Abstract
As a clean alternative fuel oil for marine engines, methanol has received increasing attention, but its low cetane number requires diesel ignition, which increases the difficulty of retrofitting existing engine fuel injection systems. Polymethoxy dimethyl ether (PODEn) is an ether fuel mixture whose chemical structural formula can be expressed as CH3O(CH2O)nCH3 (). PODE3 is the predominant component in the blend, and its properties are representative of the blend. PODE is a low-carbon fuel with a high cetane number and is easy to compression ignite, and, as such, can be used to ignite methanol in a marine diesel engine. This article explores the combustion mechanism of mixed methanol–PODE fuel using the characteristics of PODE that can be easily mixed with methanol for combustion. Taking methanol and PODE3 as representative fuels, the detailed combustion mechanism of PODE3 and the detailed combustion mechanism of methanol are simplified using a DRGEPSA (direct relationship graph with error propagation (DRGEP) and sensitivity analysis (SA)) method. Based on the target engine cylinder combustion environment, a simplified mechanism for mixed methanol–PODE fuel is obtained, and the new mechanism is validated in terms of the ignition delay period and laminar flame speed. The results indicate that the newly constructed simplified mechanism is basically consistent with the ignition delay data and flame propagation speed data measured by a rapid compression machine (RCM), laying the foundation for the application of alternative methanol fuels in marine engines.
1. Introduction
According to industrial estimates, there are 79,753 ships in total globally, of which 4338 are in China [1]. More than 90% of these ships are powered by diesel engines, mainly due to the high thermal efficiency of diesel engines. However, a serious problem with diesel engines is that they produce harmful emissions such as nitrogen oxides, particulate matter, and carbon dioxide. The International Maritime Organization (IMO) has latterly issued a series of regulations to limit the harmful emissions from ships [2]. Clean alternative fuels are currently the main way for ships to deal with these harmful emissions. Low- and zero-carbon fuels are needed to achieve carbon reduction in marine vessels [3]. Methanol has a high oxygen content of 50% and a molecular formula without carbon–carbon bonds. It produces almost no carbon smoke during combustion, which is more conducive to achieving clean and efficient combustion in engine cylinders. It is one of the green fuels recommended by the IMO. Maersk, COSCO, and other leading global shipping companies plan to use methanol as an important alternative fuel for marine engines in future ships [4,5]. However, methanol has a low cetane number and requires diesel oil to ignite it before combustion [6]. There are two ways to ignite methanol. One way is to install a methanol injection nozzle for each cylinder, which will increase the complexity of the diesel engine fuel injection system and make it difficult to retrofit existing engines [7]. The other way is to mix diesel and methanol together and inject the mixed fuel into the cylinder with a small amount of diesel oil to ignite the methanol. However, it is usually difficult to mix diesel and methanol together thoroughly, which makes combustion in the cylinder very unstable. Jamrozik et al. [8] utilized different blending ratios of diesel–methanol blends in a single-cylinder diesel engine, and found that the usable proportion of methanol in the blend was not high, as the in-cylinder pressure dropped considerably after the proportion of methanol rose to more than 30%, and the engine worked abnormally.
PODE is a low molecular weight condensation polymer that consists mainly of oxided methylene chains based on dimethoxy alkyl, and which has a high cetane number (CN) and high oxygen content and is stably miscible with methanol at room temperature. When PODE is mixed with methanol, it can solve the problems of timing ignition and stable combustion of methanol. This kind of mixed fuel, composed of methanol mixed with a certain proportion of PODE, combines the advantages of high oxygen content and high octane number from methanol with the high cetane number of PODE, and it can be directly applied to marine diesel engines without changing the engine structure. However, to date, there has been only limited research on this kind of mixed fuel, and there is no corresponding chemical kinetic combustion mechanism for combustion simulation and experimental research of this mixed fuel oil in marine diesel engines. To rectify this, experimental studies were carried out in our laboratory on mixed methanol–PODE fuels in a marine diesel engine. It was found that the diesel engine could achieve comparable power with mixed methanol–PODE fuels under low and medium loads, and harmful emissions such as nitrogen oxides, sulfur oxides, and particulate matter were significantly reduced.
It is necessary to determine suitable methanol–PODE blending ratios in order to solve the hazardous emission problem under engine high load. This requires exploration of the implementation path and combustion mechanism of mixed-fuel chemical kinetics in order to establish a control strategy for the proper combustion of mixed fuels. As the detailed mechanism is quite large and the computation is extremely large, it is necessary to construct an accurate and reliable simplified combustion mechanism for the mixed methanol–PODE fuel chemical kinetics.
Previous studies have examined PODE chemical kinetic combustion mechanisms. Sun et al. [9] first constructed a high-temperature combustion mechanism for PODE. However, due to the lack of a low-temperature mechanism, it remained impossible to accurately predict the impact of ignition delay on spray organization and the combustion process. He et al. [10] constructed a detailed mechanism of PODE (225 components, 1082 steps of reactions) under low and high temperatures based on Sun’s high-temperature model for PODE, which was also validated. However, the PODE chemical combustion mechanism is relatively large, and a mixed-fuel combustion mechanism requires reasonable simplification. This paper first simplifies the PODE mechanism of He et al. [10] by eliminating unimportant components and reactions using the DRG and DRGEP methods. Then, the remaining components and reactions are further simplified with the SA method. Finally, the RPA method is used to further simplify the PODE mechanism.
The application of blended methanol–PODE fuel in marine diesel engines requires the development and production of a safe and reliable blended fuel supply system, and the key point for the development of this fuel supply system lies in the need to master the coordinated combustion control strategy of different components of the blended fuel. CFD simulation is applied to explore the combustion law of blended methanol–PODE fuels, which provides a theoretical basis for the experimental study of combustion control strategy.
Based on this background, the main objective of this paper was to develop a simplified chemical kinetic mechanism for the combustion of blended methanol–PODE fuels suitable for CFD simulations. Therefore, in this paper, methanol and PODE3 were used as the characterizing fuels, and the detailed mechanisms of methanol and PODE were simplified and optimized using ANSYS CHEMKIN software and employing the direct relation graph (DRG) method, the direct relation graph with error propagation (DRGEP) method, the sensitivity analysis (SA) method, and the reaction path analysis (RPA) method, respectively. Finally, a simplified chemical kinetic combustion mechanism of blended methanol–PODE fuel with 137 components and 635 basic reactions was obtained. The new chemical combustion mechanism of blended methanol–PODE fuel was validated in terms of ignition delay period, laminar flame velocity, and component concentration.
2. A New Simplified Combustion Mechanism for Methanol
2.1. Simplification of Detailed Combustion Mechanism for Methanol
Based on the detailed chemical kinetics mechanism of LLNL3.1 [11] for methanol, which includes 654 components and 2827 reactions, a simplified combustion mechanism for methanol was constructed under both low and high temperatures with DRGEPSA. To construct the simplified chemical kinetics mechanism for methanol, unimportant components and reactions were firstly removed based on the DRG and DRGEP methods. Then important components and reactions were classified and the simplified methanol mechanism was further optimized. Figure 1 shows a diagram of the important oxidation reaction pathways of methanol.
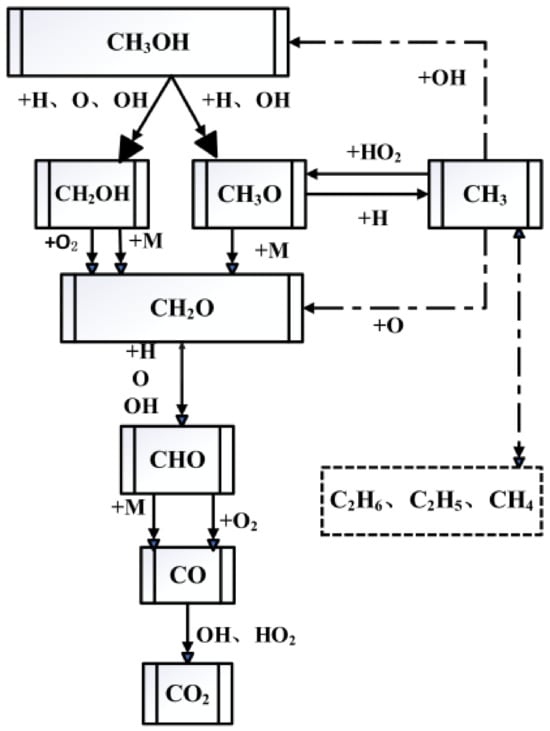
Figure 1.
Main oxidation reaction pathways of methanol.
The 4135ACa diesel engine is an inline four-cylinder, naturally aspirated, water-cooled, four-stroke marine diesel engine. There are four holes, 0.37 mm in diameter, in each injector. In our simulation, the fuel is injected at 22–24 °CA before the piston reaches TDC with 24 MPa injection pressure and 15 s injection duration.
Since normal fuel ignition in diesel engine occurs before the piston reaches Top Dead Center (TDC) in the combustion chamber, the optimized mechanism should be set based on the conditions of combustion temperature, pressure, and reactant concentration inside the chamber when the fuel is about to ignite before TDC of the diesel engine. According to the existing one-dimensional working process and three-dimensional CFD simulation results of the 4135 diesel engine, 112 initial operating conditions are set, the initial pressure (Pin) range is set to 1–4 MPa, the initial temperature range (Tin) is set to 800–2000 K, and the equivalence air/fuel ratio range is set to 0.5–2. During the iterative simplification process, the ignition delay is designated as the target parameter of methanol. Based on the simplification method mentioned above, a simplified chemical kinetics combustion mechanism of methanol is obtained, which consists of 42 components and 233 elementary reactions. Figure 2 shows a comparison between the simplified and detailed combustion mechanisms for methanol. From Figure 2, it can be seen that the simplified combustion mechanism for methanol still exhibits high prediction accuracy within a certain error range.
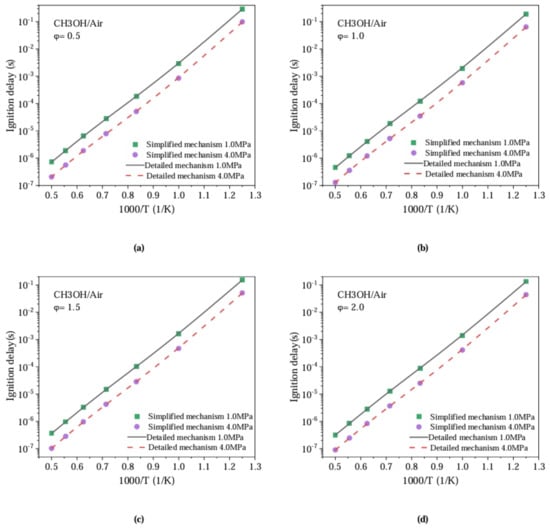
Figure 2.
Comparison of Ignition Delay between Detailed and Simplified Combustion Mechanisms for Methanol.
2.2. Optimization of Simplified Methanol Combustion Mechanism
Figure 2 shows the preliminary verification results of the detailed and simplified combustion mechanisms of methanol. There is a certain error in the predicted ignition delay of methanol between the simplified mechanism and the detailed mechanism under high temperature. Since methanol is ignited by PODE fuel in the engine cylinder, the temperature in the cylinder during methanol combustion is usually high, exceeding 1000 K (local temperature in the combustion chamber at ignition moment). Therefore, it is necessary to adjust the simplified methanol combustion mechanism with optimization methods. Firstly, a sensitivity analysis of the methanol ignition delay is conducted to determine the contribution of each reaction to the fuel ignition delay. Sensitivity analysis is conducted on the ignition delay periods of methanol at temperatures of 1600 K, 1800 K, and 2000 K. For the high-pressure environment of rich mixture combustion in the combustion chamber at the ignition moment in a 4135 diesel engine, the equivalence ratio and initial pressure selected for calculation and analysis are 0.5 and 4.0 MPa, respectively. In order to clarify the key reactions that affect the ignition delay, a normalized sensitivity coefficient is defined as [12]:
where, is the ignition delay; ki is the pre-exponential factor of the i reaction; and 2ki and 0.5ki are the ignition delays calculated by multiplying different pre-factors.
A negative sensitivity coefficient indicates that the reaction has a promoting effect on fuel ignition, and vice versa. As the adjustment of the CO-C2 components in the sub-mechanism can affect the laminar flame speed, the reaction rate coefficients for the main fuel decomposition and oxidation reactions are optimized.
Figure 3 shows the sensitivity analysis of the ignition delay of the eight main reactions in the simplified methanol combustion mechanism at a 0.5 equivalence ratio and 4.0 MPa initial pressure. In this paper, some sensitive reactions are selected for optimization and the pre-exponential factor is adjusted to further reduce the error in the simplified methanol combustion mechanism. In order to accurately adjust the pre-exponential factor [13], the relative error is defined as:
where, is the ignition delay of the simplified methanol combustion mechanism after the adjustment of the pre-factor and is the ignition delay of the detailed methanol combustion mechanism.
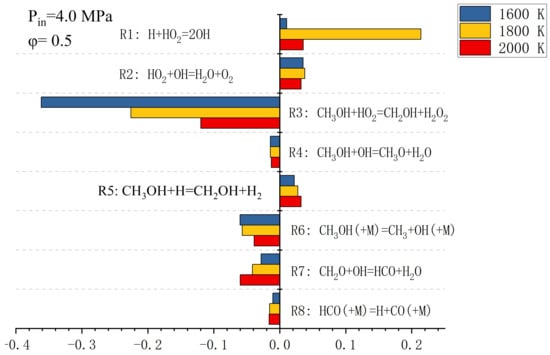
Figure 3.
Temperature sensitivity analysis at the moment of methanol ignition.
Equation (2) represents the relative error between the detailed and simplified mechanisms. Figure 4 shows the relative error for the temperature-sensitive reaction in Figure 3. It can be seen from Figure 4 that the error can be reduced by adjusting the pre-factors of different sensitive reactions under low and high temperatures.
Figure 4.
Ignition delay: relative error of methanol ignition delay.
By adjusting the pre-exponential factors of corresponding sensitive reactions (elementary reaction numbers R1, R2, R3, R4, and R8) to change the reaction rate constants, the accuracy of the simplified methanol combustion mechanism is improved. The final adjustment results are shown in Table 1.

Table 1.
Adjusted pre-exponential factors of sensitivity reactions.
3. A New Simplified Combustion Mechanism for PODE
3.1. Simplification of Detailed Combustion Mechanism for PODE
PODEn is a new type of carbon-based ether-oxygen-containing fuel, whose chemical formula can be expressed as CH3O(CH2O)nCH3 (), that is produced by polymerizing methanol in formaldehyde solution. The physicochemical properties of each component of PODE are listed in Table 2 [14]. In this paper, PODE3 is used as a characterization fuel.

Table 2.
Physical properties of each component of PODE.
The ignition environment of PODE3 in the 4135 diesel engine combustion chamber is mostly that of a medium-low temperature range of 900–1400 K. The target parameter in the simplification process is the ignition delay of the PODE. The initial conditions for the mechanism simplification were set as follows: initial pressure Pin was set at 1–1.5 MPa, initial temperature Tin was set at 900–1400 K, and air/fuel equivalence ratio Phi was set at 0.5–1.5. Under these conditions, a simplified PODE combustion mechanism was obtained consisting of 65 components and 249 reactions. Figure 5 shows a comparison of ignition delay between the detailed and simplified combustion mechanisms for PODE. It can be seen that between the lean ( < 1) and rich ( > 1) combustion ranges, the simplified PODE combustion mechanism still exhibits high prediction accuracy within a certain error range.
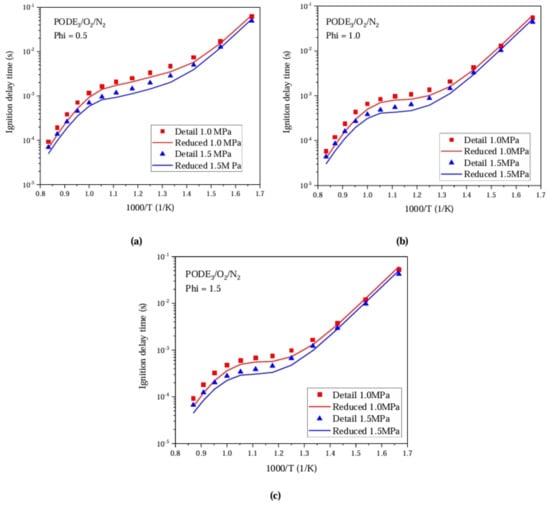
Figure 5.
Comparison of Ignition Delay between Detailed and Simplified Combustion Mechanisms for PODE.
3.2. Optimization of the Simplified PODE Combustion Mechanism
Figure 5 shows the preliminary verification of the simplified PODE combustion mechanism, which has a good agreement with the detailed mechanism in terms of ignition delay, but still has large errors at low and high temperature ranges. Therefore, it is necessary to optimize the simplified PODE combustion mechanism. Firstly, a sensitivity analysis of PODE3 ignition delay is conducted. Temperature is the most sensitive factor affecting ignition delay, while the influence of equivalence ratio and pressure vary depending on the fuels [15]. Therefore, sensitivity analysis of PODE ignition delay at temperatures of 650 K, 950 K, and 1200 K is conducted. The sensitivity coefficients of each reaction’s ignition delay refer to Formula (1).
Figure 6 shows a sensitivity analysis of the ignition delay of eight main reactions in the simplified PODE3 combustion mechanism when the equivalence ratio is 1.0, the initial pressure is 1.0 MPa, and the initial temperatures are 650 K, 950 K, 1200 K, respectively. In this paper, some sensitive reactions are selected for optimization and the pre-exponential factor (A) is adjusted to further reduce the error of the simplified PODE3 combustion mechanism. In order to accurately adjust the pre-exponential factor, the relative error is calculated according to Equation (2) adjusted for PODE.
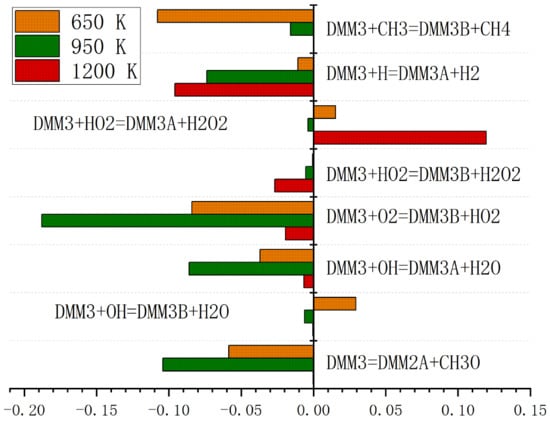
Figure 6.
Sensitivity analysis of the ignition delay of PODE.
Figure Figure 7 shows the relative error for each sensitive reaction in Figure 6. It can be seen from Figure 7, that the pre-exponential factors can be adjusted to reduce the error for different sensitive reactions under both the low and high temperatures.
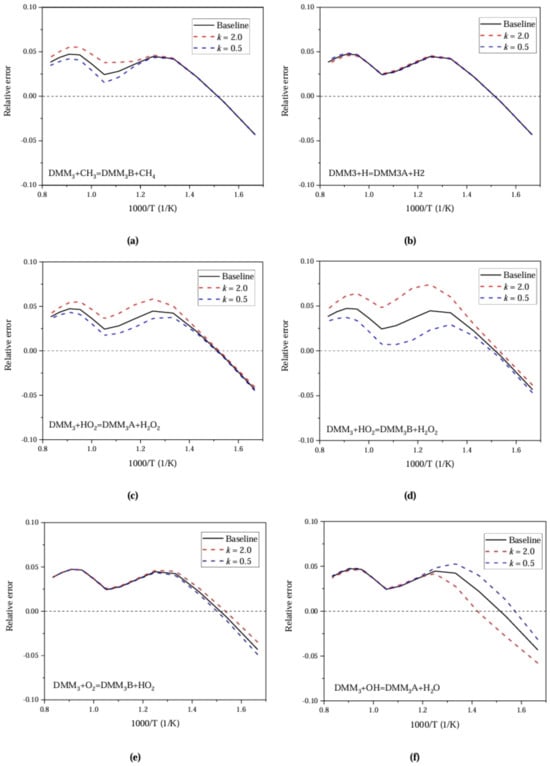
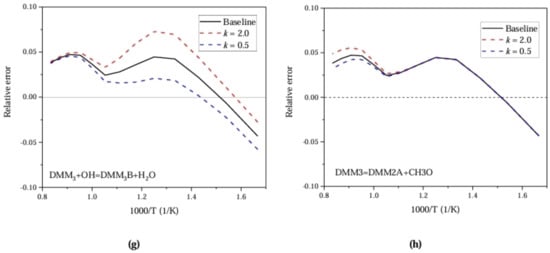
Figure 7.
Relative error with respect to ignition delay for PODE at Pin = 0.1 MPa and Phi = 1.0.
Adjusting the exponential pre-factors of corresponding sensitive reactions to change the reaction rate constants, the accuracy of the simplified PODE combustion mechanism was improved. The final adjustment results are shown in Table 3.

Table 3.
Adjustment of pre-factor for sensitive response of PODE.
4. Combination and Verification of Simplified Combustion Mechanism for Methanol/PODE Mixed Fuel
When the mixture of PODE/methanol fuel is used in a 4135 marine diesel engine, first, the PODE is compressed and ignited, which then ignites the methanol. Therefore, the simplified combustion mechanism of the mixed fuel requires the combination of the two simplified fuel combustion mechanisms. In Table 3, only the pre-exponential factors of high-carbon component units (methanol, ketohydroperoxide, etc.) in the methanol-rich combustion mechanism were adjusted, with little effect on the PODE combustion mechanism. Therefore, the three adjusted sensitive reactions can be combined to form a PODE/methanol fuel combustion mechanism. By deleting duplicated C1 and C2 in the process of reaction, keeping the basic reactions and components of PODE, and adjusting the reaction rate to optimize the mechanism, finally, a simplified combined methanol/PODE combustion mechanism is obtained with 137 components and 635 basic reaction steps.
For the newly constructed methanol–PODE simplified combustion mechanism, verification is necessary to ensure that the zero-dimensional simulation results match experimental results with fundamental experiments in flow reactors, jet stirred reactors, and shock tubes. Here, only the fuel component concentration, ignition delay, and laminar flame speed are selected to be verified.
Under existing conditions, the verification of the mixed methanol–PODE simplified combustion mechanism is carried out separately, that is, the ignition delay of each fuel is verified separately [16,17,18,19,20,21,22,23]. The ignition delay is defined as the time required for the initial temperature of methanol to rise to 400 K. A closed homogeneous reactor is used to calculate the ignition delay of the methanol fuel. Referring to the data on methanol-air mixture ignition delay obtained from shock tube experiments by Heufer K. and Ciezki H.K. et al. [24], the new proposed methanol-rich combustion mechanism for a 4135 diesel engine is verified. Figure 8 shows the comparison between the simulated data and experimental results of methanol ignition delay. It can be seen that the ignition delay predicted by the methanol-rich combustion mechanism is in good agreement under the initial pressures of 0.4927 MPa and 0.5095 MPa, with the data obtained from the shock tube experiment by Ciezki H.K. et al. So, the optimized combustion mechanism can predict the ignition data of the pilot fuel very well under fuel-rich conditions ( = 2).
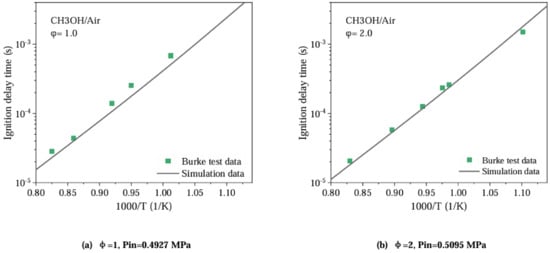
Figure 8.
Comparison between simulation and experimental results of methanol ignition delay.
Using RCM experimental data from He et al. [14] under initial pressures of 1.0 MPa and 1.5 MPa, the ignition delay of the PODE3/O2/N2 mixture at three different equivalence ratios—(a) Phi = 0.5, O2:N2 = 1:8; (b) Phi = 1.0, O2:N2 = 1:15; and (c) Phi = 1.5, O2:N2 = 1:20— was verified. It can be seen from Figure 9 that the optimized mechanism can better match the ignition delay data measured using an RCM than the original detailed PODE combustion mechanism.
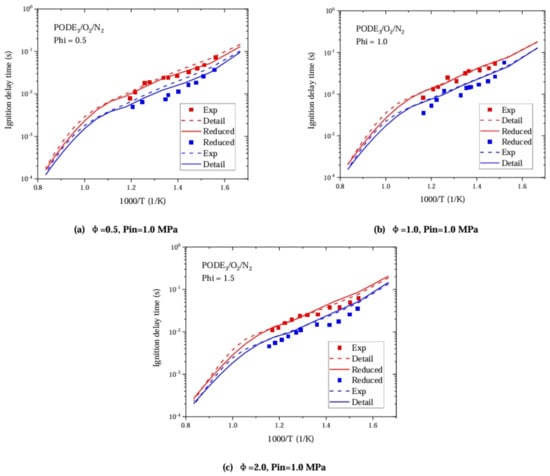
Figure 9.
Comparison between simulation and experimental results of PODE ignition delay.
Laminar flame speed is another key combustion characteristic of fuel. Therefore, the simplified combustion mechanism for PODE3 needs to be further verified with experimental results on laminar flame speed. Experiments to measure the laminar flame speed of the PODE3 and air mixture were conducted at a 0.1 MPa pressure and equivalence ratios ranging from 0.7 to 1.6 as shown in Figure 10. It can be seen that the laminar flame speed is slightly higher, based on the proposed simplified combustion mechanism, which is due to some component transport parameters in the simplified PODE3 combustion mechanism that cannot be obtained from the detailed mechanism. Therefore, some component transport parameters in the simplified combustion mechanism for PODE are derived from species with similar chemical structures in the LLNL database [25]. As the detailed mechanism [14] does not include laminar flame speed verification, the predicted results of this study are acceptable.
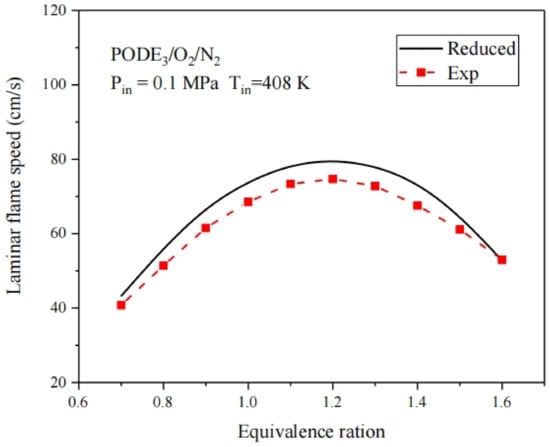
Figure 10.
Comparison between simulation and experimental results of PODE3 laminar flame speed.
Simulation with the pre-mixing model in the CHEMKIN-PRO software can further verify the simplified combustion mechanism by validating the concentrations of major intermediate products and reaction components during the combustion process. Figure 11 compares the simulated data of PODE component concentrations under 3.3 kPa pressure with the experimental data [13]. Due to probe disturbance and temperature measurement errors in the experiment, which cause measurement deviations in the concentrations of key components, the calculation errors of the concentrations of the important components in the flame zone within 3 mm of the center of the premixed flame can be neglected. It can be seen from Figure 11 that the predicted results for PODE3, O2, Ar, CO2, H2, H2O, and other major substances are consistent with the experimental results
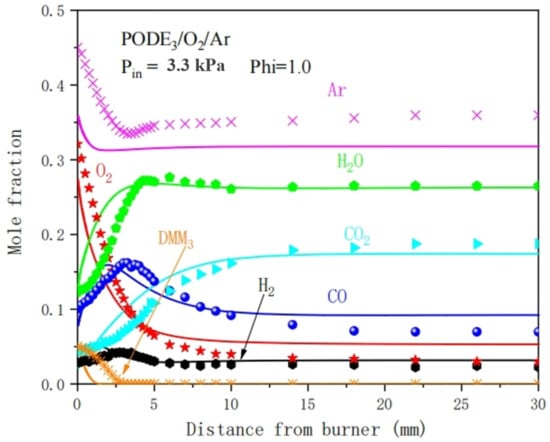
Figure 11.
Comparison between simulation and experimental results of PODE3 component concentration.
5. Conclusions
- 1.
- The detailed combustion mechanisms for methanol and PODE3 were simplified using the DRG, DRGEP, SA, and RPA methods, resulting in simplified combustion mechanisms for methanol and PODE3. Based on sensitivity analysis of the ignition delay period of the simplified mechanisms, the pre-exponential factors of key reactions were adjusted, and under the requirement of prediction error, a simplified combustion mechanism for methanol containing 42 components and 233 basic reactions and a simplified combustion mechanism for PODE containing 65 components and 249 basic reactions were finally obtained;
- 2.
- A simplified mechanism for a methanol–PODE mixed fuel combustion reaction was obtained by merging the simplified combustion mechanisms for methanol and PODE, deleting duplicate mechanisms, finally containing a mechanism with 137 components and 635 reactions. Through the verification of ignition delay, laminar flame speed and key component concentration, the results showed that the error range between the simplified and detailed combustion mechanisms meets the requirements for CFD simulation, and this simplified mechanism lays the foundation for the numerical simulation of methanol–PODE mixed fuel combustion in the cylinder.
Author Contributions
Writing—Origin Draft, C.L.; Writing—Review and Editing, Y.H. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science & Technology Commission of Shanghai Municipality and Shanghai Engineering Research Centre of Ship Intelligent Maintenance and Energy Efficiency under Grant 20DZ2252300.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank each of the authors for their contributions to this study. We would also like to thank each editor and reviewer for their constructive comments and revisions to the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- World Shipbuilding Review and Outlook 2022 [EB/OL]. Available online: http://www.eworldship.com/html/2023/ship_market_observation_0214/189731.html (accessed on 12 December 2023).
- International Maritime Organization (IMO). Pollution Prevention. [EB/OL]. Available online: https://www.imo.org/en/OurWork/Environment/Pages/Pollution-Prevention.aspx (accessed on 12 December 2023).
- International Maritime Organization (IMO). Future Fuels and Technology [EB/OL]. Available online: https://www.imo.org/en/OurWork/Environment/Pages/Future-Fuels-And-Technology.aspx (accessed on 12 December 2023).
- Ling, L. Maersk China Decarbonization Business Director Kaka: We are very Optimistic about China’s Green Methanol Market. Industry Insight. Available online: https://www.maersk.com.cn/news/articles/2023/11/22/maersk-signs-landmark-green-methanol-offtake-agreement (accessed on 6 November 2023).
- Newsletter. CMA CGM Ordered 12 24,000 TEU Methanol Dual-Fuel Container Ships. China Aviation Weekly, 2023.
- Wang, Y. Experimental Study of Direct-injected Methanol Ignited by Diesel. Ph.D Thesis, Dalian University of Technology, Dalian City, China, 2020. [Google Scholar]
- Jia, Z.Q.; Denbratt, I. Experimental investigation into the combustion characteristics of a methanol-Diesel heavy duty engine operated in RCCI mode. Fuel 2018, 226, 745–753. [Google Scholar] [CrossRef]
- Arkadiusz, J. The effect of the alcohol content in the fuel mixture on the performance and emissions of a direct injection diesel engine fueled with diesel-methanol and diesel-ethanol blends. Energy Convers. Manag. 2017, 148, 461–476. [Google Scholar]
- Sun, W.; Wang, G.; Li, S. Speciation and the laminar burning velocities of poly(oxymethylene) dimethyl ether3 (POMDME3) flames: An experimental and modeling study. Proc. Combust. Inst. 2012, 36, 1269–1278. [Google Scholar] [CrossRef]
- He, T.; Wang, Z.; You, X. A chemical kinetic mechanism for the low- and intermediate-temperature combustion of Polyoxymethylene Dimethyl Ether 3 (PODE3). Fuel 2018, 212, 223. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Kazakov, A. A comprehensive Kinetic Mechanism for CO, CH2O, and CH3OH Combustion. Int. J. Chem. Kinet. 2007, 39, 109–136. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, L.; Man, X. Experimental and Modeling Study of n-Butanol Oxidation at High Temperature. Energy Fuels 2012, 26, 3368–3380. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, G. A new skeletal mechanism for diesel-n-butanol blends combustion in engine. Fuel 2020, 264, 116856.1–116856.13. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ma, X. Homogeneous charge compression ignition (HCCI) combustion of polyoxymethylene dimethyl ethers (PODE). Fuel 2016, 183, 206–213. [Google Scholar] [CrossRef]
- Li, R.; Herreros, J.M.; Tsolakis, A. Chemical kinetic study on ignition and flame characteristic of polyoxymethylene dimethyl ether 3 (PODE3). Fuel 2020, 279, 118423. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, L.; Jia, D.; Gao, W.; Sun, P.; Ji, Q. Study on Simplifed Chemical Kinetics Mechanism of PODE/Methanol Dual Fuel. J. Eng. Thermophys. 2022, 43, 846–855. [Google Scholar]
- Natarajan, K.; Bhaskaran, K.A. An Experimental and An alytical Study of Methanol Ignition Behind Shock Waves. Combust. Flame 1981, 43, 35–49. [Google Scholar] [CrossRef]
- Tsuboi, T.; Hashimoto, K. Shock Tube Study on Homogeneous Thermal Oxidation of Methanol. Combust. Flame 1981, 42, 61–67. [Google Scholar] [CrossRef]
- Aronowitz, D.; Santoro, R.J.; Dryer, F.L. Kinetics of the Oxidation of Methanol: Experimental Results Semi Global Modeling and Mechanistic Concepts. Symp. Int. Combust. 1979, 17, 633–644. [Google Scholar] [CrossRef]
- Bowman, C.T. A Shock-Tube Investigation of the High Temperature Oxidation of Methanol. Combust. Flame 1975, 25, 343–354. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Z.; Li, B. Development of a Reduced Polyoxymethylene Dimethyl Ethers (PODEn) Mechanism for Engine Applications. Fuel 2019, 238, 208–224. [Google Scholar] [CrossRef]
- Held, T.J.; Dryer, F.L. An Experimental and Computational Study of Methanol Oxidation in the Intermediate and High-Temperature Regimes. Symp. Int. Combust. 1994, 25, 901–908. [Google Scholar] [CrossRef]
- Lin, Q.J.; Tay, K.L.; Zhou, D.Z. Development of a Compact and Robust Polyoxymethylene Dimethyl Ether3 Reaction Mechanism for Internal Combustion Engines. Energy Convers. Manag. 2019, 185, 35–43. [Google Scholar] [CrossRef]
- Heufer, K.; Ciezki, H.K. Numerical study on auto-ignition characteristics of hydrogen-enriched methane under engine-relevant conditions. Energy Convers Manag. 2019, 200, 112092. [Google Scholar]
- Pei, Y.; Mehl, M.; Liu, W. A Multicomponent Blend as a Diesel Fuel Surrogate for Compression Ignition Engine Applications. J. Eng. Gas Turbines Power 2015, 137, 111502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).