Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Suspension Calcination System
2.3. Product Analysis
2.3.1. Phase Composition and Dissolution Activity
2.3.2. Carbon Content and Decarbonization Rate
2.3.3. Kaolinite Decomposition Rate
2.3.4. System State Parameters
2.4. Performance Analysis of Cementitious Materials
3. Results and Discussion
3.1. Effects of Temperature on Product Composition and Reaction Conversion
3.1.1. Phase Composition
3.1.2. Reaction Conversion
3.2. Effect of Particle Size on Decarbonization Rate
3.3. Effect of O2 Content on Decarbonization Rate
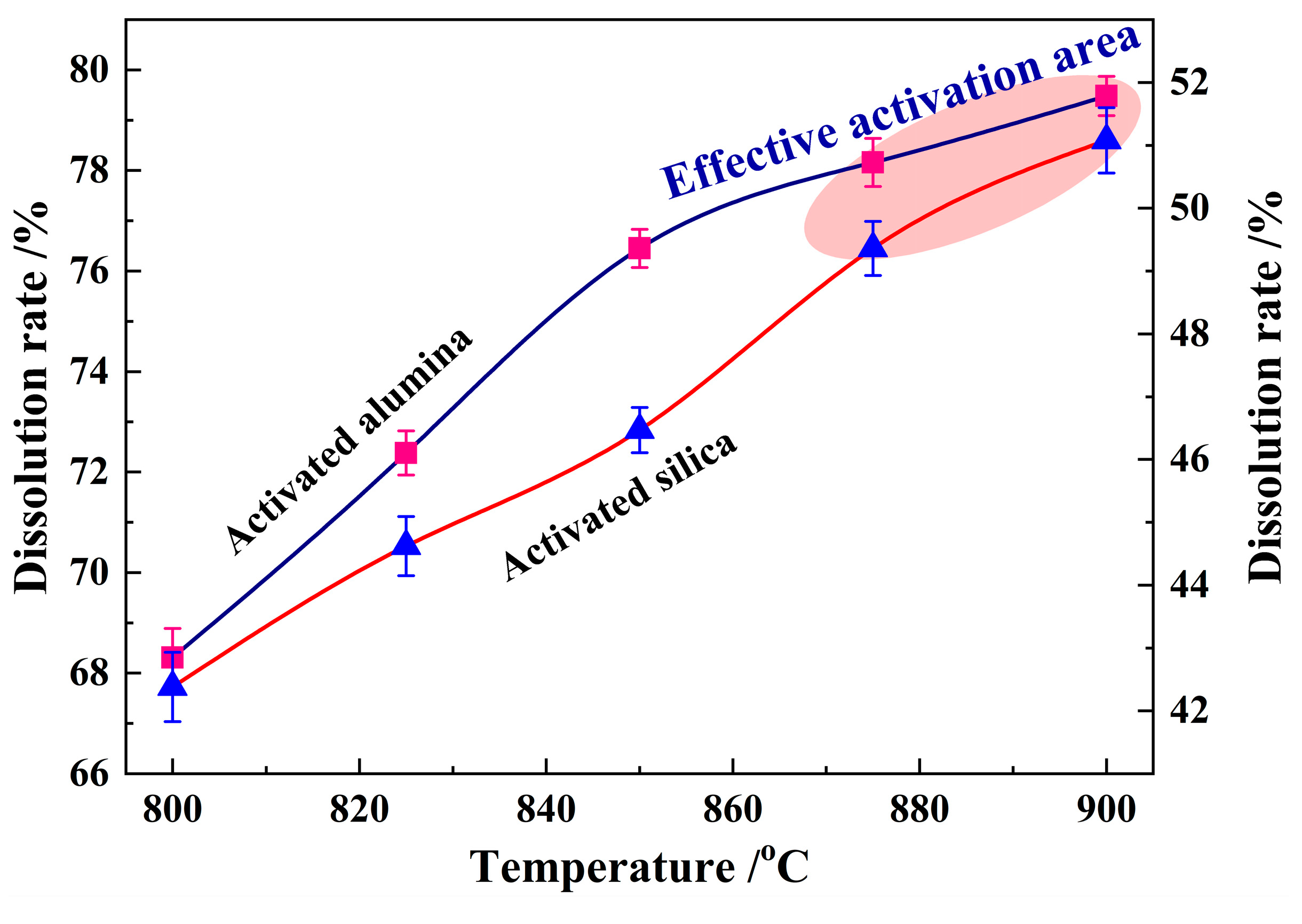
3.4. Dissolution Rate Analysis
3.5. Material and Energy Balance
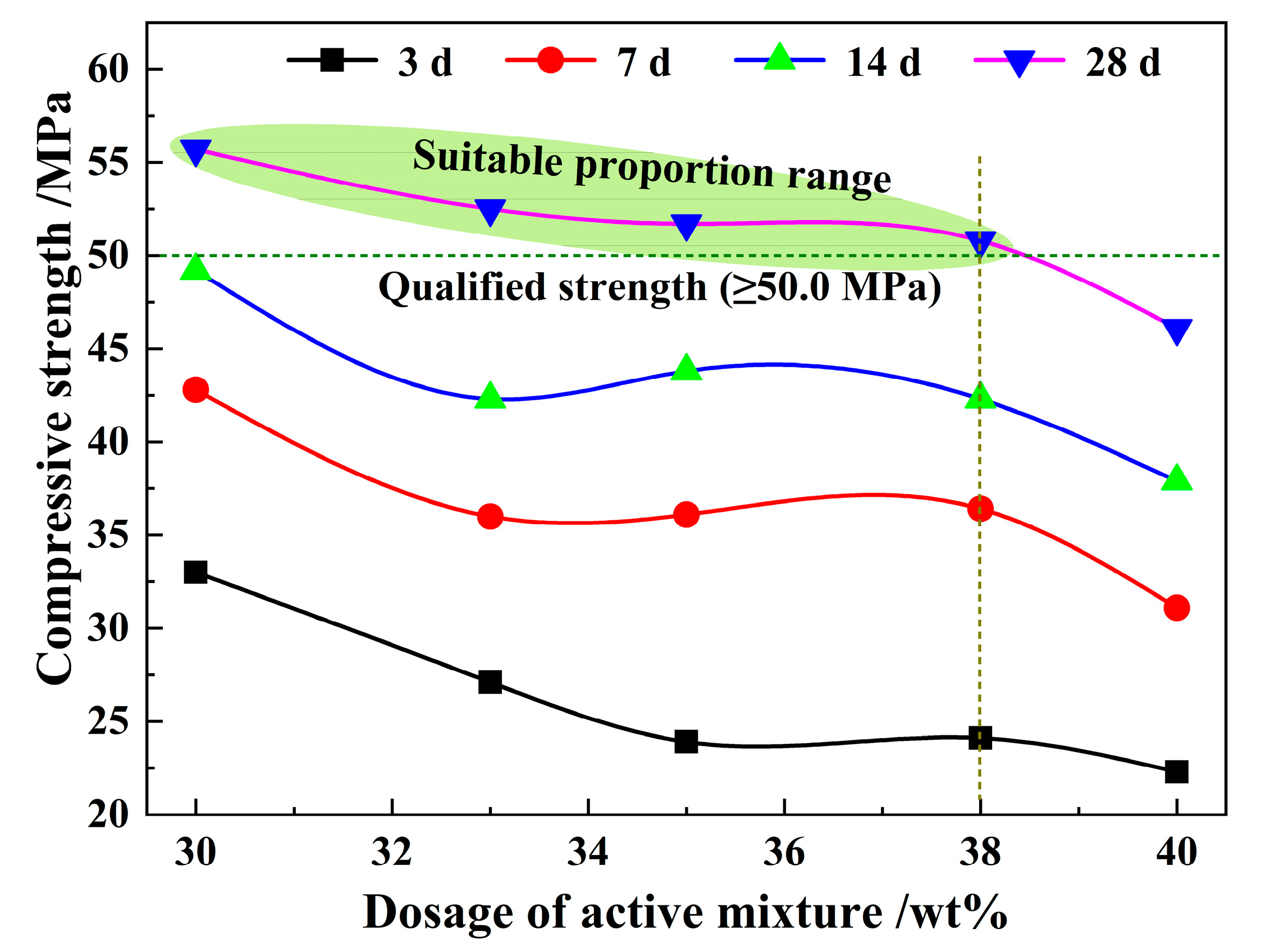
3.6. Properties of Cementitious Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q. Research progress on properties and comprehensive utilization of coal gangue. Appl. Chem. Indus. China 2023, 52, 1576–1581. [Google Scholar]
- Zhang, Y.; Ge, X.; Liu, L.; Wang, X.; Zhang, Z. Fuel nitrogen conversion and release of nitrogen oxides during coal gangue calcination. Environ. Sci. Pollut. Res. 2015, 22, 7139–7146. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Li, W.; Li, J.; Ouyang, S.; Song, T.; Lv, F.; Zhai, W.; Ma, K. Explanation of heavy metal pollution in coal mines of china from the perspective of coal gangue geochemical characteristics. Environ. Sci. Pollut. Res. 2021, 28, 65363–65373. [Google Scholar] [CrossRef]
- Li, D.; Song, X.; Gong, C.; Pan, Z. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Zunino, F.; Dhandapani, Y.; Haha, M.; Skibsted, J.; Joseph, S.; Krishnan, S.; Parashar, A.; Juenger, M.; Hanein, T.; Bernal, S.; et al. Hydration and mixture design of calcined clay blended cements: Review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 234. [Google Scholar] [CrossRef]
- Lee, S.; Moon, H.; Hooton, R.; Kim, J.P. Effect of solution concentrations and replacement levels of metakaolin on the resistance of mortars exposed to magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1314–1323. [Google Scholar] [CrossRef]
- Cassagnabère, F.; Mouret, M.; Escadeillas, G.; Broilliard, P.; Bertrand, A. Metakaolin, a solution for the precast industry to limit the clinker content in concrete: Mechanical aspects. Constr. Build. Mater. 2010, 24, 1109–1118. [Google Scholar] [CrossRef]
- Liu, X. Low-carbon utilization of coal gangue under the carbon neutralization strategy: A short review. J. Mater. Cycles. Waste. 2023, 25, 1978–1987. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Seetharaman, S.; Wang, X.; Zhang, Z. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2015, 48, 2779–2793. [Google Scholar] [CrossRef]
- Tan, W.; Wang, L.; Huang, C. Environmental effects of coal gangue and its utilization. Energ. Source. Part A 2016, 38, 3716–3721. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L.; Køhler, L.L. Experimental and modeling study of flash calcination of kaolinite rich clay particles in a gas suspension calciner. Appl. Clay Sci. 2015, 103, 10–19. [Google Scholar] [CrossRef]
- Xia, W.; Xie, G.; Peng, Y. Recent advances in beneficiation for low rank coals. Powder Technol. 2015, 277, 206–221. [Google Scholar] [CrossRef]
- Duan, C.; Zhou, C.; Dong, L.; Zhao, Y.; Liu, Q. A novel dry beneficiation technology for pyrite recovery from high sulfur gangue. J. Clean Prod. 2018, 172, 2475–2484. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitiousmaterial. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Cheng, S.; Jiu, S.; Li, H. Preparation of calcined kaolin by efficient decarburization of coal-series kaolinite in a suspended bed reactor. Processes 2022, 10, 2048. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Cheng, S.; Wu, Z.; Wang, B. Performance and hydration mechanism of modified tabia with composite-activated coal gangue. Crystals 2022, 12, 150. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2021, 318, 125999. [Google Scholar] [CrossRef]
- Chen, J.; Guan, X.; Zhu, M.; Gao, J. Mechanism on activation of coal gangue admixture. Adv. Civ. Eng. 2021, 2021, 5436482. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, L. Hardening mechanisms of alkali activated burned gangue cementitious material. Mater. Sci. Tech. 2004, 12, 597–601. [Google Scholar]
- Zhao, Y.; Qiu, J.; Ma, Z.; Sun, X. Eco-friendly treatment of coal gangue for its utilization as supplementary cementitious materials. J. Clean Prod. 2021, 285, 124834. [Google Scholar] [CrossRef]
- Gao, X.; Liu, C.; Shui, Z.; Yu, R. Effects of expansive additives on the shrinkage behavior of coal gangue based alkali activated materials. Crystals 2021, 11, 816. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, Z.; Fu, Y.; Zhou, R. FeCu-coal gangue heterogeneous activation of peracetic acid for degradation of sulfamethoxazole. J. Environ. Chem. Eng. 2023, 11, 110007. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration kinetics of cementitious materials composed of red mud and coal gangue. Int. J. Min. Met. Mater. 2016, 23, 1215–1224. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.; Sun, H.; Li, L. Investigation on the activation of coal gangue by a new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of limestone calcined clay cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, T.; Wang, M.; Wu, Y.Y. Synergic performance of low-kaolinite calcined coal gangue blended with limestone in cement mortars. Constr. Build. Mater. 2021, 300, 124012. [Google Scholar] [CrossRef]
- Jiu, S.; Wang, M.; Chen, Y.; Chen, J.; Gao, Q. Synthesis and characterization of low-carbon cementitious materials from suspended calcined coal gangue. Front. Mater. 2022, 9, 982861. [Google Scholar] [CrossRef]
- Cheng, S.; Jiu, S.; Li, H. Kinetics of Dehydroxylation and decarburization of coal series kaolinite during calcination: A novel kinetic method based on gaseous products. Materials 2021, 14, 1493. [Google Scholar] [CrossRef]
- Jiu, S.; Cheng, S.; Li, H.; Wang, L. Reaction mechanism of metakaolin materials prepared by calcining coal gangue. Mater. Res. Express. 2021, 8, 015508. [Google Scholar] [CrossRef]
- GB/T 212-2008; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China. Proximate Analysis of Coal. China Standard Publishing House: Beijing, China, 2008; pp. 1–11, ISBN 155066134278.
- Zhen, J.; Zhou, T.; Chen, X. Study on the reactivity and its fast determination method of metakaoline in synthesizing geopolymer. B. Chin. Ceram. Soc. China 2007, 26, 887–900. [Google Scholar]
- Qiu, G.; Pang, S. Spectrophotometric determination of activated aluminium in soil using eriochrome cyanine R. J. Instrum. Anal. China 1989, 8, 68–71. [Google Scholar]
- GB/T 17671-2021; The State Administration for Market Regulation (China), National Standardization Administration (China); Standardization Administration of the People’s Republic of China. Test method of cement mortar strength (ISO method). China Standard Publishing House: Beijing, China, 2021; pp. 1–17, ISBN 155066169021.
- Jiu, S.; Lin, M.; Zhao, B.; Chen, Y.; Yang, C. Pilot study on a new conveyor bed magnetization roasting process for efficient Iron extraction from low-grade siderite. Processes 2023, 11, 1020. [Google Scholar] [CrossRef]
- GB 175-2023; Standardization Administration of the People’s Republic of China. Common Portland Cement. China Standard Publishing House: Beijing, China, 2023; pp. 1–8, ISBN 173736.


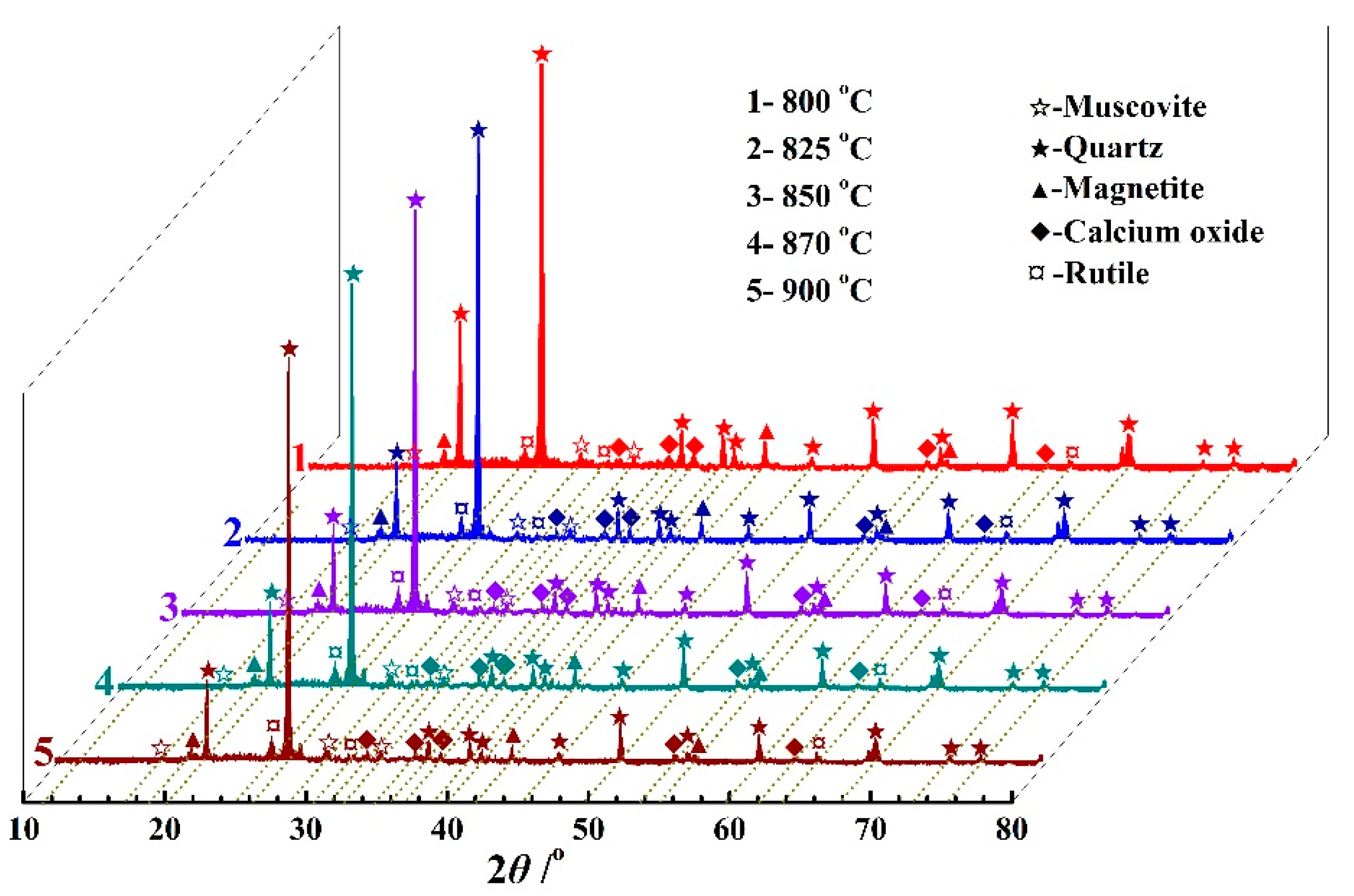
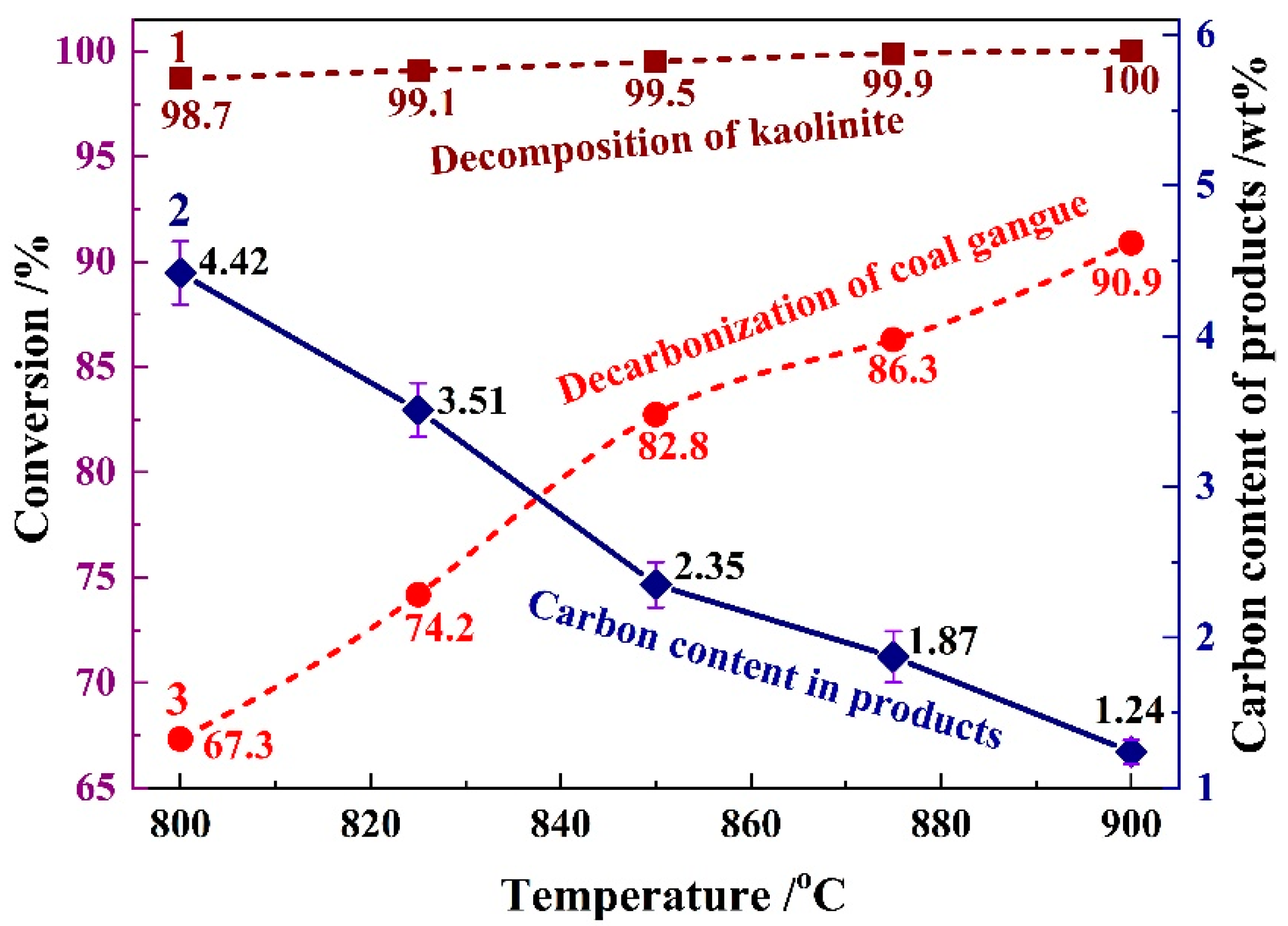
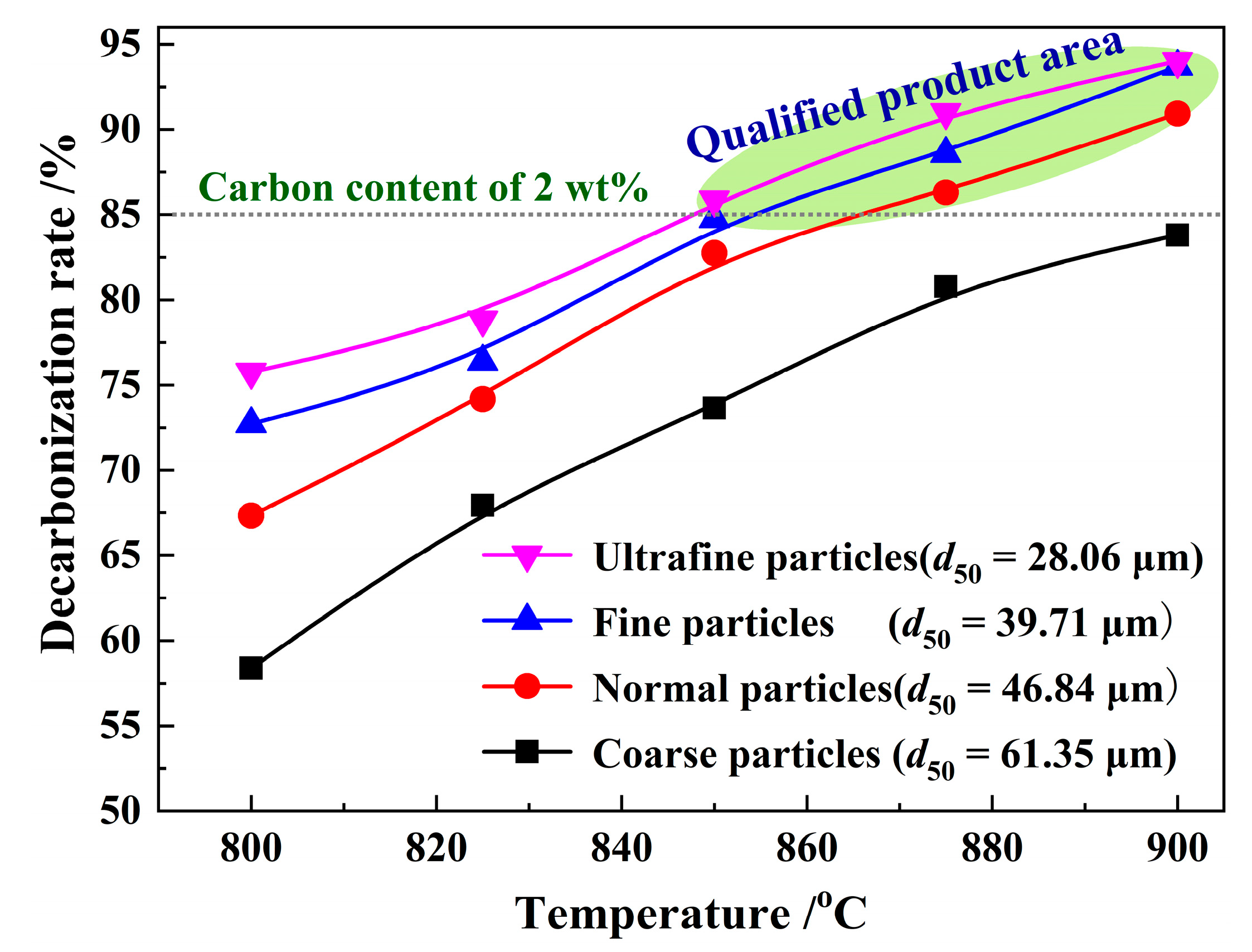
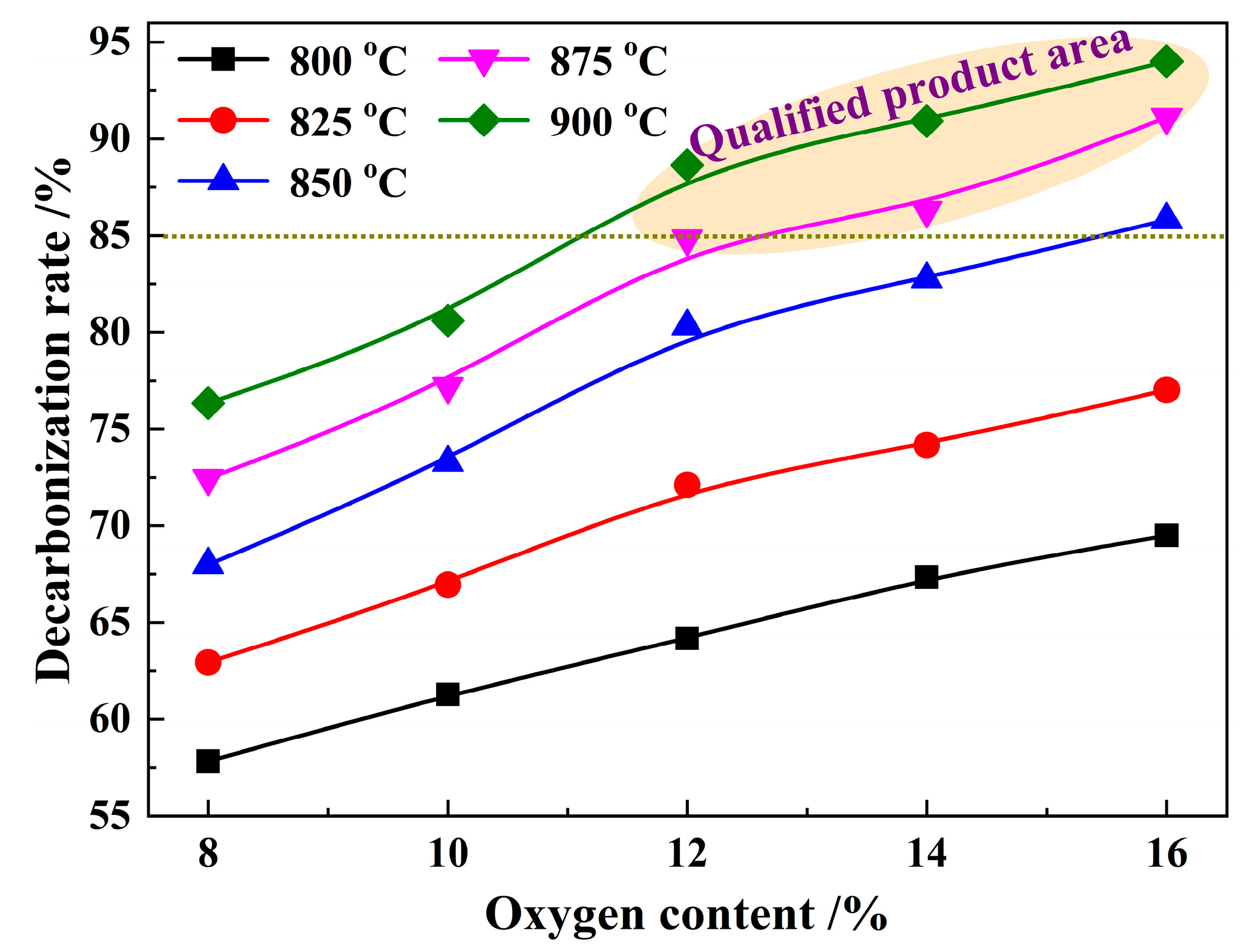

| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | MgO | Na2O | SO3 | MnO | P2O5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 49.14 | 28.14 | 15.93 | 2.08 | 1.67 | 1.05 | 0.626 | 0.312 | 0.286 | 0.286 | 0.142 | 99.66 |
| Moisture Mad (%) | Ash (wt%) | Volatile (wt%) | Fixed Carbon (wt%) | Lower Calorific Value (kJ/kg) | |||
|---|---|---|---|---|---|---|---|
| Aad | Ad | Vad | Vd | Vdaf | |||
| 0.48 | 71.82 | 72.17 | 14.62 | 15.18 | 52.79 | 13.08 | 4233.44 |
| Kaolinite | Quartz | Siderite | Calcite | Rutile | Coal |
|---|---|---|---|---|---|
| 52.31 | 11.77 | 16.98 | 2.73 | 0.77 | 13.08 |
| Serial Number | Clinker | Desulfurized Gypsum | Calcined Coal Gangue | Limestone | Mixing Material Dosage |
|---|---|---|---|---|---|
| 0 | 95 | 5 | 0 | 0 | 0 |
| 1 | 65 | 5 | 20 | 10 | 30 |
| 2 | 62 | 5 | 22 | 11 | 33 |
| 3 | 60 | 5 | 25 | 10 | 35 |
| 4 | 57 | 5 | 24 | 14 | 38 |
| 5 | 55 | 5 | 30 | 10 | 40 |
| Mass of Input | kg/kg | Percentage/(%) | Mass of Output | kg/kg | Percentage/(%) |
|---|---|---|---|---|---|
| Coal gangue | 1.000 | 19.16 | Products | 0.527 | 10.10 |
| Air | 1.848 | 35.41 | Exhaust gas | 4.371 | 83.75 |
| Pulverized coal | 0.068 | 1.30 | Fly ash | 0.321 | 6.15 |
| Air into the cooler | 2.105 | 40.33 | |||
| Air leakage | 0.198 | 3.79 | |||
| Total mass of input | 5.219 | 100.00 | Total mass of output | 5.219 | 100.00 |
| Heat of Input | kJ/kg | Percentage/(%) | Heat of Output | kJ/kg | Percentage/(%) |
|---|---|---|---|---|---|
| Sensible heat of coal gangue | 23.2 | 0.51 | Sensible heat of products | 55.1 | 1.20 |
| Combustion heat | 2860.7 | 62.36 | Sensible heat of exhaust gas | 1013.2 | 22.09 |
| Sensible heat of air | 1.7 | 0.04 | Sensible heat of fly ash | 88.0 | 1.92 |
| Sensible heat of pulverized coal | 1581.0 | 34.46 | Reaction heat absorption | 1818.9 | 39.65 |
| Sensible heat of air into the cooler | 61.4 | 1.34 | Surface heat dissipation | 1612.5 | 35.15 |
| Sensible heat of air leakage | 59.7 | 1.30 | |||
| Total heat of input | 4587.7 | 100.00 | Total heat of output | 4587.7 | 100.00 |
| Serial Number | Specific Surface Area (m2/g) | Water–Cement Ratio (%) | Initial Setting Time (min) | Final Setting Time (min) | Soundness |
|---|---|---|---|---|---|
| 0 | 378.4 | 28.1 | 204 | 224 | Qualified |
| 1 | 377.6 | 29.2 | 180 | 238 | Qualified |
| 2 | 381.8 | 29.6 | 194 | 255 | Qualified |
| 3 | 378.1 | 29.3 | 172 | 257 | Qualified |
| 4 | 378.1 | 29.4 | 217 | 275 | Qualified |
| 5 | 380.5 | 29.9 | 204 | 273 | Qualified |
| Serial Number | 3 d | 7 d | 14 d | 28 d | ||||
|---|---|---|---|---|---|---|---|---|
| Flexural Strength /(MPa) | Compressive Strength /(MPa) | Flexural Strength /(MPa) | Compressive Strength /(MPa) | Flexural Strength /(MPa) | Compressive Strength /(MPa) | Flexural Strength /(MPa) | Compressive Strength /(MPa) | |
| 0 | 8.8 | 34.8 | 9.8 | 41.6 | 10.6 | 42.0 | 11.9 | 51.4 |
| 1 | 5.9 | 33.0 | 7.1 | 42.8 | 7.5 | 49.2 | 8.1 | 55.7 |
| 2 | 4.7 | 27.1 | 6.6 | 36.0 | 7.0 | 42.3 | 8.2 | 52.5 |
| 3 | 4.9 | 23.9 | 5.8 | 36.1 | 6.8 | 43.8 | 6.8 | 51.7 |
| 4 | 4.8 | 24.1 | 6.6 | 36.4 | 6.7 | 42.3 | 7.7 | 50.8 |
| 5 | 4.7 | 22.3 | 5.5 | 31.1 | 6.5 | 37.9 | 6.9 | 46.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jiu, S.; Gao, Q.; Zhao, S.; Chen, Y.; Cheng, F.; Han, D.; Shi, R.; Yuan, K.; Li, J.; et al. Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study. Processes 2024, 12, 550. https://doi.org/10.3390/pr12030550
Zhang H, Jiu S, Gao Q, Zhao S, Chen Y, Cheng F, Han D, Shi R, Yuan K, Li J, et al. Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study. Processes. 2024; 12(3):550. https://doi.org/10.3390/pr12030550
Chicago/Turabian StyleZhang, Hongbo, Shaowu Jiu, Qianwen Gao, Sijun Zhao, Yanxin Chen, Feng Cheng, Ding Han, Ruihong Shi, Kaixin Yuan, Jiacheng Li, and et al. 2024. "Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study" Processes 12, no. 3: 550. https://doi.org/10.3390/pr12030550
APA StyleZhang, H., Jiu, S., Gao, Q., Zhao, S., Chen, Y., Cheng, F., Han, D., Shi, R., Yuan, K., Li, J., Li, Y., Wang, Z., & Zhao, B. (2024). Activation of Low-Quality Coal Gangue Using Suspension Calcination for the Preparation of High-Performance Low-Carbon Cementitious Materials: A Pilot Study. Processes, 12(3), 550. https://doi.org/10.3390/pr12030550








