Polyelectrolyte Platforms with Copper Nanoparticles as a Multifunctional System Aimed at Healing Process Support
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Polyelectrolyte Layer Coatings
- Copper colloid at 50 ppm from the bulk solution;
- CuNP solution at 1000 ppm, prepared from copper nanopowder and deionized water (MilliQ) with 0.1% Triton-X. The solution was sonicated in a sonication water bath for a total of 11 h at proper intervals to avoid overheating the solution.
2.2. Cell Culture
2.3. Cell’s Functioning Evaluation
2.4. Fluorescence Staining
2.5. Scanning Electron Microscopy Analysis
2.6. SEM-EDX Studies
2.7. Statistical Analysis
3. Results
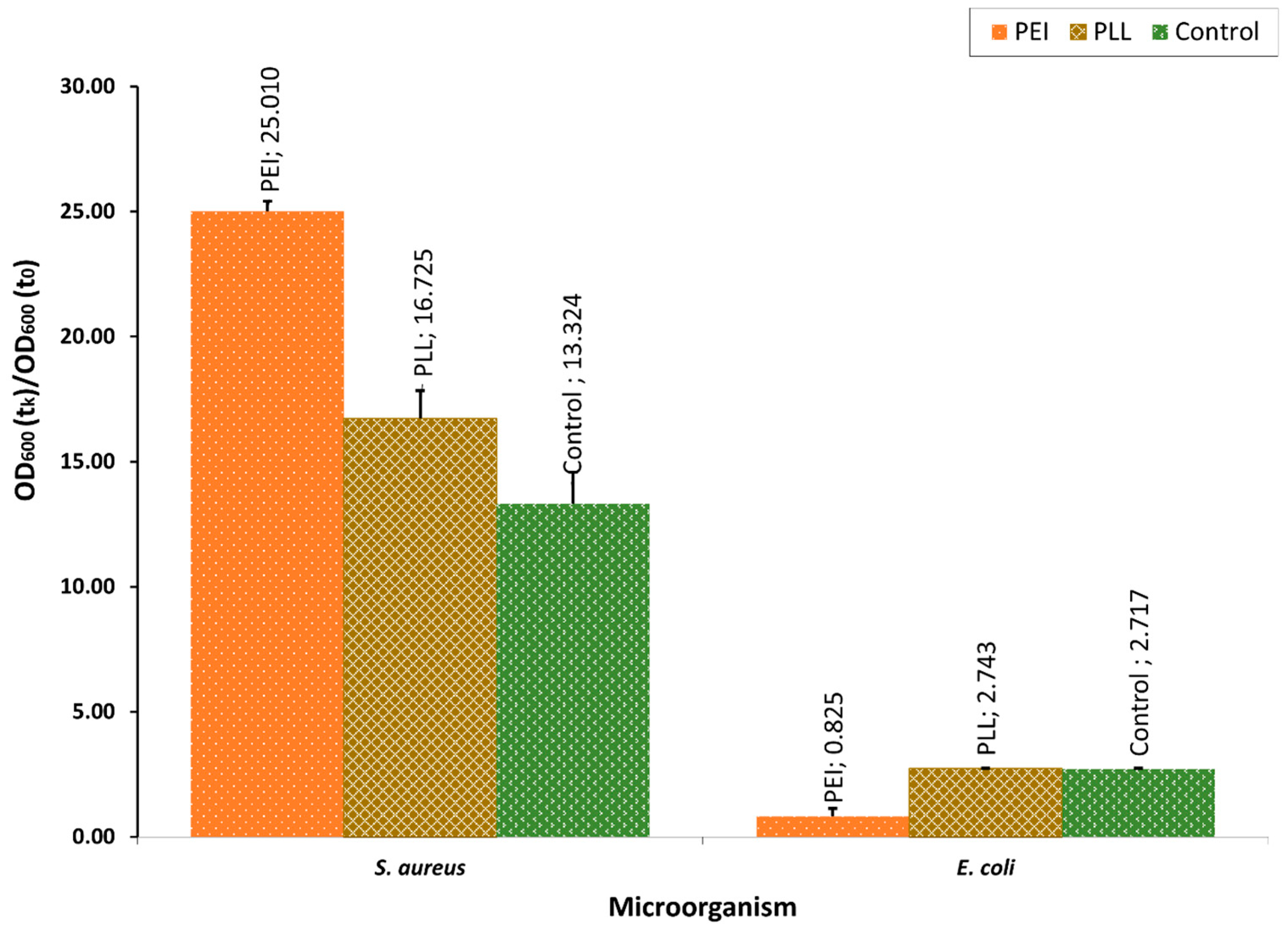
3.1. The Polyelectrolyte Layer Coatings’ Bacteriostatic Effect Evaluation
3.2. Characterization of Polyelectrolyte Layer Coatings
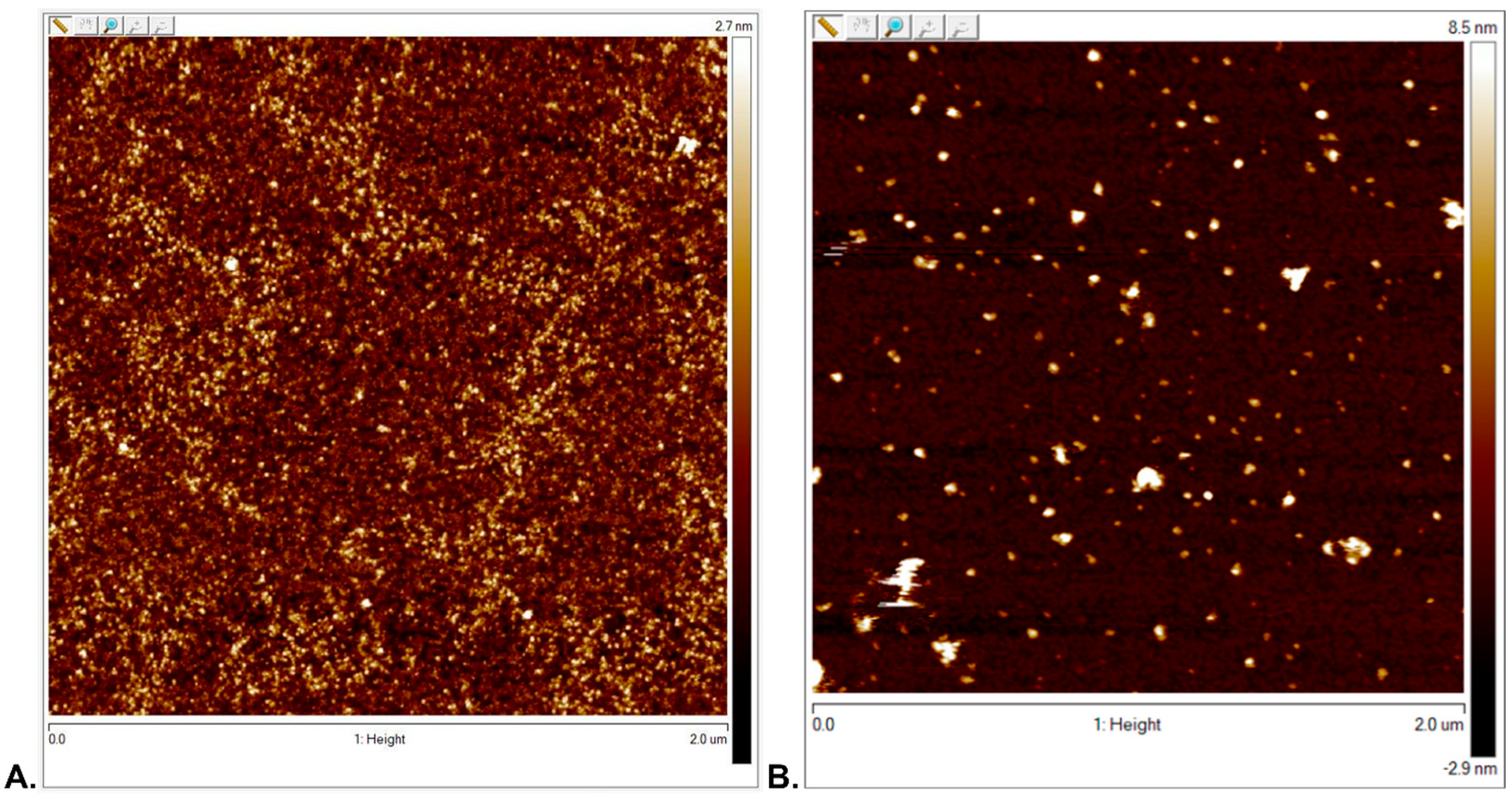
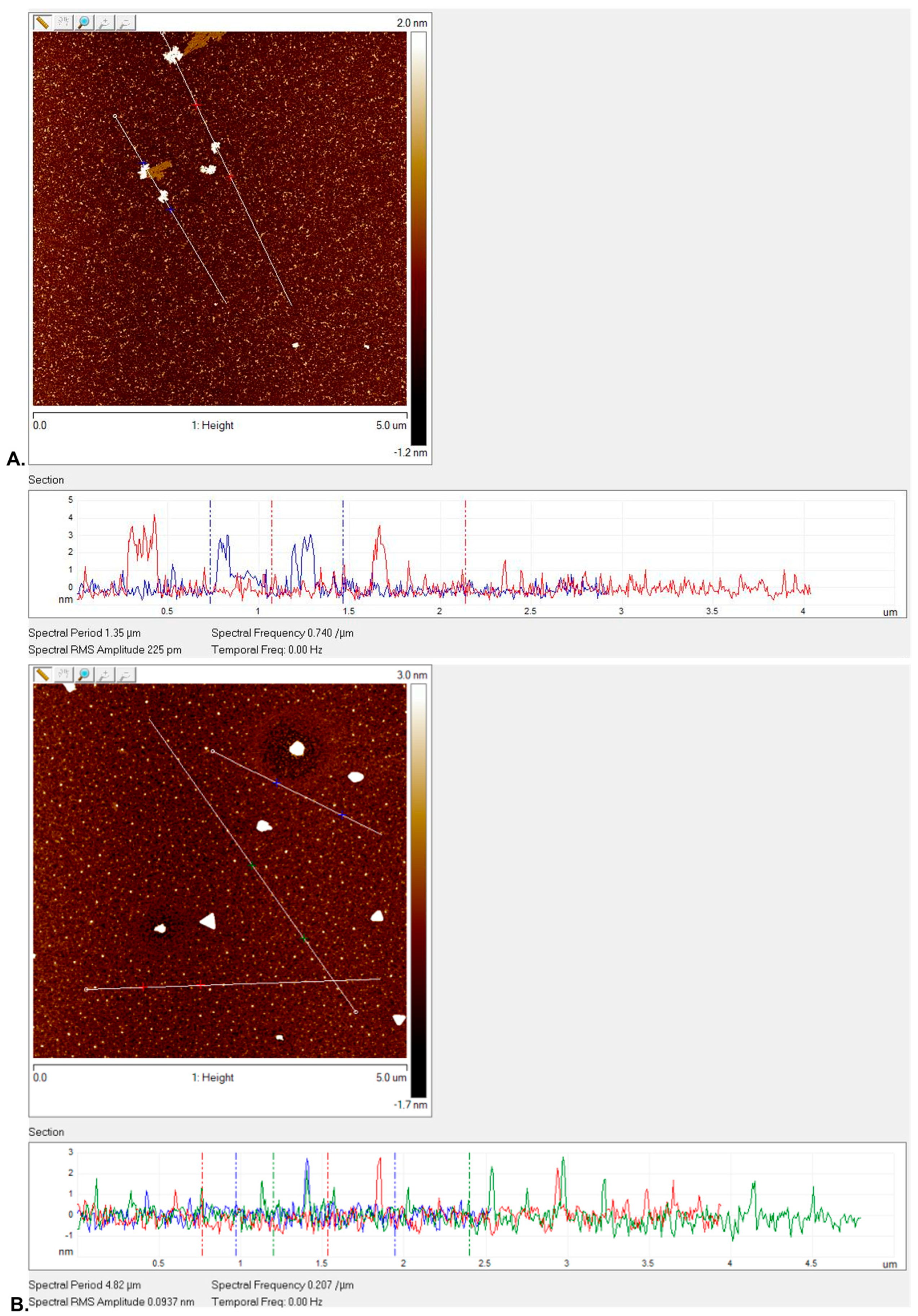
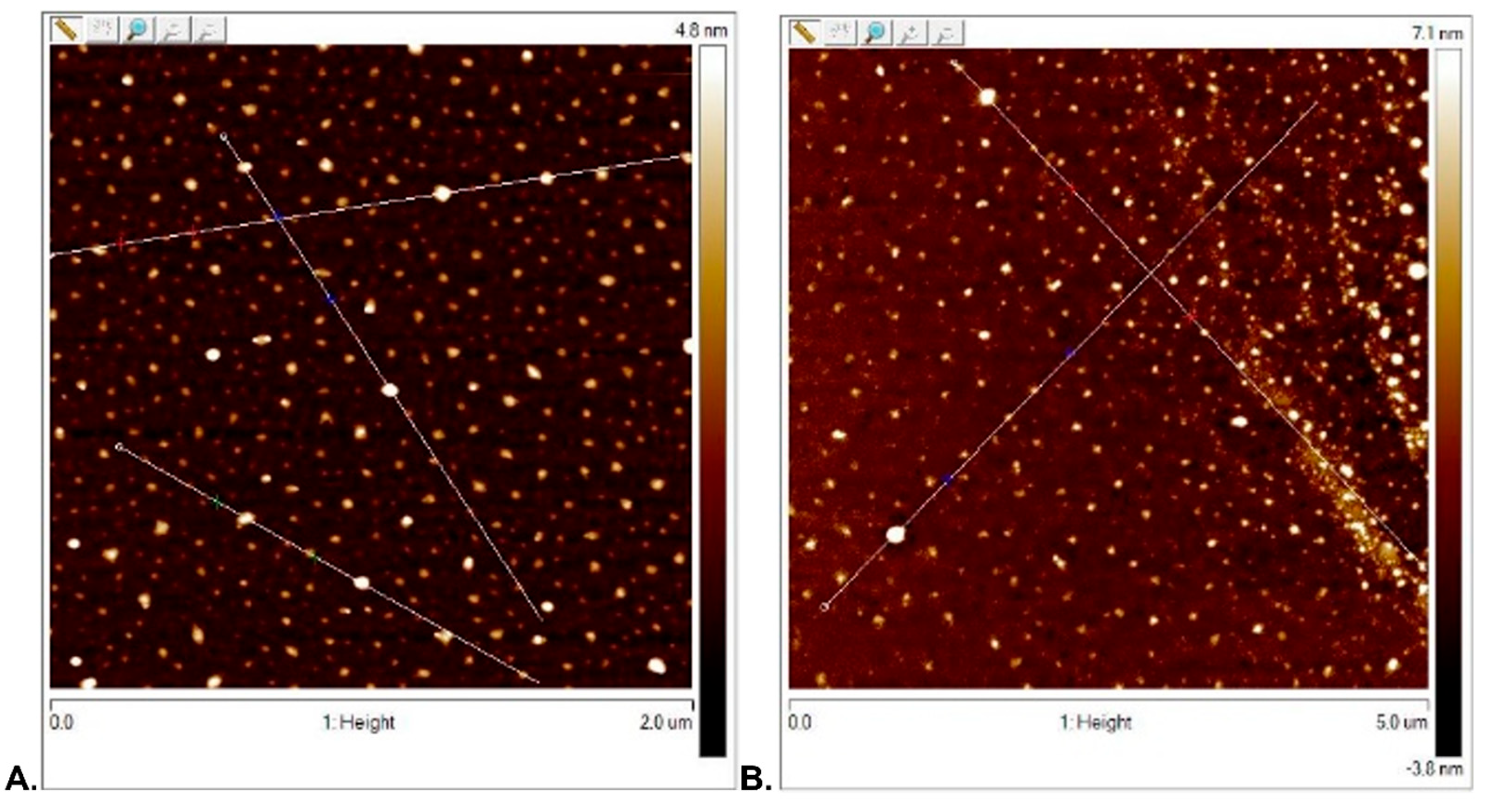
3.2.1. Surface Topography Analysis
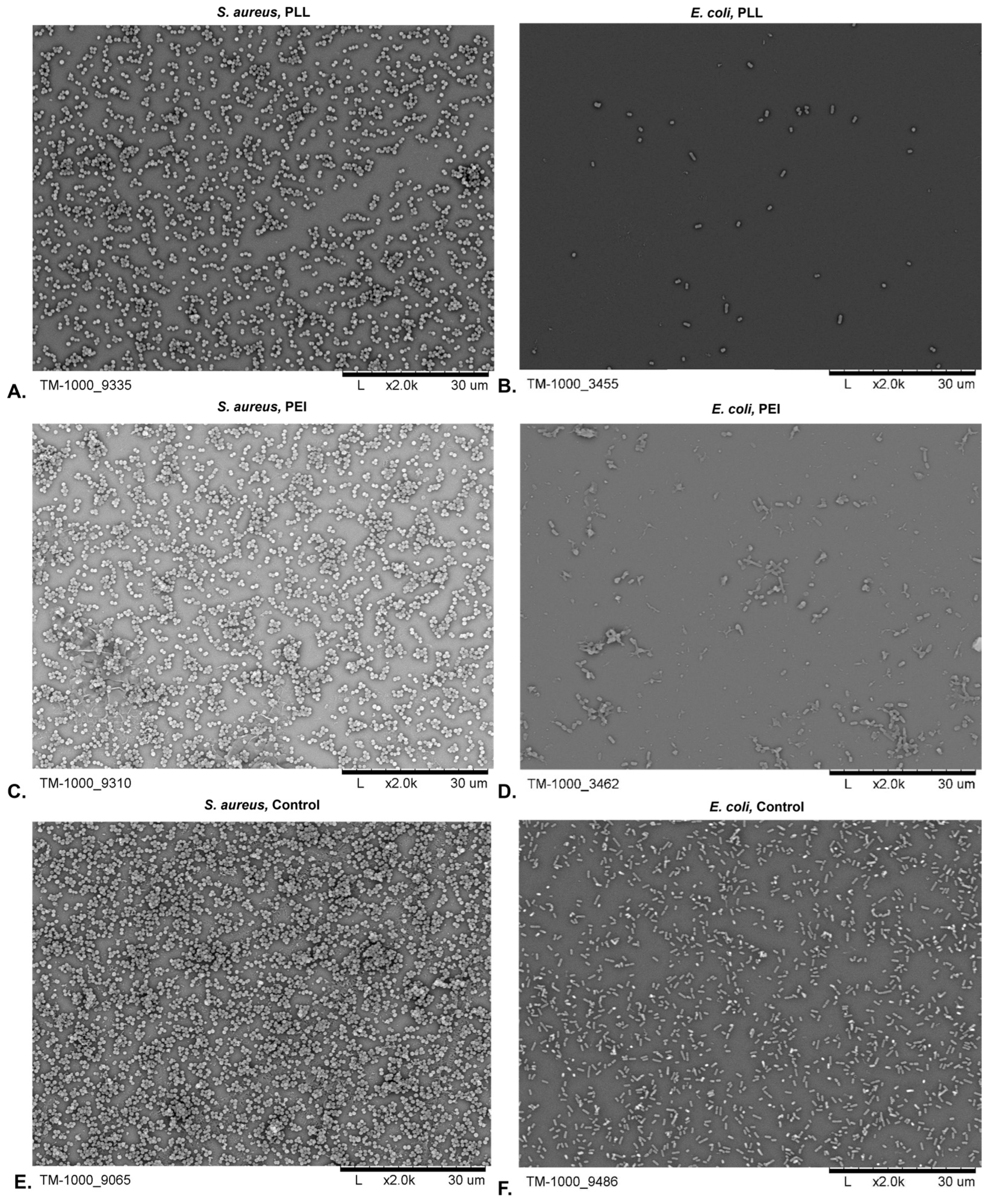
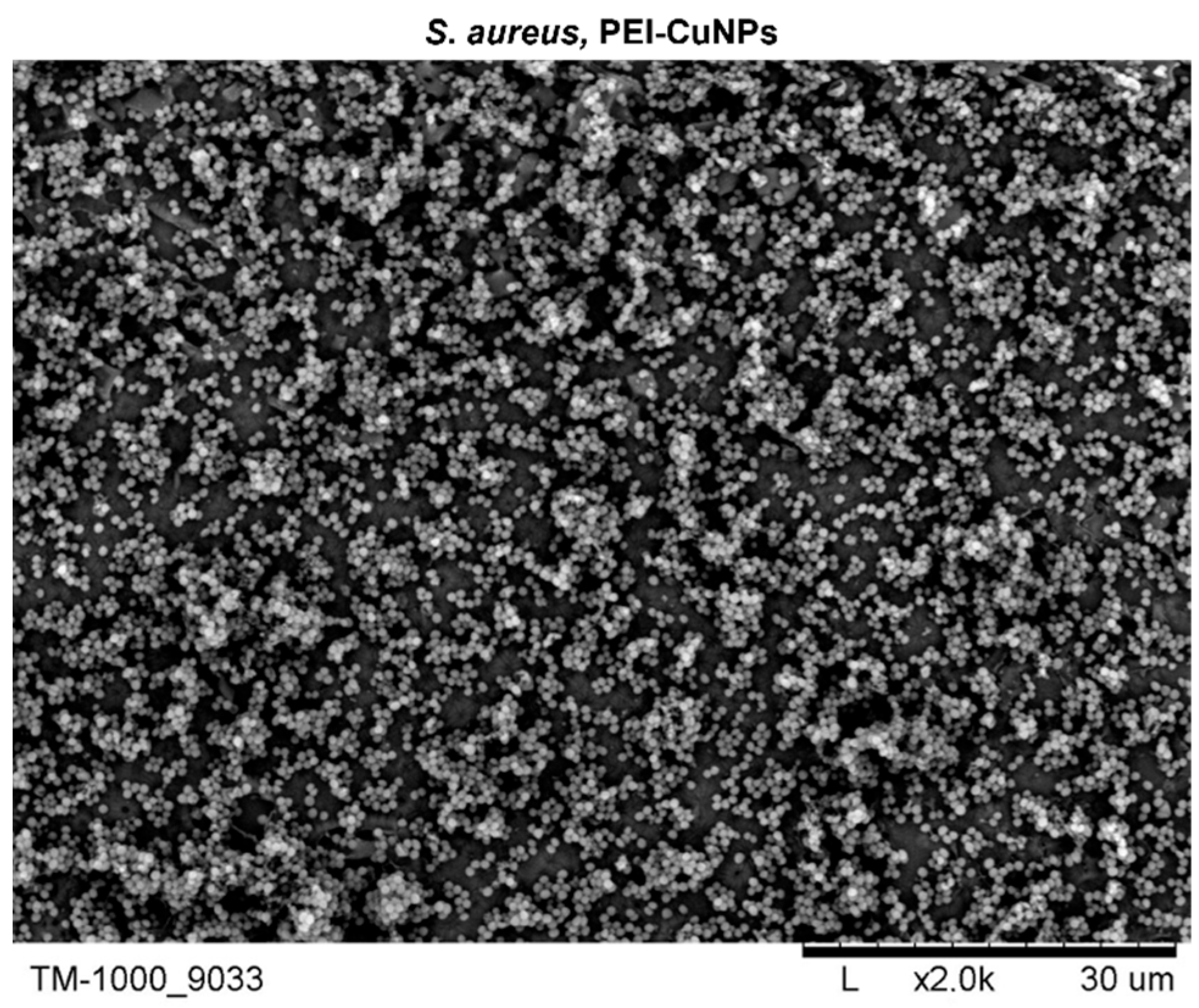
3.2.2. Surface Morphology Analysis
3.3. The Copper Nanoparticles’ Bacteriostatic Effect Evaluation
3.4. Evaluation of the CuNPs-Incorporated Layer Coatings’ Performance on E. coli
3.5. The CuNPs-Incorporated Polyelectrolyte Layer Coatings’ Evaluation
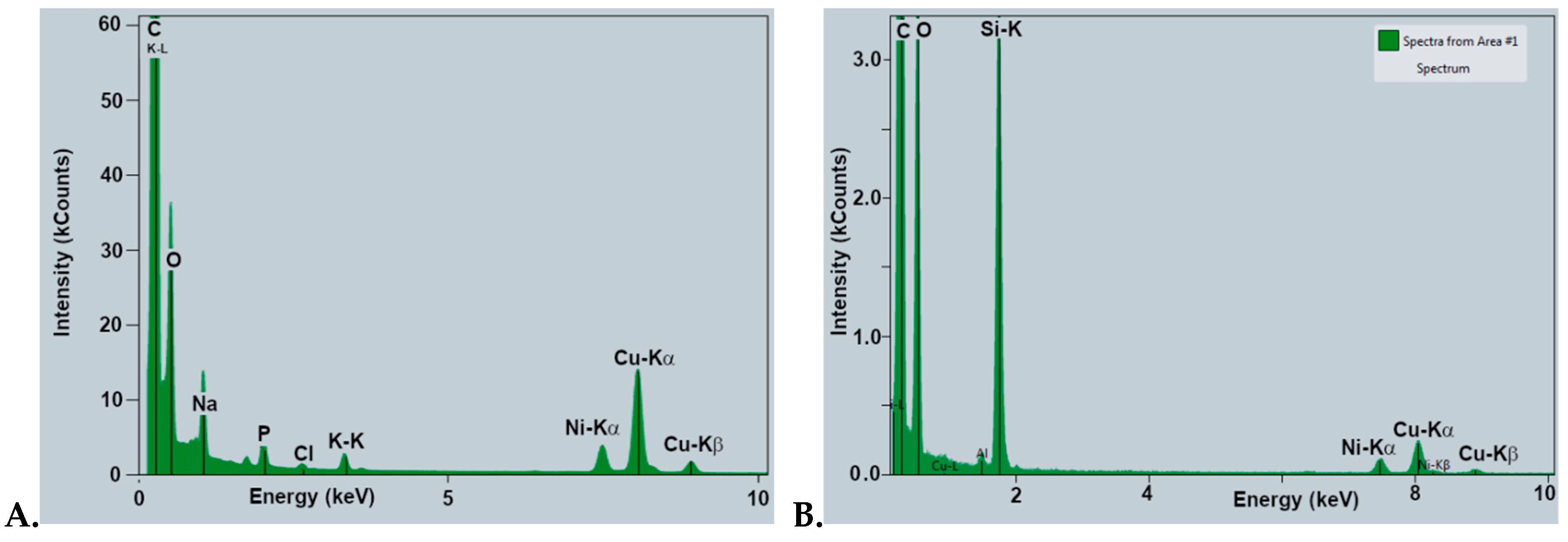
3.5.1. SEM-EDX Evaluation
3.5.2. Water Contact Angle Studies
3.6. Evaluation of the Functioning of Human Lung Cells in the Presence of the Layer Coatings Incorporating CuNPs
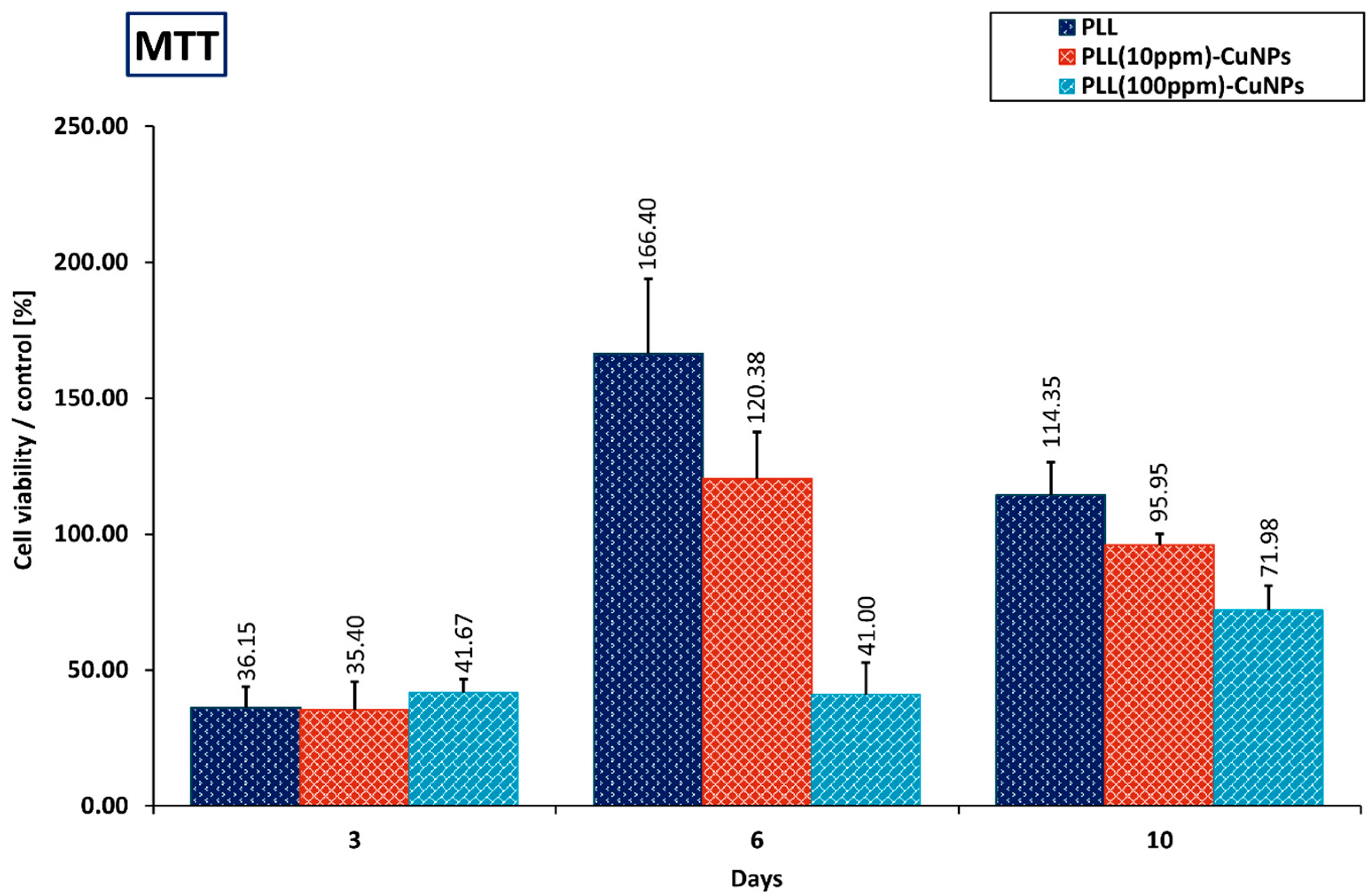
3.6.1. MTT Evaluation
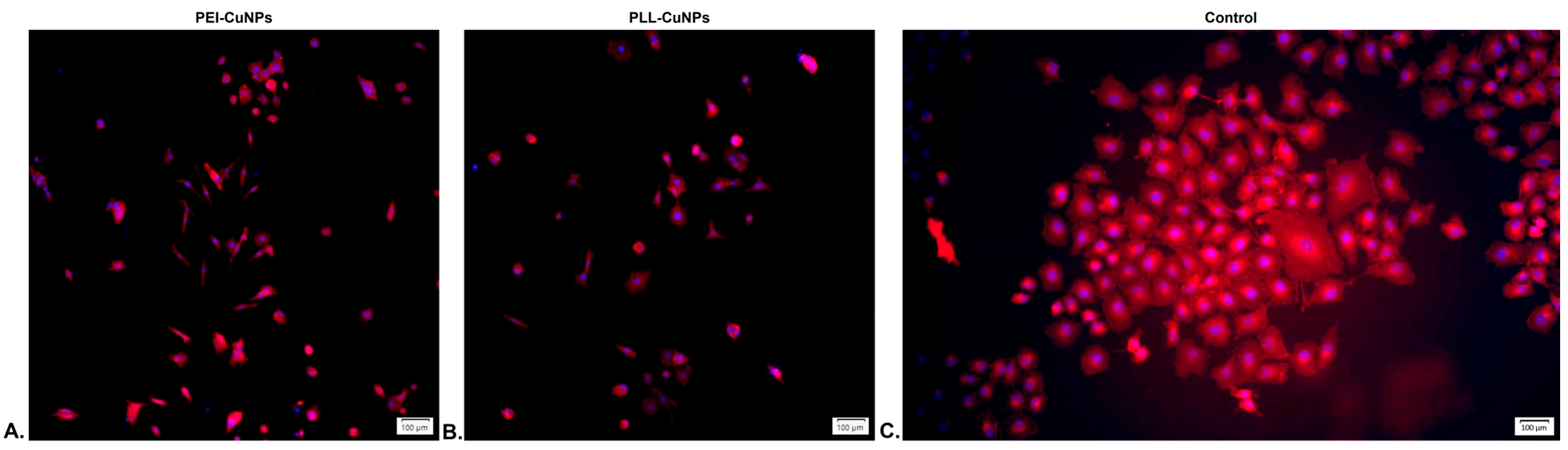
3.6.2. Fluorescence Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, N.N.; de Faria Lopes, L.; Ferreira, D.F.; de Prado, E.M.L.; Severi, J.A.; Resende, J.A.; de Paula Careta, F.; Ferreira, M.C.P.; Carreira, L.G.; de Souza, S.O.L.; et al. Polymeric films containing pomegranate peel extract based on PVA/starch/PAA blends for use as wound dressing: In vitro analysis and physicochemical evaluation. Mater. Sci. Eng. C 2020, 109, 110643. [Google Scholar] [CrossRef] [PubMed]
- Stricker, P.E.F.; de Souza Dobuchak, D.; Irioda, A.C.; Mogharbel, B.F.; Franco, C.R.C.; de Souza Almeida Leite, J.R.; de Araújo, A.R.; Borges, F.A.; Herculano, R.D.; de Oliveira Graeff, C.F.; et al. Human mesenchymal stem cells seeded on the natural membrane to neurospheres for cholinergic-like neurons. Membranes 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, D.S.M.; Mogharbel, B.F.; Irioda, A.C.; Stricker, P.E.F.; Perussolo, M.C.; Franco, C.R.C.; Chang, H.W.; Abdelwahid, E.; de Carvalho, K.A.T. Adipose-Derived Stromal Cells and Mineralized Extracellular Matrix Delivery by a Human Decellularized Amniotic Membrane in Periodontal Tissue Engineering. Membranes 2021, 11, 606. [Google Scholar] [CrossRef]
- Yang, C.; Yang, C.; Chen, Y.; Liu, J.; Liu, Z.; Chen, H.J. The trends in wound management: Sensing, therapeutic treatment, and “theranostics”. J. Sci. Adv. Mater. Devices 2023, 8, 100619. [Google Scholar] [CrossRef]
- Veiga, A.S.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Pept. Sci. 2013, 100, 637–644. [Google Scholar] [CrossRef]
- Ponco, A.; Helmiyati, H. Hydrogel of carboxymethyl cellulose and polyvinyl alcohol modified by CuNPs as antibacterial in wound dressing. AIP Conf. Proc. 2020, 2242, 040009. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ash, D.; Fouda, A.Y.; Sudhahar, V.; Kim, Y.M.; Hou, Y.; Hudson, F.Z.; Stansfield, B.K.; Caldwell, R.B.; McMenamin, M.; et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol. 2022, 24, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Khashan, K.S.; Sulaiman, G.M.; Mahdi, R. Preparation of iron oxide nanoparticles-decorated carbon nanotube using laser ablation in liquid and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium oxide nanoparticles: Synthesis methods and applications in wound healing. Mater. Today Bio 2023, 23, 100823. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, G.; Kim, H.J.; Rangarajulu, S.K. In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria. Antibiotics 2023, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.E.; Elsewedy, H.S.; Younis, N.S.; Shinu, P.; Elsawy, L.E.; Ramadan, H.A. Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Śliwiński, J.; Kamaszewski, M.; Sysa, P.; Chojnacki, M. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environ. Sci. Pollut. Res. 2018, 25, 908–915. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C.; Schencke, C.; Acevedo, F.; del Sol, M. Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds. Pharmaceutics 2023, 15, 2187. [Google Scholar] [CrossRef] [PubMed]
- Deokar, A.R.; Perelshtein, I.; Saibene, M.; Perkas, N.; Mantecca, P.; Nitzan, Y.; Gedanken, A. Antibacterial and In Vivo Studies of a Green, One-Pot Preparation of Copper/Zinc Oxide Nanoparticle-Coated Bandages. Membranes 2021, 11, 462. [Google Scholar] [CrossRef]
- Kruk, T.; Gołda-Cępa, M.; Szczepanowicz, K.; Szyk-Warszyńska, L.; Brzychczy-Włoch, M.; Kotarba, A.; Warszyński, P. Nanocomposite multifunctional polyelectrolyte thin films with copper nanoparticles as the antimicrobial coatings. Colloids Surf. B. Biointerfaces 2019, 181, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Kang, Y. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zi, L.; Cen, Y.; You, C.; Tian, M. Copper Sulfide Nanoparticles-Incorporated Hyaluronic Acid Injectable Hydrogel With Enhanced Angiogenesis to Promote Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 543970. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Rath, S.N.; Brandl, A.; Hiller, D.; Hoppe, A.; Gbureck, U.; Horch, R.E.; Boccaccini, A.R.; Kneser, U. Bioactive Copper-Doped Glass Scaffolds Can Stimulate Endothelial Cells in Co-Culture in Combination with Mesenchymal Stem Cells. PLoS ONE 2014, 9, e113319. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Cochis, A.; Kumar, A.; Arciola, C.R.; Rimondini, L.; Verné, E. Copper-Doped Bioactive Glass as Filler for PMMA-Based Bone Cements: Morphological, Mechanical, Reactivity, and Preliminary Antibacterial Characterization. Materials 2018, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mishra, A.; Singh, D.; Li, C.; Srivastava, P. In-Vitro Studies on Copper Nanoparticles and Nano-hydroxyapatite Infused Biopolymeric Composite Scaffolds for Bone Bioengineering Applications. Biotechnol. Bioprocess Eng. 2023, 28, 162–180. [Google Scholar] [CrossRef]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef]
- Gorel, O.; Hamuda, M.; Feldman, I.; Kucyn-Gabovich, I. Enhanced healing of wounds that responded poorly to silver dressing by copper wound dressings: Prospective single arm treatment study. Health Sci. Rep. 2024, 7, e1816. [Google Scholar] [CrossRef]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef]
- Lucchini, A.C.; Gachanja, N.N.; Rossi, A.G.; Dorward, D.A.; Lucas, C.D. Epithelial Cells and Inflammation in Pulmonary Wound Repair. Cells 2021, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Grzeczkowicz, A.; Lipko, A.; Kwiatkowska, A.; Strawski, M.; Bącal, P.; Więckowska, A.; Granicka, L.H. Polyelectrolyte Membrane Nanocoatings Aimed at Personal Protective and Medical Equipment Surfaces to Reduce Coronavirus Spreading. Membranes 2022, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, C.; Mallamace, D.; Neri, G.; Fazio, E. Hydrophilicity and hydrophobicity: Key aspects for biomedical and technological purposes. Phys. A Stat. Mech. Its Appl. 2021, 580, 126189. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Scheller, C.; Krebs, F.; Minkner, R.; Astner, I.; Gil-Moles, M.; Wätzig, H. Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis 2020, 41, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J.; Sharifian Gh, M.; Wu, T.; Li, Y.; Chang, C.M.; Ma, J.; Dai, H.L. Determination of bacterial surface charge density via saturation of adsorbed ions. Biophys. J. 2021, 120, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Park, J.H.; Peters, T.M.; Thorne, P.S. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air-liquid interface compared with in vivo assessment. Toxicol. Vitr. 2015, 29, 502–511. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Ebrahim, N.M.; Gaber, M.H. In-Vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J. Trace Elem. Med. Biol. 2020, 60, 126481. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef]




| Membrane | CuNPs | ColloidCuNPs |
|---|---|---|
| polyethyleneimine-based | ||
| polyethyleneimine (PEI) | No | No |
| polyethyleneimine incorporating CuNPs (PEI-CuNPs) | Yes | No |
| polyethyleneimine incorporating ColloidCuNPs (PEI-ColloidCuNPs) | No | Yes |
| poly-L-lysine-based | ||
| polylysine (PLL) | No | No |
| polylysine incorporating CuNPs (PLL-CuNPs) | Yes | No |
| polylysine incorporating CuNPs (PLL-ColloidCuNPs) | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipko, A.; Grzeczkowicz, A.; Antosiak-Iwańska, M.; Strawski, M.; Drabik, M.; Kwiatkowska, A.; Godlewska, E.; Granicka, L.H. Polyelectrolyte Platforms with Copper Nanoparticles as a Multifunctional System Aimed at Healing Process Support. Processes 2024, 12, 512. https://doi.org/10.3390/pr12030512
Lipko A, Grzeczkowicz A, Antosiak-Iwańska M, Strawski M, Drabik M, Kwiatkowska A, Godlewska E, Granicka LH. Polyelectrolyte Platforms with Copper Nanoparticles as a Multifunctional System Aimed at Healing Process Support. Processes. 2024; 12(3):512. https://doi.org/10.3390/pr12030512
Chicago/Turabian StyleLipko, Agata, Anna Grzeczkowicz, Magdalena Antosiak-Iwańska, Marcin Strawski, Monika Drabik, Angelika Kwiatkowska, Ewa Godlewska, and Ludomira H. Granicka. 2024. "Polyelectrolyte Platforms with Copper Nanoparticles as a Multifunctional System Aimed at Healing Process Support" Processes 12, no. 3: 512. https://doi.org/10.3390/pr12030512
APA StyleLipko, A., Grzeczkowicz, A., Antosiak-Iwańska, M., Strawski, M., Drabik, M., Kwiatkowska, A., Godlewska, E., & Granicka, L. H. (2024). Polyelectrolyte Platforms with Copper Nanoparticles as a Multifunctional System Aimed at Healing Process Support. Processes, 12(3), 512. https://doi.org/10.3390/pr12030512






