Solid-State Fermentation of Hyperactive Pectinase by the Novel Strain Aspergillus sp. CM96
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Chemicals
2.2. Optimization of Fermentation Substrate Composition and Condition
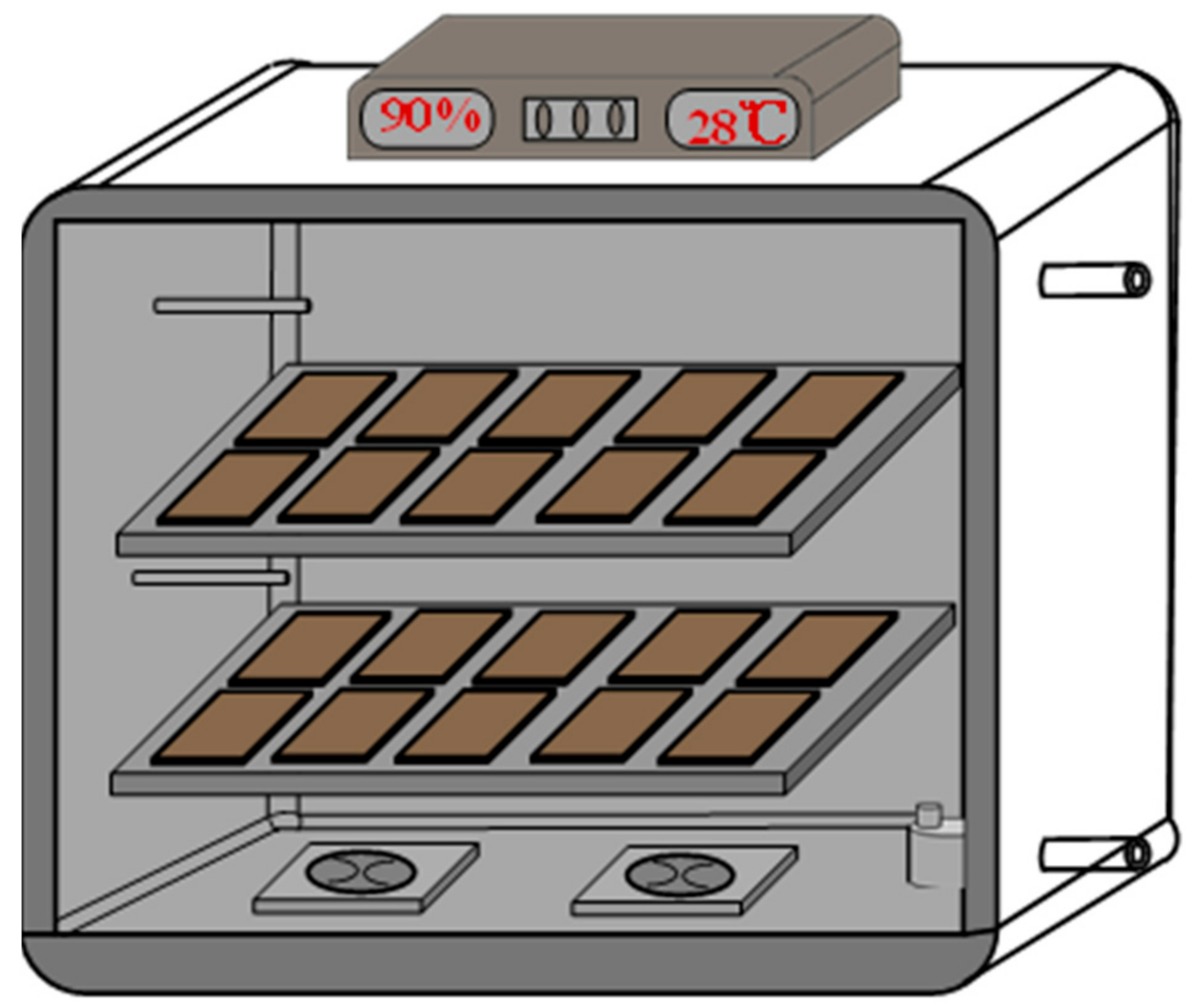
2.3. Tray Bioreactor Design and Configuration
2.4. Enzyme Fermentation and Purification
2.5. Analytical Methods
3. Results and Discussion
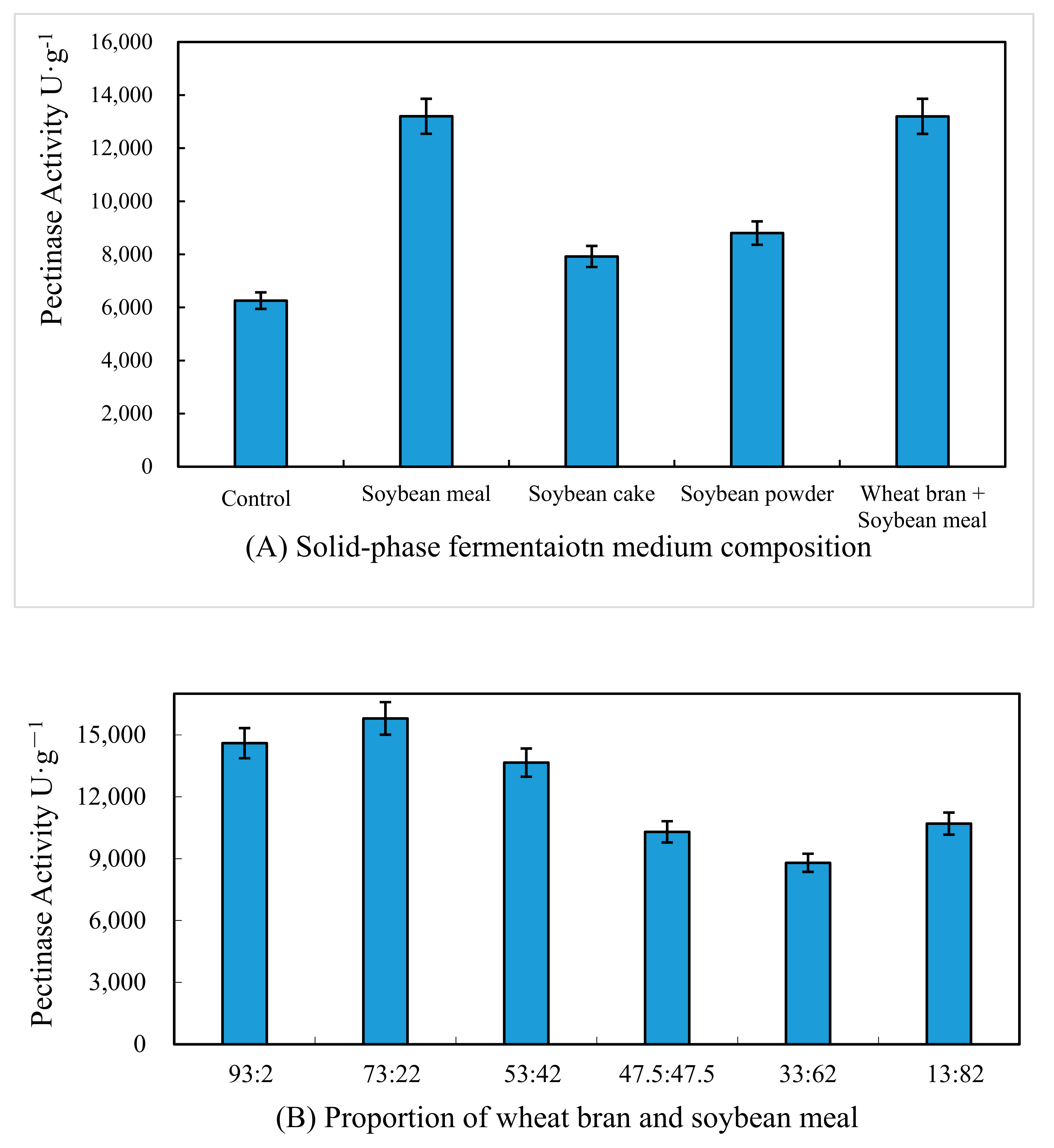
3.1. Effect of Medium Composition on Pectinase Activity
3.2. Effect of Water Content and Inoculant on Pectinase Activity
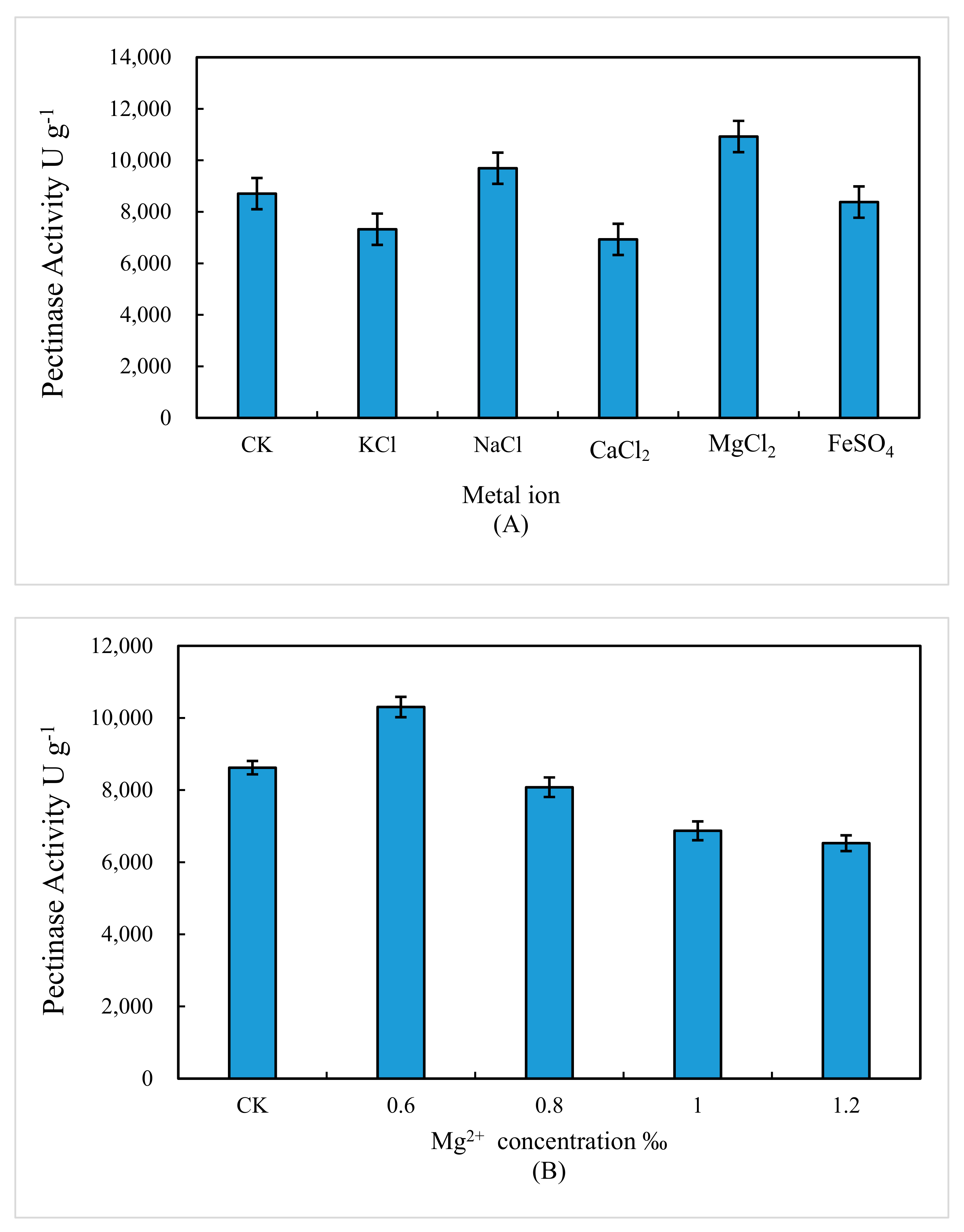
3.3. Effect of Additional Metal Ion on Pectinase Activity
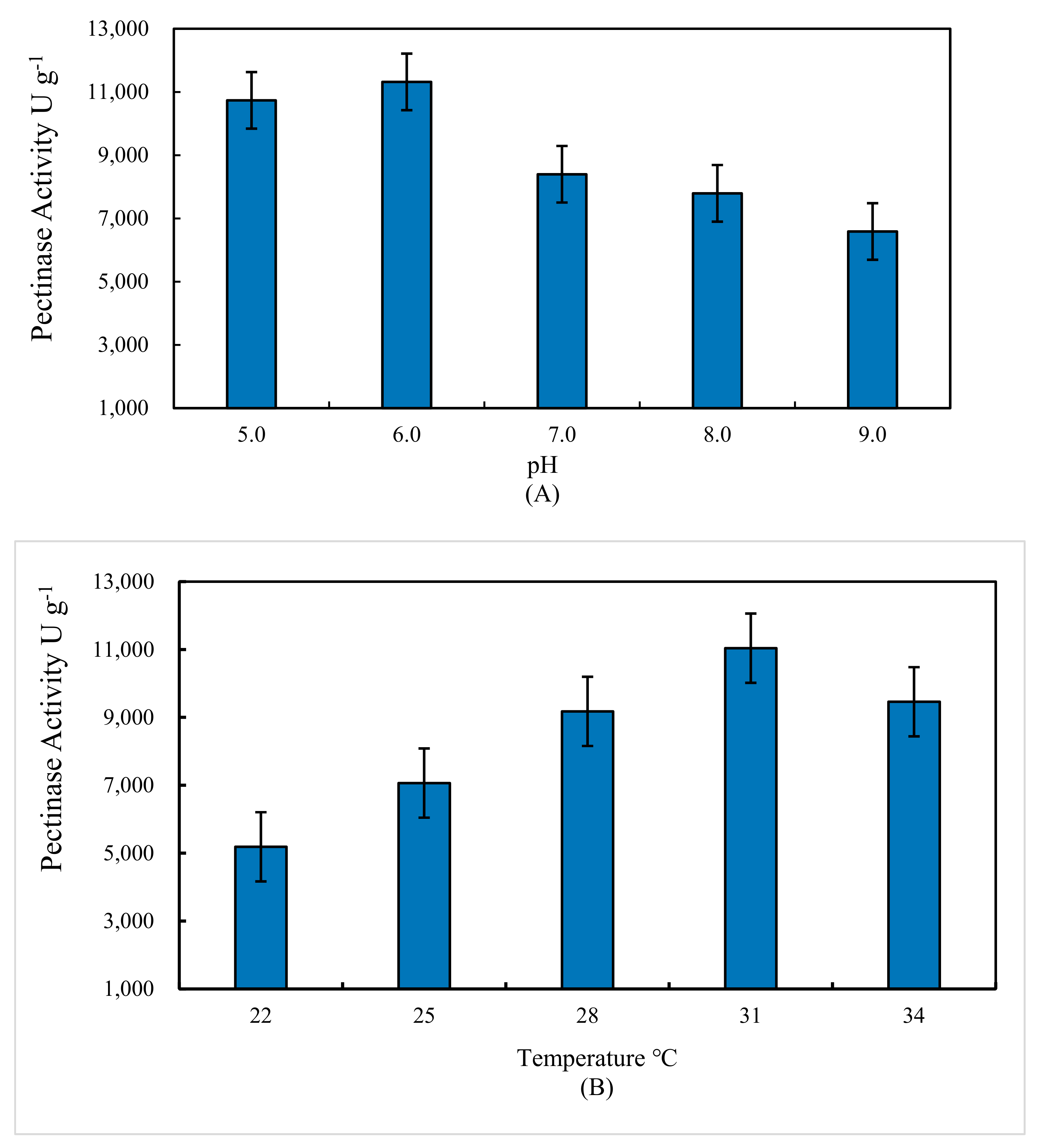
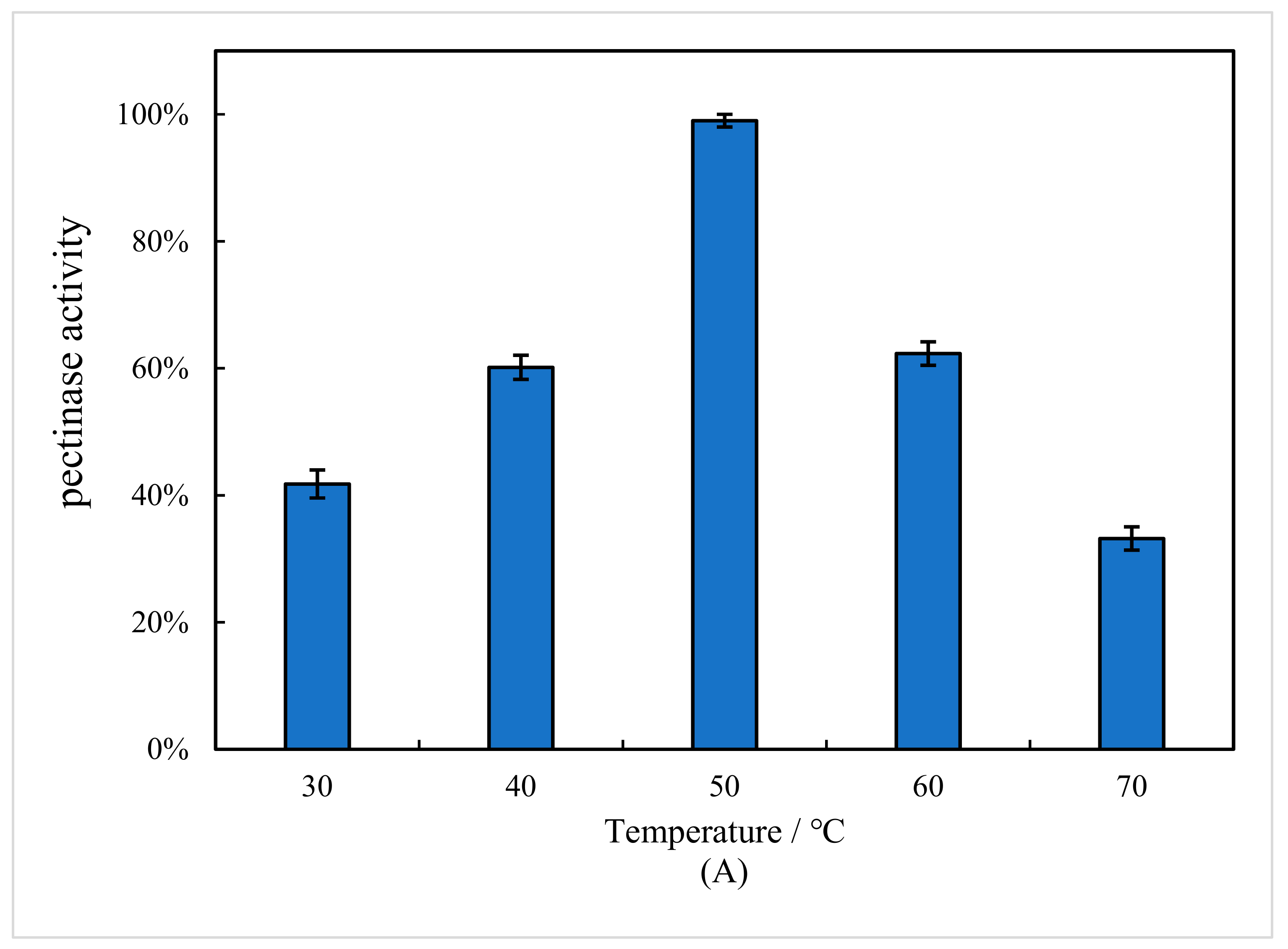
3.4. Effect of Temperature and Initial pH Value on Pectinase Activity
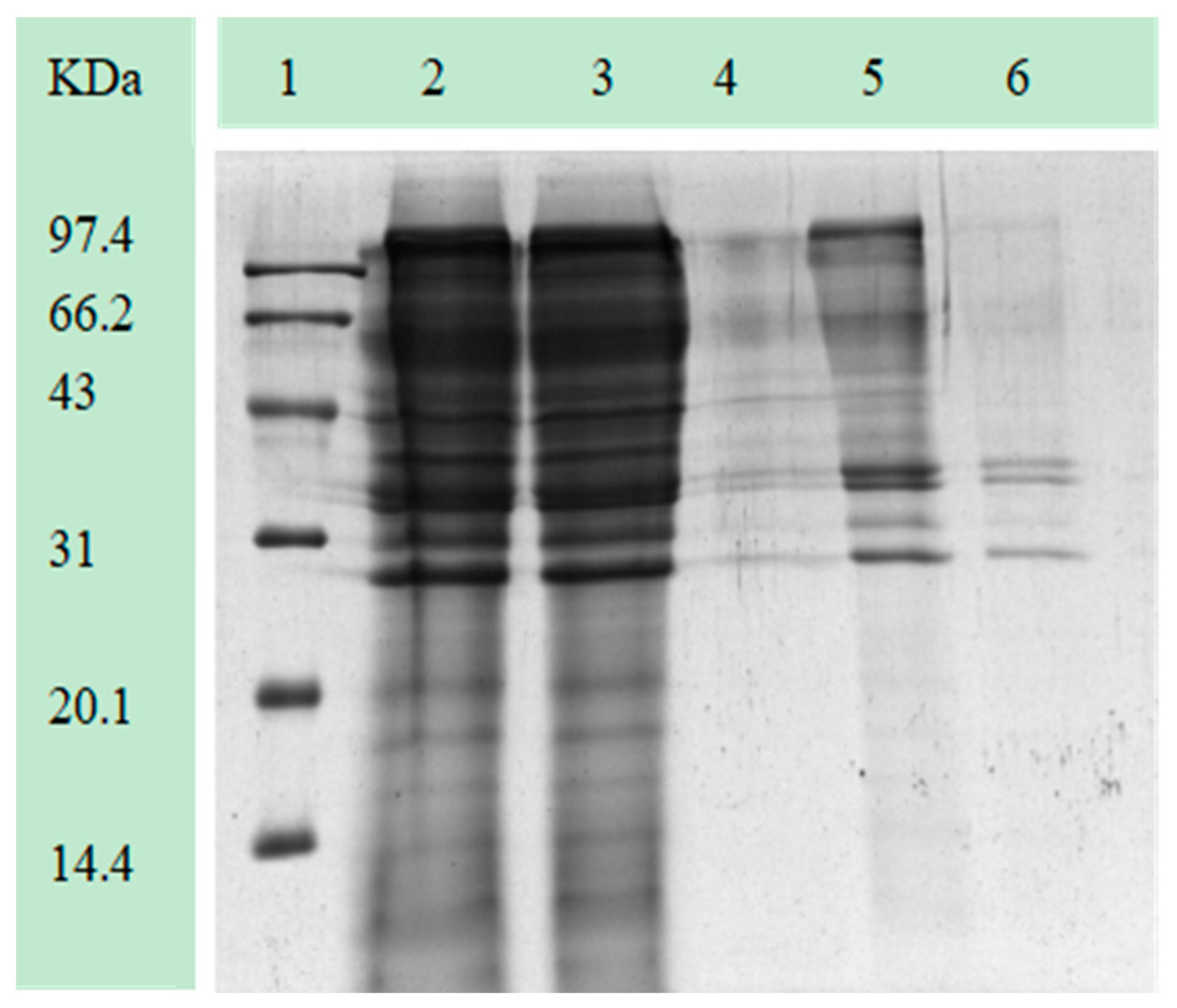
3.5. Pectinase Fermentation and Purification
3.6. Characteristics of Purified Pectinase
3.7. Characterization of Enzyme Cocktail and Its Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, J.; Kaimal, K.K.S.; Smith, M.L.; Rahman, P.K.S.M.; Chellam, P.V. Advances in Upstream and Downstream Strategies of Pectinase Bioprocessing: A Review. Int. J. Biol. Macromol. 2020, 162, 1086–1099. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Shrestha, S.; Rahman, M.S.; Qin, W. New Insights in Pectinase Production Development and Industrial Applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef]
- Abdollahzadeh, R.; Pazhang, M.; Najavand, S.; Fallahzadeh-Mamaghani, V.; Amani-Ghadim, A.R. Screening of Pectinase-Producing Bacteria from Farmlands and Optimization of Enzyme Production from Selected Strain by RSM. Folia Microbiol. 2020, 65, 705–719. [Google Scholar] [CrossRef]
- Kc, S.; Upadhyaya, J.; Joshi, D.R.; Lekhak, B.; Kumar Chaudhary, D.; Raj Pant, B.; Raj Bajgai, T.; Dhital, R.; Khanal, S.; Koirala, N.; et al. Production, Characterization, and Industrial Application of Pectinase Enzyme Isolated from Fungal Strains. Fermentation 2020, 6, 59. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Mousavi, S.M.E.; Kennedy, J.F.; Azimi, S.Z. A Health-Friendly Strategy for Covalent-Bonded Immobilization of Pectinase on the Functionalized Glass Beads. Food Bioprocess Technol. 2021, 14, 177–186. [Google Scholar] [CrossRef]
- Heerd, D.; Tari, C.; Fernández-Lahore, M. Microbial Strain Improvement for Enhanced Polygalacturonase Production by Aspergillus sojae. Appl. Microbiol. Biotechnol. 2014, 98, 7471–7481. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, M.P.M.; Mu, T.; Ma, M. Structural, Physicochemical and Emulsifying Properties of Sweet Potato Pectin Treated by High Hydrostatic Pressure and/or Pectinase: A Comparative Study. J. Sci. Food Agric. 2020, 100, 4911–4920. Available online: https://pubmed.ncbi.nlm.nih.gov/32483850/ (accessed on 20 February 2024). [CrossRef] [PubMed]
- Alazi, E.; Niu, J.; Otto, S.B.; Arentshorst, M.; Pham, T.T.M.; Tsang, A.; Ram, A.F.J. W361R Mutation in GaaR, the Regulator of D-Galacturonic Acid-Responsive Genes, Leads to Constitutive Production of Pectinases in Aspergillus Niger. Microbiologyopen 2019, 8, e00732. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent Advances in the Production Strategies of Microbial Pectinases—A Review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Do, S.; Phungviwatnikul, T.; de Godoy, M.R.C.; Swanson, K.S. Nutrient Digestibility and Fecal Characteristics, Microbiota, and Metabolites in Dogs Fed Human-Grade Foods. J. Anim. Sci. 2021, 99, skab028. [Google Scholar] [CrossRef] [PubMed]
- Duijsens, D.; Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How Postharvest Variables in the Pulse Value Chain Affect Nutrient Digestibility and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5067–5096. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, G. Enzyme Cocktail with Hyperactive Lipase through Solid-State Fermentation by the Novel Strain Penicillium sp. Y-21. Sci. Rep. 2023, 13, 14527. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Patterson, R.; Rogiewicz, A.; Woyengo, T.A. Nutrient Digestibility of Multi-Enzyme Supplemented Low-Energy and AA Diets for Grower Pigs1. J. Anim. Sci. 2019, 97, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pitarch, A.; Hermans, D.; Manzanilla, E.G.; Bindelle, J.; Everaert, N.; Beckers, Y.; Torrallardona, D.; Bruggeman, G.; Gardiner, G.E.; Lawlor, P.G. Effect of Feed Enzymes on Digestibility and Growth in Weaned Pigs: A Systematic Review and Meta-Analysis. Anim. Feed Sci. Technol. 2017, 233, 145–159. [Google Scholar] [CrossRef]
- Luo, W.; Xu, K.P.; Wang, Y.; Cai, Z.Q. Screening, fermentation optimization and enzy-matic properties of pectinase-producing strains. Food Sci. Technol. 2022, 47, 6–13. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ra, C.H. Comparison of Liquid and Solid-State Fermentation Processes for the Production of Enzymes and Beta-Glucan from Hulled Barley. J. Microbiol. Biotechnol. 2022, 32, 317–323. [Google Scholar] [CrossRef]
- Hong, T.; Liu, X.; Ji, Y.; Tan, S.; Cai, Z. Construction of Chiral Capillary Electrochromatography Microsystems Based on Aspergillus sp. CM96. Mikrochim. Acta 2023, 190, 357. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Najafpour, G.D.; Mohammadi, M. Bioconversion of Agroindustrial Wastes to Pectinases Enzyme via Solid State Fermentation in Trays and Rotating Drum Bioreactors. Biocatal. Agric. Biotechnol. 2019, 21, 101280. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Liu, X.; Zhang, P.; Yue, W.; Cai, Z. High Molecular Weight α-Galactosidase from the Novel Strain Aspergillus sp. D-23 and Its Hydrolysis Performance. Processes 2023, 11, 255. [Google Scholar] [CrossRef]
- Bora, L. Purification and Characterization of Highly Alkaline Lipase from Bacillus licheniformis MTCC 2465: And Study of Its Detergent Compatibility and Applicability. J. Surfact. Deterg. 2014, 17, 889–898. [Google Scholar] [CrossRef]
- Shi, H.; Meng, Y.; Yang, M.; Zhang, Q.; Meng, Y. Purification and Characterization of a Hydrolysis-Resistant Lipase from Aspergillus terreus. Biotechnol. Appl. Biochem. 2014, 61, 165–174. [Google Scholar] [CrossRef]
- Wu, J.W.; Hao, L.; Guo, J.H.; Li, Q.Q.; Zhao, L.L. Optimization of fermentation conditions for pectinase production by Aspergillus niger SH312-26-19. China Brew. 2021, 40, 118–122. [Google Scholar] [CrossRef]
- Pokorny, D.; Cimerman, A.; Steiner, W. Aspergillus Niger Lipases: Induction, Isolation and Characterization of Two Lipases from a MZKI A116 Strain. J. Mol. Catal. B Enzym. 1997, 2, 215–222. [Google Scholar] [CrossRef]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Yu, W.; Li, Q. The Preparation and Potential Bioactivities of Modified Pectins: A Review. Foods 2023, 12, 1016. [Google Scholar] [CrossRef]
- Du, C.; Tan, S.; Liu, L.; Zhou, Y.; Wu, P.; Zhang, G. Improving the Specific Activity and Stability of Alkaline Pectinase PEL3 through SpyTag/SpyCatcher Cyclization. Biotechnol. Lett. 2023, 45, 847–859. [Google Scholar] [CrossRef]
- Takeyama, M.M.; de Carvalho, M.C.; Carvalho, H.S.; Silva, C.R.; Uetanabaro, A.P.T.; da Costa, A.M.; Evaristo, J.A.M.; Nogueira, F.C.S.; Fai, A.E.C.; Koblitz, M.G.B. Pectinases Secretion by Saccharomyces cerevisiae: Optimization in Solid-State Fermentation and Identification by a Shotgun Proteomics Approach. Molecules 2022, 27, 4981. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ingale, S.L.; Hosseindoust, A.R.; Lee, S.H.; Lee, J.H.; Chae, B.J. Effects of Mannan Level and β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility and Blood Metabolites of Growing Pigs. Animal 2017, 11, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pitarch, A.; Manzanilla, E.G.; Gardiner, G.E.; O’Doherty, J.V.; Lawlor, P.G. Systematic Review and Meta-Analysis of the Effect of Feed Enzymes on Growth and Nutrient Digestibility in Grow-Finisher Pigs: Effect of Enzyme Type and Cereal Source. Anim. Feed Sci. Technol. 2019, 251, 153–165. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Fan, Y.F.; Cao, Y.H.; Guo, P.P.; Dong, B.; Ma, Y.X. Effects of Exogenous Phytase and Xylanase, Individually or in Combination, and Pelleting on Nutrient Digestibility, Available Energy Content of Wheat and Performance of Growing Pigs Fed Wheat-Based Diets. Asian-Australas. J. Anim. Sci. 2017, 30, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Yadav, K.; Gupta, S.; Tanveer, A.; Yadav, S.; Yadav, D. Fungal Pectinases: An Insight into Production, Innovations and Applications. World J. Microbiol. Biotechnol. 2023, 39, 305. [Google Scholar] [CrossRef] [PubMed]


| Kind of Enzyme | Activity (U·g−1) |
|---|---|
| Glucanase | 7000 ± 400 |
| Protease | 8000 ± 600 |
| Pectinase | 15,000 ± 700 |
| Cellulose | 3000 ± 200 |
| Items | Control | Treatment | t | p |
|---|---|---|---|---|
| Initial average weight (kg) | 5.74 ± 0.02 | 5.87 ± 0.01 | −60.360 | <0.01 |
| Final average weight (kg) | 16.15 ± 0.56 | 18.04 ± 0.39 | −58.063 | <0.01 |
| ADG (g/d) | 252.20 ± 1.10 | 391.61 ± 0.06 | −15 | <0.01 |
| ADFI (g/d) | 522.13 ± 0.01 | 502.31 ± 1.65 | 143.051 | <0.01 |
| FCR | 1.43 ± 0.00 | 1.36 ± 0.01 | 158.995 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wan, M.; Liu, Y.; Yang, G.; Cai, Z. Solid-State Fermentation of Hyperactive Pectinase by the Novel Strain Aspergillus sp. CM96. Processes 2024, 12, 615. https://doi.org/10.3390/pr12030615
Chen H, Wan M, Liu Y, Yang G, Cai Z. Solid-State Fermentation of Hyperactive Pectinase by the Novel Strain Aspergillus sp. CM96. Processes. 2024; 12(3):615. https://doi.org/10.3390/pr12030615
Chicago/Turabian StyleChen, Huiling, Meimei Wan, Yang Liu, Guanghua Yang, and Zhiqiang Cai. 2024. "Solid-State Fermentation of Hyperactive Pectinase by the Novel Strain Aspergillus sp. CM96" Processes 12, no. 3: 615. https://doi.org/10.3390/pr12030615
APA StyleChen, H., Wan, M., Liu, Y., Yang, G., & Cai, Z. (2024). Solid-State Fermentation of Hyperactive Pectinase by the Novel Strain Aspergillus sp. CM96. Processes, 12(3), 615. https://doi.org/10.3390/pr12030615






