Synthesis of Activated Biochar from the Bark of Moringa oleifera for Adsorption of the Drug Metronidazole Present in Aqueous Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of the Biochar
2.3. Elementary Biochar Analysis
2.4. Preparation of the Activated Biochar by H3PO4
2.5. Preparation of the Activated Biochar by KOH
2.6. Characterization
2.7. Adsorption Experiments
2.8. Study of the Influence of pH on Adsorption Tests
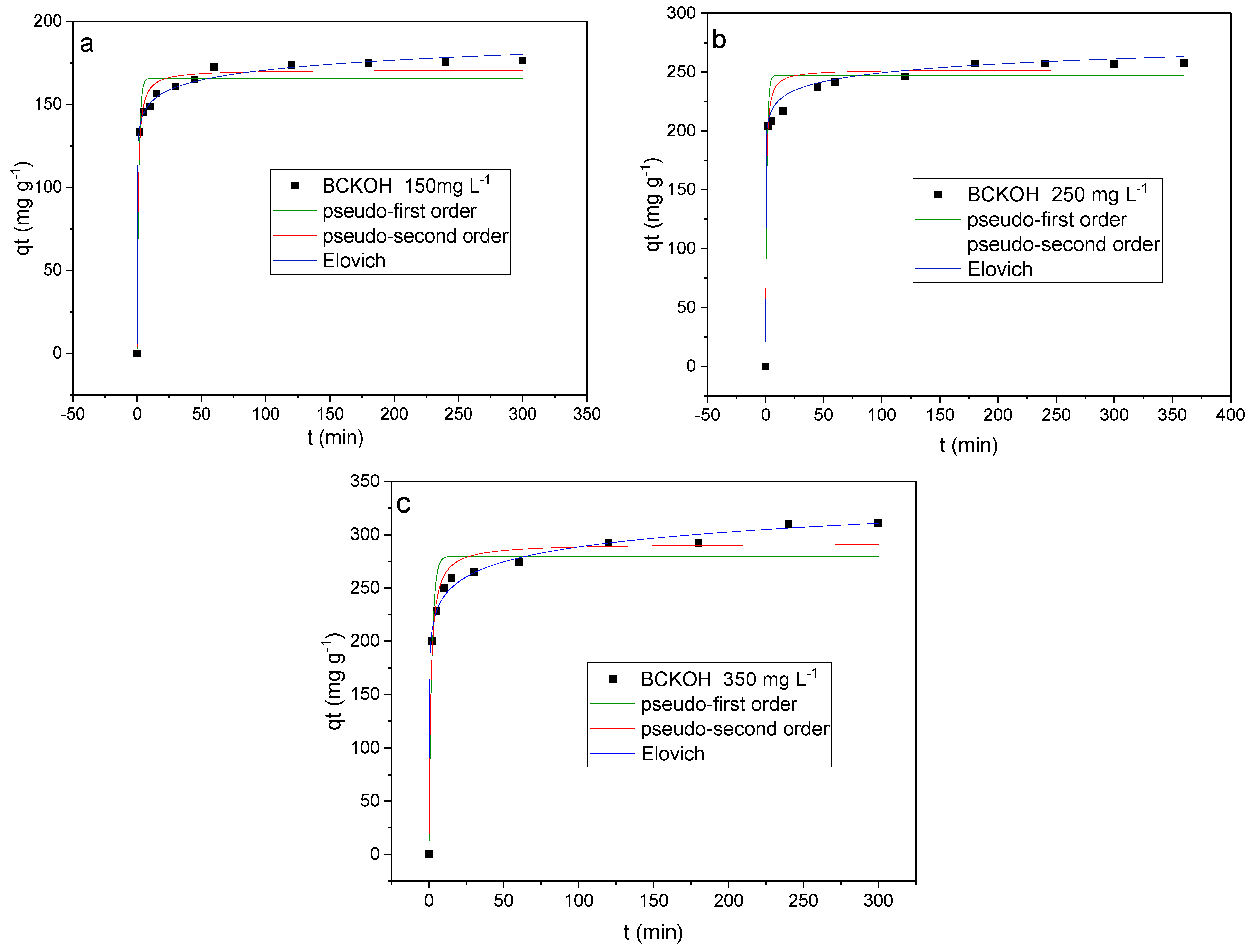
2.9. Adsorption Kinetics
- qt = amount of adsorbed adsorbate (mg g−1) at time t (min).
- qe = equilibrium and the maximum adsorption capacities (mg g−1).
- k1 = adsorption rate constant (min−1).
- k2 = adsorption rate constant (g mg−1 min−1).
- A = initial adsorption rate (mg g−1 min−1).
- Β = adsorption constant (mg g−1).
- kD = intraparticle diffusion coefficient (mg g−1 min−0.5).
- C = constant (mg g−1).
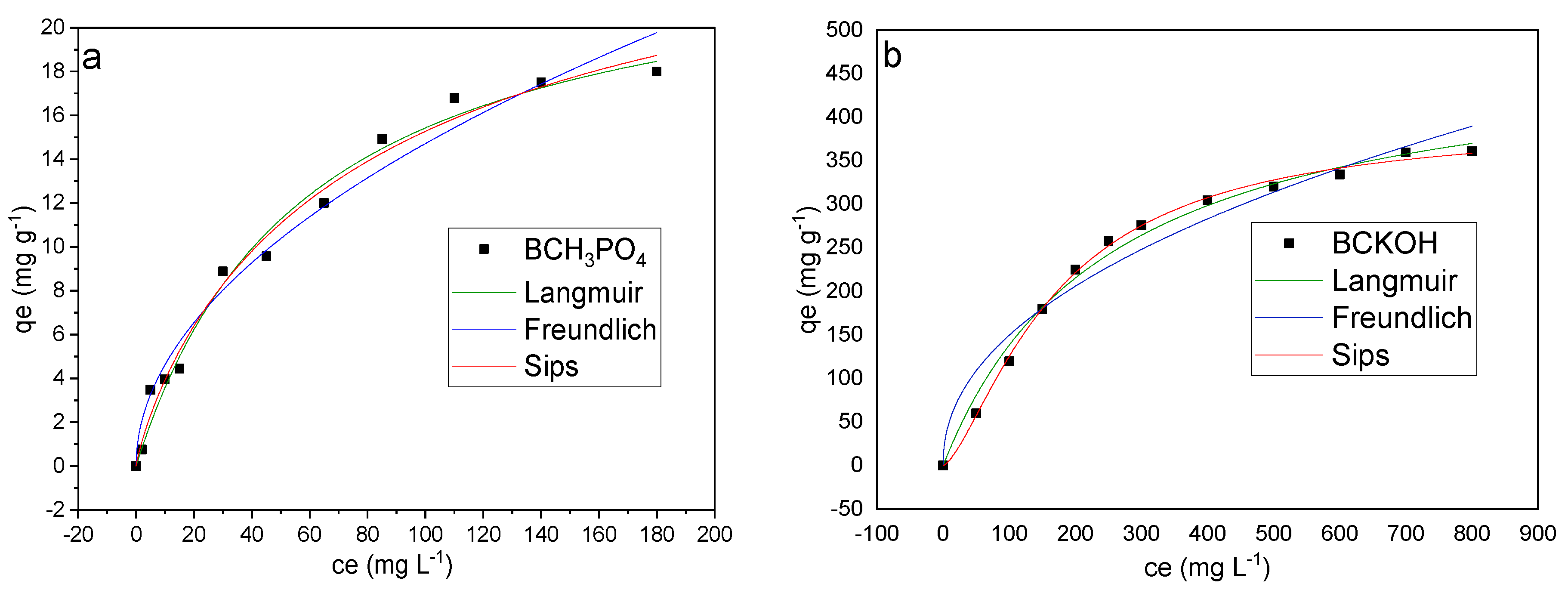
2.10. Adsorption Isotherms
- qmax = maximum adsorption capacity, mg g−1.
- qe = amount of adsorbate concentration in the solid phase in equilibrium, mg g−1.
- Ce = amount of adsorbate concentration in the liquid phase, mg L−1.
- Kl = equilibrium constant, affinity between adsorbate and adsorbent, L mg−1.
- Kf = Freundlich constant relates the adsorption capacity and number of adsorption sites, L mg−1.
- n = that relates the intensity of the adsorption process (affinity between adsorbent and adsorbate).
- Ks = Sips constant, with the adsorption energy, L mg−1.
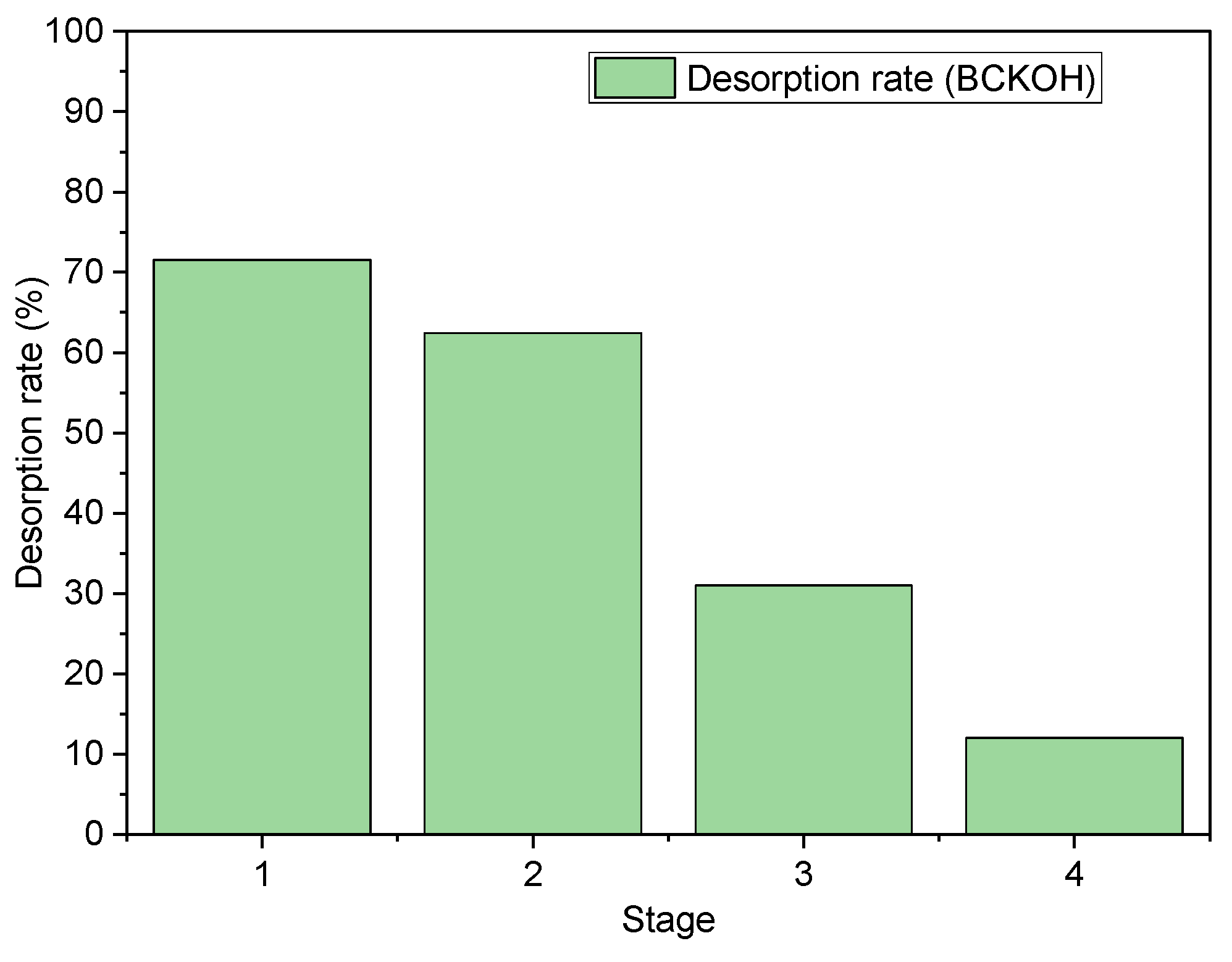
2.11. Recuperation Studies
3. Results and Discussion
3.1. Immediate Analysis and Elemental Analysis
3.2. Statistical Evaluation of the Biochars Produced under Different Investigated Factors
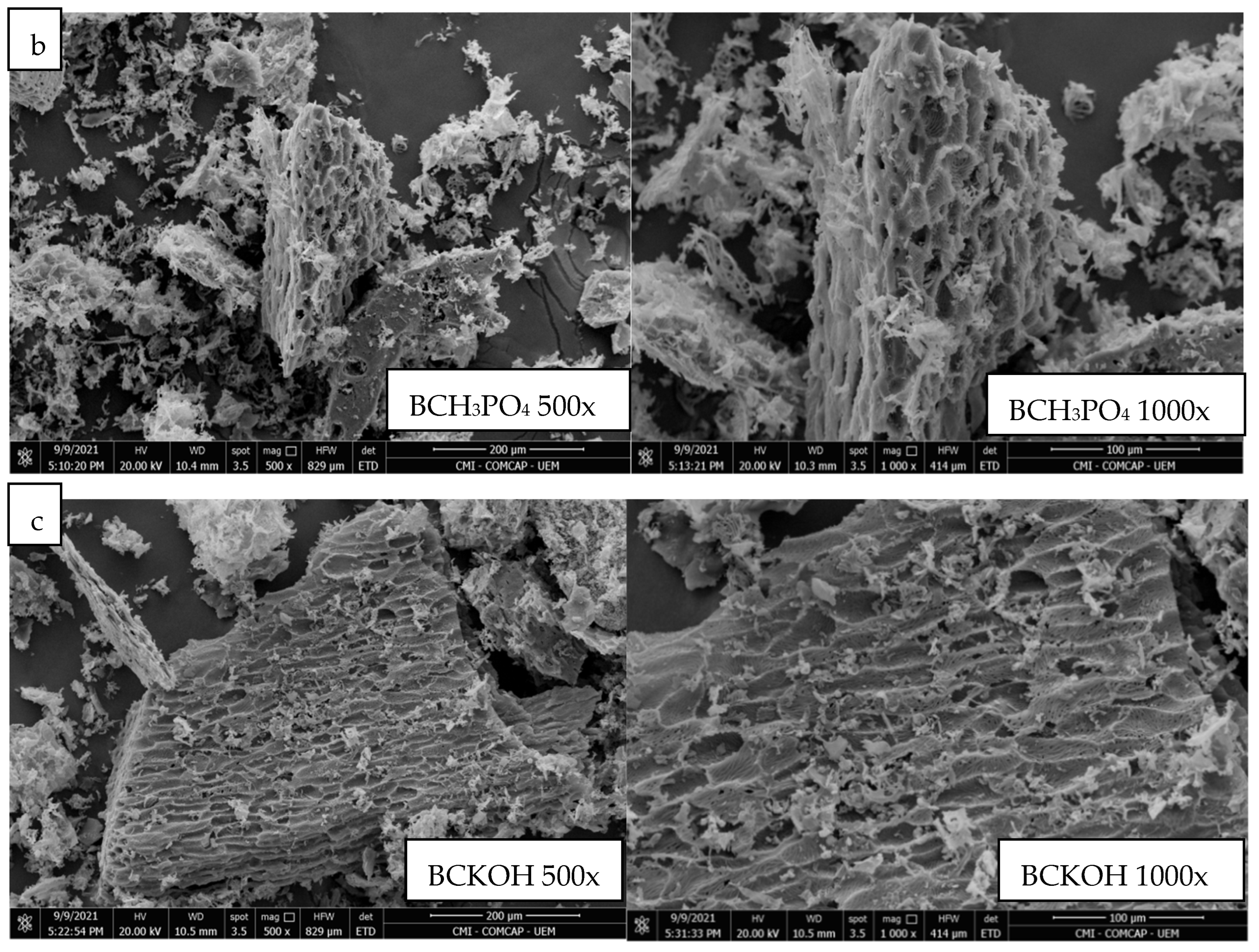
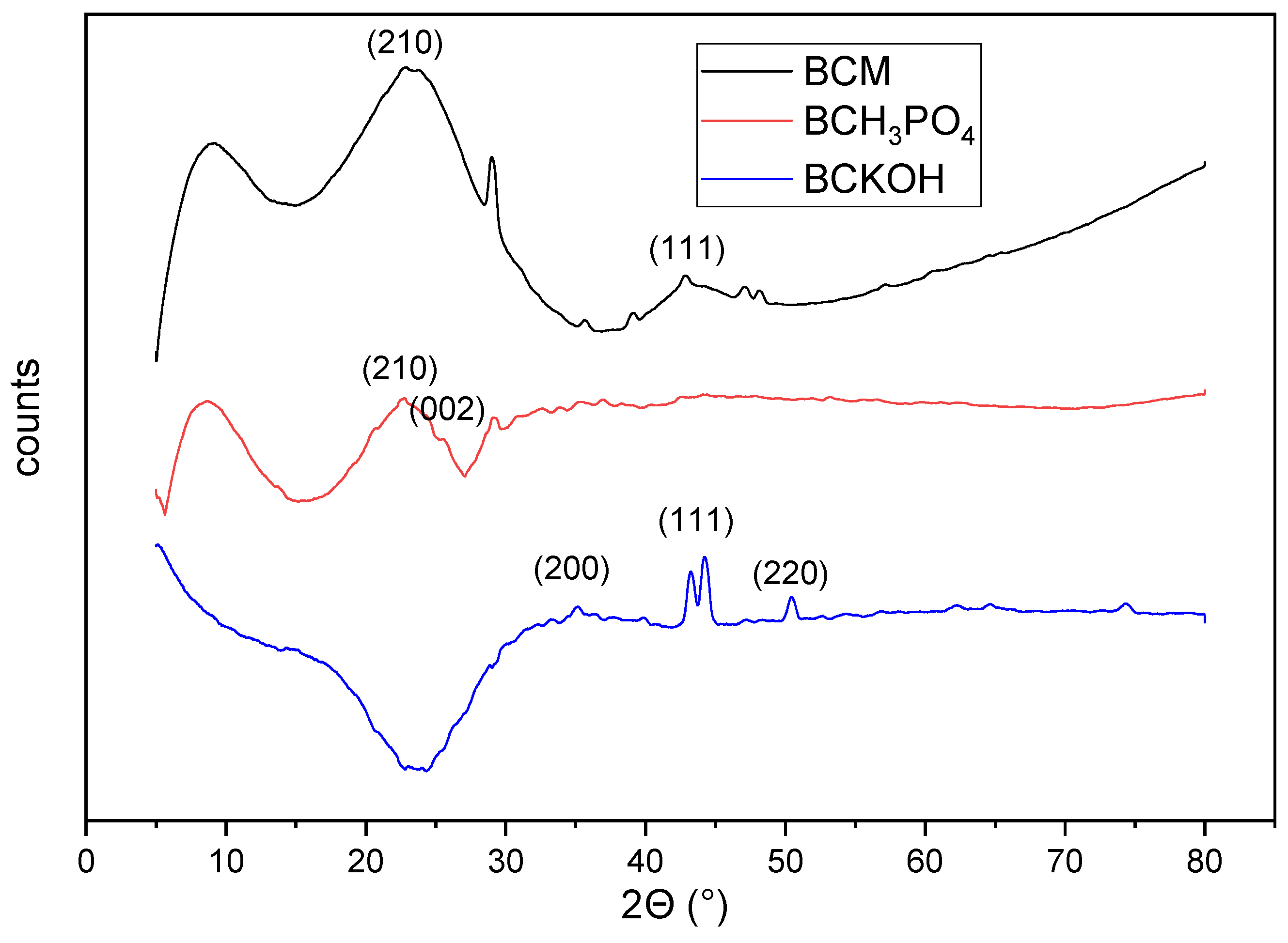
3.3. Characterization of the Biochars
3.4. Adsorption Experiments
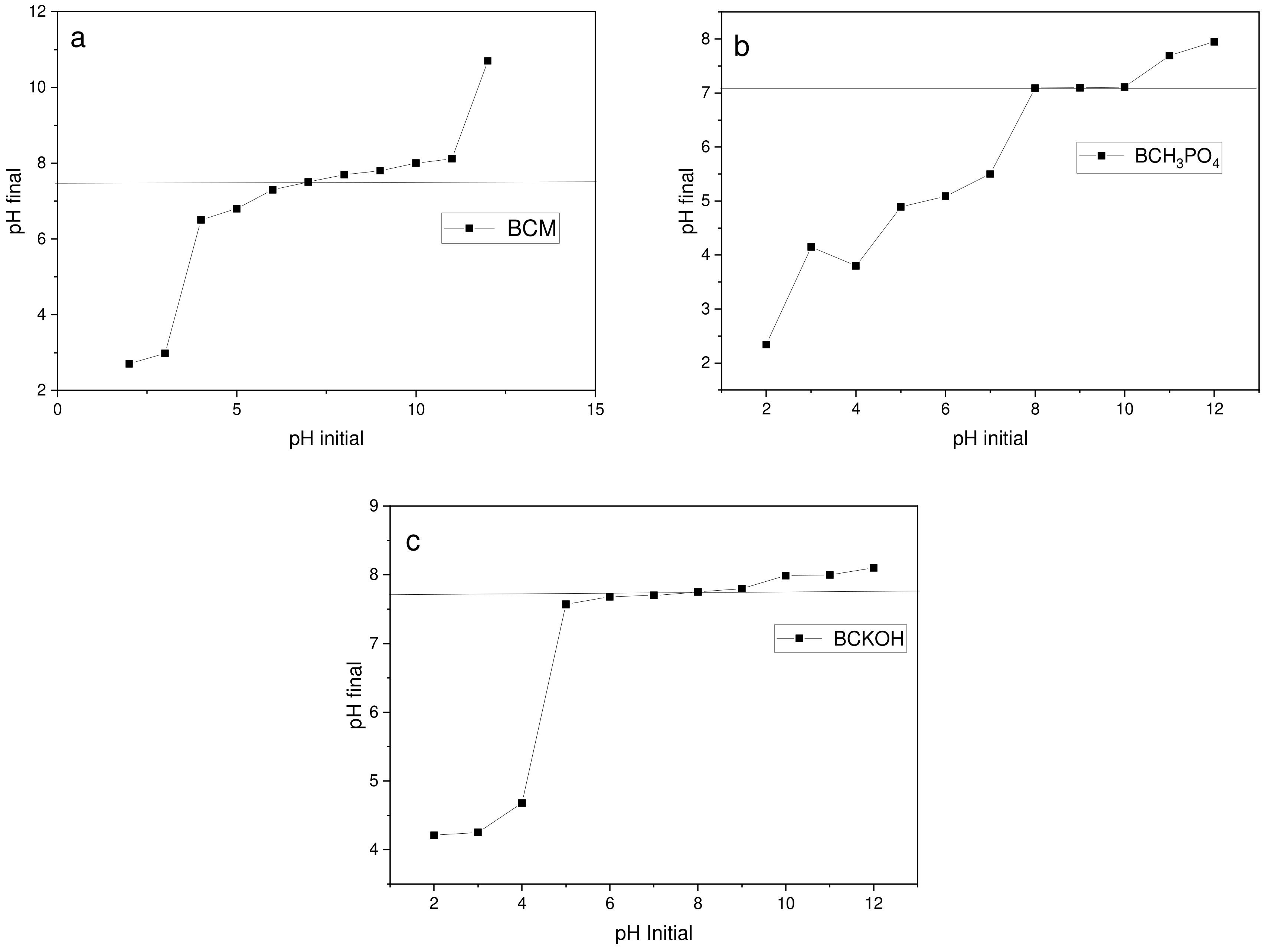
3.5. Adsorption Studies at Different pH
3.6. Adsorption Kinetics
3.7. Adsorption Isotherms
3.8. Recuperation Studies
4. Conclusions
- The biochar had a high carbon content, making it a favorable material for use as an adsorbent.
- The pH of the studied biochars was close to neutral, facilitating their use for various contaminants.
- TGA and DTG demonstrated that activated biochars have thermal stability.
- The FTIR spectra indicated the presence of lignin- and cellulose-based compounds in the biochar before activation, and after adsorption, the formation and displacement of functional groups were observed, indicating the possibility of chemisorption.
- Adsorption tests showed that activation with H3PO4 and KOH improved the adsorption characteristics of Metronidazole.
- In the kinetic study, the experimental data were better fitted to the Elovich model, indicating that chemisorption occurs.
- The adsorption mechanism of the activated biochars was favorable, reaching an adsorption capacity of 18 mg g−1 for BCH3PO4 and 366.49 mg g−1 for BCKOH and better fitting the Sips isotherm.
- BCKOH showed the best results in the adsorption studies, making it promising to study other contaminants.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, A.; Dindorkar, S.S.; Ramisetti, S.B. Adsorption Behaviour of Boron Nitride Nanosheets towards the Positive, Negative and the Neutral Antibiotics: Insights from First Principle Studies. J. Water Process Eng. 2022, 46, 102555. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, S.G.; Chang, C.C. Emerging Pollutants—Part II: Treatment. Water Environ. Res. 2018, 90, 1792–1820. [Google Scholar] [CrossRef]
- Gomes, A.R.; Justino, C.; Rocha-Santos, T.; Freitas, A.C.; Duarte, A.C.; Pereira, R. Review of the Ecotoxicological Effects of Emerging Contaminants to Soil Biota. J. Environ. Sci. Health Part A 2017, 52, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Saki, K.; Rafieian-Kopaei, M.; Karamati, S.A.; Eftekhari, Z.; Jelodari, M. The Most Common Herbal Medicines Affecting Sarcomastigophora Branches: A Review Study. Asian Pac. J. Trop. Med. 2014, 7, S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Mabe, K.; Okuda, M.; Kikuchi, S.; Amagai, K.; Yoshimura, R.; Kato, M.; Sakamoto, N.; Asaka, M. Randomized Controlled Trial: PPI-Based Triple Therapy Containing Metronidazole versus Clarithromycin as First-Line Treatment for Helicobacter pylori in Adolescents and Young Adults in Japan. J. Infect. Chemother. 2018, 24, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, N.A.; Nashee, H.; Elgendy, A.A.; Rabee, I.; El Amir, A. Encapsulation of Low Metronidazole Dose in Poly (D,L-Lactide-Co-Glycolide) (PLGA) Nanoparticles Improves Giardia Intestinalis Treatment. Infect. Chemother. 2020, 52, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. Biochar: Production, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Cao, Q.; Huang, Z.; Liu, S.; Wu, Y. Potential of Punica Granatum Biochar to Adsorb Cu(II) in Soil. Sci. Rep. 2019, 9, 11116. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of Copper Ions from Aqueous Solution Using Low Temperature Biochar Derived from the Pyrolysis of Municipal Solid Waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Duan, S.; Wang, Y.; Liu, X.; Shao, D.; Hayat, T.; Alsaedi, A.; Li, J. Removal of U(VI) from Aqueous Solution by Amino Functionalized Flake Graphite Prepared by Plasma Treatment. ACS Sustain. Chem. Eng. 2017, 5, 4073–4085. [Google Scholar] [CrossRef]
- Hernandez-Mena, L.; Pecora, A.; Beraldo, A. Slow Pyrolysis of Bamboo Biomass: Analysis of Biochar Properties. Chem. Eng. Trans. 2014, 37, 115–120. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Yao, P.; Chen, Y.; Xu, X.; Li, T.; Gong, G. Removal of Pb(II) from Aqueous Solutions by Phytolacca americana L. Biomass as a Low Cost Biosorbent. Arab. J. Chem. 2018, 11, 99–110. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible Role of Biomass Types and Its Compositions on the Formation of Persistent Free Radicals in Biochar: Insight into the Influences on Fenton-like Process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- França, F.R.M.; dos Santos Freitas, L.; Ramos, A.L.D.; da Silva, G.F.; Brandão, S.T. Storage and Oxidation Stability of Commercial Biodiesel Using Moringa oleifera Lam as an Antioxidant Additive. Fuel 2017, 203, 627–632. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, R.; Kachhwaha, S.; Kothari, S.L. Nutritional and Medicinal Applications of Moringa oleifera Lam.—Review of Current Status and Future Possibilities. J. Herb. Med. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Raman, J.K.; Alves, C.M.; Gnansounou, E. A Review on Moringa Tree and Vetiver Grass—Potential Biorefinery Feedstocks. Bioresour. Technol. 2018, 249, 1044–1051. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávila, G.C.G. Moringa Plants: Bioactive Compounds and Promising Applications in Food Products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef]
- Belhamdi, B.; Merzougui, Z.; Laksaci, H.; Trari, M. The Removal and Adsorption Mechanisms of Free Amino Acid L-Tryptophan from Aqueous Solution by Biomass-Based Activated Carbon by H3PO4 Activation: Regeneration Study. Phys. Chem. Earth Parts A/B/C 2019, 114, 102791. [Google Scholar] [CrossRef]
- De Araújo, T.P.; Quesada, H.B.; dos Santos, D.F.; da Silva Fonseca, B.C.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen Removal by Calcium Alginate/Activated Hydrochar Composite Beads: Batch and Fixed-Bed Studies. Int. J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.O.; Regalbuto, J.R. The Engineering of Pt/Carbon Catalyst Preparation for Application on Proton Exchange Fuel Cell Membrane (PEFCM); University of Illinois: Chicago, IL, USA, 2004. [Google Scholar]
- González-García, P. Activated Carbon from Lignocellulosics Precursors: A Review of the Synthesis Methods, Characterization Techniques and Applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- dos Santos Bispo, M.; dos Santos, J.P.L.; dos Santos, L.C.L.; dos Santos Freitas, L.; Bispo, D.F.; da Silva, G.F. Synthesis and Characterization of Activated Carbon of Moringa oleifera Lam Pod and Evaluation of Its Performance in the Removal of Oils and Greases. J. Environ. Chem. Eng. 2021, 9, 105965. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point Zero Charge: Usefulness in Sorption Studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Herath, A.; Layne, C.A.; Perez, F.; Hassan, E.B.; Pittman, C.U.; Mlsna, T.E. KOH-activated high surface area Douglas Fir biochar for adsorbing aqueous Cr(VI), Pb(II) and Cd(II). Chemosphere 2021, 269, 128409. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac Removal from Water by Adsorption on Moringa oleifera Pods and Activated Carbon: Mechanism, Kinetic and Equilibrium Study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- de Araújo, T.P.; de Oliveira Tavares, F.; Vareschini, D.T.; Barros, M.A.S.D. Biosorption Mechanisms of Cationic and Anionic Dyes in a Low-Cost Residue from Brewer’s Spent Grain. Environ. Technol. 2021, 42, 2925–2940. [Google Scholar] [CrossRef]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Removal of Tartrazine from Aqueous Solutions Using Adsorbents Based on Activated Carbon and Moringa oleifera Seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.M.; Mitan, N.M.M.; Dimin, M.F. Characterization of Biochar Derived from Rubber Wood Sawdust through Slow Pyrolysis on Surface Porosities and Functional Groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef]
- de Souza dos Santos, G.E.; Ide, A.H.; Duarte, J.L.S.; McKay, G.; Silva, A.O.S.; Meili, L. Adsorption of Anti-Inflammatory Drug Diclofenac by MgAl/Layered Double Hydroxide Supported on Syagrus coronata Biochar. Powder Technol. 2020, 364, 229–240. [Google Scholar] [CrossRef]
- Paunovic, O.; Pap, S.; Maletic, S.; Taggart, M.A.; Boskovic, N.; Turk Sekulic, M. Ionisable emerging pharmaceutical adsorption onto microwave functionalised biochar derived from novel lignocellulosic waste biomass. J. Colloid Interface Sci. 2019, 547, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Jawaid, M.; Firoozian, P.; Rashid, U.; Islam, A.; Akil, H.M. Activated Carbon from Various Agricultural Wastes by Chemical Activation with KOH: Preparation and Characterization. J. Biobased Mater. Bioenergy 2013, 7, 708–714. [Google Scholar] [CrossRef]
- Quesada, H.B.; De Araújo, T.P.; Cusioli, L.F.; De Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Evaluation of Novel Activated Carbons from Chichá-Do-Cerrado (Sterculia striata St. Hil. et Naud) Fruit Shells on Metformin Adsorption and Treatment of a Synthetic Mixture. J. Environ. Chem. Eng. 2021, 9, 104914. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.H.; Show, P.L. Biochar Production via Pyrolysis of Citrus Peel Fruit Waste as a Potential Usage as Solid Biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit Waste as Feedstock for Recovery by Pyrolysis Technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Gehrke, V.; Maron, G.K.; da Silva Rodrigues, L.; Alano, J.H.; de Pereira, C.M.P.; Orlandi, M.O.; Carreño, N.L.V. Facile Preparation of a Novel Biomass-Derived H3PO4 and Mn(NO3)2 Activated Carbon from Citrus bergamia Peels for High-Performance Supercapacitors. Mater. Today Commun. 2021, 26, 101779. [Google Scholar] [CrossRef]
- Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein Fractionation of Seeds of Moringa oleifera Lam and Its Application in Superficial Water Treatment. Sep. Purif. Technol. 2017, 180, 114–124. [Google Scholar] [CrossRef]
- Alves, V.N.; Mosquetta, R.; Coelho, N.M.M.; Bianchin, J.N.; Di Pietro Roux, K.C.; Martendal, E.; Carasek, E. Determination of Cadmium in Alcohol Fuel Using Moringa oleifera Seeds as a Biosorbent in an On-Line System Coupled to FAAS. Talanta 2010, 80, 1133–1138. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Fagundes-Klen, M.R.; Nishi, L.; Valverde, K.C.; Cavalcanti, E.B.; dos Santos, O.A.; Bergamasco, R. Potential Effect of Chemical and Thermal Treatment on the Kinetics, Equilibrium, and Thermodynamic Studies for Atrazine Biosorption by the Moringa oleifera Pods. Can. J. Chem. Eng. 2017, 95, 961–973. [Google Scholar] [CrossRef]
- Nasri, N.S.; Basri, H.; Garba, A. Synthesis and Characterization of Low-Cost Porous Carbon from Palm Oil Shell via K2CO3 Chemical Activation Process. Appl. Mech. Mater. 2015, 735, 36–40. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Al-Muhtaseb, A.H. Biochar Production from Waste Rubber-Wood-Sawdust and Its Potential Use in C Sequestration: Chemical and Physical Characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Teixeira, J.A.; van der Mei, H.C.; Oliveira, R. Physicochemical and Functional Characterization of a Biosurfactant Produced by Lactococcus lactis 53. Colloids Surf. B Biointerfaces 2006, 49, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Princípios de Bioquímica de Lehninger, 5th ed.; Artmed: Porto Alegre, Brazil, 2011. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agricultural Residues—Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Guan, B. Adsorption of Nitrobenzene from Aqueous Solution on Activated Sludge Modified by Cetyltrimethylammonium Bromide. J. Hazard. Mater. 2010, 183, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, X.; Ge, C.; Müller, K.; Yu, H.; Huang, P.; Li, J.; Tsang, D.C.W.; Bolan, N.S.; Rinklebe, J.; et al. Sorption of Norfloxacin, Sulfamerazine and Oxytetracycline by KOH-Modified Biochar under Single and Ternary Systems. Bioresour. Technol. 2018, 263, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, A.P.; Gonçalves, A.C., Jr.; Strey, L.; Rubio, F.; Schwantes, D.; Casarin, J. Biosorption and Removal of Chromium from Water by Using Moringa Seed Cake (Moringa oleifera Lam.). Quim. Nova 2013, 36, 1104–1110. [Google Scholar] [CrossRef]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and Characterization of Activated Carbon Produced from Kenaf Core Fiber Using H3PO4 Activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Tee, E.; Tallo, I.; Kurig, H.; Thomberg, T.; Jänes, A.; Lust, E. Huge Enhancement of Energy Storage Capacity and Power Density of Supercapacitors Based on the Carbon Dioxide Activated Microporous SiC-CDC. Electrochim. Acta 2015, 161, 364–370. [Google Scholar] [CrossRef]
- Chen, Y.; Syed-Hassan, S.S.A.; Xiong, Z.; Li, Q.; Hu, X.; Xu, J.; Ren, Q.; Deng, Z.; Wang, X.; Su, S.; et al. Temporal and Spatial Evolution of Biochar Chemical Structure during Biomass Pellet Pyrolysis from the Insights of Micro-Raman Spectroscopy. Fuel Process. Technol. 2021, 218, 106839. [Google Scholar] [CrossRef]
- Liu, X.; He, C.; Yu, X.; Bai, Y.; Ye, L.; Wang, B.; Zhang, L. Net-like Porous Activated Carbon Materials from Shrimp Shell by Solution-Processed Carbonization and H3PO4 Activation for Methylene Blue Adsorption. Powder Technol. 2018, 326, 181–189. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Wang, J.; Zhu, T.; Ye, M. Effects of Activated Carbon Properties on Chlorobenzene Adsorption and Adsorption Product Analysis. Chem. Eng. J. 2014, 236, 506–512. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New Insights on the Structural Evolution of Biomass Char upon Pyrolysis as Revealed by the Raman Spectroscopy and Elemental Analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Zuo, S.; Yang, J.; Liu, J. Effects of the Heating History of Impregnated Lignocellulosic Material on Pore Development during Phosphoric Acid Activation. Carbon 2010, 48, 3293–3295. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.C.; Lv, Y.K.; Bao, W.R. Process Effects on Activated Carbon with Large Specific Surface Area from Corn Cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Aarab, N.; Hsini, A.; Essekri, A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Removal of an Emerging Pharmaceutical Pollutant (Metronidazole) Using PPY-PANi Copolymer: Kinetics, Equilibrium and DFT Identification of Adsorption Mechanism. Groundw. Sustain. Dev. 2020, 11, 100416. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Rodríguez-Ramos, I.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Villela-Martínez, D.E. Effect of Surface Area and Physical–Chemical Properties of Graphite and Graphene-Based Materials on Their Adsorption Capacity towards Metronidazole and Trimethoprim Antibiotics in Aqueous Solution. Chem. Eng. J. 2020, 402, 126155. [Google Scholar] [CrossRef]
- Hendrasarie, N.; Maria, S.H. Combining Grease Trap and Moringa Oleifera as Adsorbent to Treat Wastewater Restaurant. S. Afr. J. Chem. Eng. 2021, 37, 196–205. [Google Scholar] [CrossRef]
- Rudzinski, W.; Everett, D.H. Adsorption of Gases on Heterogeneous Surfaces; Academic Press: London, UK, 1992. [Google Scholar]
- Habibi, A.; Belaroui, L.S.; Bengueddach, A.; López Galindo, A.; Sainz Díaz, C.I.; Peña, A. Adsorption of Metronidazole and Spiramycin by an Algerian Palygorskite. Effect of Modification with Tin. Microporous Mesoporous Mater. 2018, 268, 293–302. [Google Scholar] [CrossRef]
- Araujo, L.A.; Bezerra, C.O.; Cusioli, L.F.; Silva, M.F.; Nishi, L.; Gomes, R.G.; Bergamasco, R. Moringa oleifera Biomass Residue for the Removal of Pharmaceuticals from Water. J. Environ. Chem. Eng. 2018, 6, 7192–7199. [Google Scholar] [CrossRef]
- Kariim, I.; Abdulkareem, A.S.; Abubakre, O.K. Development and Characterization of MWCNTs from Activated Carbon as Adsorbent for Metronidazole and Levofloxacin Sorption from Pharmaceutical Wastewater: Kinetics, Isotherms and Thermodynamic Studies. Sci. Afr. 2020, 7, e00242. [Google Scholar] [CrossRef]
- Xavier, C.S.F.; Vieira, F.F.; Alves, M.P.; de Sousa, J.T. Use of Sugarcane Bagasse in the Adsorption of Textile Dyes in Aqueous Solutions. Res. Soc. Dev. 2021, 10, e59110716974. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in Water Pollution Research: Removal of Biologically Resistant Pollutant from Waste Water by Adsorption. In Proceedings of the 1st International Conference on Water Pollution; Pergamon Press: Oxford, UK, 1962; Volume 2, pp. 231–266. [Google Scholar]
- Juang, R.S.; Wu, F.C.; Tseng, R.L. Characterization and Use of Activated Carbons Prepared from Bagasses for Liquid-Phase Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2002, 201, 191–199. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L. The Adsorption of Basic Dyes from Aqueous Solution on Modified Peat–Resin Particle. Water Res. 2003, 37, 1535–1544. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambrigde University: New York, NY, USA, 1997. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Kazemian, H.; Ghahramani, E.; Amrane, A.; Sivasankar, V.; Zarrabi, M. Defluoridation of Water via Light Weight Expanded Clay Aggregate (LECA): Adsorbent Characterization, Competing Ions, Chemical Regeneration, Equilibrium and Kinetic Modeling. J. Taiwan Inst. Chem. Eng. 2014, 45, 1821–1834. [Google Scholar] [CrossRef]
- Kalhori, E.M.; Al-Musawi, T.J.; Ghahramani, E.; Kazemian, H.; Zarrabi, M. Enhancement of the Adsorption Capacity of the Light-Weight Expanded Clay Aggregate Surface for the Metronidazole Antibiotic by Coating with MgO Nanoparticles: Studies on the Kinetic, Isotherm, and Effects of Environmental Parameters. Chemosphere 2017, 175, 8–20. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Microporous Activated Carbon from Siris Seed Pods by Microwave-Induced KOH Activation for Metronidazole Adsorption. J. Anal. Appl. Pyrolysis 2013, 99, 101–109. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Ocampo-Pérez, R.; Leyva-Ramos, R.; Rivera-Utrilla, J. Removal of the Antibiotic Metronidazole by Adsorption on Various Carbon Materials from Aqueous Phase. J. Colloid Interface Sci. 2014, 436, 276–285. [Google Scholar] [CrossRef]
- Ma, F.; Peng, C.; Hou, D.; Wu, B.; Zhang, Q.; Li, F.; Gu, Q. Citric Acid Facilitated Thermal Treatment: An Innovative Method for the Remediation of Mercury Contaminated Soil. J. Hazard. Mater. 2015, 300, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Bulmău, C.; Mărculescu, C.; Lu, S.; Qi, Z. Analysis of Thermal Processing Applied to Contaminated Soil for Organic Pollutants Removal. J. Geochem. Explor. 2014, 147, 298–305. [Google Scholar] [CrossRef]






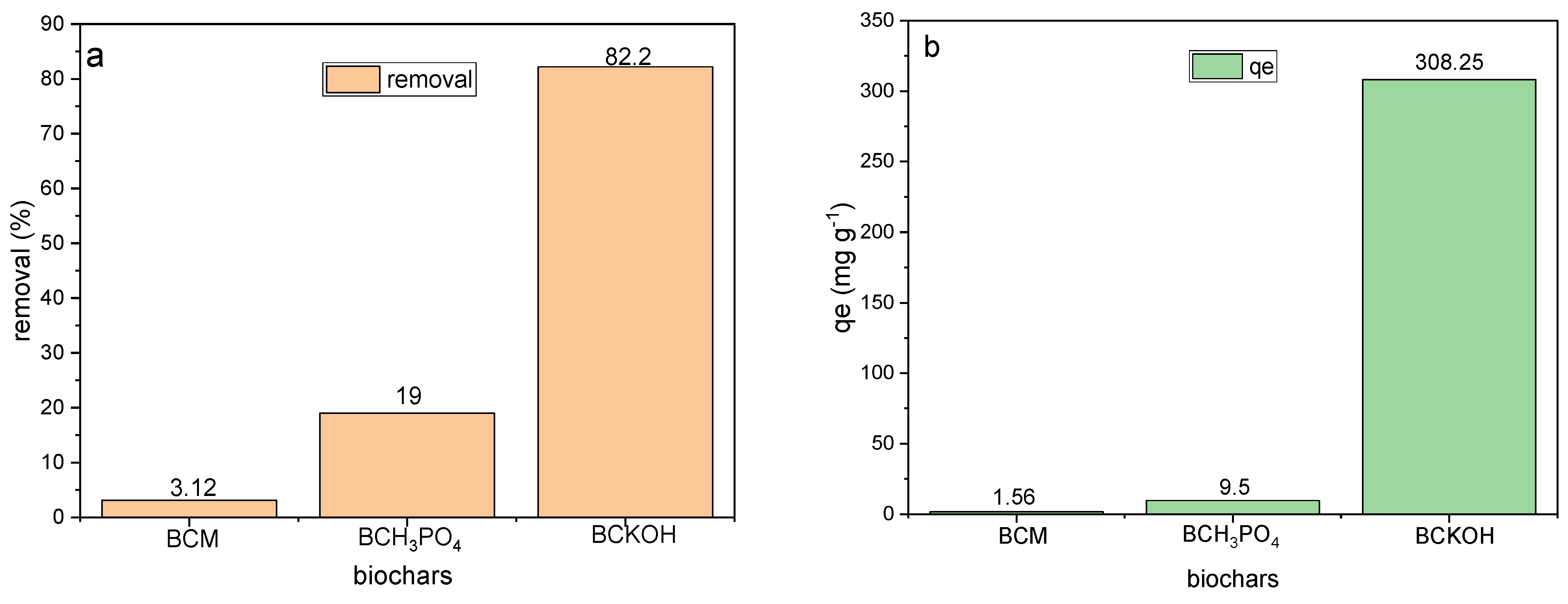




| Technique | Methodology | Equipment |
|---|---|---|
| pHpzc | Methodology based on the “11 points” method proposed by [22] | - |
| SEM | The High and Low Vacuum | FEI Quanta 250 (Hillsboro, OR, USA) |
| XRD | The analysis conditions used a 40 KV copper tube lamp and a 30 mA current at a preset counting time of 120.32 s with the initial 2Θ of 5° and 80° at the end. The X’Pert HighScore 2.1.1 software database was used to understand the analysis results. | X-ray diffractometry (BRUKER) equipment |
| TGA and DTG | - | SDT Q600 V20.9 Build 20 (TA instruments, New Castle, DE, USA) |
| Nitrogen adsorption/desorption | Where the samples were dried at 150 °C for 3 h. The specific surface (SBET) and pore volume were determined using the Brunauer–Emmett-Teller (BET) method, where the total pore volume (Vp) was calculated by the P/Po ratio. | Micromeritics, ASAP 2020 PLUS (Norcross, GA, USA) |
| FTIR | Spectral range of 4000 to 400 cm−1, with a resolution of 4 cm−1 and 124 scans. | Bruker’s Vertex 70v |
| RAMAN | Spectral range of 800–1800 cm−1, with the laser at a wavelength of 532 nm with a power of 20 mW, at a resolution of 9–15 cm−1. The baseline has been corrected using OPUS 7.5 software (Bruker, Ettlingen, Alemanha). | Bruker’s Micro Raman Senterra |
| Race Order | Temperature °C | Heating Rate °C Min−1 | Residence Time (Min) | C% | Yield (%) |
|---|---|---|---|---|---|
| 2 | 500 (−1) | 10 (−1) | 40 (−1) | 72.97 | 35.0 |
| 7 | 700 (1) | 10 (−1) | 40 (−1) | 76.63 | 32.69 |
| 1 | 600 (0) | 15 (0) | 60 (0) | 77.34 | 31.92 |
| 3 | 700 (1) | 10 (−1) | 80 (1) | 77.48 | 30.76 |
| 4 | 500 (−1) | 20 (1) | 80 (1) | 75.29 | 38.07 |
| 6 | 500 (−1) | 20 (1) | 40 (−1) | 73.62 | 36.92 |
| 5 | 700 (1) | 20 (1) | 40 (−1) | 78.39 | 31.53 |
| 9 | 500 (−1) | 10 (−1) | 80 (1) | 73.80 | 31.53 |
| 8 | 700 (1) | 20 (1) | 80 (1) | 77.44 | 36.53 |
| Yield | |||||
|---|---|---|---|---|---|
| Factor | Sum of Squares | Degrees of Freedom | Mean Square | F-Statistic | p-Value |
| (1) Temperature | 49.75031 | 1 | 49.75031 | 19.88611 | 0.046786 |
| (2) Heating rate | 1.22461 | 1 | 1.22461 | 0.48950 | 0.556575 |
| (3) Time | 0.07031 | 1 | 0.07031 | 0.02811 | 0.882281 |
| 1 and 2 | 1.85281 | 1 | 1.85281 | 0.74060 | 0.480160 |
| 1 and 3 | 2.58781 | 1 | 2.58781 | 1.03440 | 0.416142 |
| 2 and 3 | 0.32401 | 1 | 0.32401 | 0.12951 | 0.753386 |
| Error | 5.00352 | 2 | 2.50176 | ||
| TSQ | 60.81340 | 8 | |||
| Carbon Content | |||||
|---|---|---|---|---|---|
| Factor | Sum of Squares | Degrees of Freedom | Mean Square | F-Statistic | p-Value |
| (1) Temperature | 37.97561 | 1 | 37.97561 | 37.52505 | 0.025629 |
| (2) Heating rate | 1.43651 | 1 | 1.43651 | 1.41947 | 0.355707 |
| (3) Time | 0.44651 | 1 | 0.44651 | 0.44121 | 0.574870 |
| 1 and 2 | 0.03001 | 1 | 0.03001 | 0.02966 | 0.879122 |
| 1 and 3 | 1.54001 | 1 | 1.54001 | 1.52174 | 0.342658 |
| 2 and 3 | 0.17701 | 1 | 0.17701 | 0.17491 | 0.716411 |
| Error | 2.02401 | 2 | 1.01201 | ||
| TSQ | 43.62969 | 8 | |||
| BCM | BCH3PO4 | BCKOH | |
|---|---|---|---|
| SBET (m² g−1) | 1.78 | 21.37 | 1135.14 |
| Vp (cm3 g−1) | 0 | 0.01 | 0.61 |
| SMESOP (m² g−1) | 0.17 | 13.76 | 358.71 |
| VMICROP (cm3 g−1) | 0 | 0 | 0.37 |
| VMESOP (cm3 g−1) | 0 | 0.01 | 0.24 |
| Biochar | Band D | Band G | R | ||
|---|---|---|---|---|---|
| Raman Shift (cm−1) | Intensity | Raman Shift (cm−1) | Intensity | ID/IG | |
| BCM | 1330 | 1763 | 1576 | 2141 | 0.823 |
| BCH3PO4 | 1330 | 284 | 1584 | 372 | 0.763 |
| BCKOH | 1330 | 335 | 1583 | 360 | 0.93 |
| Model | Metronidazole BCH3PO4 | Metronidazole BCKOH | |||||
|---|---|---|---|---|---|---|---|
| 30 mg L−1 | 45 mg L−1 | 60 mg L−1 | 150 mg L−1 | 250 mg L−1 | 350 mg L−1 | ||
| Pseudo-first order | qt (mg g−1) | 0.239 | 0.269 | 0.382 | 0.750 | 0.842 | 0.524 |
| k1 (min−1) | 7.13 | 7.80 | 7.5 | 165.84 | 243.2 | 279.8 | |
| R² | 0.77 | 0.90 | 0.89 | 0.958 | 0.954 | 0.935 | |
| Adj. R² | 0.749 | 0.89 | 0.885 | 0.954 | 0.95 | 0.927 | |
| Pseudo-second order | qt (mg g−1) | 0.045 | 0.053 | 0.075 | 0.00816 | 0.0068 | 0.0029 |
| k2 (g mg−1 min−1) | 7.52 | 8.17 | 7.82 | 170.9 | 248.5 | 291.8 | |
| R2 | 0.88 | 0.97 | 0.96 | 0.985 | 0.977 | 0.976 | |
| Adj. R² | 0.873 | 0.967 | 0.96 | 0.983 | 0.974 | 0.973 | |
| Elovich | A (g mg−1 min−1) | 74.49 | 298.1 | 1675 | 3.266 | 1.78 | 3.21 |
| B (mg g−1) | 1.29 | 1.35 | 1.673 | 0.11 | 0.086 | 0.049 | |
| R2 | 0.987 | 0.988 | 0.993 | 0.996 | 0.998 | 0.995 | |
| Adj. R² | 0.986 | 0.986 | 0.992 | 0.996 | 0.997 | 0.995 | |
| BCH3PO4 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | ||||||
| kdif (mg g−1 min−0.5) | C (mg g−1) | R² | kdif (mg g−1 min−0.5) | C (mg g−1) | R² | kdif (mg g−1 min−0.5) | C (mg g−1) | R² |
| 0.574 | 6.12 | 0.879 | 3.368 | 0.20 | 0.985 | 0.55 | 6.13 | 0.91 |
| BCKOH | ||||||||
| Stage 1 | Stage 2 | Stage 3 | ||||||
| kdif (mg g−1 min−0.5) | C (mg g−1) | R² | kdif (mg g−1 min−0.5) | C (mg g−1) | R² | kdif (mg g−1 min−0.5) | C (mg g−1) | R² |
| 118.05 | 20.29 | 0.886 | 11.97 | 207.1 | 0.937 | 0.6 | 264.9 | 0.92 |
| BCH3PO4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Freundlich | Sips | Langmuir | |||||||
| Kf (L mg−1) | n | R² | Ks (L mg−1) | n | Qmax (mg g−1) | R² | Kl (L mg−1) | Qmax (mg g−1) | R² |
| 1.44 | 1.985 | 0.976 | 0.011 | 0.76 | 28.64 | 0.988 | 0.017 | 24.49 | 0.987 |
| BCKOH | |||||||||
| Freundlich | Sips | Langmuir | |||||||
| Kf (L mg−1) | n | R² | Ks (L mg−1) | n | Qmax (mg g−1) | R² | Kl (L mg−1) | Qmax (mg g−1) | R² |
| 17.97 | 2.17 | 0.957 | 0.0058 | 1.45 | 396.17 | 0.998 | 0.0039 | 486.01 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, C.H.; de Araújo, T.P.; de Souza, A.T.; Scaliante, M.H.N.O.; Martins Moreira, W. Synthesis of Activated Biochar from the Bark of Moringa oleifera for Adsorption of the Drug Metronidazole Present in Aqueous Medium. Processes 2024, 12, 560. https://doi.org/10.3390/pr12030560
da Silva CH, de Araújo TP, de Souza AT, Scaliante MHNO, Martins Moreira W. Synthesis of Activated Biochar from the Bark of Moringa oleifera for Adsorption of the Drug Metronidazole Present in Aqueous Medium. Processes. 2024; 12(3):560. https://doi.org/10.3390/pr12030560
Chicago/Turabian Styleda Silva, Caio Henrique, Thiago Peixoto de Araújo, Alexandre Teixeira de Souza, Mara Heloisa Neves Olsen Scaliante, and Wardleison Martins Moreira. 2024. "Synthesis of Activated Biochar from the Bark of Moringa oleifera for Adsorption of the Drug Metronidazole Present in Aqueous Medium" Processes 12, no. 3: 560. https://doi.org/10.3390/pr12030560
APA Styleda Silva, C. H., de Araújo, T. P., de Souza, A. T., Scaliante, M. H. N. O., & Martins Moreira, W. (2024). Synthesis of Activated Biochar from the Bark of Moringa oleifera for Adsorption of the Drug Metronidazole Present in Aqueous Medium. Processes, 12(3), 560. https://doi.org/10.3390/pr12030560







