Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides
Abstract
1. Introduction
2. Experimental
2.1. Support Material Synthesis
2.2. Nitrogen Doping and Catalyst Synthesis
3. Results and Discussion
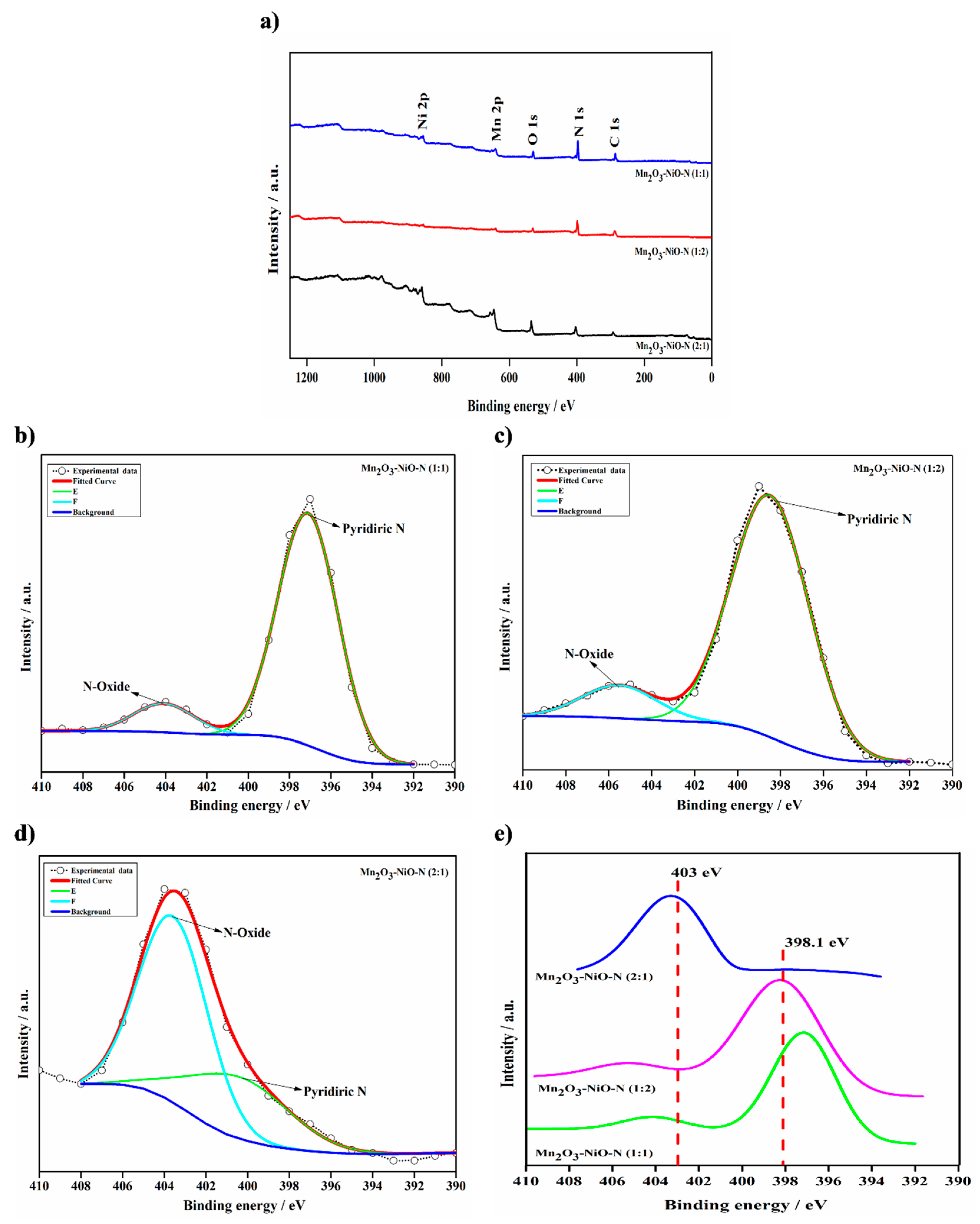
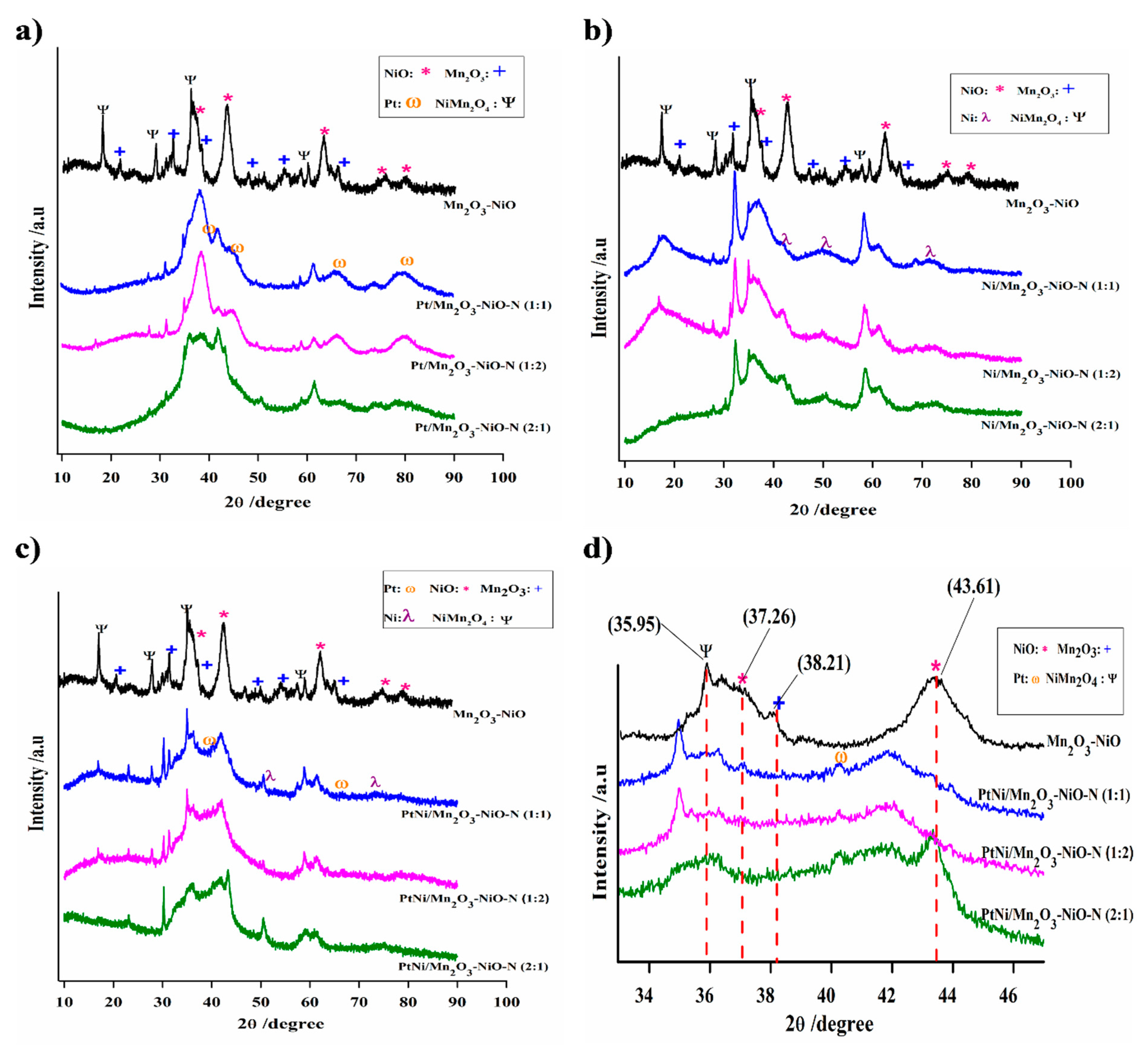
3.1. Catalyst Characterization
3.2. Double-Layer Capacitance Investigation
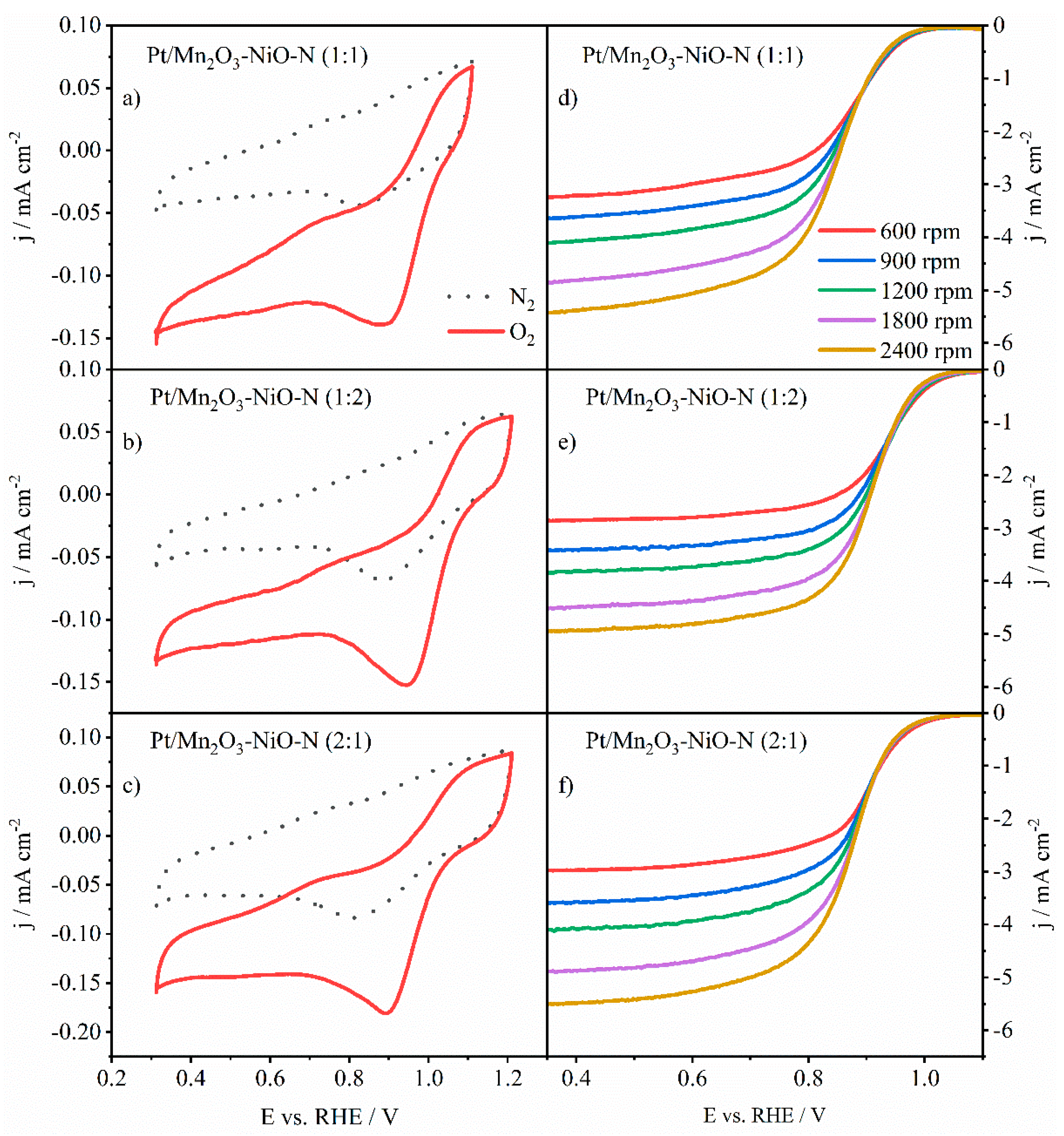
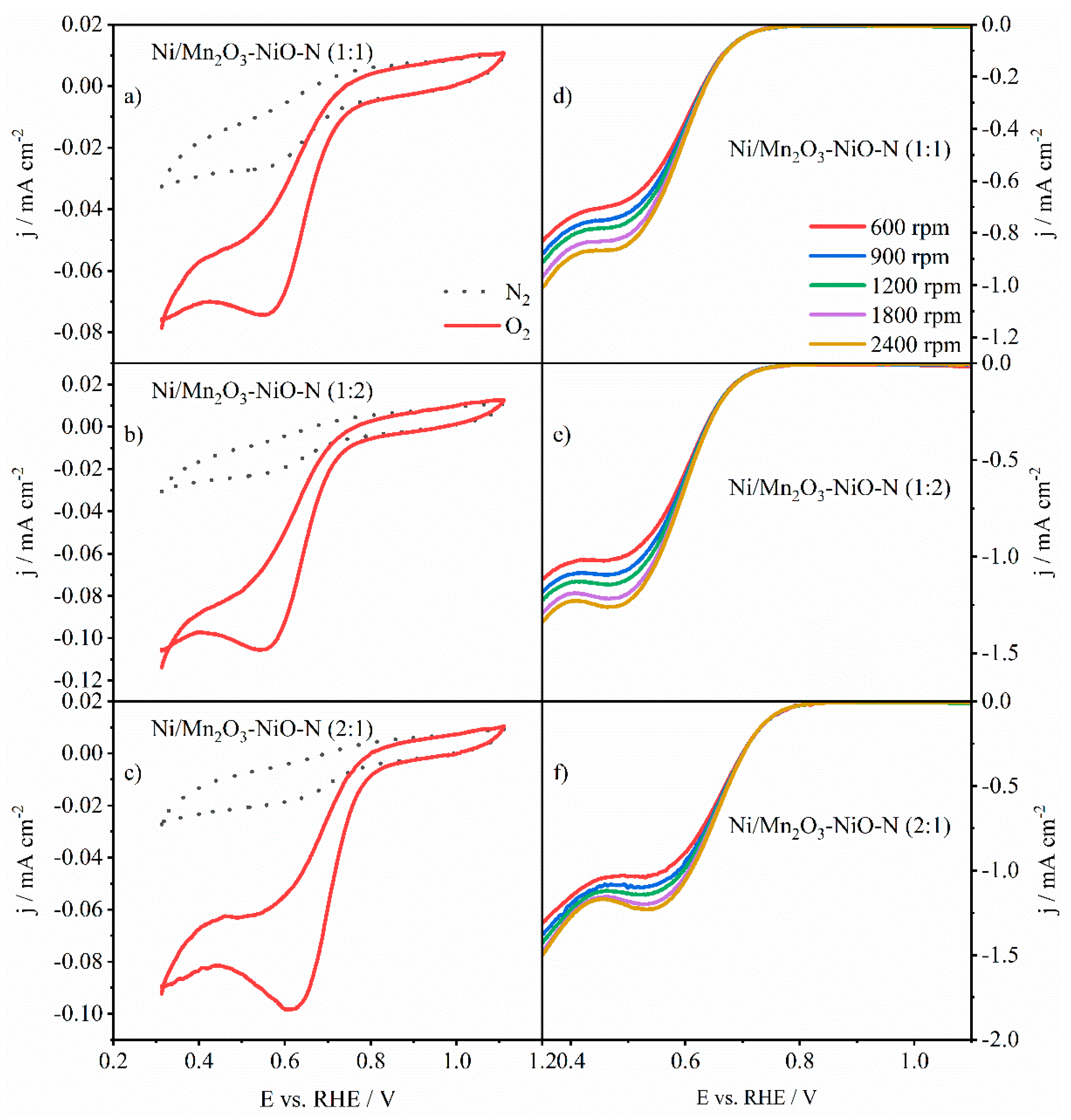
3.3. Activity towards ORR
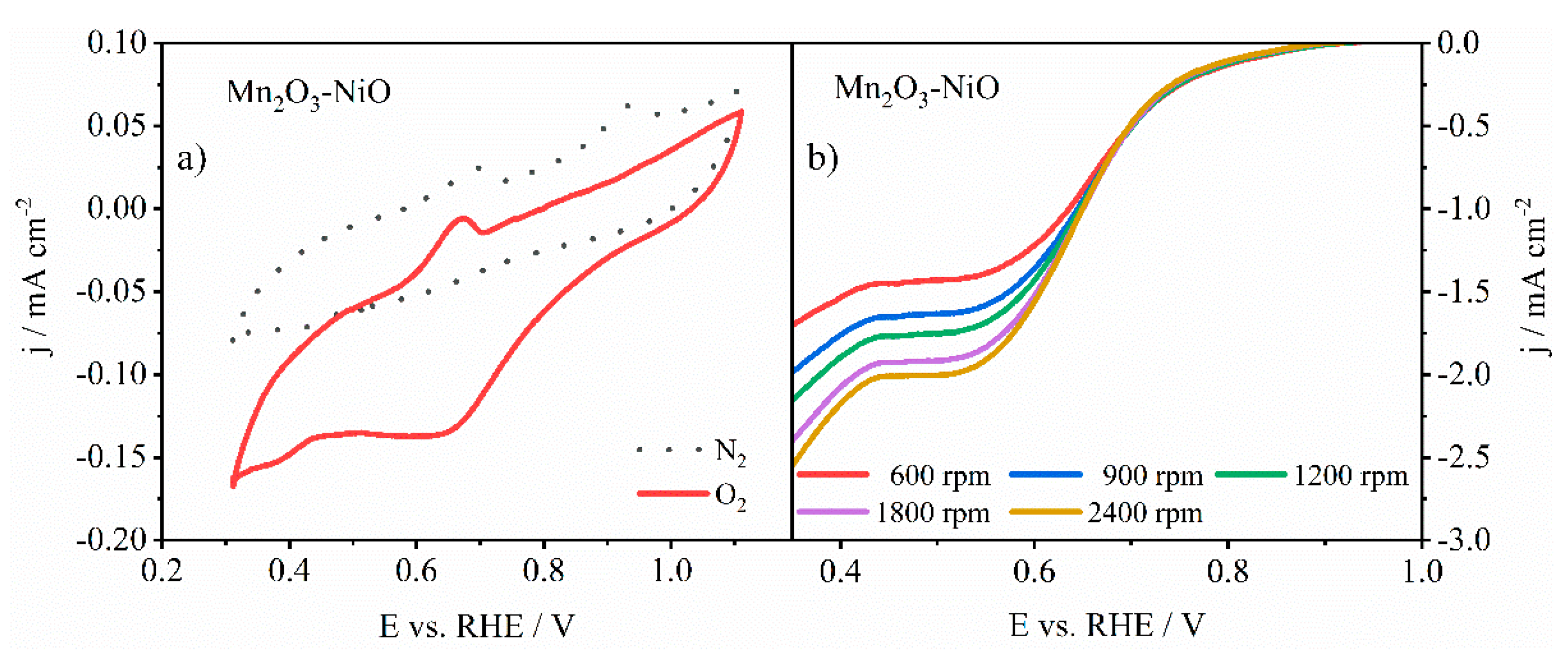
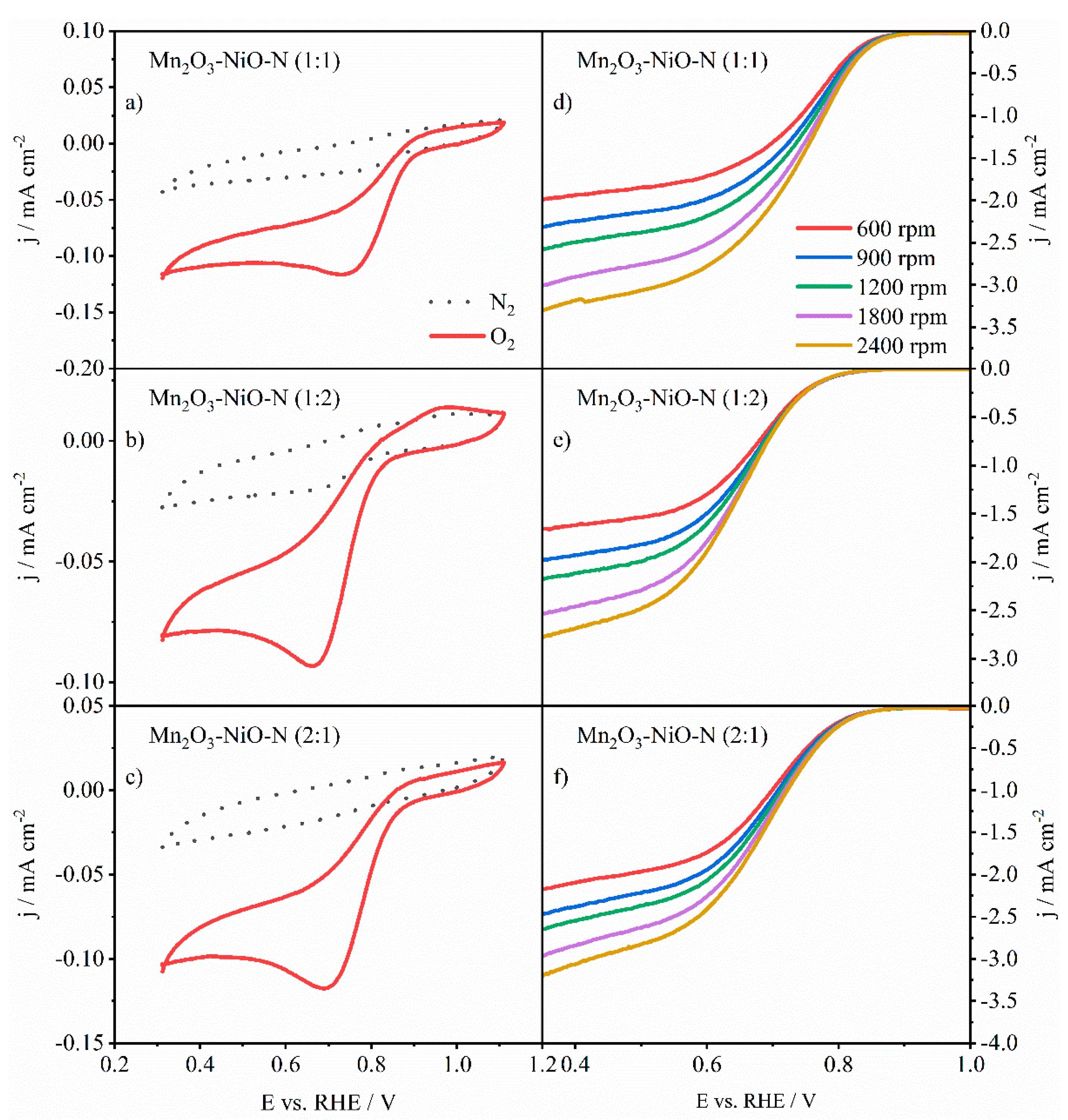
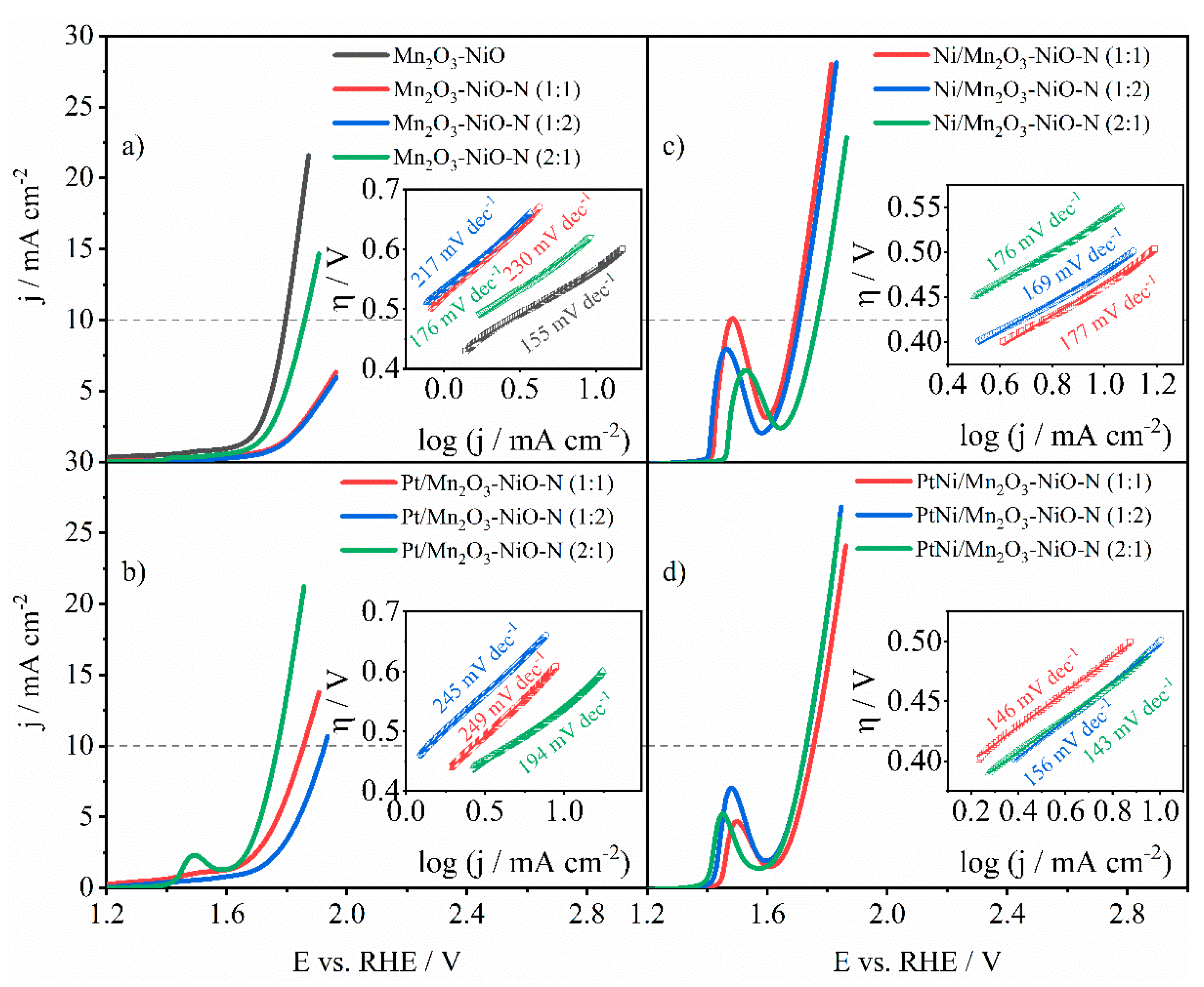
3.3.1. ORR Activity of Pure and N-Doped Mn2O3-NiO
3.3.2. ORR Activity of Pt-Decorated N-Doped Mn2O3-NiO
3.3.3. ORR Activity of Ni-Decorated N-Doped Mn2O3-NiO
3.3.4. ORR Activity of PtNi-Decorated N-Doped Mn2O3-NiO
3.4. OER Performance
3.5. Bifunctional Performance Assessment
| Material | ORR Parameters | OER Parameters | ΔE */V | Electrolyte | Source | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| jd/mA cm−2 | jk/mA cm−2 | E1/2/V | b/mV dec−1 | n | η10/V | b/mV dec−1 | ||||
| Mn2O3-NiO | −1.93 | −0.67 | 0.66 | 151 | 2.90 | 0.57 | 155 | 1.14 | 0.1 M KOH | This work |
| Mn2O3-NiO-N (1:1) | −2.75 | −1.65 | 0.75 | 82 | 2.73 | - | 230 | - | 0.1 M KOH | This work |
| Mn2O3-NiO-N (1:2) | −2.29 | −1.26 | 0.67 | 90 | 2.35 | - | 217 | - | 0.1 M KOH | This work |
| Mn2O3-NiO-N (2:1) | −2.62 | −1.00 | 0.70 | 99 | 3.63 | 0.63 | 176 | 1.16 | 0.1 M KOH | This work |
| Pt/Mn2O3-NiO-N (1:1) | −4.69 | −2.97 | 0.87 | 90 | 3.88 | 0.63 | 249 | 0.99 | 0.1 M KOH | This work |
| Pt/Mn2O3-NiO-N (1:2) | −4.43 | −3.68 | 0.92 | 92 | 3.84 | 0.70 | 245 | 0.94 | 0.1 M KOH | This work |
| Pt/Mn2O3-NiO-N (2:1) | −4.81 | −6.71 | 0.89 | 96 | 3.72 | 0.54 | 194 | 0.88 | 0.1 M KOH | This work |
| Ni/Mn2O3-NiO-N (1:1) | −0.83 | −0.22 | 0.60 | 86 | 2.24 | 0.46 | 177 | 1.09 | 0.1 M KOH | This work |
| Ni/Mn2O3-NiO-N (1:2) | −1.21 | −0.32 | 0.60 | 100 | 3.17 | 0.48 | 169 | 1.11 | 0.1 M KOH | This work |
| Ni/Mn2O3-NiO-N (2:1) | −1.18 | −0.29 | 0.65 | 87 | 3.97 | 0.54 | 176 | 1.12 | 0.1 M KOH | This work |
| PtNi/Mn2O3-NiO-N (1:1) | −1.64 | −0.45 | 0.65 | 129 | 3.82 | 0.53 | 146 | 1.11 | 0.1 M KOH | This work |
| PtNi/Mn2O3-NiO-N (1:2) | −2.94 | −1.37 | 0.77 | 67 | 3.38 | 0.50 | 156 | 0.96 | 0.1 M KOH | This work |
| PtNi/Mn2O3-NiO-N (2:1) | −2.93 | −1.28 | 0.70 | 67 | 3.52 | 0.50 | 143 | 1.03 | 0.1 M KOH | This work |
| FeCo@N-HC | −5.80 | - | 0.85 | 97 | 3.98 | 0.32 | 92 | 0.70 | 0.1 M KOH | [15] |
| NiFeCoNC | −6.00 | - | 0.84 | - | 4.00 | 0.36 | - | 0.75 | 0.1 M KOH | [16] |
| LaMnNiCoO3 (1:2:3) | −5.90 | - | 0.75 | 80 | 3.99 | 0.37 | - | 0.85 | 0.1 M KOH | [17] |
| Ni0.33Co0.67Ox | −5.80 | - | 0.81 | - | 3.89 | 0.28 | 76 | 0.70 | 0.1 M KOH | [18] |
| MnFe2O4 | −4.70 | - | 0.71 | 71 | 3.88 | 0.58 | - | 1.10 | 0.1 M KOH | [51] |
| NiFe2O4 | −4.95 | - | 0.68 | 57 | 3.76 | 0.41 | - | 0.96 | 0.1 M KOH | [51] |
| NiO-Mn2O3-CDs | −6.00 | - | 0.84 | 126 | 3.85 | 0.30 | 141 | 0.69 | 0.1 M KOH | [52] |
| Pt/Mn2O3-NiO | −4.48 | −4.34 | 0.79 | 62 and 109 | 3.73 | 0.54 | 154 | 0.98 | 0.1 M KOH | [19] |
| PtNi/Mn2O3-NiO | −4.32 | −3.10 | 0.79 | 63 and 103 | 3.99 | 0.53 | 140 | 0.97 | 0.1 M KOH | [19] |
| IrO2 | - | - | - | - | - | 0.36 | 84 | - | 0.1 M KOH | [53] |
| RuO2 | - | - | - | - | - | 0.40 | - | - | 0.1 M KOH | [16] |
| Pt/C (40 wt.%) | −6.44 | 14.90 | 0.86 | 79 and 60 | 3.97 | 0.58 | 198 | 0.95 | 0.1 M KOH | [19] |
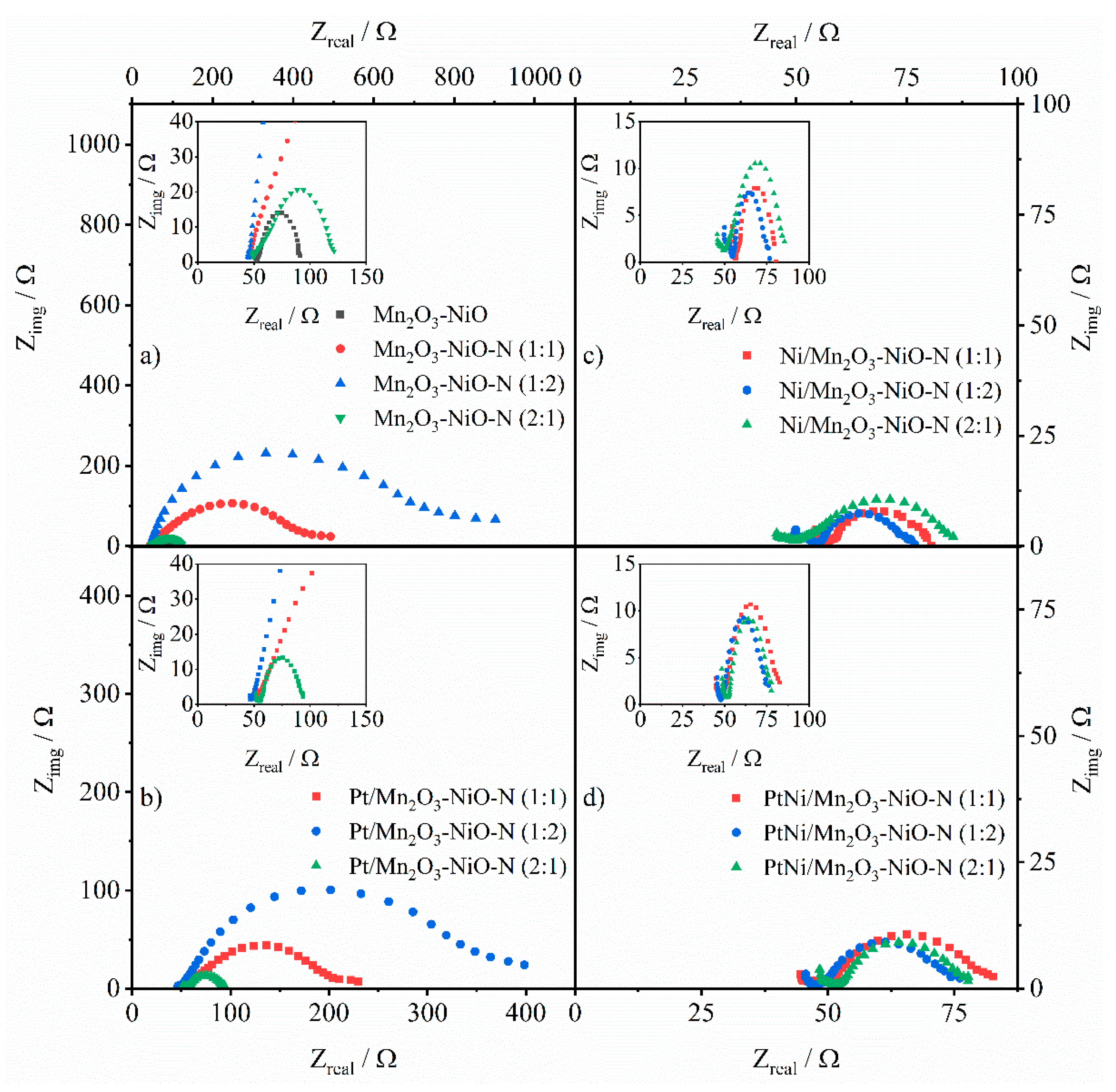
3.6. EIS and Stability Study of Tested Electrocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.theworldcounts.com (accessed on 9 October 2023).
- Utz, R.C.; Teledyne Energy Systems, Inc. Study of Unitized Regenerative Fuel Cell Systems for Aircraft Applications; Federal Aviation Administration. William J. Hughes Technical Center: Egg Harbor Township, NJ, USA, 2022. [CrossRef]
- Ball, M.; Weeda, M. The hydrogen economy—Vision or reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The hydrogen economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part B: Unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 775–795. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.H.; Kim, T.H.; Park, K.W.; Jung, S.; Kurkuri, M.D.; Jung, H.Y. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrogen Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Adzic, R. Recent Advances in the Kinetics of Oxygen Reduction; Brookhaven National Laboratory: Upton, NY, USA, 1996. [CrossRef]
- Miles, M.H.; Klaus, E.A.; Gunn, B.P.; Locker, J.R.; Serafin, W.E.; Srinivasan, S. The oxygen evolution reaction on platinum, iridium, ruthenium and their alloys at 80 °C in acid solutions. Electrochim. Acta 1978, 23, 521–526. [Google Scholar] [CrossRef]
- Wen, X.; Bai, L.; Li, M.; Guan, J. Ultrafine iridium oxide supported on carbon nanotubes for efficient catalysis of oxygen evolution and oxygen reduction reactions. Mater. Today Energy 2018, 10, 153–160. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: An application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem.—Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Han, Z.; Lin, S.Y.; Feng, J.J.; Zhang, L.; Zhang, Q.L.; Wang, A.J. Transitional metal alloyed nanoparticles entrapped into the highly porous N-doped 3D honeycombed carbon: A high-efficiency bifunctional oxygen electrocatalyst for boosting rechargeable Zn-air batteries. Int. J. Hydrogen Energy 2021, 46, 19385–19396. [Google Scholar] [CrossRef]
- Kisand, K.; Sarapuu, A.; Kikas, A.; Kisand, V.; Rähn, M.; Treshchalov, A.; Käärik, M.; Piirsoo, H.M.; Aruväli, J.; Paiste, P.; et al. Bifunctional multi-metallic nitrogen-doped nanocarbon catalysts derived from 5-methylresorcinol. Electrochem. Commun. 2021, 124, 106932. [Google Scholar] [CrossRef]
- Sun, J.; Du, L.; Sun, B.; Han, G.; Ma, Y.; Wang, J.; Huo, H.; Zuo, P.; Du, C.; Yin, G. A bifunctional perovskite oxide catalyst: The triggered oxygen reduction/evolution electrocatalysis by moderated Mn-Ni co-doping. J. Energy Chem. 2021, 54, 217–224. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, G.; Wang, X.; Jin, M.; Akinoglu, E.M.; Luo, D.; Shui, L. Bimetallic hollow tubular NiCoOx as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution reaction. ACS Appl. Mater. Interfaces 2021, 13, 7334–7342. [Google Scholar] [CrossRef]
- Mladenović, D.; Santos, D.M.F.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Tailoring metal-oxide-supported PtNi as bifunctional catalysts of superior activity and stability for unitised regenerative fuel cell applications. Electrochem. Commun. 2021, 124, 106963. [Google Scholar] [CrossRef]
- Kotta, A.; Ansari, S.A.; Parveen, N.; Fouad, H.; Alothman, O.Y.; Khaled, U.; Seo, H.K.; Ansari, S.G.; Ansari, Z.A. Mechanochemical synthesis of melamine doped TiO2 nanoparticles for dye sensitized solar cells application. J. Mater. Sci. Mater. Electron. 2018, 29, 9108–9116. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, Q.; Mossin, S.; Nielsen, D.; Kong, M.; Jiang, L.; Yang, J.; Ren, S.; Wen, J. Promotional effects of nitrogen doping on catalytic performance over manganese-containing semi-coke catalysts for the NH3-SCR at low temperatures. J. Hazard. Mater. 2020, 387, 121704. [Google Scholar] [CrossRef] [PubMed]
- Aykut, Y.; Yurtcan, A.B. Catalyst development for viability of electrochemical hydrogen purifier and compressor (EHPC) technology. Int. J. Hydrogen Energy 2022, 47, 19619–19632. [Google Scholar] [CrossRef]
- Mladenović, D.; Daş, E.; Santos, D.M.F.; Yurtcan, A.B.; Šljukić, B. Highly efficient oxygen electrode obtained by sequential deposition of transition metal-platinum alloys on graphene nanoplatelets. Materials 2023, 16, 3388. [Google Scholar] [CrossRef]
- Luan, V.H.; Tien, H.N.; Hur, S.H.; Han, J.H.; Lee, W. Three-dimensional porous nitrogen-doped NiO nanostructures as highly sensitive NO2 sensors. Nanomaterials 2017, 7, 313. [Google Scholar] [CrossRef]
- Ievtushenko, A.; Khyzhun, O.; Shtepliuk, I.; Tkach, V.; Lazorenko, V.; Lashkarev, G. X-ray photoelectron spectroscopy study of nitrogen and aluminum-nitrogen doped ZnO films. Acta Phys. Pol. A 2013, 124, 858–861. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Zhang, G.; Huang, J.; Liu, P.; Antonietti, M.; Wang, X. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution. J. Mater. Chem. 2011, 21, 13032–13039. [Google Scholar] [CrossRef]
- Yang, G.; Yan, W.; Wang, J.; Yang, H. Fabrication and formation mechanism of Mn2O3 hollow nanofibers by single-spinneret electrospinning. CrystEngComm 2014, 16, 6907–6913. [Google Scholar] [CrossRef]
- Taghizadeh, F. The study of structural and magnetic properties of NiO nanoparticles. Opt. Photonics J. 2016, 6, 164–169. [Google Scholar] [CrossRef]
- Abel, M.J.; Pramothkumar, A.; Archana, V.; Senthilkumar, N.; Jothivenkatachalam, K.; Prince, J.J. Facile synthesis of solar light active spinel nickel manganite (NiMn2O4) by co-precipitation route for photocatalytic application. Res. Chem. Intermed. 2020, 46, 3509–3525. [Google Scholar] [CrossRef]
- Liu, Z.; Hong, L.; Tham, M.P.; Lim, T.H.; Jiang, H. Nanostructured Pt/C and Pd/C catalysts for direct formic acid fuel cells. J. Power Sources 2006, 161, 831–835. [Google Scholar] [CrossRef]
- Tientong, J.; Garcia, S.; Thurber, C.R.; Golden, T.D. Synthesis of nickel and nickel hydroxide nanopowders by simplified chemical reduction. J. Nanotechnol. 2014, 2014, 193162. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy storage data reporting in perspective—Guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Oxygen reduction reaction on Pt and Pt bimetallic surfaces: A selective review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Shao, M.H.; Liu, P.; Adzic, R.R. Superoxide anion is the intermediate in the oxygen reduction reaction on platinum electrodes. J. Am. Chem. Soc. 2006, 128, 7408–7409. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation. Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Li, D.; Batchelor-McAuley, C.; Compton, R.G. Some thoughts about reporting the electrocatalytic performance of nanomaterials. Appl. Mater. Today 2020, 18, 100404. [Google Scholar] [CrossRef]
- Sokolov, S.V.; Sepunaru, L.; Compton, R.G. Taking cues from nature: Hemoglobin catalysed oxygen reduction. Appl. Mater. Today 2017, 7, 82–90. [Google Scholar] [CrossRef]
- Mladenović, D.; Vujković, M.; Mentus, S.; Santos, D.M.F.; Rocha, R.P.; Sequeira, C.A.C.; Figueiredo, J.L.; Šljukić, B. Carbon-supported Mo2C for oxygen reduction reaction electrocatalysis. Nanomaterials 2020, 10, 1805. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped graphene-supported transition-metals carbide electrocatalysts for oxygen reduction reaction. Sci. Rep. 2015, 5, 10389. [Google Scholar] [CrossRef]
- Milikić, J.; Vasić, M.; Amaral, L.; Cvjetićanin, N.; Jugović, D.; Hercigonja, R.; Šljukić, B. NiA and NiX zeolites as bifunctional electrocatalysts for water splitting in alkaline media. Int. J. Hydrogen Energy 2018, 43, 18977–18991. [Google Scholar] [CrossRef]
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Ferrer, P.; García, Á.; Torrero, J.; Gianolio, D.; Fierro, J.L.G.; Peña, M.A.; Alonso, J.A. Role of lattice oxygen content and Ni geometry in the oxygen evolution activity of the Ba-Ni-O system. J. Power Sources 2018, 404, 56–63. [Google Scholar] [CrossRef]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A structurally versatile nickel phosphite acting as a robust bifunctional electrocatalyst for overall water splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef]
- Kumar, J.P.; Giri, S.D.; Sarkar, A. Mesoporous NiO with different morphology: Synthesis, characterization and their evaluation for oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 15639–15649. [Google Scholar] [CrossRef]
- Da Zhang, W.; Hu, Q.T.; Wang, L.L.; Gao, J.; Zhu, H.Y.; Yan, X.; Gu, Z.G. In-situ generated Ni-MOF/LDH hetero-structures with abundant phase interfaces for enhanced oxygen evolution reaction. Appl. Catal. B 2021, 286, 119906. [Google Scholar] [CrossRef]
- Fang, L.; Niu, S.; Wang, S.; Lu, Y.; Cheng, Y. Enhanced oxygen reduction catalytic performance of PtNi alloy through modulating metal–support interaction. Appl. Catal. A Gen. 2024, 669, 119511. [Google Scholar] [CrossRef]
- Hyun, K.; Lee, J.H.; Yoon, C.W.; Cho, Y.H.; Kim, L.H.; Kwon, Y. Improvement in oxygen reduction activity of polypyrrole-coated PtNi alloy catalyst prepared for proton exchange membrane fuel cells. Synth. Met. 2014, 190, 48–55. [Google Scholar] [CrossRef]
- Si, C.; Zhang, Y.; Zhang, C.; Gao, H.; Ma, W.; Lv, L.; Zhang, Z. Mesoporous nanostructured spinel-type MFe2O4 (M = Co, Mn, Ni) oxides as efficient bi-functional electrocatalysts towards oxygen reduction and oxygen evolution. Electrochim. Acta 2017, 245, 829–838. [Google Scholar] [CrossRef]
- Shao, C.; Liao, F.; Zhu, W.; Zhang, Y.; Ma, M.; Yang, J.; Yin, K.; Shao, M.; Jiang, B. Carbon dots bridge NiO and Mn2O3 as highly efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Appl. Surf. Sci. 2022, 596, 153642. [Google Scholar] [CrossRef]
- Paul, A.; Silva, T.A.R.; Soliman, M.M.A.; Karačić, J.; Šljukić, B.; Alegria, E.C.B.A.; Khan, R.A.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Benzimidazole Schiff base copper (II) complexes as catalysts for environmental and energy applications: VOC oxidation, oxygen reduction and water splitting reactions. Int. J. Hydrogen Energy 2022, 47, 23175–23190. [Google Scholar] [CrossRef]
- Swierk, J.R.; Klaus, S.; Trotochaud, L.; Bell, A.T.; Tilley, T.D. Electrochemical study of the energetics of the oxygen evolution reaction at nickel iron (oxy)hydroxide catalysts. J. Phys. Chem. C 2015, 119, 19022–19029. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Nadesan, J.C.B.; Tseung, A.C.C. Oxygen evolution on nickel oxide electrodes. J. Electrochem. Soc. 1985, 132, 2957–2959. [Google Scholar] [CrossRef]
- Martins, M.; Milikić, J.; Šljukić, B.; Soylu, G.S.P.; Yurtcan, A.B.; Bozkurt, G.; Santos, D.M.F. Mn2O3-MO (MO = ZrO2; V2O5, WO3) supported PtNi nanoparticles: Designing stable and efficient electrocatalysts for oxygen reduction and borohydride oxidation. Microporous Mesoporous Mater. 2019, 273, 286–293. [Google Scholar] [CrossRef]
- Guo, D.J.; Cui, S.K.; Cheng, D.; Zhang, P.; Jiang, L.; Zhang, C.C. One-pot synthesis of PtNi alloy nanoflowers supported on multi-walled carbon nanotubes with superior electrocatalytic activity for the oxygen reduction. J. Power Sources 2014, 255, 157–162. [Google Scholar] [CrossRef]




| Sample | BET Surface Area (m2 g−1) | BJH Adsorption Cumulative Pore Volume (cm3 g−1) | BJH Desorption Cumulative Pore Volume (cm3 g−1) | BJH Adsorption Average Pore Width (nm) | BJH Desorption Average Pore Width (nm) |
|---|---|---|---|---|---|
| Mn2O3-NiO | 73.11 | 0.465 | 0.465 | 23.0 | 16.5 |
| Mn2O3-NiO-N (1:1) | 16.19 | 0.143 | 0.143 | 31.3 | 22.8 |
| Mn2O3-NiO-N (1:2) | 3.16 | 0.058 | 0.063 | 31.2 | 21.3 |
| Mn2O3-NiO-N (2:1) | 37.52 | 0.219 | 0.220 | 24.6 | 20.4 |
| Material | Rs/Ω | Rct/Ω |
|---|---|---|
| Mn2O3-NiO | 52.6 | 38.3 |
| Mn2O3-NiO-N (1:1) | 45.7 | 380 |
| Mn2O3-NiO-N (1:2) | 45.1 | 710 |
| Mn2O3-NiO-N (2:1) | 49.0 | 71.0 |
| Pt/Mn2O3-NiO-N (1:1) | 53.0 | 144 |
| Pt/Mn2O3-NiO-N (1:2) | 47.5 | 306 |
| Pt/Mn2O3-NiO-N (2:1) | 55.5 | 38.5 |
| Ni/Mn2O3-NiO-N (1:1) | 56.1 | 24.4 |
| Ni/Mn2O3-NiO-N (1:2) | 54.6 | 22.1 |
| Ni/Mn2O3-NiO-N (2:1) | 50.0 | 35.4 |
| PtNi/Mn2O3-NiO-N (1:1) | 48.5 | 32.2 |
| PtNi/Mn2O3-NiO-N (1:2) | 47.6 | 27.5 |
| PtNi/Mn2O3-NiO-N (2:1) | 51.8 | 25.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, D.; Aykut, Y.; Yurtcan, A.B.; Soylu, G.S.P.; Santos, D.M.F.; Miljanić, Š.; Šljukić, B. Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides. Processes 2024, 12, 453. https://doi.org/10.3390/pr12030453
Mladenović D, Aykut Y, Yurtcan AB, Soylu GSP, Santos DMF, Miljanić Š, Šljukić B. Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides. Processes. 2024; 12(3):453. https://doi.org/10.3390/pr12030453
Chicago/Turabian StyleMladenović, Dušan, Yasemin Aykut, Ayşe B. Yurtcan, Gulin S. P. Soylu, Diogo M. F. Santos, Šćepan Miljanić, and Biljana Šljukić. 2024. "Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides" Processes 12, no. 3: 453. https://doi.org/10.3390/pr12030453
APA StyleMladenović, D., Aykut, Y., Yurtcan, A. B., Soylu, G. S. P., Santos, D. M. F., Miljanić, Š., & Šljukić, B. (2024). Optimizing Oxygen Electrode Bifunctionality with Platinum and Nickel Nanoparticle-Decorated Nitrogen-Doped Binary Metal Oxides. Processes, 12(3), 453. https://doi.org/10.3390/pr12030453









