The Aerobic Granules Process for Wastewater Treatment: From Theory to Engineering
Abstract
1. Introduction
2. The Development of Aerobic Granular Technology
2.1. Aerobic Granulation in Sequencing Batch Reactors
2.2. Aerobic Granulation in Continuous-Flow Reactors
3. Mechanism of Aerobic Granulation
4. Characteristics of Aerobic Granules
- It possesses a spherical and uniform shape with a distinct, smooth exterior;
- It exhibits a tightly packed and robust microbial constitution;
- It is sufficiently large to be visible as individual entities in the mixed liquor during both the mixing and settling phases.
- Sludge has a high biomass retention capability caused by its large size and fast settling velocity.
- It is capable of enduring high organic loading rates.
- It is resilient against the toxicity substances present in wastewater.
5. Structure and Strength of Aerobic Granule
6. Diversity of Aerobic Granules
7. Factors Affecting the Formation and Structure of Aerobic Granule
7.1. Shear Force
7.2. Settling Time
7.3. Organic Loading Rate
7.4. Substrate Composition
7.5. EPS
7.6. Hydraulic Retention Time
7.7. Dissolved Oxygen
7.8. Aerobic Starvation
7.9. Trace Elements
8. Application of Aerobic Granulation Technology
9. The Engineering of Aerobic Granular Sludge Technology
10. Conclusions and Challenges in Future
- (1)
- The process of aerobic granule formation remains largely unknown. Various theories have been proposed to explain this phenomenon, yet conclusive experimental support is lacking.
- (2)
- Achieving low substrate concentrations during steady-state operations to enable feast/famine conditions in CAGS is difficult. Research into how CAGS’s operational parameters affect the feast/famine ratio is scarce, necessitating future investigations into its impact on granule stability.
- (3)
- Employing a particle-size-based selection pressure could potentially facilitate CAGS granulation. However, research in this area is still in its infancy, and such a selection pressure might compromise CAGS stability in the long term.
- (4)
- While most CAGS setups incorporate a settling tank or clarifier for granule recycling, the recycling process can lead to granule disintegration. This calls for the creation of more sophisticated CAGS reactors with optimized recycling mechanisms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALR | Airlift reactor |
| CFR | Continuous-flow reactor |
| COD | Chemical oxygen demand |
| EPSs | Extracellular polymeric substances |
| HRT | Hydraulic retention time |
| MBR | Membrane bioreactor |
| OLR | Organic loading rate |
| RFBR | Reverse flow baffled reactor |
| SBR | Sequential batch reactor |
| SVI | Sludge volume index |
| WWTP | Wastewater treatment plan |
References
- Lee, D.-J.; Chen, Y.-Y.; Show, K.-Y.; Whiteley, C.G.; Tay, J.-H. Advances in aerobic granule formation and granule stability in the course of storage and reactor operation. Biotechnol. Adv. 2010, 28, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.G.; Tay, J.H. Characterization of microbial granulation process during UASB start-up. Water Res. 1997, 31, 1573–1580. [Google Scholar] [CrossRef]
- Alves, M.; Cavaleiro, A.J.; Ferreira, E.C.; Amaral, A.L.; Mota, M.; da Motta, M.; Vivier, H.; Pons, M.N. Characterization by image analysis of anaerobic sludge under shock conditions. Water Sci. Technol. 2000, 41, 207–214. [Google Scholar] [CrossRef][Green Version]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.A.; Yerushalmi, L.; Guiot, S.R. A market study on the anaerobic waste-water treatment systems. Water Air Soil Pollut. 2003, 143, 179–192. [Google Scholar] [CrossRef]
- Mishima, K.; Nakamura, M. Self-immobilization of aerobic activated sludge–a pilot study of the aerobic upflow sludge blanket process in municipal sewage treatment. Water Sci. Technol. 1991, 23, 981–990. [Google Scholar] [CrossRef]
- Morgenroth, E.; Sherden, T.; Van Loosdrecht, M.; Heijnen, J.; Wilderer, P. Aerobic granular sludge in a sequencing batch reactor. Water Res. 1997, 31, 3191–3194. [Google Scholar] [CrossRef]
- Beun, J.; Hendriks, A.; van Loosdrecht, M.; Morgenroth, E.; Wilderer, P.; Heijnen, J. Aerobic granulation in a sequencing batch reactor. Water Res. 1999, 33, 2283–2290. [Google Scholar] [CrossRef]
- Peng, D.C.; Bernet, N.; Delgenes, J.P.; Moletta, R. Aerobic granular sludge—A case report. Water Res. 1999, 33, 890–893. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L. Wastewater Engineering, Treatment, Disposal, Reuse, 3rd ed.; McGraw-Hill Book Company: New York, NY, USA, 1991. [Google Scholar]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.-Z.; Morgan, G.; Iorhemen, O.T. A review of the state of development of aerobic granular sludge technology over the last 20 years: Full-scale applications and resource recovery. Case Stud. Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.-H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Li, X.Q.; Li, J.A. Review on Pilot-scale study and application progress of the aerobic granular sludge technology. China Water Wastewater 2020, 36, 30–37. [Google Scholar]
- Royal Haskoning DHV. Available online: https://global.royalhaskoningdhv.com/nereda/projects (accessed on 30 December 2023).
- Shin, H.-S.; Lim, K.-H.; Park, H.-S. Effect of shear stress on granulation in oxygen aerobic upflow sludge bed reactors. Water Sci. Technol. 1992, 26, 601–605. [Google Scholar] [CrossRef]
- de Beer, D.; Heuvel, J.C.v.D.; Ottengraf, S.P.P. Microelectrode measurements of the activity distribution in nitrifying bacterial aggregates. Appl. Environ. Microbiol. 1993, 59, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Tijhuis, L.; Huisman, J.L.; Hekkelman, H.D.; van Loosdrecht, M.C.M.; Heijnen, J.J. Formation of nitrifying biofilms on small suspended particles in airlift reactors. Biotechnol. Bioeng. 1995, 47, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, A.; Ding, L.; Sellamuthu, B.; Perreault, J. Aerobic sludge granulation in a Reverse Flow Baffled Reactor (RFBR) operated in continuous-flow mode for wastewater treatment. Sep. Purif. Technol. 2015, 149, 437–444. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Badgley, B.D.; Sung, S.; Zhang, H.; He, Z. Nitrogen removal by granular nitritation–anammox in an upflow membrane-aerated biofilm reactor. Water Res. 2016, 94, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Lan, G.H.; Zeng, P. Excessive precipitation of CaCO3 as aragonite in a continuous aerobic granular sludge reactor. Appl. Microbiol. Biotechnol. 2015, 99, 8225–8234. [Google Scholar] [CrossRef]
- Huang, Q.J.; Wang, S.F.; Wu, N.; He, H.; Yu, W.T.; Cong, L. Research progress on aerobic granular sludge technology under continuous flow condition. Chem. Bioeng. 2016, 33, 12–14. [Google Scholar]
- Tay, J.H.; Liu, Q.S.; Liu, Y. Characteristics of aerobic granules grown on glucose and acetate in sequential aerobic sludge blanket reactors. Environ. Technol. 2002, 23, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The effects of shear force on the formation, structure and metabolism of aerobic granules. Appl. Microbiol. Biot. 2001, 57, 227–233. [Google Scholar]
- Liu, Y.; Tay, J.-H. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 2002, 36, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.-F.; Tay, J.-H.; Liu, Q.-S.; Qin, L.; Li, Y. Cell hydrophobicity is a triggering force of biogranulation. Enzym. Microb. Technol. 2004, 34, 371–379. [Google Scholar] [CrossRef]
- Barr, J.J.; Cook, A.E.; Bond, P.L. Granule formation mechanisms within an aerobic wastewater system for phosphorus removal. Appl. Environ. Microbiol. 2010, 76, 7588–7597. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Wu, P.; Xu, Y.Z.; Li, Y.H.; Shen, Y.L. Formation Mechanism of Aerobic Granular Sludge and Removal Efficiencies in Integrated ABR-CSTR Reactor. Huanjing Kexue 2015, 36, 2947–2953. [Google Scholar] [PubMed]
- van Dijk, E.J.; Haaksman, V.A.; van Loosdrecht, M.C.; Pronk, M. On the mechanisms for aerobic granulation—Model based evaluation. Water Res. 2022, 216, 118365. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-G.; Zhu, N.-W.; Liu, J.-S.; Wang, Z.-P.; Cai, W.-M. Characteristics of aerobic granules grown on glucose a sequential batch shaking reactor. J. Environ. Sci.-China 2004, 16, 624–626. [Google Scholar] [PubMed]
- Tsuneda, S.; Ejiri, Y.; Nagano, T.; Hirata, A. Formation mechanism of nitrifying granules observed in an aerobic upflow fluidized bed (AUFB) reactor. Water Sci. Technol. 2004, 49, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Nilsen, p.; Maulidiany, N.D. Thermal pretreatment to enhance biogas production of waste aerobic granular sludge with and without calcium phosphate precipitates. Chemosphere 2019, 234, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Wang, J.L.; Wen, X.H.; Qian, Y. The formation and characteristics of aerobic granules in sequencing batch reactor (SBR) by seeding anaerobic granules. Process. Biochem. 2005, 40, 5–11. [Google Scholar]
- Zheng, Y.-M.; Yu, H.-Q.; Sheng, G.-P. Physical and chemical characteristics of granular activated sludge from a sequencing batch airlift reactor. Process. Biochem. 2005, 40, 645–650. [Google Scholar] [CrossRef]
- Bengtsson, S.; de Blois, M.; Wilén, B.-M.; Gustavsson, D. A comparison of aerobic granular sludge with conventional and compact biological treatment technologies. Environ. Technol. 2019, 40, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Kehrein, P.; van Loosdrecht, M.; Osseweijer, P.; Posada, J. Exploring resource recovery potentials for the aerobic granular sludge process by mass and energy balances–energy, biopolymer and phosphorous recovery from municipal wastewater. Environ. Sci. Water Res. Technol. 2020, 6, 2164–2179. [Google Scholar] [CrossRef]
- Szabó, E.; Liébana, R.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.-M. Microbial Population Dynamics and Ecosystem Functions of Anoxic/Aerobic Granular Sludge in Sequencing Batch Reactors Operated at Different Organic Loading Rates. Front. Microbiol. 2017, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Gao, J.-F.; Pan, K.-L.; Li, D.-C.; Zhang, L.-F.; Wang, S.-J. Shifts in bacterial community composition and abundance of nitrifiers during aerobic granulation in two nitrifying sequencing batch reactors. Bioresour. Technol. 2018, 251, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liébana, R.; Modin, O.; Persson, F.; Szabó, E.; Hermansson, M.; LiÉ, R.; Bana; Wilen, B.-M. Combined Deterministic and Stochastic Processes Control Microbial Succession in Replicate Granular Biofilm Reactors. Environ. Sci. Technol. 2019, 53, 4912–4921. [Google Scholar] [CrossRef]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; Gonzalez-Lopez, J.; Pfetzing, P.; Gonzalez-Martinez, A. Performance and microbial community structure of aerobic granular bioreactors at different operational temperature. J. Water Process. Eng. 2020, 33, 101110. [Google Scholar] [CrossRef]
- Layer, M.; Adler, A.; Reynaert, E.; Hernandez, A.; Pagni, M.; Morgenroth, E.; Holliger, C.; Derlon, N. Organic substrate diffusibility governs microbial community composition, nutrient removal performance and kinetics of granulation of aerobic granular sludge. Water Res. X 2019, 4, 100033. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Liu, Q.-S.; Tay, J.-H.; Liu, Y. Growth kinetics of aerobic granules developed in sequencing batch reactors. Lett. Appl. Microbiol. 2004, 38, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Beun, J.; van Loosdrecht, M.; Heijnen, J. Aerobic granulation in a sequencing batch airlift reactor. Water Res. 2002, 36, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Ivanov, V.; Pan, S.; Tay, S.-L. Specific layers in aerobically grown microbial granules. Lett. Appl. Microbiol. 2002, 34, 254–257. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.Q.; Li, J.; Ma, R.R.; Zeng, P.; Ng, C.A.; Liu, F.H. The dynamic shift of bacterial communities in hybrid anaerobic baffled reactor (ABR)—Aerobic granules process for berberine pharmaceutical wastewater treatment. Processes 2022, 10, 2506. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Kong, Y.H.; Zhang, R.; Zhang, X.; Wong, F.S.; Tay, J.H.; Zhu, J.R.; Jiang, W.J. Microbial population dynamics of granular aerobic sequencing batch reactors during start-up and steady state periods. Water Sci. Technol. 2010, 62, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbeck, N.; Erley, R.; Wilderer, P. Aerobic granular sludge in an SBR-system treating wastewater rich in particulate matter. Water Sci. Technol. 2004, 49, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; You, S.; Guo, H.; Ma, F.; Xiao, X.; Zhang, J.; Ma, X. Response of aerobic granular sludge to loading shock: Performance and proteomic study. Chem. Eng. J. 2022, 444, 136458. [Google Scholar] [CrossRef]
- Etterer, T.; Wilderer, P.A. Generation and properties of aerobic granular sludge. Water Sci. Technol. 2001, 43, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.-L.; Ivanov, V.; Yi, S.; Zhuang, W.-Q.; Tay, J.-H. Presence of anaerobic bacteroides in aerobically grown microbial granules. Microb. Ecol. 2002, 44, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Tay, S.-L.; Ivanov, V.; Pan, S.; Jiang, H.-L.; Liu, Q.-S. Biomass and porosity profiles in microbial granules used for aerobic wastewater treatment. Lett. Appl. Microbiol. 2003, 36, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Lan, G.H.; Zeng, P. Size-dependent calcium carbonate precipitation induced microbiologically in aerobic granules. Chem. Eng. J. 2016, 285, 341–348. [Google Scholar] [CrossRef]
- Tay, J.; Liu, Q.; Liu, Y. The effect of upflow air velocity on the structure of aerobic granules cultivated in a sequencing batch reactor. Water Sci. Technol. 2004, 49, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Wang, Y.; Liu, Y.Q.; Li JLiu, F.H.; Chang, M.; Zhang, Y.Z. The Impact of berberine pharmaceutical wastewater on aerobic granules formation: Change of granules’ size. Processes 2022, 10, 792. [Google Scholar] [CrossRef]
- Meyer, R.L.; Saunders, A.M.; Zeng, R.J.X.; Keller, J.; Blackall, L.L. Microscale structure and function of anaerobic-aerobic granules containing glycogen accumulating organisms. FEMS Microbiol. Ecol. 2003, 45, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Nagano, T.; Hoshino, T.; Ejiri, Y.; Noda, N.; Hirata, A. Characterization of nitrifying granules produced in an aerobic upflow fluidized bed reactor. Water Res. 2003, 37, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.Q.; Mao, S.; Steinberg, C.E.W.; Duan, W.; Chen, F. Reproducibility of aerobic granules in treating low-strength and low C/N ratio wastewater and associated microbial community structure. Processes. 2022, 10, 444. [Google Scholar] [CrossRef]
- Aqeel, H.; Basuvaraj, M.; Hall, M.; Neufeld, J.D.; Liss, S.N. Microbial dynamics and properties of aerobic granules developed in a laboratory-scale sequencing batch reactor with an intermediate filamentous bulking stage. Appl. Microbiol. Biotechnol. 2016, 100, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Maszenan, A.; Tay, S.-L. A culture-independent approach for studying microbial diversity in aerobic granules. Water Sci. Technol. 2003, 47, 283–290. [Google Scholar]
- Jiang, H.-L.; Tay, J.-H.; Maszenan, A.M.; Tay, S.T.-L. Bacterial diversity and function of aerobic granules engineered in a sequencing batch reactor for phenol degradation. Appl. Environ. Microbiol. 2004, 70, 6767–6775. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Liu, Y.; Tay, J.-H. Development and characteristics of phosphorus-accumulating microbial granules in sequencing batch reactors. Appl. Microbiol. Biotechnol. 2003, 62, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Y.-M.; Yang, S.-F.; Tay, J.-H. A balanced model for biofilms developed at different growth and detachment forces. Process. Biochem. 2003, 38, 1761–1765. [Google Scholar] [CrossRef]
- Yang, S.-F.; Tay, J.-H.; Liu, Y. A novel granular sludge sequencing batch reactor for removal of organic and nitrogen from wastewater. J. Biotechnol. 2003, 106, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Tay, J.-H.; Tay, S.-L. Aggregation of immobilized activated sludge cells into aerobically grown microbial granules for the aerobic biodegradation of phenol. Lett. Appl. Microbiol. 2002, 35, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Pan, S.; He, Y.; Tay, S.T.L. Effect of organic loading rate on aerobic granulation. ii: Characteristics of aerobic granules. J. Environ. Eng. 2004, 130, 1102–1109. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Tay, J.H. Fast formation of aerobic granules by combining strong hydraulic selection pressure with overstressed organic loading rate. Water. Res.. 2015, 80, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Tay, J.; Moy, B.; Ivanov, V.; Tay, S. Size-effect on the physical characteristics of the aerobic granule in a SBR. Appl. Microbiol. Biotechnol. 2003, 60, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhang, X.; Zhang, R.; Liu, W.T.; Tay, J.H. Effects of hydraulic retention time on aerobic granulation and granule growth kinetics at steady state with a fast start-up strategy. Appl. Microbiol. Biotechnol. 2016, 100, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Tay, J.H.; Liu, Y. Substrate concentration-independent aerobic granulation in sequential aerobic sludge blanket reactor. Environ. Technol. 2003, 24, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.-P.; Tay, J.-H.; Toh, S.-K.; Liu, Y.; Tay, S.-L. High organic loading influences the physical characteristics of aerobic sludge granules. Lett. Appl. Microbiol. 2002, 34, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Tay, J.-H.; Liu, Y.; Tay, S.T.-L. Ca2+ augmentation for enhancement of aerobically grown microbial granules in sludge blanket reactors. Biotechnol. Lett. 2003, 25, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Liu, Q.-S.; Liu, Y. Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor. J. Appl. Microbiol. 2001, 91, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lv, Y.; Cao, M.; Zeng, H.; Zhang, J. Optimized hydraulic retention time for phosphorus and COD removal from synthetic domestic sewage with granules in a continuous-flow reactor. Bioresour. Technol. 2016, 216, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Tay, J.-H.; He, Y.-X.; Tay, S.-L. The effect of hydraulic retention time on the stability of aerobically grown microbial granules. Lett. Appl. Microbiol. 2004, 38, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Yang, S.F.; Liu, Y. Hydraulic selection pressure-induced nitrifying granulation in sequencing batch reactors. Appl. Microbiol. Biot. 2002, 59, 332–337. [Google Scholar]
- Corsino, S.; Campo, R.; Di Bella, G.; Torregrossa, M.; Viviani, G. Study of aerobic granular sludge stability in a continuous-flow membrane bioreactor. Bioresour. Technol. 2016, 200, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Lu, H.; Yao, L.; Leng, L.; Guan, L. Rapid formation of aerobic granular sludge and its mechanism in a continuous-flow bioreactor. Appl. Biochem. Biotechnol. 2016, 181, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.L.; Yang, X.; Lee, D.J.; Sun, S.P.; Liu, X.; Zhang, P. Influence of hydraulic retention time on partial nitrification of continuous-flow aerobic granular-sludge reactor. Environ. Technol. 2014, 35, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Kent, T.R.; Bott, C.B.; Wang, Z.-W. State of the art of aerobic granulation in continuous flow bioreactors. Biotechnol. Adv. 2018, 36, 1139–1166. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Liu, Q.-S.; Liu, Y. The role of cellular polysaccharides in the formation and stability of aerobic granules. Lett. Appl. Microbiol. 2001, 33, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Ejiri, Y.; Ogiwara, M.; Nagano, T.; Hirata, A. Characterization of Nitrifying Granules Produced in an Aerobic Upflow Fluidized Bed Reactor; Institute of Water Quality Control and Waste Management: Munich, Germany, 2004. [Google Scholar]
- McSwain, B.; Irvine, R.; Wilderer, P. The effect of intermittent feeding on aerobic granule structure. Water Sci. Technol. 2004, 49, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Chen, Y.-Y. Magnesium carbonate precipitate strengthened aerobic granules. Bioresour. Technol. 2015, 183, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Liu, Q.-S.; Liu, Y. Aerobic granulation in sequential sludge blanket reactor. Water Sci. Technol. 2002, 46, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Tay, J.H.; Tay, S.T.L. Changes in structure, activity and metabolism of aerobic granules as a microbial response to high phenol loading. Appl. Microbio. Biotechnol. 2004, 63, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Beun, J.J.; Heijnen, J.J.; van Loosdrecht, M.C.M. N-Removal in a granular sludge sequencing batch airlift reactor. Biotechnol. Bioeng. 2001, 75, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Dulekgurgen, E.; Ovez, S.; Artan, N.; Orhon, D. Enhanced biological phosphate removal by granular sludge in a sequencing batch reactor. Biotechnol. Lett. 2003, 25, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Lan, G.H.; Zeng, P. Resistance and resilience of nitrifying bacteria in aerobic granules to pH shock. Lett. Appl. Microbiol. 2015, 61, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Tay, J.H. The competition between flocculent sludge and aerobic granules during the long-term operation period of granular sludge sequencing batch reactor. Environ. Technol. 2012, 33, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Liu, Y.Q.; Tay, J.H.; Ning, P. Rapid formation of nitrifying granules treating high-strength ammonium wastewater in a sequencing batch reactor. Appl. Microbiol. Biotechnol. 2015, 99, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Maulidiany, N.; Zeng, P.; Heo, S. Decolourization of azo, anthraquinone and triphenylmethane dyes using aerobic granules: Acclimatization and long-term stability. Chemosphere 2021, 263, 128312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Moy, B.Y.P.; Tay, J.H. COD removal and nitrification of low-strength domestic wastewater in aerobic granular sludge sequencing batch reactors. Enzyme Microb. Tech. 2007, 42, 23–28. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Moy, B.; Kong, Y.H.; Tay, J.H. Formation, physical characteristics and microbial community structure of aerobic granules in a pilot-scale sequencing batch reactor for real wastewater treatment. Enzym. Microb. Technol. 2010, 46, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Chao, L.; Yunxia, Z.; Hongzhi, S.; Xin, L.; Li, G. Pilot test of domestic wastewater treatment in anaerobic/aerobic granular sludge SBR system. Chin. J. Environ. Eng. 2010, 4, 1276–1282. (In Chinese) [Google Scholar]
- Tu, X.; Su, B.-S.; Kong, Y.-H.; Zhu, J.-R. Cultivation of aerobic granules in a large pilot SBR with domestic sewage. Environ. Sci. 2010, 31, 2118–2123. (In Chinese) [Google Scholar]
- Tu, X.; Su, B.-S.; Kong, Y.-H.; Zhu, J.-R. Characteristics of extracellular fluorescent substances of aerobic granular sludge in pilot-scale sequencing batch reactor. J. Cent. South Univ. Technol. 2010, 17, 522–528. [Google Scholar] [CrossRef]
- Liu, S.; Mei, Z.; Xie, W.; Ni, B.; Li, W.; Yu, H. Cultivation and granulation process of aerobic granular sludge applied to treat municipal wastewater. Res. Environ. Sci. 2010, 23, 918–923. (In Chinese) [Google Scholar]
- Liu, Y.-Q.; Kong, Y.; Tay, J.-H.; Zhu, J. Enhancement of start-up of pilot-scale granular SBR fed with real wastewater. Sep. Purif. Technol. 2011, 82, 190–196. [Google Scholar] [CrossRef]
- Jungles, M.K.; Figueroa, M.; Morales, N.; del Río, Á.V.; da Costa, R.H.R.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Start up of a pilot scale aerobic granular reactor for organic matter and nitrogen removal. J. Chem. Technol. Biotechnol. 2011, 86, 763–768. [Google Scholar] [CrossRef]
- Li, Z.-H.; Fu, J.-F.; Li, S.; Liu, Z.-K.; Ji, X.-Q.; Wang, X.-C. Pilot study on aerobic granular sludge for treating comprehensive municipal wastewater. China Water Wastewater 2011, 27, 4–8. [Google Scholar]
- Wei, D.; Si, W.; Zhang, Y.; Qiao, Z.; Yao, Z.; Zhao, W.; Zhao, J.; Chen, G.; Wei, Q.; Du, B. Aerobic granulation and nitrogen removal with the effluent of internal circulation reactor in start-up of a pilot-scale sequencing batch reactor. Bioprocess Biosyst. Eng. 2012, 35, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Su, B.S.; Cui, X.J.; Zhu, J.R. Optimal cultivation and characteristics of aerobic granules with typical domestic sewage in an alternating anaerobic/aerobic sequencing batch reactor. Bioresour. Technol. 2012, 110, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Isanta, E.; Suárez-Ojeda, M.E.; del Río, Á.V.; Morales, N. Long term operation of a granular sequencing batch reactor at pilot scale treating a low-strength wastewater. Chem. Eng. J. 2012, 198–199, 163–170. [Google Scholar] [CrossRef]
- Wei, D.; Qiao, Z.; Zhang, Y.; Hao, L.; Si, W.; Du, B.; Wei, Q. Effect of COD/N ratio on cultivation of aerobic granular sludge in a pilot scale sequencing batch reactor. Appl. Microbiol. Biotechnol. 2013, 97, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Song, Y.; Zeng, P.; Duan, L.; Xiao, S. Characterization of bacterial communities in hybrid upflow anaerobic sludge blanket (UASB)—Membrane bioreactor (MBR) process for berberine antibiotic wastewater treatment. Bioresour. Technol. 2013, 142, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.; Figueroa, M.; Fra-Vázquez, A.; del Río, A.V.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Operation of an aerobic granular pilot scale SBR plant to treat swine slurry. Process Biochem. 2013, 48, 1216–1221. [Google Scholar] [CrossRef]
- Yang, S.F.; Zhang, J.J.; Zou, G.L.; Du, Z.L. Formation and characterization of aerobic granules in a pilot-scale reactor for real wastewater treatment. Environ. Sci. 2014, 35, 1850–1856. (In Chinese) [Google Scholar]
- Long, B.; Yang, C.Z.; Pu, W.H.; Yang, J.K.; Jiang, G.S.; Dan, J.F.; Li, C.Y.; Liu, F.B. Rapid cultivation of aerobic granular sludge in a pilot scale sequencing batch reactor. Bioresour. Technol. 2014, 166, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.B.; Ma, J.J.; Li, J.; Chen, T.; Zhou, Y.N.; Xie, K.; Huang, G.X. Evaluation of operation efficiency of pilot-scale SBR with aerobic granular sludge. China Water Wastewater 2014, 30, 87–90. (In Chinese) [Google Scholar]
- Rocktäschel, T.; Klarmann, C.; Ochoa, J.; Boisson, P.; Sørensen, K.; Horn, H. Influence of the granulation grade on the concentration of suspended solids in the effluent of a pilot scale sequencing batch reactor operated with aerobic granular sludge. Sep. Purif. Technol. 2015, 142, 234–241. [Google Scholar] [CrossRef]
- de Kreuk, M.K. Aerobic Granular Sludge: Scaling up a New Technology. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2006. [Google Scholar]
- Sajjad, M.; Kim, I.S.; Kim, K.S. Development of a novel process to mitigate membrane fouling in a continuous sludge system by seeding aerobic granules at pilot plant. J. Membr. Sci. 2016, 497, 90–98. [Google Scholar] [CrossRef]
- Guimarães, L.B.; Mezzari, M.P.; Daudt, G.C.; da Costa, R.H. Microbial pathways of nitrogen removal in aerobic granular sludge treating domestic wastewater. J. Chem. Technol. Biotechnol. 2017, 92, 1756–1765. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Wang, Z.W. Continuous-flow aerobic granulation in plug-flow bioreactors fed with real domestic wastewater. Sci. Total Environ. 2019, 688, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Hasebe, Y.; Furusawa, K.; Shiomi, H.; Inoue, D.; Ike, M. Enhancement of nutrient removal in an activated sludge process using aerobic granular sludge augmentation strategy with ammonium-based aeration control. Chemosphere 2023, 340, 139826. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, T.; Tian, Y.; Li, H.; Wan, J.; Wang, Y. Efficient partial nitrification with hybrid nitrifying granular sludge based on a simultaneous fill/draw SBR mode. Chemosphere 2023, 313, 137579. [Google Scholar] [CrossRef] [PubMed]
- RHDHV. Available online: https://www.royalhaskoningdhv.com/en-gb/nereda/nereda-plants (accessed on 30 December 2023).
- Sepúlveda-Mardones, M.; Campos, J.L.; Magrí, A.; Vidal, G. Moving forward in the use of aerobic granular sludge for municipal wastewater treatment: An overview. Rev. Environ. Sci. Bio/Technol. 2019, 18, 741–769. [Google Scholar] [CrossRef]
- Regmi, P.; Sturm, B.; Hiripitiyage, D.; Keller, N.; Murthy, S.; Jimenez, J. Combining continuous flow aerobic granulation using an external selector and carbon-efficient nutrient removal with AvN control in a full-scale simultaneous nitrification-denitrification process. Water Res. 2022, 210, 117991. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, K.; Zhang, K.; Liu, R.; Zheng, P. Full-scale upgrade activated sludge to continuous-flow aerobic granular sludge: Implementing microaerobic-aerobic configuration with internal separators. Water Res. 2024, 248, 120870. [Google Scholar] [CrossRef] [PubMed]
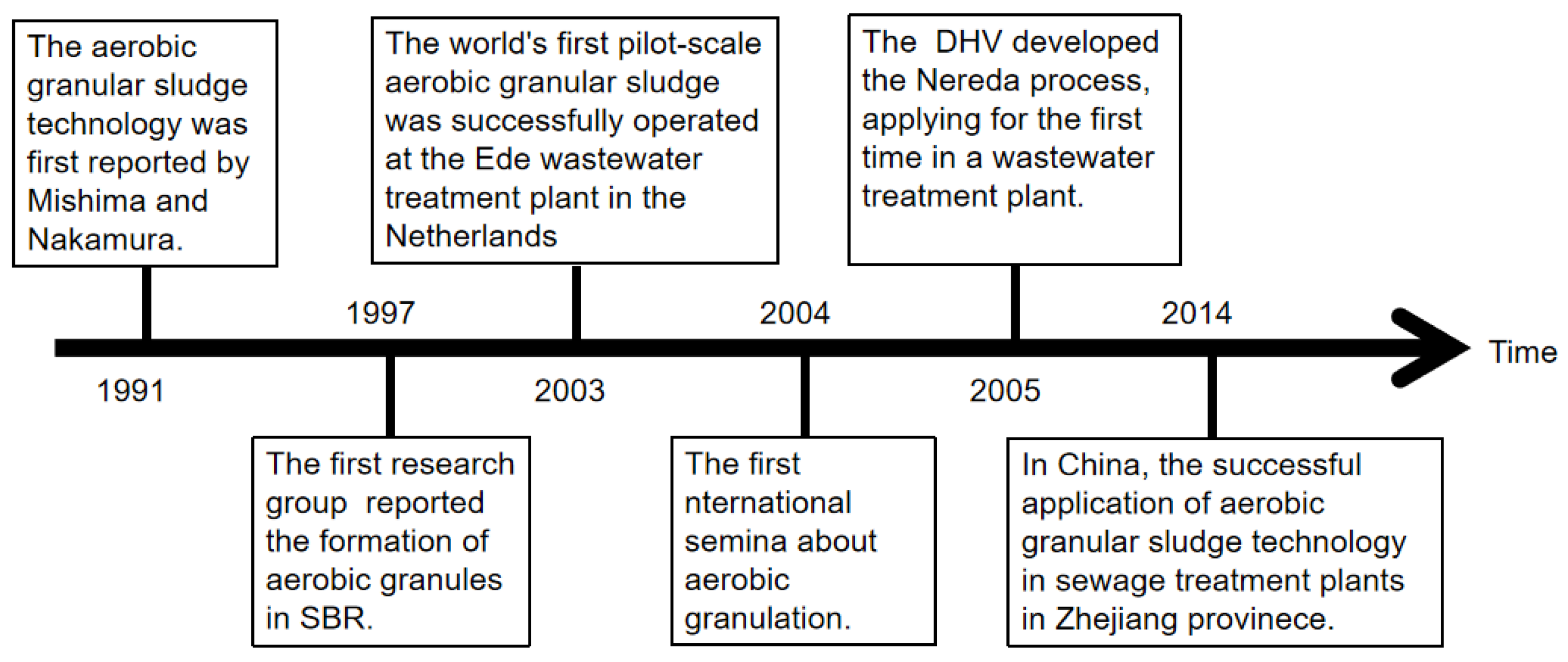
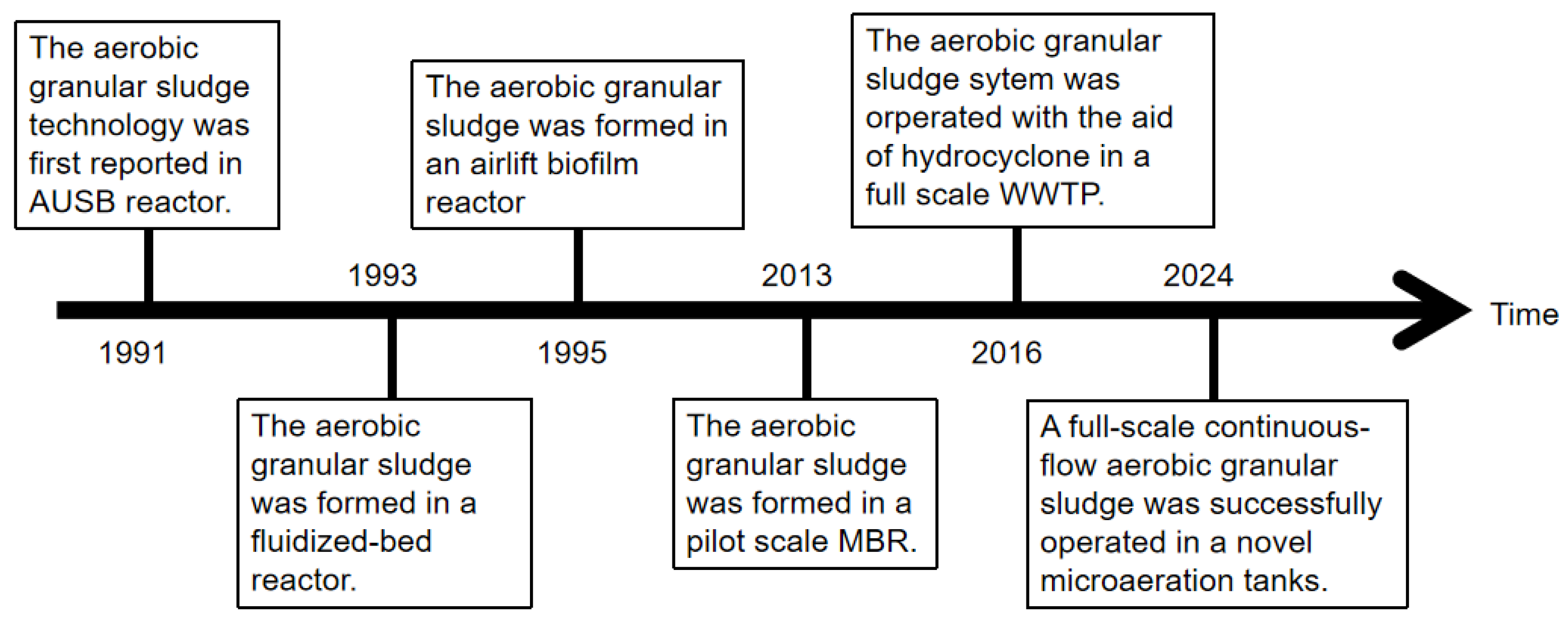
| Year | Researcher | The Proposed Mechanism | Reference |
|---|---|---|---|
| 1999 | Beun et al. | (1) Aerobic granulation started with fungi. (2) Fungi easily formed pellets. When the pellets grew up and lysed, (3) the pellets broke apart, and only the adequately dense colonies were able to settle successfully. (4) As time progressed, these colonies expanded and transformed into newly formed granules. | [8] |
| 2001 | Tay et al. | With the condition of sequential operation, aerobic granules originate from seed sludge, progress to compact aggregates, then develop into granular sludge, and ultimately evolve into mature granules. | [23] |
| 2002 | Liu and Tay | (1) Cells moving randomly and colliding; (2) cells moving randomly and colliding; (3) continued irreversible clustering and expansion within the matrix of extracellular polymeric substances (EPSs); and (4) the formation of shape and structure, influenced by shear forces. | [24] |
| 2004 | Liu et al. | Hydraulic pressure is a key factor in the formation of biogranules, whereas the hydrophobicity of cells markedly contributes to the development of granules. Furthermore, the formation of aerobic granules results from the combined efforts of different functional groups and their interactions with the ambient environment. | [25] |
| 2010 | Barr et al. | (1) A single microbial colony can gradually expand to form compact and smooth granules. (2) The aggregation of multiple independent microbial colonies can lead to the formation of relatively loose granules. | [26] |
| 2015 | Wu et al. | Under continuous flow conditions, the formation of aerobic granules is critically dependent on two key factors: a high organic loading rate and intense selection pressure. | [27] |
| 2022 | Edward et al. | (1) Selection of microorganisms, (2) targeted substrate utilization, (3) enhancing substrate transport into the biofilm, (4) specific feeding strategies, (5) substrates that either form or do not form particles, (6) breakdown of granules. | [28] |
| Reactor | Diameter (mm) | SVI (mL g−1) | MLSS (g L−1) | Specific Gravity | Settling Velocity (m h−1) | Substrate | Reference |
|---|---|---|---|---|---|---|---|
| SBR | 0.6–1.4 | 30–40 | 5 | 1.021 | 22–60 | Synthetic wastewater with sodium acetate as the main carbon source, COD: 500 mg L−1 | [41] |
| SBAR | 0.3–3 | 7–10 | - | - | Influence synthetic wastewater with an acetate concentration of 18.3 Cmmol L-1 | [42] | |
| SBR | 0.3–0.5 | 80–100 | - | - | - | Acetate | [9] |
| SBR | 2 | 172 | 2 | 1.0038 | - | Phenol | [43] |
| SBR | 2.8 | 73 | 7.9 | 1.0068 | - | Phenol with Ca2+ | [43] |
| CFR system with baffled bubble column | 0.2–2 | 33.5 | 2.8–5.8 | - | - | Synthetic wastewater with sodium acetate as the main carbon source, COD:1500 mg L−1 | [44] |
| CFR system with multiple serial chambers | 0.13 | 43 | 3.0 | - | - | Municipal (30%) and industrial (70%) wastewater | [45] |
| CFR system with MBR | 0.1–1.0 | 25–40 | 10 | - | 15–25 | Synthetic wastewater with glucose as the main carbon source, COD: 100–300 mg L−1 | [46] |
| Year | Reactor | Working Volume (m3) | Inoculation Sludge | Influence | Flow Pattern | MLSS (g/L) | Diameter of Granules (mm) | SVI (mL/g) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2003 | SBR | 1.5 | Sludge from municipal wastewater treatment plant | Sewage | Intermittent flow | 9–10 | >0.6 | 60 | [90] |
| 2010 | SBR | 0.03 | Sludge from municipal wastewater treatment plant, MLSS: 2 g L−1, SVI: 145 mL g−1 | 40% sewage + 60% industrial wastewater, COD: 360–1832 mg L−1, NH4+-N: 37.5–108.5 mg L−1 | Intermittent flow | 20 | 0.8 | 30 | [91,92] |
| 2010 | SBR | 0.226 | Sludge from municipal wastewater treatment plant, MLSS: 2.6 g L−1, SVI: 120–160 mL g−1 | Municipal wastewater, COD: 91.3–157.1 mg L−1, NH4+-N: 39.4–68.2 mg L−1 | Intermittent flow | 4.0 | 2.45 | 45–55 | [93] |
| 2010 | SBR | 6.0 | Activated sludge, MLSS: 3.0 g L−1 | Municipal wastewater, COD: 200–350 mg L−1, NH4+-N: 15–40 mg L−1 | Intermittent flow | 8.0 | 0.33 | 30 | [94] |
| 2010 | SBR | 1.0 | Sludge from municipal wastewater treatment plant, MLSS: 5.0–7.0 g L−1, SVI: 75 mL g−1 | Municipal wastewater, COD: 100–400 mg L−1, NH4+-N: 10–40 mg L−1 | Intermittent flow | 8.0 | 0.8 | 40 | [95] |
| 2011 | SBR | 0.032 | Sludge from municipal wastewater treatment plant, MLSS: 2.6 g L−1, SVI: 180 mL g−1 | 40% sewage + 60% industrial wastewater, COD: 250–1800 mg L−1, NH4+-N: 39–93 mg L−1 | Intermittent flow | 7.0–9.0 | 1.976 | 25–85 | [96] |
| 2011 | SBR | 0.1 | Sludge from municipal wastewater treatment plant, MLSS: 3.7g L−1, SVI: 190 mL g−1 | The synthetic wastewater (acetate) | Intermittent flow | 3.5 | 3.5 | - | [97] |
| 2011 | SBR | 5.95 | Sludge from municipal wastewater treatment plant, MLSS of 2.7 g L−1 | Sewage with industrial wastewater, COD: 271–1839 mg L−1, NH4+-N: 16.98–214 mg L−1 | Intermittent flow | 2.236 | - | 65.02 | [98] |
| 2012 | SBR | 1.47 | Sludge from soy protein wastewater treatment plant with SVI of 125.6 mL g−1 | Soy protein wastewater anaerobic digest effluent with COD of 800–1800 mg L−1, NH4+-N of 80–160 mg L−1 | Intermittent flow | - | 0.5–1.0 | - | [99] |
| 2012 | SBR | 0.085 | Anaerobic digest sludge, MLSS of 20 g L−1 | Sewage with COD of 200–320 mg L−1, TN of 38–55 mg L−1 | Intermittent flow | 5.9 | 0.75 | 20–35 | [100] |
| 2012 | SBR | 0.1 | - | The synthetic wastewater (Sodium acetate) with COD of 400 mg L−1, NH4+-N of 40 mg L−1 | Intermittent flow | 12±4 | 2.4 | 13±6 | [101] |
| 2013 | SBR | 1.47 | Activated sludge with MLSS of 2.8 g L−1, SVI of 105.51 mL g−1 | Soy protein wastewater anaerobic digest effluent with COD of 700–2400 mg L−1, NH4+-N of 200 mg L−1 | Intermittent flow | 7.02 | 1.2–2.0 | 42.99 | [102] |
| 2013 | MBR | 0.06 | Sludge from pharmaceutical wastewater treatment plant | Berberine wastewater with COD of 1717–4393 mg L−1, NH4+-N of 91.8–158.7 mg L−1 | Continuous flow | 7.0 | 0.1–1.0 | 90 | [103] |
| 2013 | SBR | 0.1 | Sludge from municipal wastewater treatment plant | Swine wastewater | Intermittent flow | 11–13 | 2.0–2.8 | - | [104] |
| 2014 | SBR | 3.5 | Sludge from municipal wastewater treatment plant with MLSS of 4.581 g L−1 | Municipal wastewater, COD of 100–450 mg L−1, NH4+-N of 20–30 mg L−1 | Intermittent flow | 1.2 | 1.0 | - | [105] |
| 2014 | SBR | 0.105 | Sludge cultivated in lab with MLSS of 3.0 g L−1 | The synthetic wastewater (Sodium acetate) with COD of 8000 mg L−1 | Intermittent flow | 5.0 | 1.58 | 80 | [106] |
| 2014 | SBR | 20 | Activated sludge with MLSS of 3.8 g/L, SVI of 78 mL g−1 | 30% sewage + 70% industrial wastewater with COD of 500–1000 mg L−1, NH4+-N of 30–80 mg L−1 | Intermittent flow | 8.55 | 0.3 | 38 | [107] |
| 2015 | SBR | 4 | Sludge from enhanced biological phosphorus removal treatment | Sewage with Sodium acetate | intermittent flow | 12 | 1.1 | - | [108] |
| 2016 | SBR | 1.5 | Activated sludge | Sewage | intermittent flow | 9–10 | >0.6 | 60 | [109] |
| 2016 | MBR | 14 | Sludge from municipal wastewater treatment plant with SVI of 210 mL g−1 | Municipal wastewater, COD of 300 ± 25 mg L−1, TN of 30 ± 5 mg L−1 | intermittent flow | 7 | 0.2 | 30 | [110] |
| 2017 | SBR | 0.098 | Sludge from municipal wastewater treatment plant with VSS of 3.2 g/L, SVI of 220.2 mL g−1 | Sewage with COD of 150–450 mg L−1, NH4+-N of 36–68 mg L−1 | intermittent flow | - | 0.29 | 67 | [111] |
| 2017 | SBR | 0.16 | Sludge from municipal wastewater treatment plant with MLSS of 6.5 g L−1 | Municipal wastewater, COD of 300 mg L−1, NH4+-N of 43–52 mg L−1 | intermittent flow | 12.19 | 1.269 | 21.31 | [112] |
| 2019 | CSTR | 0.128 | Activated sludge with 4.284 g L−1 | Sewage, COD of 200–400 mg L−1, NH4+-N 0f 10–35 mg L−1, TN of 30–55 mg L−1 and TP of 1–5 mg L−1 | Continuous flow | 4.1–5.8 | 3.4 | 64 | [113] |
| 2022 | RFBR | Activated sludge | - | Continuous flow | 1.3 | - | 43 | [18] | |
| 2023 | SFD-SBR | Sidestream reactor: 1.4 m3, mainstream reactor: 14 m3 | Sludge from municipal wastewater treatment plant | The municipal wastewater, TOC of 48–59 mg L−1 | Continuous flow | 3 | - | 80 | [114] |
| 2023 | CFR | 0.2 | Sludge from municipal wastewater treatment plant with MLSS of 2.5 g L−1 | The municipal wastewater, COD of 161–1145 mg L−1; TN of 14–103 mg L−1 and TP of 2.5–19 mg L−1 | Continuous flow | 6.1 | 0.5–1.0 | 40 | [115] |
| Year | Location | Name | Granulation Strategy | Reactor Type | Wastewater Treatment Plant Capacity (m3/d) | Inoculation Sludge | Influence | MLSS (g/L) | Diameter of Granules (mm) | SVI (mL/g) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | South Africa | Gansbaai WWTP | SBR (Nereda) | Column | 4000 | Activated Sludge | Sewage, COD: 1265 mg L−1, NH4+-N: 175 mg L−1, TP: 19 mg L−1 | - | - | - | [117] |
| 2010 | Zhejiang Province | Yancang WWTP | SBR | Column | 50,000 | Activated Sludge | 70% industrial wastewater + 30%municipal wastewater, COD: 200–700 mg L−1, NH4+-N: 28–40 mg L−1, TP: 2–4 mg L−1 | - | 0.5 | 47 | [13] |
| 2013 | The Netherlands | Garmerwolde | SBR (Nereda) | Column | 13,000 | Activated Sludge | Sewage, COD: 146–715 mg L−1, NH4+-N: 13.4–56.5 mg L−1, TN: 14–81 mg L−1, TP: 1.9–9.7 mg L−1 | 8.5 | 1 | 35 | [117] |
| 2014 | Portugal | Frielas WWTP | SBR (Nereda) | Column | 70,000 | Activated Sludge | Sewage | 6–8 | - | 40 | [117] |
| 2022 | The James R. Dilorio Water Recla mation Facility | Colorado, USA | Hydrocyclone-based wasting helped improve settling characteristics | Several tanks | 60,000 | Sludge from municipal wastewater treatment plant | Sewage | 2.2 | >0.2 | 83 | [118] |
| 2024 | WWTP in | Hebei province, China | A novel microaerobic–aerobic configuration with internal separators | Several tanks | 25,000 | Sludge form Municipal wastewater treatment plant | 30% sewage + 70% industrial wastewater, COD: 200–700 mg L−1, NH4+-N: 28–40 mg L−1 | 20 | 0.138, granules larger than 200 μm constituting 28.9% | 51.4 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, P.; Liu, Y.-Q.; Li, J.; Liao, M. The Aerobic Granules Process for Wastewater Treatment: From Theory to Engineering. Processes 2024, 12, 707. https://doi.org/10.3390/pr12040707
Zeng P, Liu Y-Q, Li J, Liao M. The Aerobic Granules Process for Wastewater Treatment: From Theory to Engineering. Processes. 2024; 12(4):707. https://doi.org/10.3390/pr12040707
Chicago/Turabian StyleZeng, Ping, Yong-Qiang Liu, Juan Li, and Miao Liao. 2024. "The Aerobic Granules Process for Wastewater Treatment: From Theory to Engineering" Processes 12, no. 4: 707. https://doi.org/10.3390/pr12040707
APA StyleZeng, P., Liu, Y.-Q., Li, J., & Liao, M. (2024). The Aerobic Granules Process for Wastewater Treatment: From Theory to Engineering. Processes, 12(4), 707. https://doi.org/10.3390/pr12040707







