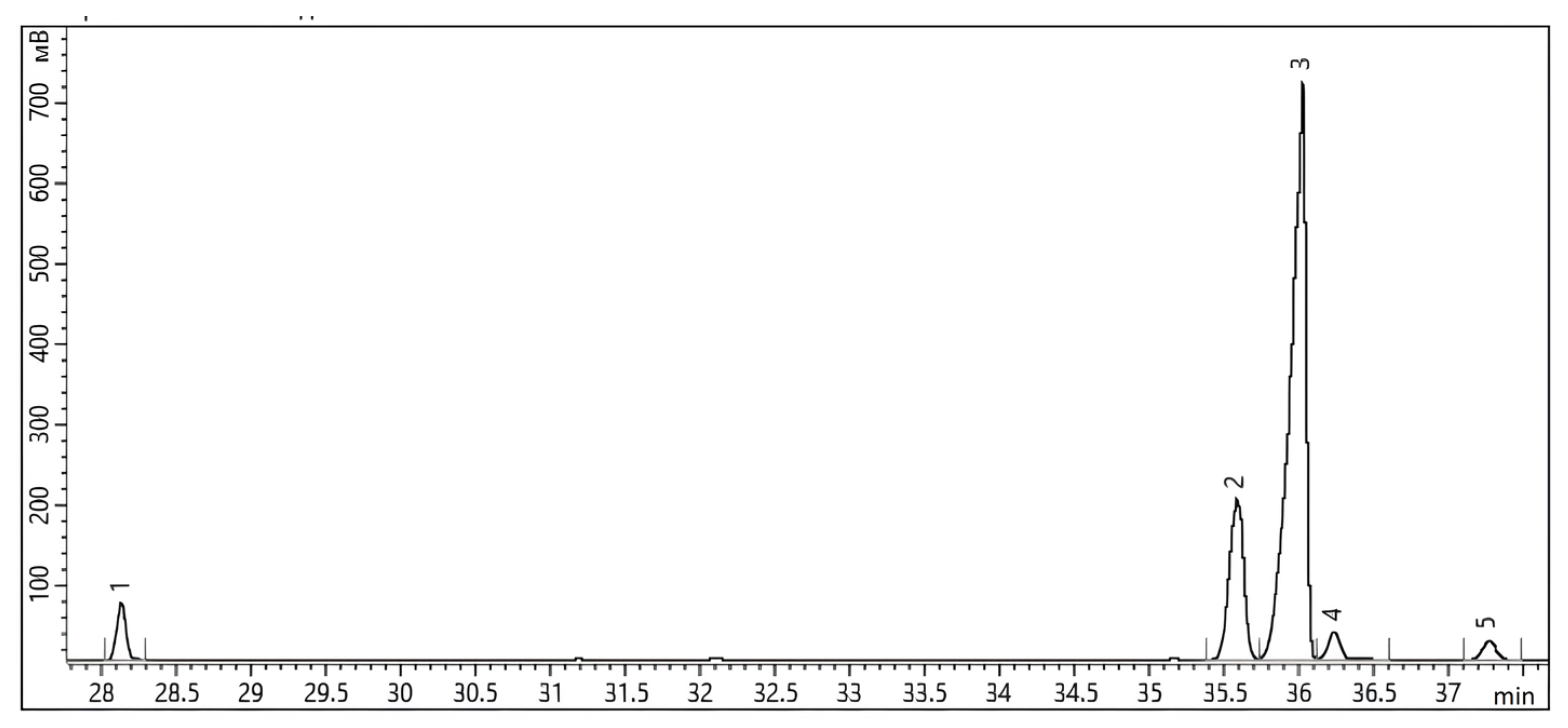
2.6. Analysis of FAEE Composition
2.7. Analysis of the Precipitate in the Reaction Mixture
2.8. Removal of Excess Alcohol
2.9. Removal of the Catalyst from the Ether Fraction
2.9.1. Neutralization with Sulfuric Acid
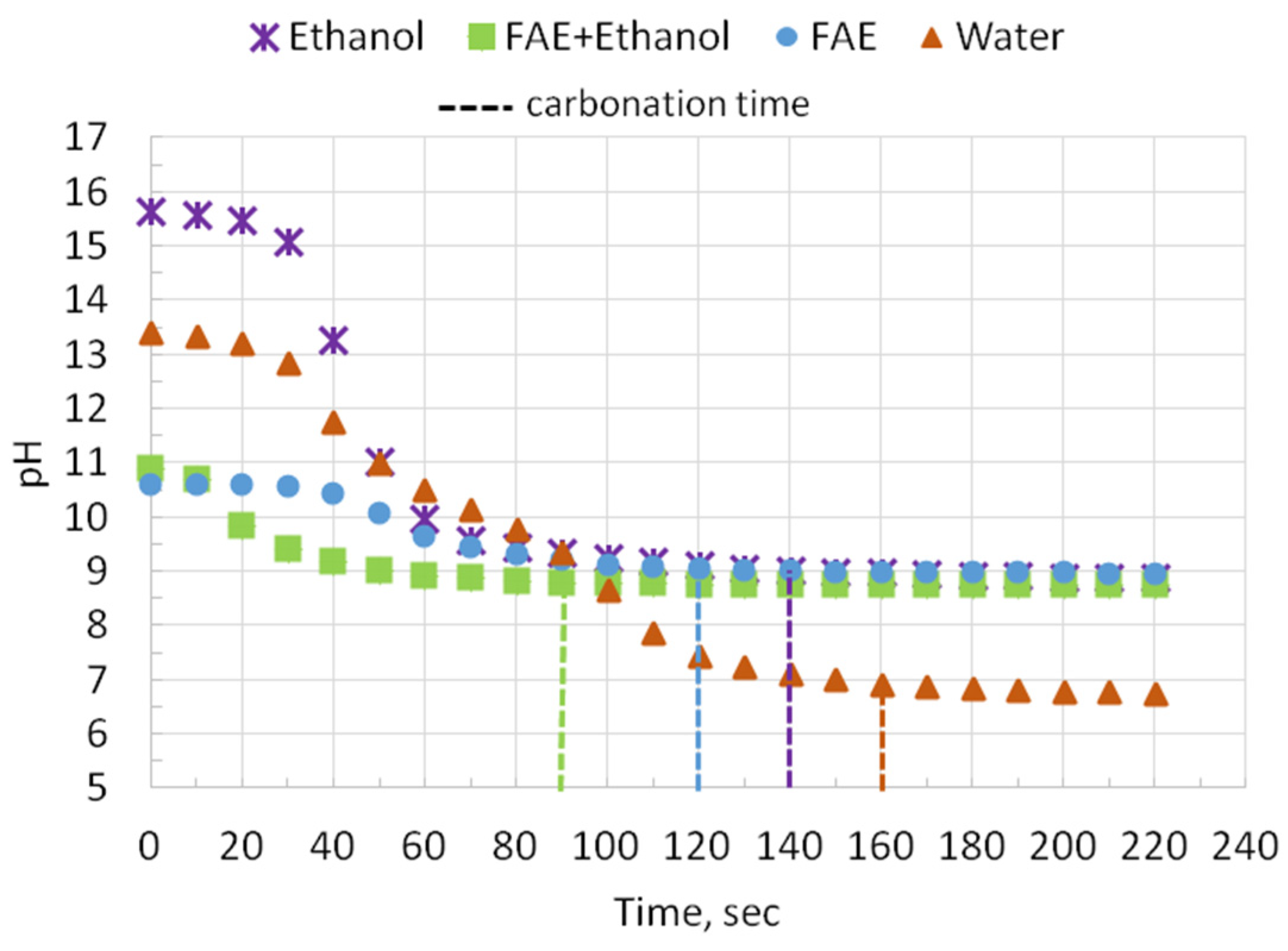
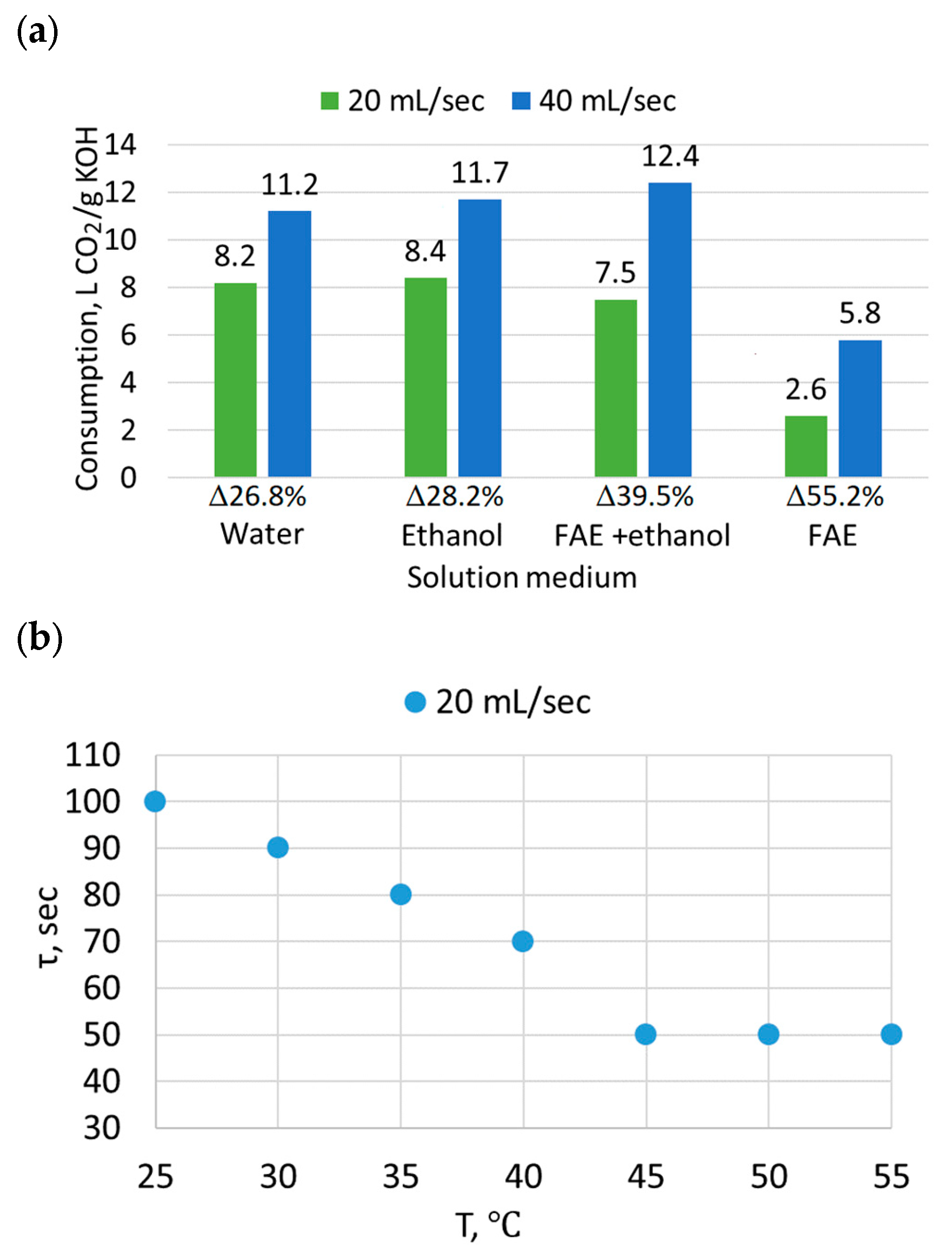
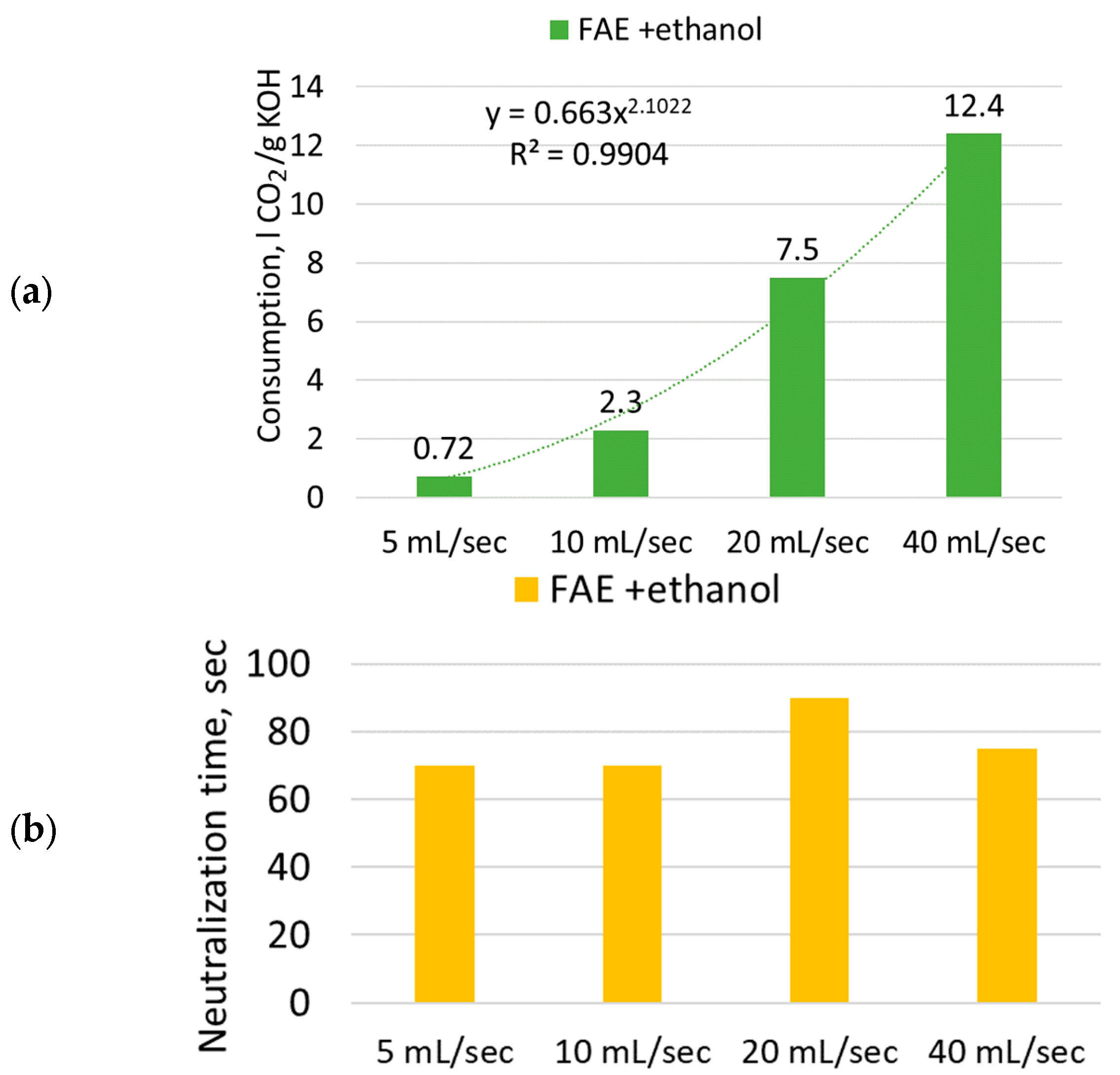
2.9.2. Neutralization via CO2 Barbotage
2.10. Removal of Potassium Salts from the Ether Fraction
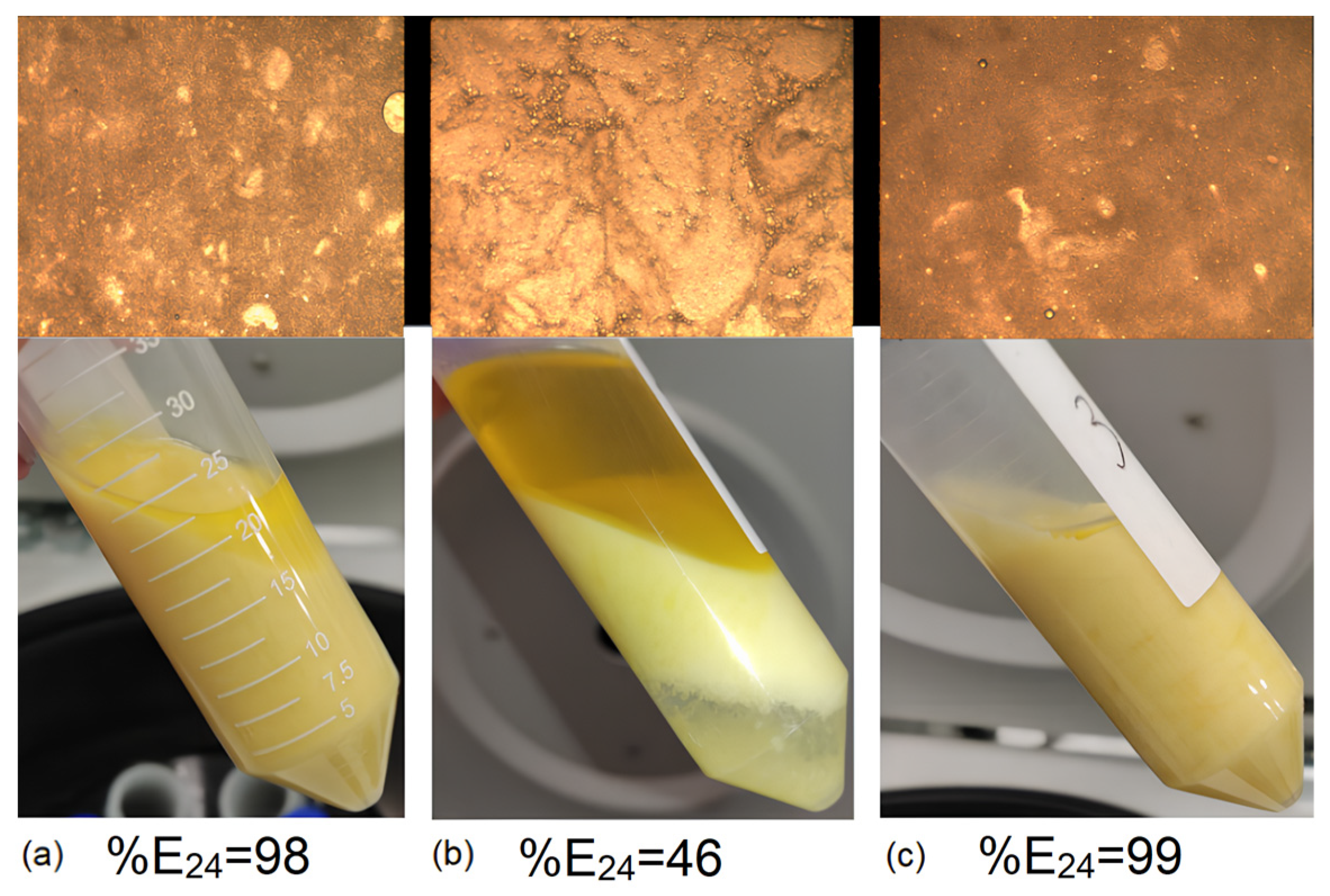
2.11. Analysis of the Emulsion Properties of the Ester Fraction in the Purification Stage
2.11.1. Analysis of Emulsion Layer Height and Stratification Time
2.11.2. Microscopic Emulsion Analysis
2.12. Analysis of FAEE
2.13. Analysis of Biodiesel Quality
2.14. Statistical Analysis
3. Results and Discussions
3.1. Perfect Mixing (PMR) and Plug Flow (PFR) Reactors
3.2. Qualitative Analysis of the Precipitate after Synthesis
3.3. Ethanol Separation
3.4. Material Balance
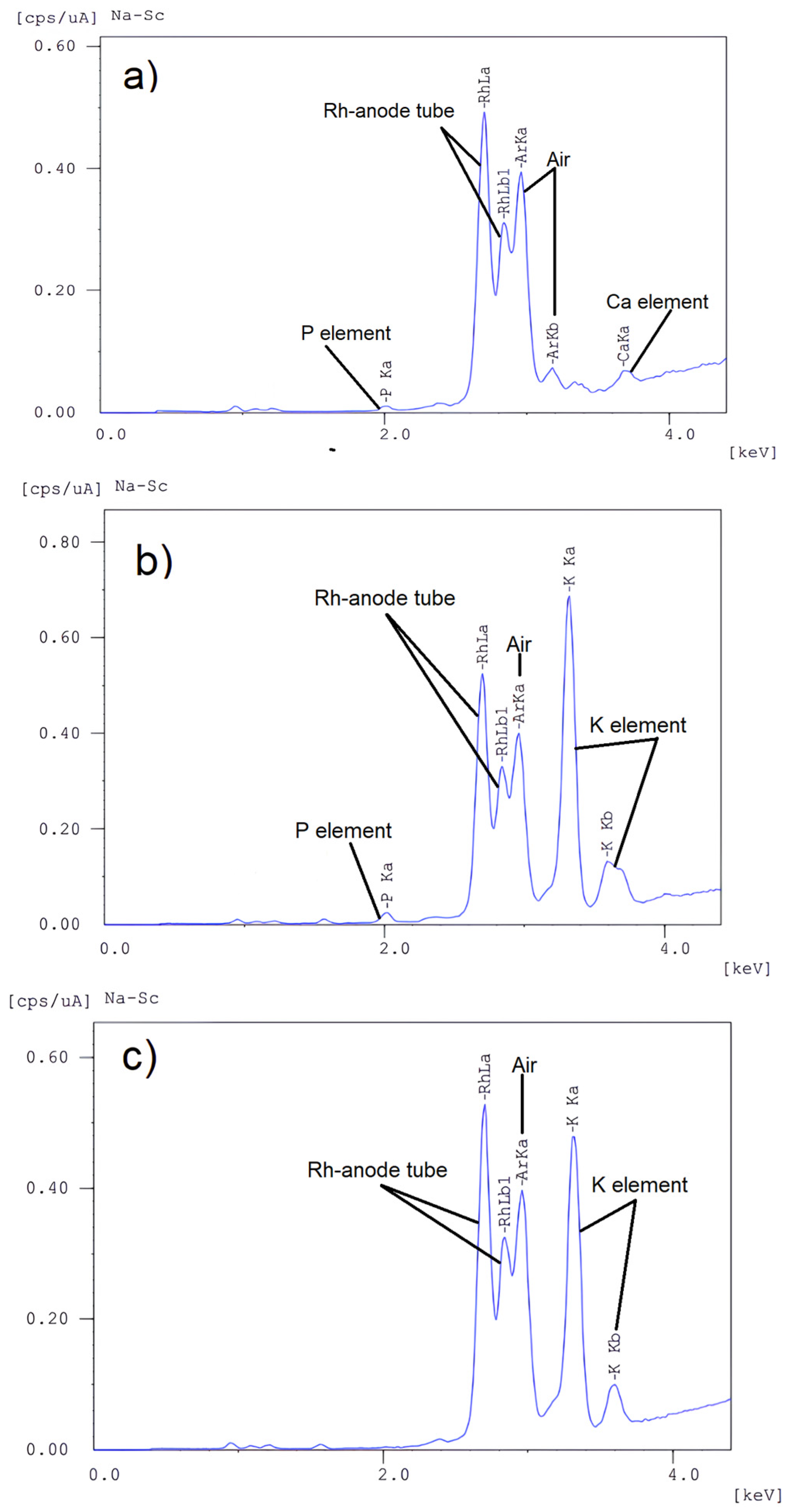
3.5. Catalyst Removal
3.6. Emulsion Properties of Esters
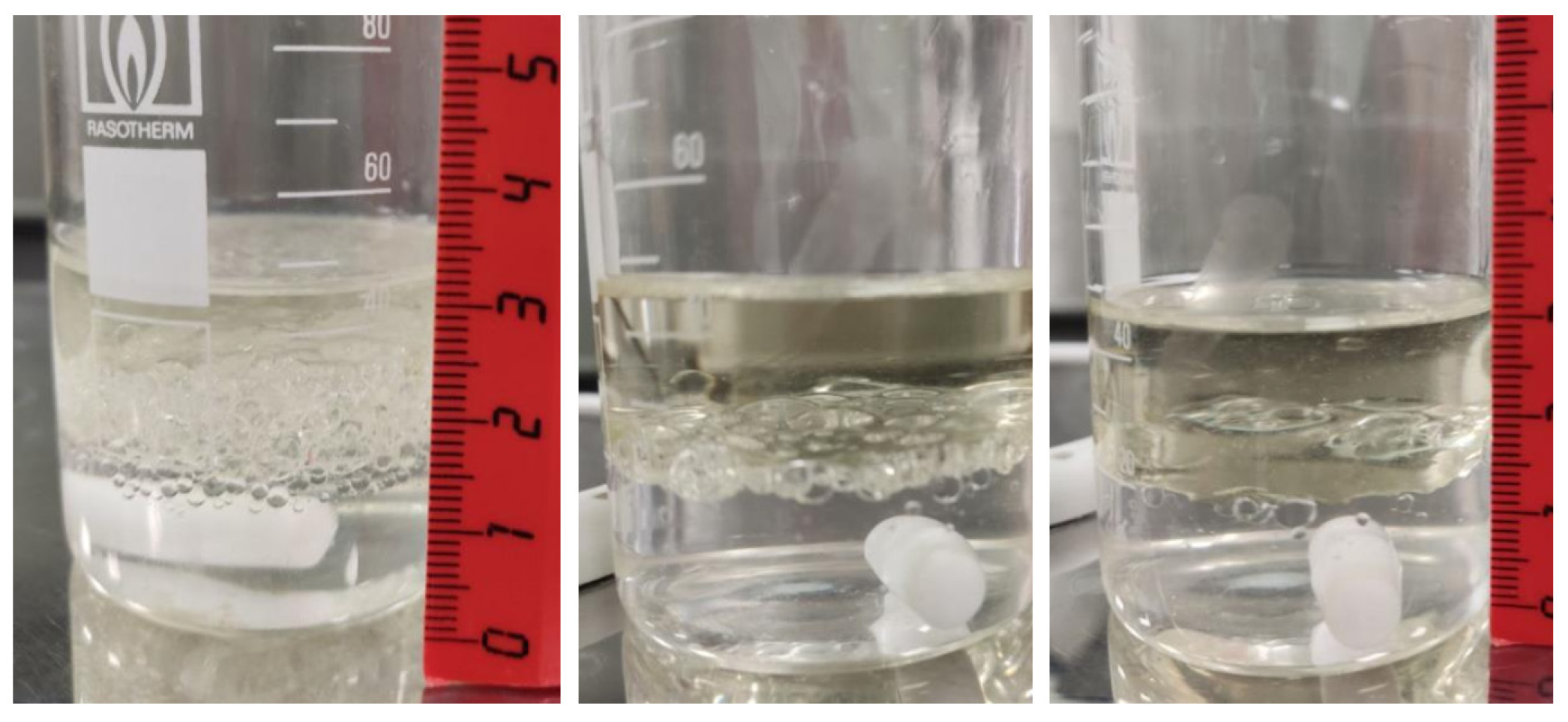
3.7. Delamination Time and Temperature
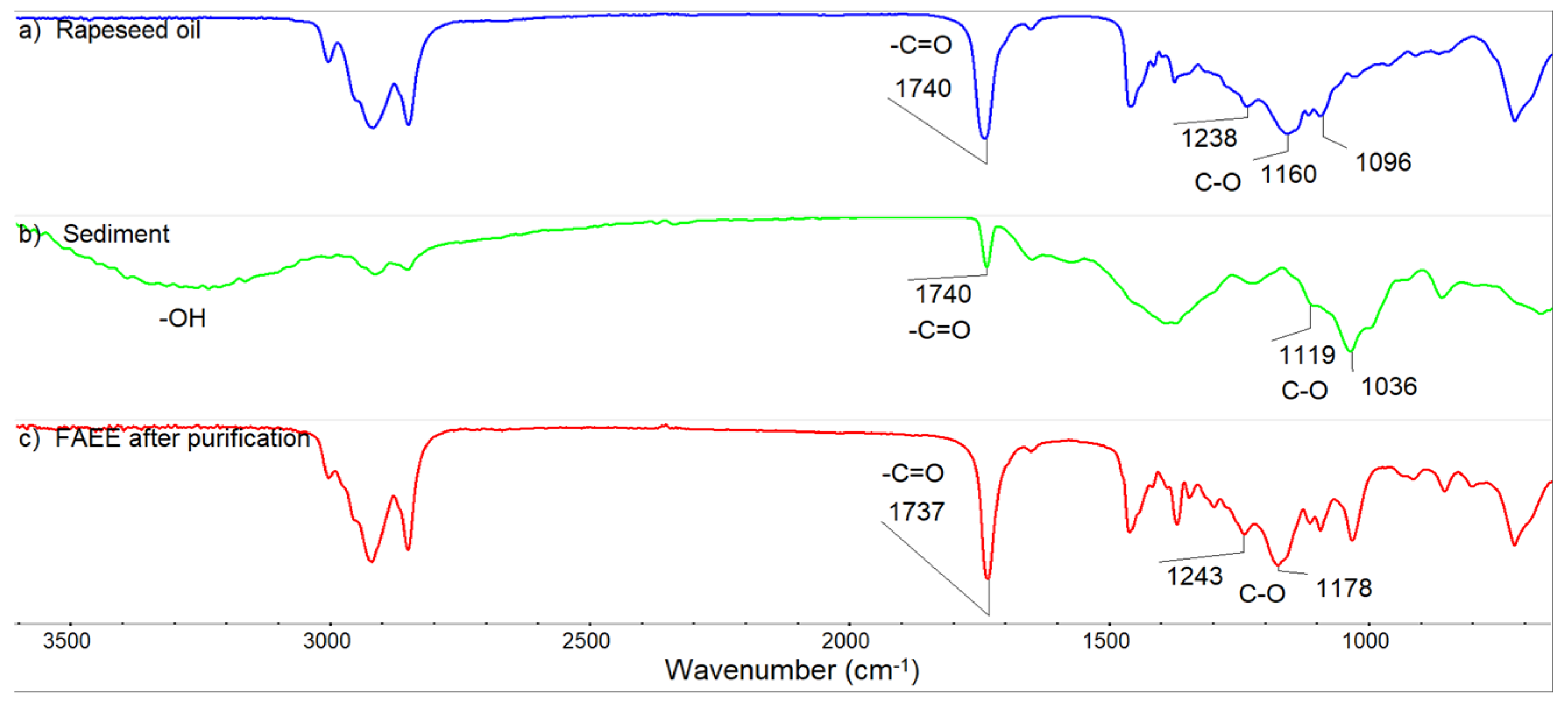
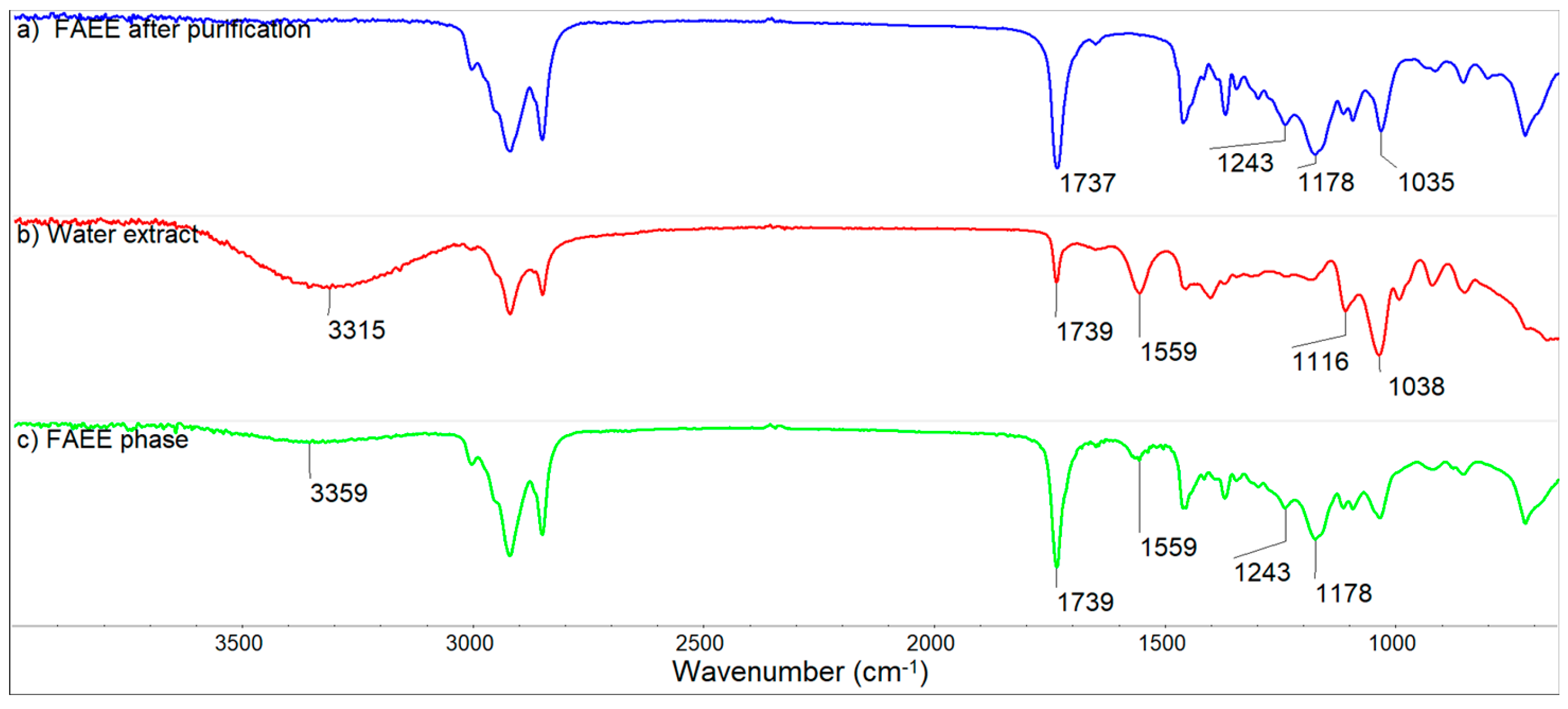
3.8. FT IR and GCMS Analysis of FAEE
3.9. Biodiesel Quality
3.10. Process Flow
4. Conclusions
- (1)
- Synthesis of fatty acid esters in a plug flow reactor showed positive results when unrefined rapeseed oil was used as a feedstock. At the oil/ethanol volume ratio of 1:3, KOH concentration of 0.5% and reactor residence time of 6 minutes, a product yield of 86.7%mol were achieved. In this case, the synthesis unit productivity was 5.0 L/h or 4.07 kg/h in laboratory conditions, which in terms of FAEE phase equals 1.0 kg/h. The scaling of the process in PMR did not give such a result, which becomes a strong argument in favor of PFR application.
- (2)
- Vacuum distillation of ethanol at residual pressure up to 20 kPa, as well as atmospheric evaporation in a mild temperature regime can be identified as rational methods of separation of excess ethanol within the investigated technological scheme.
- (3)
- Neutralization of the catalyst in FAEE phase by CO2 carbonization is considered as an alternative to the wet method of neutralization with acid solutions. On the one hand, this method eliminates the need to utilize acid waste water in the production process scheme. On the other hand, the use of carbonization in the FAEE purification process will increase CO2 consumption in the biodiesel production chain.
- (4)
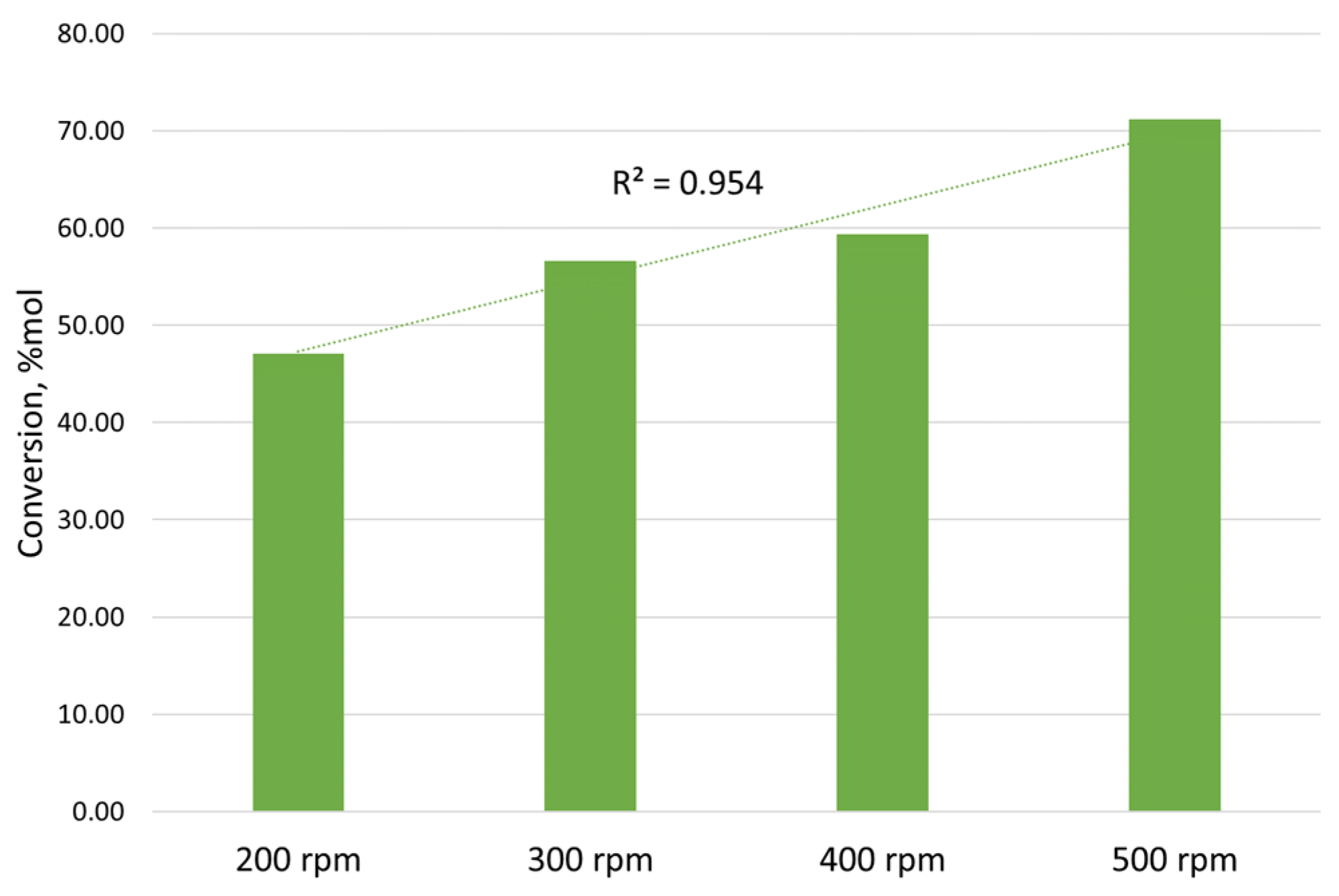
- For the speed range of 400–450 rpm, providing the necessary phase contact area for extraction, heating the mixture to a temperature of 40–45 °C would be reasonable.
- (5)
- FTIR analysis of FAEE phase before and after purification testifies to successful purification of FAEE from impurities of catalyst and soap.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swindell, W.R.; Bojanowski, K.; Singh, P.; Randhawa, M.; Chaudhuri, R.K. Bakuchiol and Ethyl (Linoleate/Oleate) Synergistically Modulate Endocannabinoid Tone in Keratinocytes and Repress Inflammatory Pathway MRNAs. JID Innov. 2023, 3, 100178. [Google Scholar] [CrossRef] [PubMed]
- Kislyak, O.A.; Starodubova, A.V. Modern Views on the Possibility of Using Ethyl Esters of Ω3 Polyunsaturated Fatty Acids 90% in the Treatment and Prevention of Cardiovascular Diseases and CHF. Atmos. Cardiol. News 2011, 3, 28–32. [Google Scholar]
- Gautam, S.; Guria, C.; Rajak, V.K. A State of the Art Review on the Performance of High-Pressure and High-Temperature Drilling Fluids: Towards Understanding the Structure-Property Relationship of Drilling Fluid Additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Ahmed, A.; Elkatatny, S.; Al-Afnan, S. Applications of Biodiesel in Drilling Fluids. Geofluids 2021, 2021, 5565897. [Google Scholar] [CrossRef]
- Tóth, Á.D.; Mike-Kaszás, N.; Bartus, G.; Hargitai, H.; Szabó, Á.I. Surface Modification of Silica Nanoparticles with Ethyl Oleate for the Purpose of Stabilizing Nanolubricants Used for Tribological Tests. Ceramics 2023, 6, 980–993. [Google Scholar] [CrossRef]
- Zulkifli, N.W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of Bio-Based Lubricant Derived from Different Chemically Modified Fatty Acid Methyl Ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- Sverguzova, S.; Sapronova, Z.; Zubkova, O.; Svyatchenko, A.; Shaikhieva, K.; Voronina, Y. Electric Steelmaking Dust as a Raw Material for Coagulant Production. J. Min. Inst. 2023, 260, 279–288. [Google Scholar] [CrossRef]
- Zubkova, O.; Alexeev, A.; Polyanskiy, A.; Karapetyan, K.; Kononchuk, O.; Reinmöller, M. Complex Processing of Saponite Waste from a Diamond-Mining Enterprise. Appl. Sci. 2021, 11, 6615. [Google Scholar] [CrossRef]
- Kondrasheva, N.; Eremeeva, A. Production of Biodiesel Fuel from Vegetable Raw Materials. J. Min. Inst. 2023, 260, 248–256. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Zhukov, V.S.; Popov, G.G. Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline. Energies 2023, 16, 5585. [Google Scholar] [CrossRef]
- Tayana, E.; Litvinova, D.V.S. Lightweight Ash-Based Concrete Production as a Promising Way of Technogenic Product Utilization (on the Example of Sewage Treatment Waste). J. Min. 2023, 264, 906–918. [Google Scholar]
- Litvinova, T.E.; Suchkov, D.V. Comprehensive Approach to the Utilisation of Technogenic Waste from the Mineral Resource Complex. Min. Informational Anal. Bull. 2022, 6, 331–348. [Google Scholar] [CrossRef]
- Cheremisina, O.; Litvinova, T.; Sergeev, V.; Ponomareva, M.; Mashukova, J. Application of the Organic Waste-Based Sorbent for the Purification of Aqueous Solutions. Water 2021, 13, 3101. [Google Scholar] [CrossRef]
- Korshunov, G.I.; Eremeeva, A.M.; Drebenstedt, C. Justification of the Use of a Vegetal Additive to Diesel Fuel as a Method of Protecting Underground Personnel of Coal Mines from the Impact of Harmful Emissions of Diesel-Hydraulic Locomotives. J. Min. Inst. 2021, 247, 39–47. [Google Scholar] [CrossRef]
- Tcvetkov, P. Engagement of Resource-Based Economies in the Fight against Rising Carbon Emissions. Energy Rep. 2022, 8, 874–883. [Google Scholar] [CrossRef]
- Kovalskaya, K.V.; Gorlanov, E.S. Al–Ti–B Master Alloys: Structure Formation in Modified Alloys. Tsvetnye Met. 2022, 57–64. [Google Scholar] [CrossRef]
- Vinogradova, A.; Gogolinskii, K.; Umanskii, A.; Alekhnovich, V.; Tarasova, A.; Melnikova, A. Method of the Mechanical Properties Evaluation of Polyethylene Gas Pipelines with Portable Hardness Testers. Inventions 2022, 7, 125. [Google Scholar] [CrossRef]
- Litvinenko, V.; Bowbrick, I.; Naumov, I.; Zaitseva, Z. Global Guidelines and Requirements for Professional Competencies of Natural Resource Extraction Engineers: Implications for ESG Principles and Sustainable Development Goals. J. Clean. Prod. 2022, 338, 130530. [Google Scholar] [CrossRef]
- Buslaev, G.; Lavrik, A.; Lavrik, A.; Tcvetkov, P. Hybrid System of Hydrogen Generation by Water Electrolysis and Methane Partial Oxidation. Int. J. Hydrogen. Energy 2023, 48, 24166–24179. [Google Scholar] [CrossRef]
- Litvinenko, V.; Petrov, E.; Vasilevskaya, D.; Yakovenko, A.; Naumov, I.; Ratnikov, M. Assessment of the Role of the State in the Management of Mineral Resources. J. Min. Inst. 2022, 259, 95–111. [Google Scholar] [CrossRef]
- Povarov, V.; Efimov, I. Use of the UNIFAC Model in the Calculation of Physicochemical Properties of Ecotoxicants for Technological and Ecoanalytical Purposes. J. Min. Inst. 2023, 260, 238–247. [Google Scholar] [CrossRef]
- Vasilyeva, M.A.; Volchikhina, A.A.; Kuskildin, R.B. Improvement of Water Segregation in Backfilling. Min. Informational Anal. Bull. 2023, 4, 125–139. [Google Scholar] [CrossRef]
- Iusovskii, A.; Boldushevskii, R.; Mozhaev, A.; Shmelkova, O.; Pavlycheva, E.; Koklyukhin, A.; Nikulshin, P. Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils. Energies 2023, 16, 5859. [Google Scholar] [CrossRef]
- Iuzmukhametova, R.; Boldushevskii, R.; Shmelkova, O.; Khamzin, Y.; Minaev, A.; Nikulshin, P. Adsorptive Treatment of Residues on Macroporous Adsorbent for Marine Fuel Production Scheme on Refinery. J. Mar. Sci. Eng. 2023, 11, 525. [Google Scholar] [CrossRef]
- Permyakova, I.A.; Vol’khin, V.V.; Kazakov, D.A.; Kaczmarski, K.; Kudryashova, O.S.; Sukhoplecheva, E.A. Phase Equilibria in Triacylglycerols—Ethanol—Oleic Acid—Athyl Oleate Quasi-Quaternary System. Eurasian Chem.-Technol. J. 2014, 16, 257–264. [Google Scholar] [CrossRef]
- Permyakova, I.A.; Vol’khin, V.V.; Medvedeva, O.S. Extraction in Emulsion Mode of Higher Fatty Acids from Vegetable Oils by Methanol or Ethanol in Esters Production. Russ. J. Appl. Chem. 2020, 93, 437–448. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Ndeddy Aka, R.J. Recent Development of Advanced Processing Technologies for Biodiesel Production: A Critical Review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Charykova, O.G.; Otinova, M.E.; Tiutiunikov, A.A. Key Directions of Agricultural Export Development in Russian Regions. Econ. Reg. 2022, 18, 193–207. [Google Scholar] [CrossRef]
- Susanu, I. Regulation of Liquid Biofuel Market in Russia and World. Trade Policy 2019, 1, 60–88. [Google Scholar] [CrossRef]
- Sergeev, V.; Balandinsky, D.; Romanov, G.; Rukin, G. Sorption Treatment of Water from Chromium Using Biochar Material. Arab. J. Basic Appl. Sci. 2023, 30, 299–306. [Google Scholar] [CrossRef]
- Petrov, D.S.; Korotaeva, A.E.; Pashkevich, M.A.; Chukaeva, M.A. Assessment of Heavy Metal Accumulation Potential of Aquatic Plants for Bioindication and Bioremediation of Aquatic Environment. Environ. Monit. Assess 2023, 195, 122. [Google Scholar] [CrossRef]
- Wu, H.; Colson, G.; Escalante, C.; Wetzstein, M. An Optimal U.S. Biodiesel Fuel Subsidy. Energy Policy 2012, 48, 601–610. [Google Scholar] [CrossRef]
- Phomsoda, K.; Puttanapong, N.; Piantanakulchai, M. Assessing Economic Impacts of Thailand’s Fiscal Reallocation between Biofuel Subsidy and Transportation Investment: Application of Recursive Dynamic General Equilibrium Model. Energies 2021, 14, 4248. [Google Scholar] [CrossRef]
- Riayatsyah, T.M.I.; Ong, H.C.; Chong, W.T.; Aditya, L.; Hermansyah, H.; Mahlia, T.M.I. Life Cycle Cost and Sensitivity Analysis of Reutealis Trisperma as Non-Edible Feedstock for Future Biodiesel Production. Energies 2017, 10, 877. [Google Scholar] [CrossRef]
- Polyakov, A.A.; Gorlanov, E.S.; Mushihin, E.A. Analytical Modeling of Current and Potential Distribution over Carbon and Low-Consumable Anodes during Aluminum Reduction Process. J. Electrochem. Soc. 2022, 169, 053502. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A.; et al. Current Challenge and Innovative Progress for Producing HVO and FAME Biodiesel Fuels and Their Applications. Waste Biomass Valorization 2023, 14, 505–521. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Rekhletskaya, E.S.; Makhova, U.A.; Kapustin, V.M.; Mukhina, D.Y.; Abdellatief, T.M.M. Technological Potential Analysis and Vacant Technology Forecasting in Properties and Composition of Low-Sulfur Marine Fuel Oil (VLSFO and ULSFO) Bunkered in Key World Ports. J. Mar. Sci. Eng. 2022, 10, 1828. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Schipachev, A. Manifestation of Incompatibility of Marine Residual Fuels: A Method for Determining Compatibility, Studying Composition of Fuels and Sediment. J. Min. Inst. 2022, 257, 843–852. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Denisov, K.; Zhurkevich, A.; Islamov, S. Reduction of Sulphur in Marine Residual Fuels by Deasphalting to Produce VLSFO. J. Mar. Sci. Eng. 2022, 10, 1765. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Transesterification for Biodiesel-a Review. Int. J. Adv. Trends Comput. Sci. Eng. 2019, 8, 46–50. [Google Scholar] [CrossRef]
- Deng, X.; Han, J.; Yin, F. Net Energy, CO2 Emission and Land-Based Cost-Benefit Analyses of Jatropha Biodiesel: A Case Study of the Panzhihua Region of Sichuan Province in China. Energies 2012, 5, 2150–2164. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally Friendly Diesel Fuel Obtained from Vegetable Raw Materials and Hydrocarbon Crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- Loktionova, E.V.; Sysa, O.K.; Loktionov, V.A. Extraction of Methylene Blue Coloring Agent from Model Solutions with a Plant-Origin Sorbent. J. Phys. Conf. Ser. 2021, 1926, 012018. [Google Scholar] [CrossRef]
- Duryagin, V.; Nguyen Van, T.; Onegov, N.; Shamsutdinova, G. Investigation of the Selectivity of the Water Shutoff Technology. Energies 2022, 16, 366. [Google Scholar] [CrossRef]
- Petukhova, Y.V.; Kudinova, A.A.; Bobrysheva, N.P.; Osmolowsky, M.G.; Alekseeva, E.V.; Levin, O.V.; Osmolovskaya, O.M. Capping Agents as a Novel Approach to Control VO2 Nanoparticles Morphology in Hydrothermal Process: Mechanism of Morphology Control and Influence on Functional Properties. Mater. Sci. Eng. B 2020, 255, 114519. [Google Scholar] [CrossRef]
- Feshchenko, R.Y.; Erokhina, O.O.; Litavrin, I.O.; Ryaboshuk, S.V. Improvement of Oxidation Resistance of Arc Furnace Graphite Electrodes. Chernye Met. 2023, 31–36. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J.V. Waste Cooking Oil as a Promising Source for Bio Lubricants- A Review. J. Indian Chem. Soc. 2023, 100, 100820. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Ashrafur Rahman, S.M.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Eze, V.C.; Harvey, A.P.; Phan, A.N. Determination of the Kinetics of Biodiesel Saponification in Alcoholic Hydroxide Solutions. Fuel 2015, 140, 724–730. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A Review of the Feedstocks, Catalysts, and Intensification Techniques for Sustainable Biodiesel Production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel Separation and Purification: A Review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Colombo, K.; Ender, L.; Santos, M.M.; Chivanga Barros, A.A. Production of Biodiesel from Soybean Oil and Methanol, Catalyzed by Calcium Oxide in a Recycle Reactor. S Afr. J. Chem. Eng. 2019, 28, 19–25. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of Soybean Oil to Biodiesel Using CaO as a Solid Base Catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An Overview on the Recent Advancements of Sustainable Heterogeneous Catalysts and Prominent Continuous Reactor for Biodiesel Production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Enhancement of Biodiesel Synthesis Using Co-Solvent in a Packed-Microchannel. J. Ind. Eng. Chem. 2017, 51, 162–171. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Oladepo, S.A.; Ganiyu, S.A. Biodiesel Production from Waste Cooking Oil via β-Zeolite-Supported Sulfated Metal Oxide Catalyst Systems. ACS Omega 2023, 8, 23720–23732. [Google Scholar] [CrossRef]
- Spetsov, E.A.; Artyushevskiy, D.I.; Konoplin, R.R.; Sizyakov, V.M. Phase Composition of Aluminium Hydroxides and Its Calculation Based on Thermal Analysis Data. Tsvetnye Met. 2023, 37–44. [Google Scholar] [CrossRef]
- Monika; Banga, S.; Pathak, V.V. Biodiesel Production from Waste Cooking Oil: A Comprehensive Review on the Application of Heterogenous Catalysts. Energy Nexus 2023, 10, 100209. [Google Scholar] [CrossRef]
- Oloyede, C.T.; Jekayinfa, S.O.; Alade, A.O.; Ogunkunle, O.; Otung, N.A.U.; Laseinde, O.T. Exploration of Agricultural Residue Ash as a Solid Green Heterogeneous Base Catalyst for Biodiesel Production. Eng. Rep. 2023, 5, e12585. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current Progress and Perspective of Heterogeneous Nanocatalytic Transesterification towards Biodiesel Production from Edible and Inedible Feedstock: A Review. Energy Convers Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Brondani, M.; dos Santos, M.S.N.; Oro, C.E.D.; Wancura, G.C.; Tres, M.V.; Oliveira, J.V. Demystifying the Enzymatic Biodiesel: How Lipases Are Contributing to Its Technological Advances. Renew. Energy 2023, 216, 119085. [Google Scholar] [CrossRef]
- Lebedev, A.B.; Shuiskaya, V.S. Influence of Composition and Cooling Rate of Alumocalcium Slag on Its Crumblability. Izvestiya Ferrous Metall. 2022, 65, 806–813. [Google Scholar] [CrossRef]
- Rekhletskaya, E.S.; Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Kapustin, V.M.; Abdellatief, T.M.M.; Potanin, D.A.; Smirnov, V.A.; Geng, T.; Abdelkareem, M.A.; et al. Unraveling the Superior Role of Characterizing Methyl Ester of Isohexene as an Innovative High-Octane Gasoline Mixing Component. Energy Fuels 2022, 36, 11829–11838. [Google Scholar] [CrossRef]
- Akubude, V.C.; Jaiyeoba, K.F.; Oyewusi, T.F.; Abbah, E.C.; Oyedokun, J.A.; Okafor, V.C. Overview on Different Reactors for Biodiesel Production; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Díaz, L.; Escalante, D.; Rodríguez, K.E.; Kuzmina, Y.; González, L.A. Response Surface Methodology for Continuous Biodiesel Production from Jatropha Curcas Oil Using Li/Pumice as Catalyst in a Packed-Bed Reactor Assisted with Diethyl Ether as Cosolvent. Chem. Eng. Process. Process Intensif. 2022, 179, 109065. [Google Scholar] [CrossRef]
- Sergeev, V.V.; Cheremisina, O.V.; Fedorov, A.T.; Gorbacheva, A.A.; Balandinsky, D.A. Interaction Features of Sodium Oleate and Oxyethylated Phosphoric Acid Esters with the Apatite Surface. ACS Omega 2022, 7, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Moyo, L.B.; Iyuke, S.E.; Muvhiiwa, R.F.; Simate, G.S.; Hlabangana, N. Application of Response Surface Methodology for Optimization of Biodiesel Production Parameters from Waste Cooking Oil Using a Membrane Reactor. S Afr. J. Chem. Eng. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Lu, P.; Yuan, Z.; Li, L.; Wang, Z.; Luo, W. Biodiesel from Different Oil Using Fixed-Bed and Plug-Flow Reactors. Renew. Energy 2010, 35, 283–287. [Google Scholar] [CrossRef]
- Likozar, B.; Pohar, A.; Levec, J. Transesterification of Oil to Biodiesel in a Continuous Tubular Reactor with Static Mixers: Modelling Reaction Kinetics, Mass Transfer, Scale-up and Optimization Considering Fatty Acid Composition. Fuel Process. Technol. 2016, 142, 326–336. [Google Scholar] [CrossRef]
- Ali, M.M.; Gheni, S.A.; Ahmed, S.M.R.; Hmood, H.M.; Hassan, A.A.; Mohammed, H.R.; Mohammed, S.T.; Karakullukcu, N.T. Catalytic Production of Biodiesel from Waste Cooking Oil in a Two-Phase Oscillatory Baffled Reactor: Deactivation Kinetics and ANN Modeling Study. Energy Convers. Manag. X 2023, 19, 100383. [Google Scholar] [CrossRef]
- Galusnyak, S.C.; Petrescu, L.; Cormos, C.C. Classical vs. Reactive Distillation Technologies for Biodiesel Production: An Environmental Comparison Using LCA Methodology. Renew. Energy 2022, 192, 289–299. [Google Scholar] [CrossRef]
- Mondal, B.; Jana, A.K. Optimal Reflux Splitting Reactive Distillation for Algal Biodiesel Production: Waste Heat Recovery through Vapor Recompression and Organic Rankine Cycle. Sep. Purif. Technol. 2022, 292, 121007. [Google Scholar] [CrossRef]
- Gaurav, A.; Ng, F.T.T.; Rempel, G.L. A New Green Process for Biodiesel Production from Waste Oils via Catalytic Distillation Using a Solid Acid Catalyst—Modeling, Economic and Environmental Analysis. Green Energy Environ. 2016, 1, 62–74. [Google Scholar] [CrossRef]
- Mondal, B.; Rangaiah, G.P.; Jana, A.K. Optimizing Algal Biodiesel Production from a Novel Reactive Distillation Based Unit: Reducing CO2 Emission and Cost. Chem. Eng. Process. Process Intensif. 2022, 176, 108948. [Google Scholar] [CrossRef]
- Purwanto, P.; Buchori, L.; Istadi, I. Reaction Rate Law Model and Reaction Mechanism Covering Effect of Plasma Role on the Transesterification of Triglyceride and Methanol to Biodiesel over a Continuous Flow Hybrid Catalytic-Plasma Reactor. Heliyon 2020, 6, e05164. [Google Scholar] [CrossRef]
- Sawangkeaw, R.; Bunyakiat, K.; Ngamprasertsith, S. Continuous Production of Biodiesel with Supercritical Methanol: Optimization of a Scale-up Plug Flow Reactor by Response Surface Methodology. Fuel Process. Technol. 2011, 92, 2285–2292. [Google Scholar] [CrossRef]
- Madhawan, A.; Arora, A.; Das, J.; Kuila, A.; Sharma, V. Microreactor Technology for Biodiesel Production: A Review. Biomass Convers Biorefin. 2018, 8, 485–496. [Google Scholar] [CrossRef]
- Chimezie, E.C.; Wang, Z.; Yu, Y.; Nonso, U.C.; Duan, P.G.; Kapusta, K. Yield Optimization and Fuel Properties Evaluation of the Biodiesel Derived from Avocado Pear Waste. Ind. Crops. Prod. 2023, 191, 115884. [Google Scholar] [CrossRef]
- Khelafi, M.; Djaafri, M.; Kalloum, S.; Atelge, M.R.; Abut, S.; Dahbi, A.; Bekirogullari, M.; Atabani, A.E. Effect of Stirring Speeds on Biodiesel Yield Using an Innovative Oscillatory Reactor and Conventional STR (A Comparative Study). Fuel 2022, 325, 124856. [Google Scholar] [CrossRef]
- Vellaiyan, S.; Kandasamy, M.; Devarajan, Y. Optimization of Bauhinia Parviflora Biodiesel Production for Higher Yield and Its Compatibility Assessment with Water and Di-Tert-Butyl Peroxide Emulsion. Waste Manag. 2023, 162, 63–71. [Google Scholar] [CrossRef]
- Sverchkov, I.P.; Gembitskaya, I.M.; Povarov, V.G.; Chukaeva, M.A. Method of Reference Samples Preparation for X-Ray Fluorescence Analysis. Talanta 2023, 252, 123820. [Google Scholar] [CrossRef]
- Kosolapova, S.M.; Smal, M.S.; Rudko, V.A.; Pyagay, I.N. A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil. Processes 2023, 11, 1534. [Google Scholar] [CrossRef]
- Moskvina, E.N.; Cornena, E.P.; Zolochevsky, V.T.; Timofeenko, T.I. Metal Composition of Phospholipids of Rapeseed Oils. News Universities. Food Technol. 1990, 76–77. [Google Scholar]
- Tarasevich, B.N.; IR Spectra of Main Classes.Reference Materials. Lomonosov Moscow State University; 2012. Available online: https://www.chem.msu.su/rus/teaching/tarasevich/Tarasevich_IR_tables_29-02-2012.pdf (accessed on 10 April 2022).
- Harutyunyan, N.S.; Kornena, E.P. Phospholipids of Vegetable Oils; Shcherbakov, V.G., Nechaev, A.P., Eds.; Agropromizdat: Moscow, Russia, 1986; Volume 256. [Google Scholar]
- Knerelman, E.I. Comparative Features of the Infrared Spectra C18-Carboxic Acids, Their Methyl Esters (Biodiesel) and Triglycerides (Vegetable Oils). Bull. Kazan Technol. Univ. 2008, 6, 68–78. [Google Scholar]
- Sultanova, G.I.; Sayfetdinova, G.A.; Rakhmatullina, A.P.; Akhmedyanova, R.A.; Liakumovich, A.G. Effect of Potassium Salts of Stearic and Oleic Acids on the Emulsion Copolymerization of Styrene and Alpha-Methylstyrene. Bull. Kazan Technol. Univ. 2006, 2, 67–71. [Google Scholar]
- Evdokimov, A.N.; Kurzin, A.V.; Sivakov, A.A.; Golikova, V.S. Solubility in Alcohols and Alcoholysis Reactions of Carbonates, Sulfides, Cyanides, and Phosphates of Alkali Metals. ChemChemTech 2018, 61, 14–23. [Google Scholar] [CrossRef]

| Property | Result | Units | Method |
|---|---|---|---|
| Acid value | 5.28 | mg KOH/g Oil | ISO 660-83 |
| Acid content | 2.57 | %wt | ISO 660-83 |
| Moisture | 0.063 | %wt | ISO 662-2019 |
| Fatty Acid | Conventional Designation | %mol | M (FAT), g/mol |
|---|---|---|---|
| Myristic acid | C14:0 | 0.08 | 723.1 |
| Pentadecanoic acid | C15:0 | 0.03 | 765.2 |
| 7,10-Hexadecadienoic acid | C16:2 | 0.09 | 795.2 |
| Palmitoleic acid | C16:1 | 0.41 | 801.2 |
| Palmitic acid | C16:0 | 7.69 | 807.2 |
| cis-10-Heptadecenoic acid | C17:1 | 0.11 | 843.3 |
| Margaric acid | C17:0 | 0.08 | 849.3 |
| Linoleic acid | C18:2 | 7.60 | 879.3 |
| Oleic acid | C18:1 | 75.25 | 885.3 |
| Stearic acid | C18:0 | 3.67 | 891.4 |
| Gondoic acid | C20:1 | 2.45 | 969.5 |
| Arachidic acid | C20:0 | 0.97 | 975.5 |
| Erucic acid | C22:1 | 0.69 | 1053.6 |
| Behenic acid | C22:0 | 0.49 | 1059.7 |
| Nervonic acid | C24:1 | 0.18 | 1137.8 |
| Lignoceric acid | C24:0 | 0.21 | 1143.8 |
| Total | 100.00 | 884.4 |
| Components, % | |||
|---|---|---|---|
| K | P | Ca | |
| Rapeseed oil | 0.007 | 0.051 | 0.009 |
| Unfiltered FAEE | 0.536 | 0.047 | 0.033 |
| Filtered FAEE | 0.369 | bdl | bdl |
| Reactor | Input, %wt | Output, %wt | |||||
|---|---|---|---|---|---|---|---|
| Oil | Ethanol | Total Input | Reaction Mix | Sediment | Loses | Total Output | |
| PFR | 28.0 | 72.0 | 100 | 98.1 | 0.1 | 1.7 | 100 |
| PMR | 28.1 | 71.9 | 100 | 96.0 | 0.3 | 3.7 | 100 |
| Reactor | Input, %wt | Output, %wt | |||||
|---|---|---|---|---|---|---|---|
| Distillation Residue | |||||||
| Total Input | Ethanol | FAEE Phase | Glycerol Phase | Loses | Total Output | FAEE Yield | |
| PFR | 100 | 58.7 | 24.4 | 5.7 | 11.2 | 100 | 86.7 |
| PMR | 100 | 60.5 | 19.8 | 10.8 | 8.9 | 100 | 73.2 |
| Property | Result | EN 14214 Limits | Units |
|---|---|---|---|
| Ester content | 97.83 | 96.5 min | % (mol/mol) |
| Density, 15 °C | 877 | 860–900 | kg/m3 |
| Kinematic viscosity, 40 °C | 3.9 | 3.5–5.0 | mm2/s |
| Sulfur content | bdl * | 10 max | mg/kg |
| Water content | 254 | 500 max | mg/kg |
| Linolenic acid ester content | bdl | 12 max | % (mol/mol) |
| Group I metals (Na, K) | bdl | 5 max | mg/kg |
| Group II metals (Ca, Mg) | bdl | 5 max | mg/kg |
| Phosphorous content | bdl | 10 max | mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosolapova, S.M.; Smal, M.S.; Pyagay, I.N.; Rudko, V.A. The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor. Processes 2024, 12, 788. https://doi.org/10.3390/pr12040788
Kosolapova SM, Smal MS, Pyagay IN, Rudko VA. The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor. Processes. 2024; 12(4):788. https://doi.org/10.3390/pr12040788
Chicago/Turabian StyleKosolapova, Sofia M., Makar S. Smal, Igor N. Pyagay, and Viacheslav A. Rudko. 2024. "The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor" Processes 12, no. 4: 788. https://doi.org/10.3390/pr12040788
APA StyleKosolapova, S. M., Smal, M. S., Pyagay, I. N., & Rudko, V. A. (2024). The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor. Processes, 12(4), 788. https://doi.org/10.3390/pr12040788









