Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material
Abstract
1. Introduction
2. Experiment
3. Results and Discussion
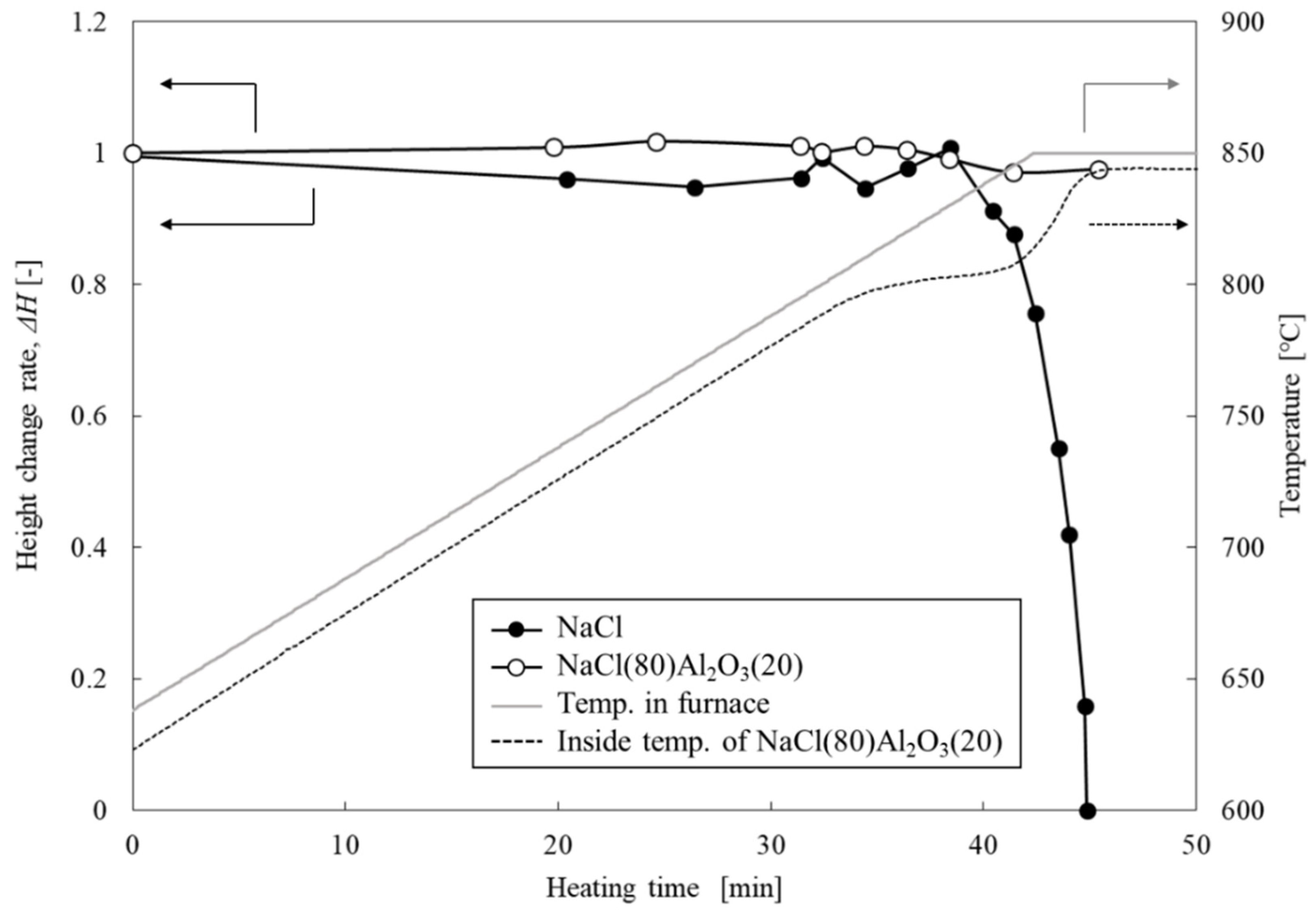
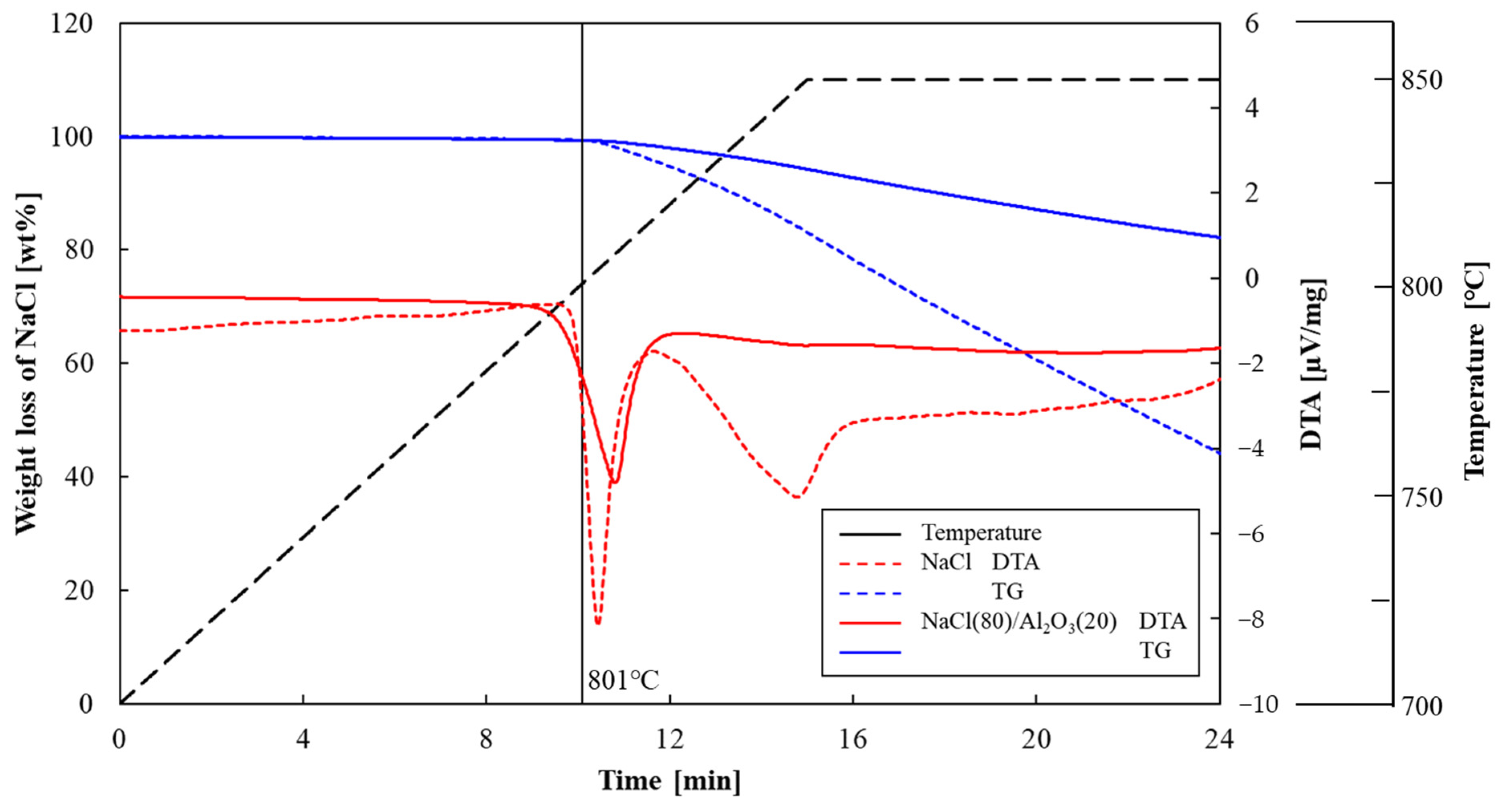
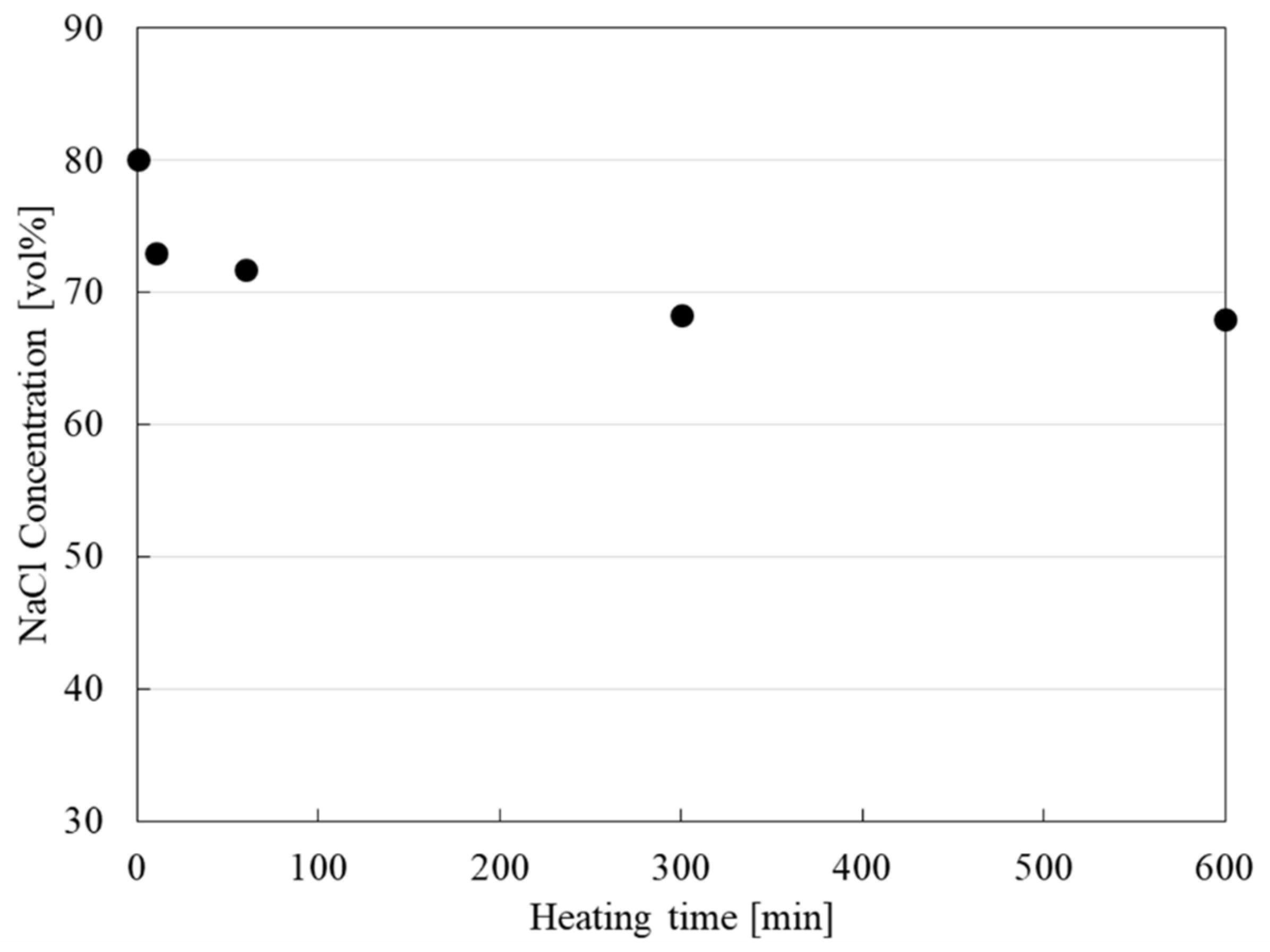
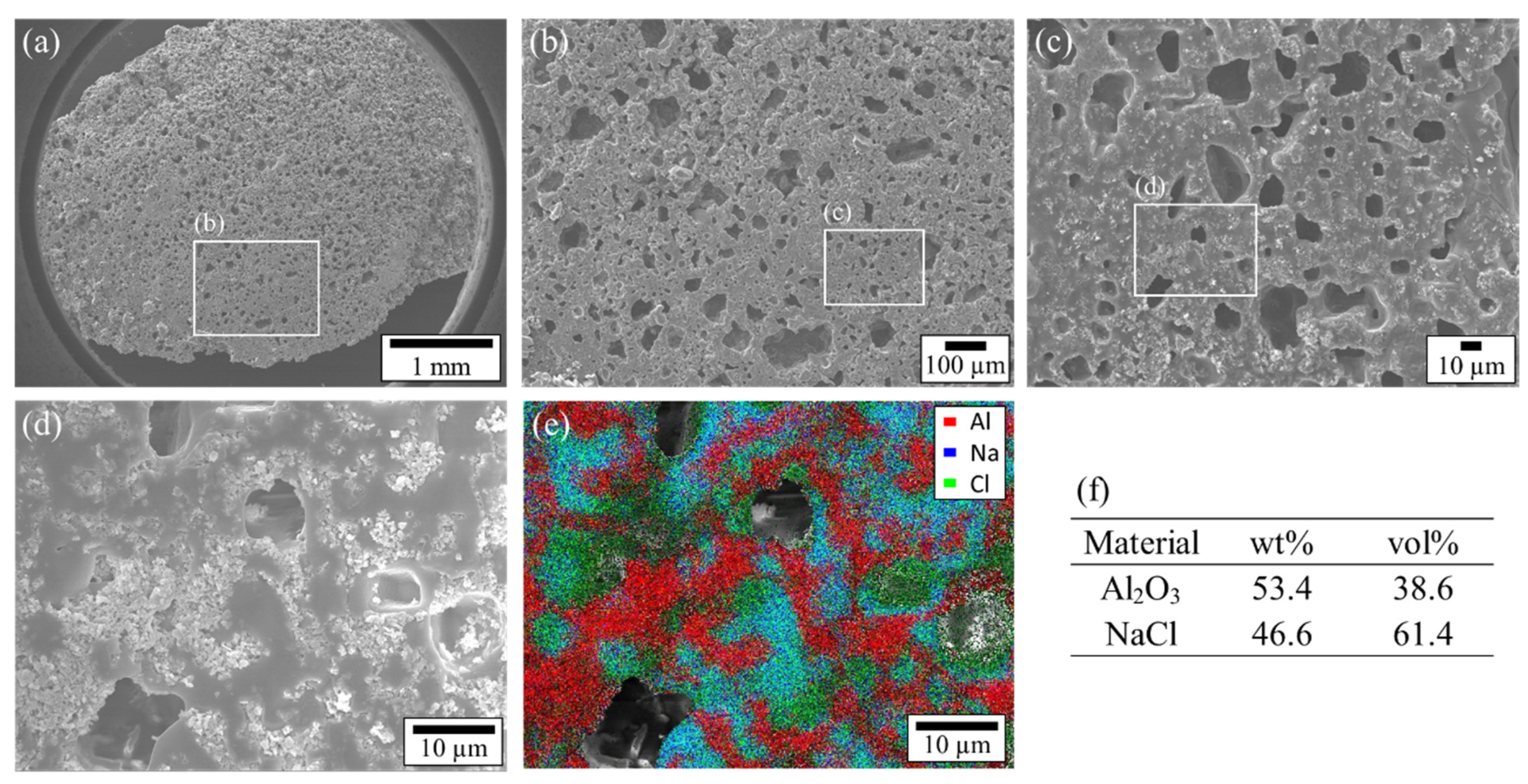
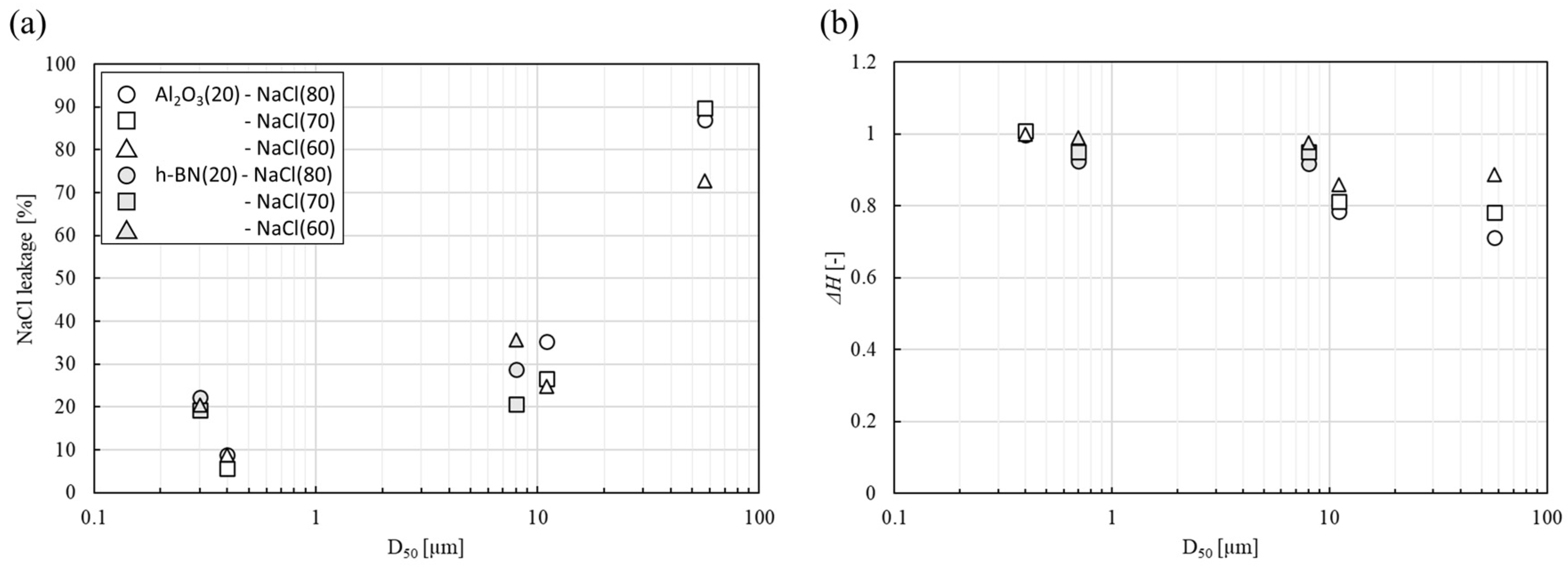
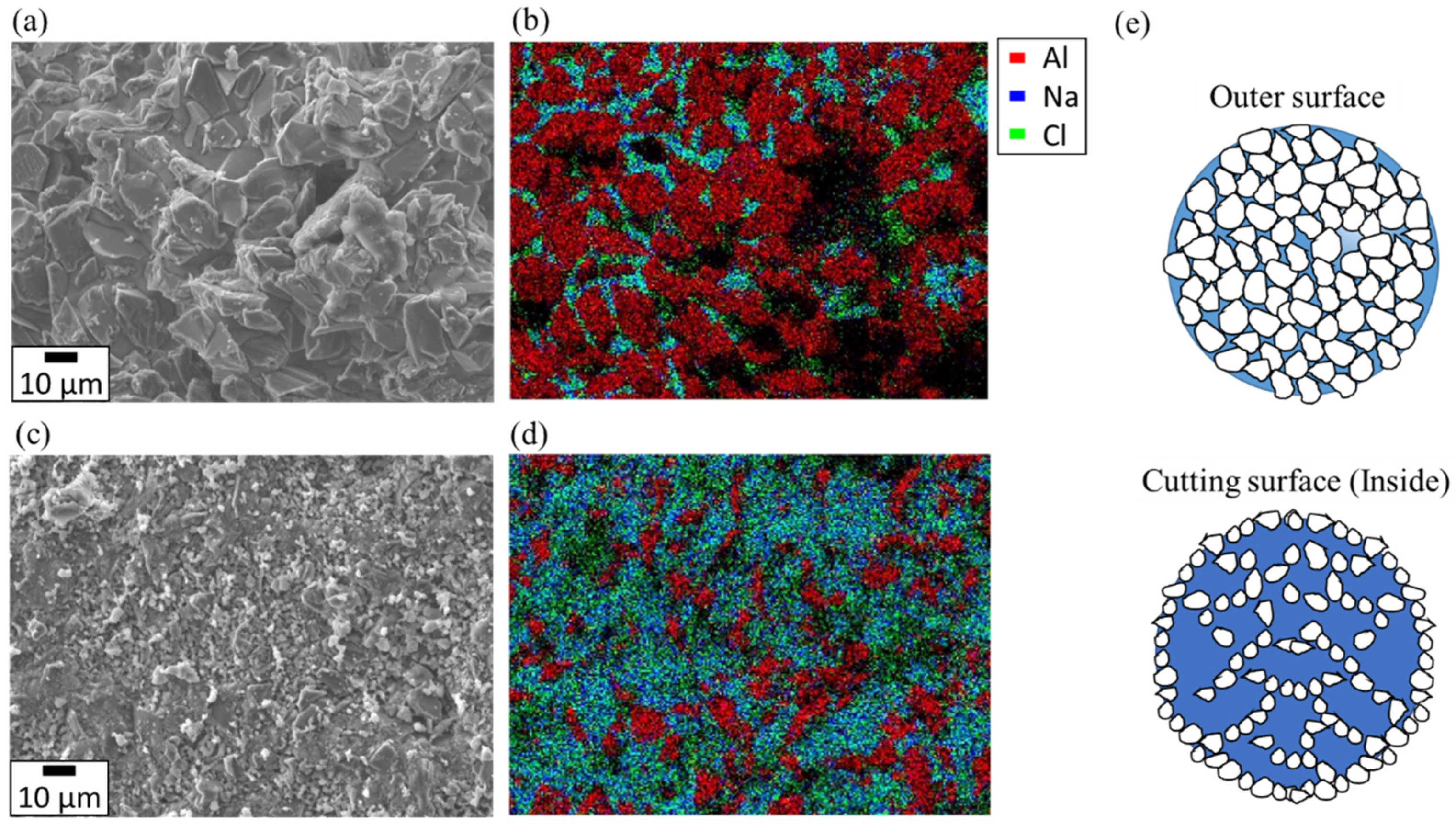
3.1. Shape-Retention Performance and Heat-Storage Property of NaCl/Al2O3-PC Structure
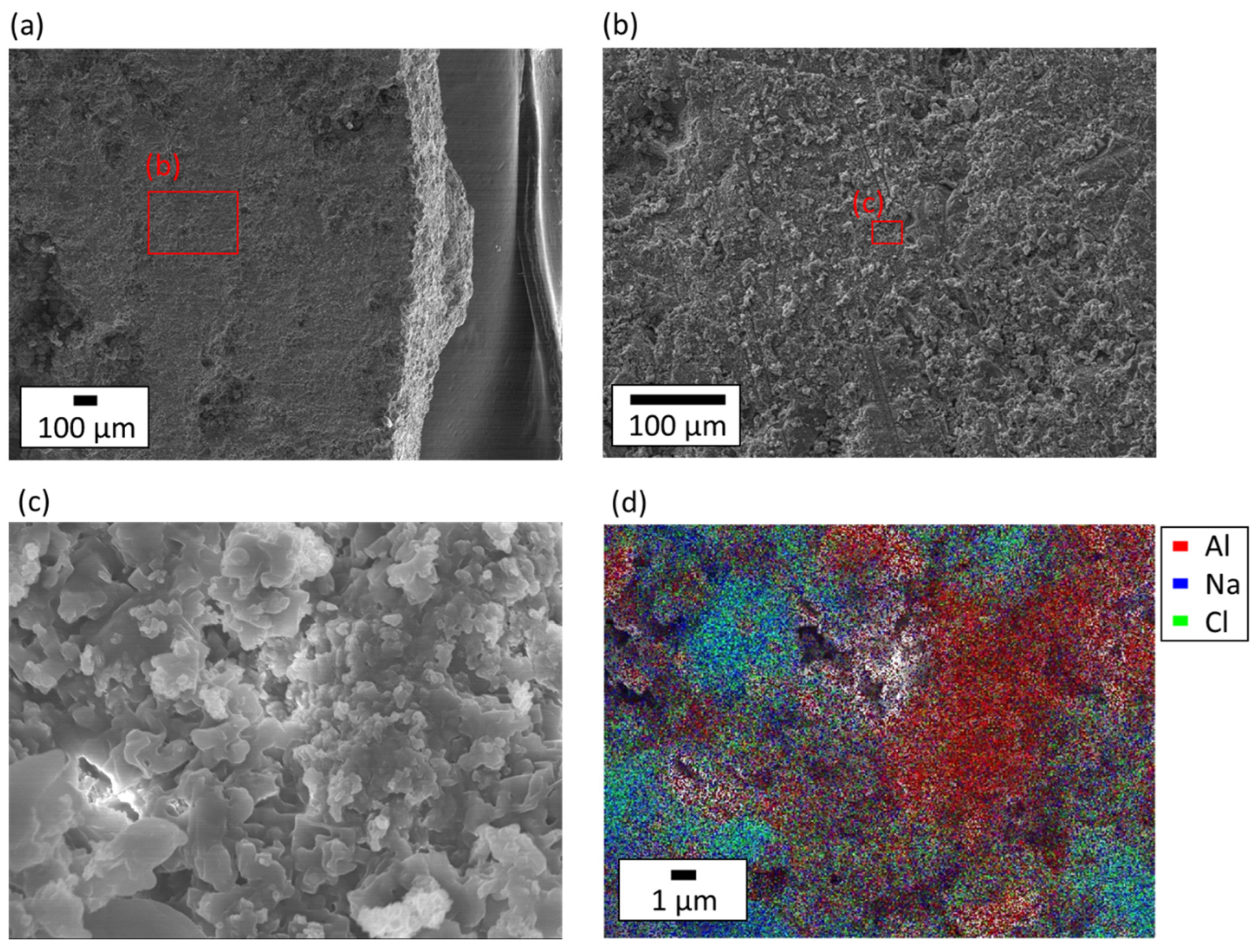
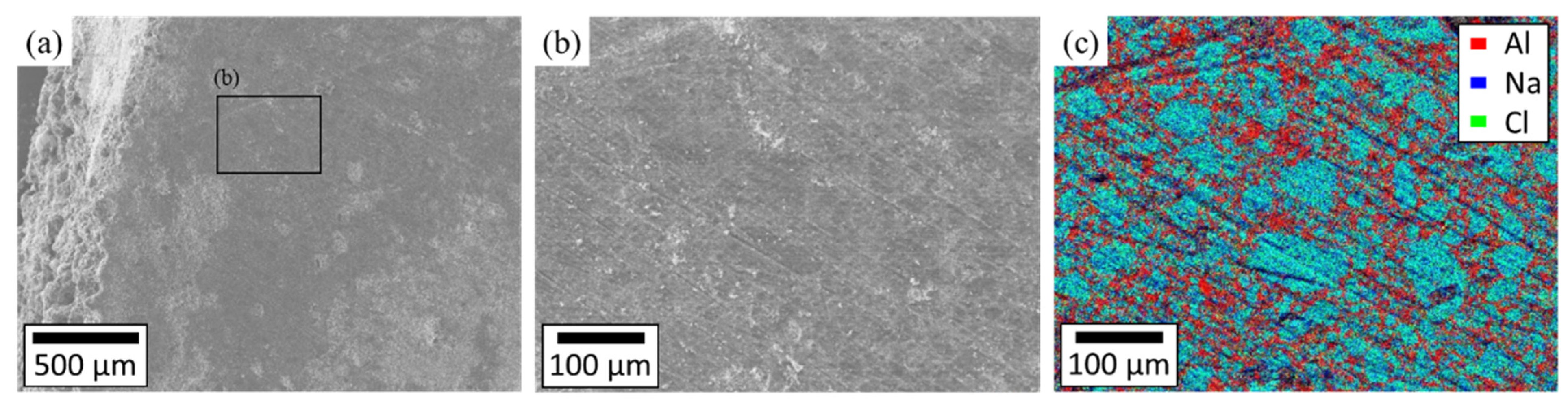
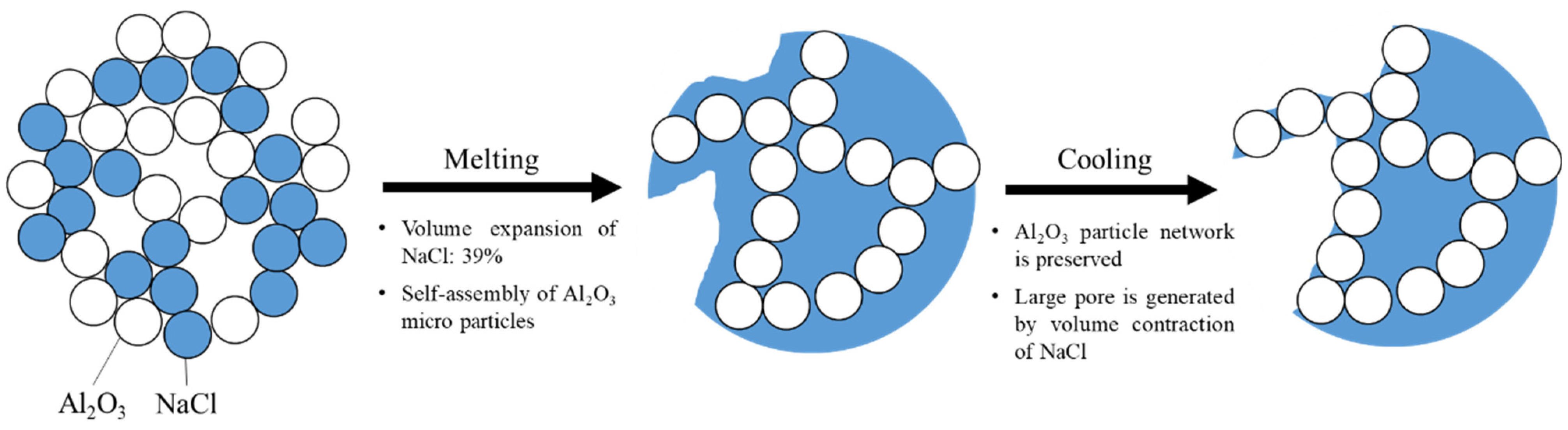
3.2. The Mechanism of the Shape Retention of NaCl/Al2O3 PC Ball
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, S. Medium- and high-temperature latent heat thermal energy storage: Material database, system review, and corrosivity assessment. Int. J. Energy Res. 2019, 43, 621–661. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Myers, P.D.; Goswami, D.Y. Thermal energy storage using chloride salts and their eutectics. Appl. Therm. Eng. 2016, 109, 889–900. [Google Scholar] [CrossRef]
- Qin, Y.; Leng, G.; Yu, X.; Cao, H.; Qiao, G.; Dai, Y.; Zhang, Y.; Ding, Y. Sodium sulfate-diatomite composite materials for high temperature thermal energy storage. Powder Technol. 2015, 282, 37–42. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Huang, G.; Kubo, S.; Shu, P. Characterization and thermal performance of nitrate mixture/SiC ceramic honeycomb composite phase change materials for thermal energy storage. Appl. Therm. Eng. 2015, 81, 193–197. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Y.; Jacob, R.D.; Bruno, F.; Li, S. Novel Na2SO4-NaCl-ceramic composites as high temperature phase change materials for solar thermal power plants (Part I). Sol. Energy Mater. Sol. Cells 2018, 178, 74–83. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, B.; Lin, J.; Zhang, F.; Wang, H.; Zhu, Z.; Dai, Z. Encapsulation of high-temperature inorganic phase change materials using graphite as heat transfer enhancer. Renew. Energy 2019, 133, 240–247. [Google Scholar] [CrossRef]
- El Karim, Y.; Grosu, Y.; Faik, A.; Lbibb, R. Investigation of magnesium-copper eutectic alloys with high thermal conductivity as a new PCM for latent heat thermal energy storage at intermediate-high temperature. J. Energy Storage 2019, 26, 100974. [Google Scholar] [CrossRef]
- Ge, Z.; Ye, F.; Cao, H.; Leng, G.; Qin, Y.; Ding, Y. Carbonate-salt-based composite materials for medium- and high-temperature thermal energy storage. Particuology 2014, 15, 77–81. [Google Scholar] [CrossRef]
- Wickramaratne, C.; Dhau, J.S.; Kamal, R.; Myers, P.; Goswami, D.Y.; Stefanakos, E. Macro-encapsulation and characterization of chloride based inorganic Phase change materials for high temperature thermal energy storage systems. Appl. Energy 2018, 221, 587–596. [Google Scholar] [CrossRef]
- Brock, L.R. The wettability of sapphire, polycrystalline alumina, and quartz by molten metal halide salts. J. Phys. Chem. Solids 2005, 66, 484–487. [Google Scholar] [CrossRef]
- Janz, G.J.; Gardner, G.L.; Krebs, U.; Tomkins, R.P.T. Molten Salts: Volume 4, Part 1, Fluorides and Mixtures Electrical Conductance, Density, Viscosity, and Surface Tension Data. J. Phys. Chem. Ref. Data 1974, 3, 1–115. [Google Scholar] [CrossRef]
- Gusev, S.A.; Protsenko, P.V.; Skvortsova, Z.N. The effect of the degree of ionicity of ceramic materials on their wettability by melted sodium chloride. Colloid J. 2016, 78, 47–51. [Google Scholar] [CrossRef]
- Barthwal, M.; Dhar, A.; Powar, S. Effect of Nanomaterial Inclusion in Phase Change Materials for Improving the Thermal Performance of Heat Storage: A Review. ACS Appl. Energy Mater. 2021, 4, 7462–7480. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Z.; Feng, Y.; Lin, L. Insights into the thermophysical properties and heat conduction enhancement of NaCl-Al2O3 composite phase change material by molecular dynamics simulation. Int. J. Heat Mass Transf. 2022, 198, 123422. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Q.; Wang, X.; Sun, S.; Bai, J.; Cui, D.; Pan, S.; Sheng, H. Effect of Al2O3 nanoparticle dispersion on the thermal properties of a eutectic salt for solar power applications: Experimental and molecular simulation studies. Energy 2024, 288, 129785. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, J.; Li, L.; Sun, B.; Bao, X.; Zhang, H. Current understanding and applications of the cold sintering process. Front. Chem. Sci. Eng. 2019, 13, 654–664. [Google Scholar] [CrossRef]
- Liu, M.; Jin, Q.; Shen, P. Cold sintering of NaNO3/MgO heat-storage composite. Ceram. Int. 2020, 46 Pt A, 28955–28960. [Google Scholar] [CrossRef]
- Zhu, J.; Li, R.; Zhou, W.; Zhang, H.; Cheng, X. Fabrication of Al2O3-NaCl composite heat storage materials by one-step synthesis method. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 950–954. [Google Scholar] [CrossRef]
- Suleiman, B.; Yu, Q.; Ding, Y.; Li, Y. Fabrication of form stable NaCl-Al2O3 composite for thermal energy storage by cold sintering process. Front. Chem. Sci. Eng. 2019, 13, 727–735. [Google Scholar] [CrossRef]
- Zhou, X.; Yamashita, S.; Kubota, M.; Kita, H. Encapsulated Copper-Based Phase-Change Materials for High-Temperature Heat Storage. ACS Omega 2022, 7, 5442–5452. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Al Kindi, R. Feasibility Study of Regenerative Burners in Aluminum Holding Furnaces. JOM 2014, 66, 1603–1611. [Google Scholar] [CrossRef]
- Zanganeh, G.; Pedretti, A.; Haselbacher, A.; Steinfeld, A. Design of packed bed thermal energy storage systems for high-temperature industrial process heat. Appl. Energy 2015, 137, 812–822. [Google Scholar] [CrossRef]
- Bayón, R.; Rojas, E. Simulation of thermocline storage for solar thermal power plants: From dimensionless results to prototypes and real-size tanks. Int. J. Heat Mass Transf. 2013, 60, 713–721. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Huang, G.; Shu, P.; Kiriki, H.; Kubo, S.; Ohno, K.; Kawai, T. Eutectic compound (KNO3/NaNO3:PCM) quasi-encapsulated into SiC-honeycomb for suppressing natural convection of melted PCM. Int. J. Energy Res. 2015, 39, 789–804. [Google Scholar] [CrossRef]
- Lee, M.N.; Chan, H.K.; Mohraz, A. Characteristics of pickering emulsion gels formed by droplet bridging. Langmuir 2012, 28, 3085–3091. [Google Scholar] [CrossRef]





| 640 °C | 801 °C | 850 °C | 850 °C-10 min | |
|---|---|---|---|---|
| NaCl(80)/ Al2O3(20) | 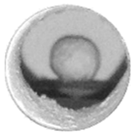 |  |  |  |
| NaCl |  | 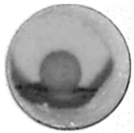 | 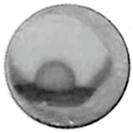 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Fuhai, B.; Shenghao, L.; Kita, H.; Hong, F. Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material. Processes 2024, 12, 465. https://doi.org/10.3390/pr12030465
Yamashita S, Fuhai B, Shenghao L, Kita H, Hong F. Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material. Processes. 2024; 12(3):465. https://doi.org/10.3390/pr12030465
Chicago/Turabian StyleYamashita, Seiji, Bao Fuhai, Liao Shenghao, Hideki Kita, and Fangjun Hong. 2024. "Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material" Processes 12, no. 3: 465. https://doi.org/10.3390/pr12030465
APA StyleYamashita, S., Fuhai, B., Shenghao, L., Kita, H., & Hong, F. (2024). Novel Sodium Chloride/Aluminum Oxide Powder-Composite Structure with High Shape-Retention Performance for the Encapsulation of a High-Temperature Phase-Change Material. Processes, 12(3), 465. https://doi.org/10.3390/pr12030465








