Smart Strategic Management for the Cold Plasma Process Using ORP Monitoring and Total Organic Carbon Correlation
Abstract
1. Introduction
2. Materials and Methods
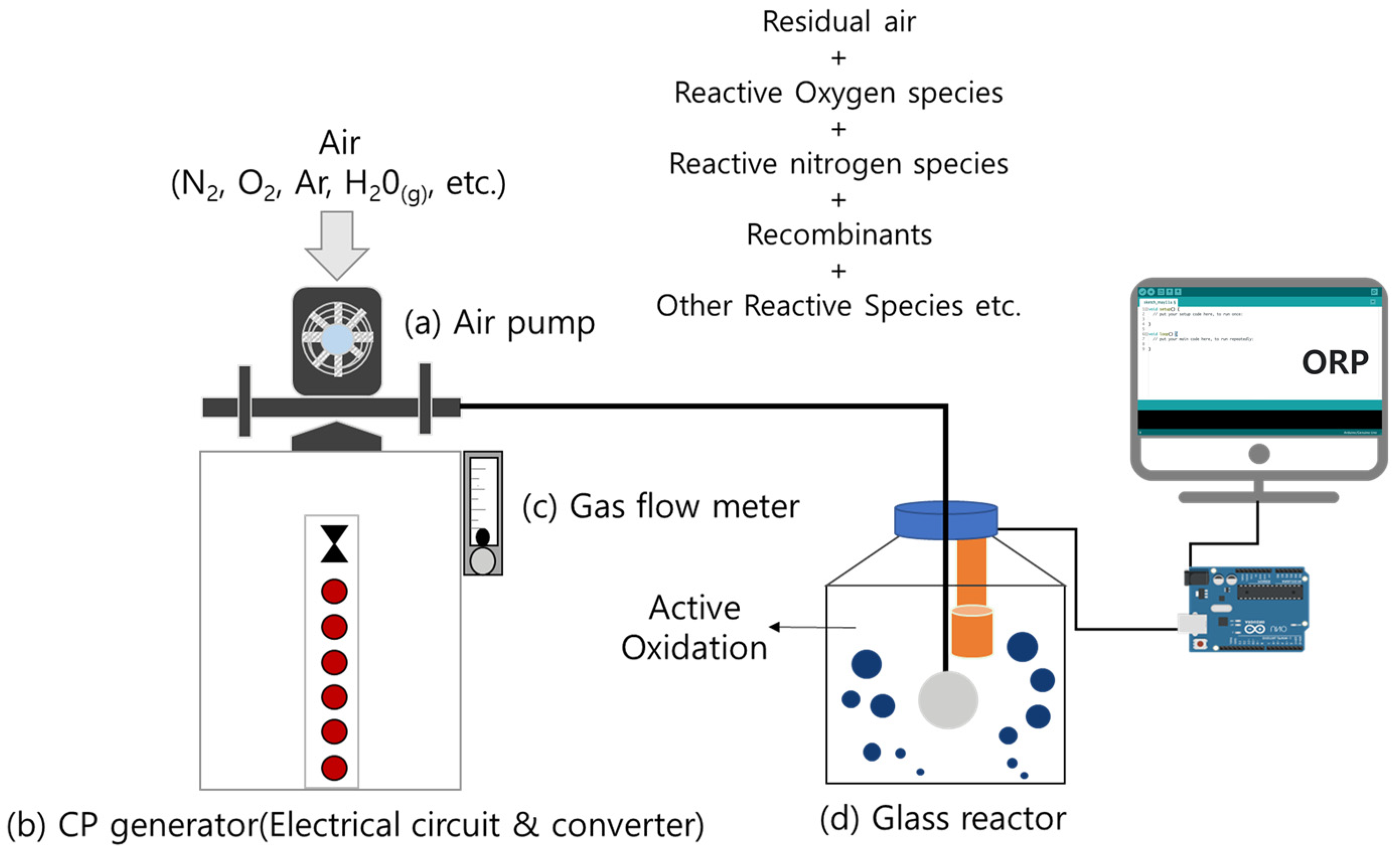
2.1. Experimental Configuration and Setups
2.2. Quantification of Hydroxyl Radical
2.3. SDBS Removal Using CP
2.4. Unsupervised and Supervised Learning in Machine Learning
3. Results and Discussion
3.1. Evidence of Hydroxyl Radical Formation in CP According to Variations in Air Flow Rate
3.2. SDBS Degradation by CP According to Air Flow Rate
3.2.1. SDBS and TOC Removal in CP Treatment
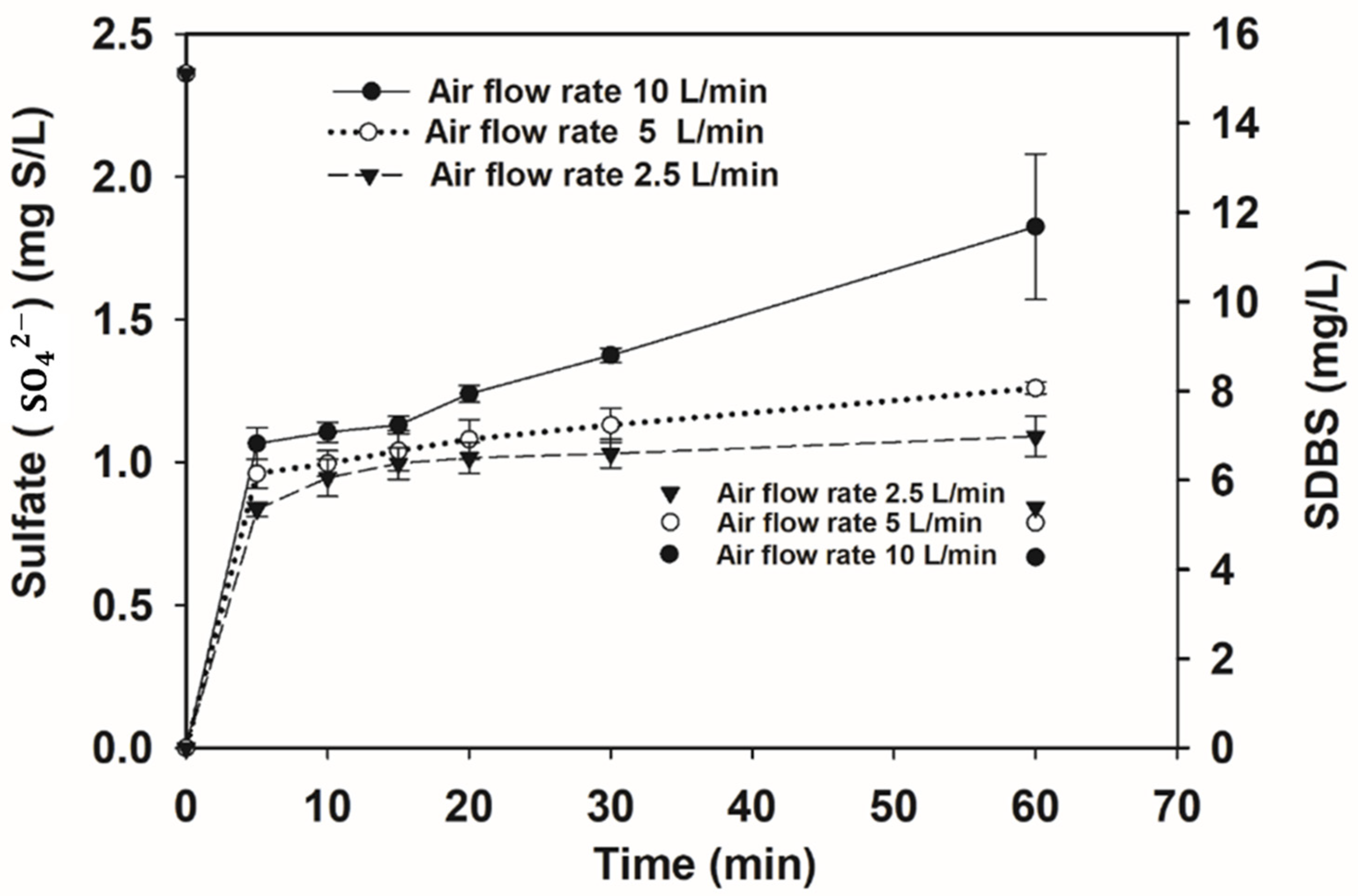
3.2.2. Sulfate Concentration Variations during CP Treatment
3.2.3. Evaluation of Acute Toxicity on the SDBS
3.3. Performances of Machine Learning
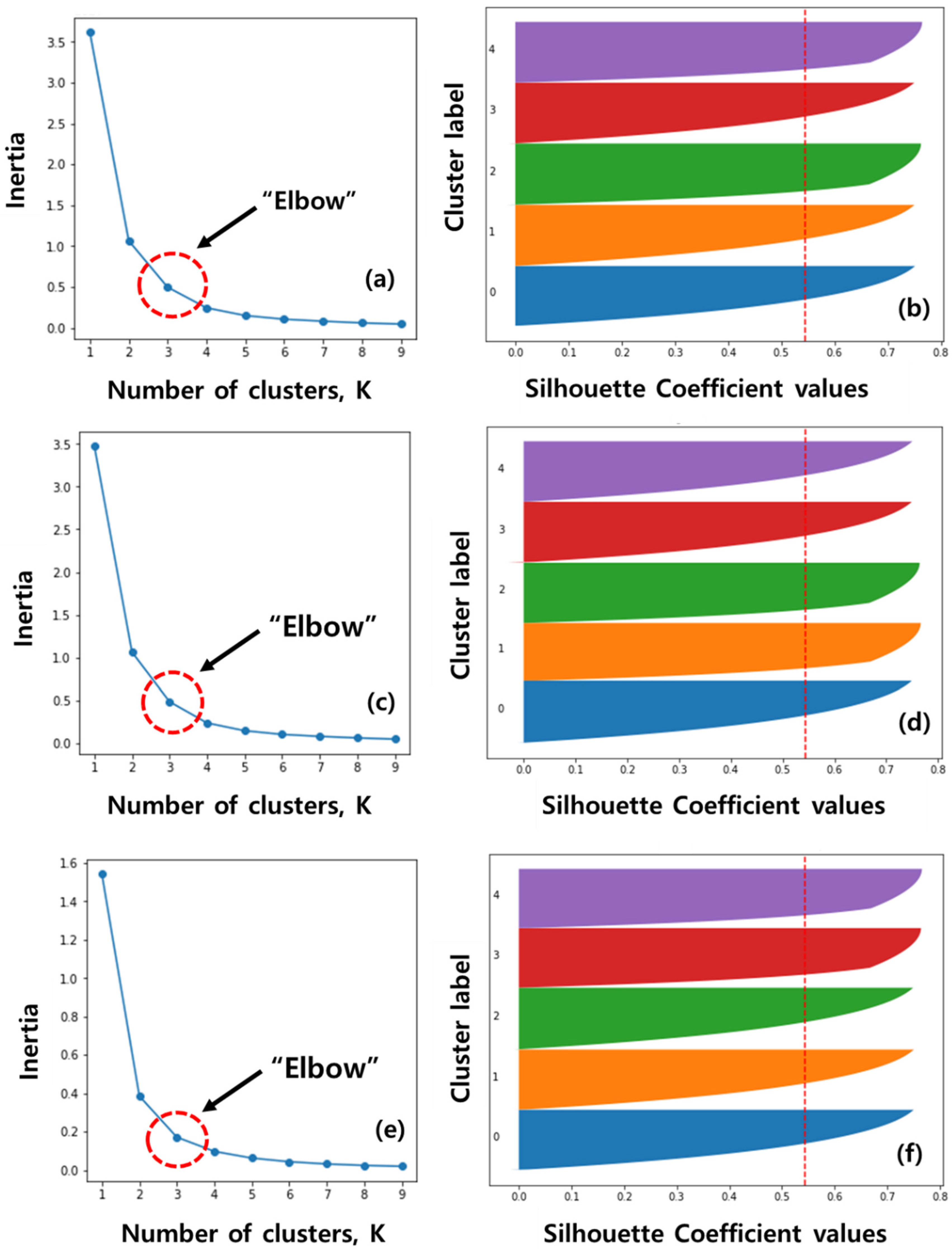
3.3.1. Unsupervised Learning in Machine Learning
3.3.2. Supervised Learning in Machine Learning
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Sun, H.; He, F.; Choi, W. Production of reactive oxygen species by the reaction of periodate and hydroxylamine for rapid removal of organic pollutants and waterborne bacteria. Environ. Sci. Technol. 2020, 54, 6427–6437. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.; Milosavljevic, V.; Bourke, P.; O’Regan, F.; Cullen, P. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Won, C.-H.; Hong, Y.-P.; Lee, I.H.; Kim, H.-W. Energy-effective elimination of harmful microcystins by a non-thermal plasma process. Chemosphere 2021, 284, 131338. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-F.; Chen, H.-W.; Cheng, W.-P.; Lin, Y.-J.; Huang, C.-L. Monitoring of ORP, pH and DO in heterogeneous Fenton oxidation using nZVI as a catalyst for the treatment of azo-dye textile wastewater. J. Taiwan Inst. Chem. Eng. 2014, 45, 947–954. [Google Scholar] [CrossRef]
- Suslow, T.V. Oxidation-Reduction Potential (ORP) for Water Disinfection Monitoring, Control, and Documentation; University of California, Division of Agriculture and Natural Resources: Davis, CA, USA, 2004. [Google Scholar]
- Tanwar, P.; Nandy, T.; Ukey, P.; Manekar, P. Correlating on-line monitoring parameters, pH, DO and ORP with nutrient removal in an intermittent cyclic process bioreactor system. Bioresour. Technol. 2008, 99, 7630–7635. [Google Scholar] [CrossRef]
- Son, C.-Y.; Kang, J.-K.; Lee, Y.-J.; Lee, C.-G.; Kim, J.; Park, S.-J.; Cho, Y.-M.; Shin, G.-A. Degradation of residual dyes in actual textile wastewater using UV/H 2 O 2: Evaluation of the impact of operating variables through multi-layer perceptron analysis. Environ. Eng. Res. 2024, 29, 230716. [Google Scholar] [CrossRef]
- Mahesh, B. Machine learning algorithms-a review. Int. J. Sci. Res. 2020, 9, 381–386. [Google Scholar]
- Aytaç, E. Unsupervised learning approach in defining the similarity of catchments: Hydrological response unit based k-means clustering, a demonstration on Western Black Sea Region of Turkey. Int. Soil Water Conserv. Res. 2020, 8, 321–331. [Google Scholar] [CrossRef]
- Bonaccorso, G. Machine Learning Algorithms; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Osisanwo, F.; Akinsola, J.; Awodele, O.; Hinmikaiye, J.; Olakanmi, O.; Akinjobi, J. Supervised machine learning algorithms: Classification and comparison. Int. J. Comput. Trends Technol. 2017, 48, 128–138. [Google Scholar]
- Rauf, A.; Sheeba, S.M.; Khusro, S.; Javed, H. Enhanced k-mean clustering algorithm to reduce number of iterations and time complexity. Middle East J. Sci. Res. 2012, 12, 959–963. [Google Scholar]
- Likas, A.; Vlassis, N.; Verbeek, J.J. The global k-means clustering algorithm. Pattern Recognit. 2003, 36, 451–461. [Google Scholar] [CrossRef]
- Tang, R.; Fong, S.; Yang, X.-S.; Deb, S. Integrating nature-inspired optimization algorithms to K-means clustering. In Proceedings of the Seventh International Conference on Digital Information Management (ICDIM 2012), Macao, China, 22–24 August 2012; pp. 116–123. [Google Scholar]
- Sinaga, K.P.; Yang, M.-S. Unsupervised K-means clustering algorithm. IEEE Access 2020, 8, 80716–80727. [Google Scholar] [CrossRef]
- Zada, I.; Ali, S.; Khan, I.; Hadjouni, M.; Elmannai, H.; Zeeshan, M.; Serat, A.M.; Jameel, A. Performance evaluation of simple K-mean and parallel K-mean clustering algorithms: Big data business process management concept. Mob. Inf. Syst. 2022, 2022, 1277765. [Google Scholar] [CrossRef]
- Ezugwu, A.E.; Ikotun, A.M.; Oyelade, O.O.; Abualigah, L.; Agushaka, J.O.; Eke, C.I.; Akinyelu, A.A. A comprehensive survey of clustering algorithms: State-of-the-art machine learning applications, taxonomy, challenges, and future research prospects. Eng. Appl. Artif. Intell. 2022, 110, 104743. [Google Scholar] [CrossRef]
- Ahmad, A.; Dey, L. A k-mean clustering algorithm for mixed numeric and categorical data. Data Knowl. Eng. 2007, 63, 503–527. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Chen, C.; Yu, S.; Wang, X. Counterion effects of nickel and sodium dodecylbenzene sulfonate adsorption to multiwalled carbon nanotubes in aqueous solution. Carbon 2008, 46, 1741–1750. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Han, W.; Tan, J.; Peng, L.; Liu, L.; Zhou, X.; Zhang, W.; Shi, B. Ecotoxicity and micellization behavior of anionic surfactant sodium dodecylbenzene sulfonate (SDBS) and its mixtures with nonionic surfactant fatty alcohol-polyoxyethylene ether (AEO). Aquat. Toxicol. 2019, 216, 105313. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Badamasi, Y.A. The working principle of an Arduino. In Proceedings of the 2014 11th International Conference on Electronics, Computer and Computation (ICECCO), Abuja, Nigeria, 29 September–1 October 2014; pp. 1–4. [Google Scholar]
- Nayyar, A.; Puri, V. A review of Arduino board’s, Lilypad’s & Arduino shields. In Proceedings of the 2016 3rd International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 16–18 March 2016; pp. 1485–1492. [Google Scholar]
- Arduino LLC. Arduino. Available online: https://search.iczhiku.com/paper/TFzDJhGhd6VMaDsI.pdf (accessed on 21 February 2024).
- Ismailov, A.S.; Jo‘Rayev, Z.B. Study of arduino microcontroller board. Sci. Educ. 2022, 3, 172–179. [Google Scholar]
- Abdullahi, S.I.; Habaebi, M.H.; Gunawan, T.S.; Islam, M.R. Miniaturized Water Flow and Level Monitoring System for Flood Disaster Early Warning. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012019. [Google Scholar]
- Badmus, K.; Tijani, J.; Eze, C.P.; Fatoba, O.; Petrik, L.F. Quantification of radicals generated in a sonicator. Anal. Bioanal. Chem. Res. 2016, 3, 139–147. [Google Scholar]
- Villeneuve, L.; Alberti, L.; Steghens, J.-P.; Lancelin, J.-M.; Mestas, J.-L. Assay of hydroxyl radicals generated by focused ultrasound. Ultrason. Sonochem. 2009, 16, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, L.; Zhang, Y.; Sun, H. Significantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye. J. Mater. 2020, 6, 256–262. [Google Scholar] [CrossRef]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Measurement of OH radicals in aqueous solution produced by atmospheric-pressure LF plasma jet. Int. J. Plasma Environ. Sci. Technol. 2012, 6, 166–171. [Google Scholar]
- Sahni, M.; Locke, B.R. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Gao, J.; Li, X.; Zhou, Z.; Wang, N.; Du, P.; Zhang, T.; Feng, J. Degradation of sodium dodecyl benzenesulfonate by vacuum ultraviolet irradiation. J. Water Process Eng. 2020, 34, 101172. [Google Scholar] [CrossRef]
- Marutho, D.; Handaka, S.H.; Wijaya, E. The determination of cluster number at k-mean using elbow method and purity evaluation on headline news. In Proceedings of the 2018 International Seminar on Application for Technology of Information and Communication, Semarang, Indonesia, 21–22 September 2018; pp. 533–538. [Google Scholar]
- Yu, S.I.; Rhee, C.; Cho, K.H.; Shin, S.G. Comparison of different machine learning algorithms to estimate liquid level for bioreactor management. Environ. Eng. Res. 2023, 28, 220037. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; von Sonntag, C. OH radical formation by ultrasound in aqueous solutions Part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Application of chemical dosimetry to hydroxyl radical measurement during underwater discharge. J. Phys. Conf. Ser. 2013, 418, 012102. [Google Scholar] [CrossRef]
- Beltran, F.J.; Garcia-Araya, J.F.; Alvarez, P.M. Sodium dodecylbenzenesulfonate removal from water and wastewater. 1. Kinetics of decomposition by ozonation. Ind. Eng. Chem. Res. 2000, 39, 2214–2220. [Google Scholar] [CrossRef]
- Méndez-Díaz, J.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Bautista-Toledo, M. Effectiveness of different oxidizing agents for removing sodium dodecylbenzenesulphonate in aqueous systems. Water Res. 2009, 43, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yang, H. Research on K-value selection method of K-means clustering algorithm. J 2019, 2, 226–235. [Google Scholar] [CrossRef]
- Kodinariya, T.M.; Makwana, P.R. Review on determining number of Cluster in K-Means Clustering. Int. J. 2013, 1, 90–95. [Google Scholar]
- Shi, C.; Wei, B.; Wei, S.; Wang, W.; Liu, H.; Liu, J. A quantitative discriminant method of elbow point for the optimal number of clusters in clustering algorithm. Eurasip J. Wirel. Commun. Netw. 2021, 2021, 31. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical power for cluster analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef] [PubMed]




| Component | Unit | Average | Standard Deviation | Relative Standard Deviation | Variance of Estimate |
|---|---|---|---|---|---|
| pH | - | 7.2 | 0.026 | 0.4 | 0.0007 |
| TOC (Total organic carbon) | mg C/L | 6.19 | 0.01 | 0.2 | 0.0001 |
| TC (Total carbon) | mg C/L | 6.41 | 0.01 | 0.2 | 0.0001 |
| TIC (Total inorganic carbon) | mg C/L | 0.22 | 0.002 | 1.0 | 0.0000 |
| Flow Rate (L/min) | Initial Value (ppm) | CP Treated Value (ppm) | Removal Efficiency (%) |
|---|---|---|---|
| 2.5 | 5.39 | 64.3 ± 0.02 | |
| 5.0 | 15.11 | 5.04 | 66.6 ± 0.01 |
| 10.0 | 4.27 | 71.7 ± 0.01 |
| Air Flow Rate | Regression Model | r | R2 | p-Value | SE 1 | DF 2 |
|---|---|---|---|---|---|---|
| 10 L/min | 0.913 | 0.83 | 0.03 * | 0.56 | 4 | |
| 5 L/min | 0.946 | 0.89 | 0.02 * | 0.33 | 4 | |
| 2.5 L/min | 0.888 | 0.78 | 0.04 * | 0.17 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, I.; Kim, H.-J.; Kim, H.-W. Smart Strategic Management for the Cold Plasma Process Using ORP Monitoring and Total Organic Carbon Correlation. Processes 2024, 12, 471. https://doi.org/10.3390/pr12030471
Lee Y, Lee I, Kim H-J, Kim H-W. Smart Strategic Management for the Cold Plasma Process Using ORP Monitoring and Total Organic Carbon Correlation. Processes. 2024; 12(3):471. https://doi.org/10.3390/pr12030471
Chicago/Turabian StyleLee, YeonA, Inho Lee, Hee-Jun Kim, and Hyun-Woo Kim. 2024. "Smart Strategic Management for the Cold Plasma Process Using ORP Monitoring and Total Organic Carbon Correlation" Processes 12, no. 3: 471. https://doi.org/10.3390/pr12030471
APA StyleLee, Y., Lee, I., Kim, H.-J., & Kim, H.-W. (2024). Smart Strategic Management for the Cold Plasma Process Using ORP Monitoring and Total Organic Carbon Correlation. Processes, 12(3), 471. https://doi.org/10.3390/pr12030471






