Abstract
Conventional fluid loss additives have difficultly controlling the water loss of cement–metakaolin slurry with semi-saturated brine cement slurry and limiting it to less than 50 mL (30 min)−1. This paper describes the development of an anti-salt fluid loss additive for metakaolin–cement systems. This study adopted the aqueous solution polymerization method; selected four kinds of monomers, namely 2-Acrylamido-2-methylpropane sulfonic acid (AMPS), N,N-Dimethylacrylamide (DMAA), acrylamide (AM), and methyl acrylate (MA); and performed a single-factor experiment on the proportion of monomer, reaction temperature, initiator dosage, and developed fluid loss additive, which has a high salt tolerance and temperature tolerance. This fluid loss additive can resist salt until saturation, and it can control fluid loss in 24 mL·(30 min)−1 when its dosage is 2%. The fluid loss additive can achieve the effect of fluid loss reduction by increasing the filtrate viscosity, forming a flexible elastic adsorption layer via adsorption, and blocking mud cake pores.
1. Introduction
In deep and geothermal wells, cement has durability problems that may lead to seal failure. Volcanic ash materials such as metakaolin have excellent properties, such as low porosity and high temperature resistance, and react with cement to form highly crystalline C-S-H [1]. Therefore, materials such as metakaolin are potential substitutes for ordinary Portland cement [2,3]. Alkaline excitation of metakaolin using only calcium hydroxide resulted in the low compressive strength of geopolymer specimens [4]. To enhance the compressive strength of the metakaolin specimens, salts such as sodium silicate need to be added to the slurry. And the salts lead to an increase in water loss from the cement paste slurry. When using sodium silicate and sodium hydroxide for alkaline excitation, with an alkaline activator dosage of 40%, the water loss of the slurry obtained from the formulation is 93 mL/30 min [5]. Therefore, there is a need to regulate the salt resistance of cement slurry containing metakaolin.
The main difficulties are analyzed as follows: Designing anti-salt slurry is difficult; the performance of cement slurry becomes worse after being eroded by salt [6,7]. And the anti-salt ability of common admixtures makes it difficult to meet the requirements, especially the fluid loss additive. Excessive fluid loss of cement slurry will lead to low cement cementation strength [8], which cannot meet the requirements of periodic strong injection.
At present, acrylamide and acrylic acid are mainly used as monomers to synthesize anti-salt fluid loss additives. Tang Xin et al. prepared a new fluid loss additive, WSP, by using AMPS, AA, DMAA, and SA, which could control the water loss of cement paste to 58 mL at a dosage of 3% in a freshwater cement paste, and 90 mL at a dosage of 5% in a 37% brine cement paste [9]. Guo Jintang et al. synthesized a novel fluid loss additive HTF-200C using AMPS and DMAA as raw materials. In freshwater cement slurry, the water loss of the slurry can be controlled within 100 mL when the dosage of HTF-200C is 2.5%. In 18% brine cement paste, when the dosage of HTF-200C is 4%, the water loss of cement paste can be controlled within 100 mL [10]. The conventional fluid loss additive does not meet the standard requirements of API fluid loss of cement slurry less than or equal to 50 mL [11].
At present, anti-salt fluid loss additive cannot meet the cementing requirements. If the fluid loss of slurry is too large, the slurry filtrate will flow along the interface between the cement slurry and salt paste layer, forming a potential channel of oil and gas channelization. From the perspective of inhibiting salt paste dissolution and improving cementing quality, it is urgent to develop a kind of salt-resistant and temperature-resistant fluid loss additive. At the same time, there is a lack of fluid loss additives that are suitable for volcanic ash materials such as metakaolin.
Fluid loss additives reduce water loss by adsorbing on the cement surface to form a dense structure [11,12]. In adsorbed monomers, itaconic acid is not considered because it has strong retarding properties. Maleic anhydride not only has relatively strong adsorption; its cyclic structure can also enhance the rigidity of the polymer molecular chain and improve the temperature resistance of the polymer molecular chain, so maleic anhydride (MA) is selected as the adsorption monomer [13].
In order to improve the salt resistance of fluid loss additive, at the same time, introduce anionic groups (sulfonic group and carboxyl) and rigid monomers (DMAA) [14].
By optimizing the ratio of various monomers and synthetic conditions, anti-salt fluid loss additive ADAM-J was developed, and ADAM-J had been used to characterize the analysis, performance evaluation of the resistance to salt, and fluid loss mechanism analysis. When the mass ratio of cement to metakaolin is 7:3, the dosage of water is 44% of the total mass of cement and metakaolin, and the dosage of the fluid loss additive ADAM-J is 2%, so it is possible to control the loss of water of fresh water cement paste within 12 mL·(30 min)−1, the loss of water of semi-saturated brine cement paste 24 mL·(30 min)−1, and the loss of water of saturated brine cement paste 24 mL·(30 min)−1. According to the analysis, the mechanism of fluid loss additive ADAM-J is as follows: negatively charged copolymer molecular chain wrapped in the surface of the cement particles, under pressure, the long chain structure of the fluid loss additive is decomposed to form, filling some of the pores of the filter cake and connecting channels, so that it is more difficult for free water to pass through the mud cake, so as to achieve the purpose of fluid loss.
2. Experiment
2.1. Experimental Material
Cement (G-grade, high-quality oil well resistance cement produced by Jiahua Special Cement Co., Ltd., Leshan, China, industrial grade); deionized water (laboratory self-made); sodium bisulfite (White Lion Chemical Reagent Co. Ltd., Pingxiang, China, AR); potassium persulfate (Shanghai Sinopharm Group, Shanghai, China, AR); AM (Jiangxi Changjiu Agro-Chemical Co., Nanchang, China, industrial grade); AMPS (Jiangxi Changjiu Agro-Chemical Co., industrial grade); MA (Shanghai Sinopharm Group, AR); DMAA (Shanghai Sinopharm Group, GR); metakaolin (Jiangyin Guangyuan Superfine Powder Co., Jiangyin, China, industrial grade); and sodium silicate hydrate (Shanghai Sinopharm Group, AR). Chemical composition and physical properties of cement are shown in Table 1. Chemical composition and physical properties of MK are shown in Table 2.

Table 1.
Chemical composition and physical properties of cement.

Table 2.
Chemical composition and physical properties of metakaolin.
2.2. Experimental Method
2.2.1. The Preparation of Cement Slurry and the Test of Compressive Strength of Set Cement
The experimental methods, such as the preparation of cement slurry and the test of the compressive strength of cement, were tested according to the GB/T19139-2012 [15] standard of oil well cement. The preparation process of cement–metakaolin mixed slurry was as follows: Firstly, sodium silicate, fluid loss additive ADAM-J, and other admixtures were added to water and stirred well. Subsequently, cement and metakaolin were added and mixed in a constant speed mixer at a low speed of 3000–4000 r/min for 15 s and then 10,000–12,000 r/min for 35 s. The amount of water added was 44% of the total mass of the metakaolin and cement.
2.2.2. Polymer Synthesis Method
In order to facilitate the preparation of cement slurry, water-soluble admixtures are mostly used. Therefore, the synthesis of fluid loss additive in this paper adopts the method of solution polymerization. The product can be directly used as fluid loss additive, or the product can be dried and crushed (powder is easy to transport) for use. The concentration of the monomer was controlled by 20%. Potassium persulfate and sodium bisulfite were selected as the initiator system in this paper (potassium persulfate: sodium bisulfite = 1:1), and the initiator dosage range was 0.12–0.20%. The synthesis temperature ranges from 45 °C to 65 °C, and the pH of the polymer system ranges from 3 to 11.
2.2.3. Characterization and Analysis Method of Fluid Loss Additive ADAM-J
- (1)
- Infrared analysis method of fluid loss additive ADAM-J
The structure of white powder was characterized by using a Thermo Scientific Nicolet iS10 Fourier-transform infrared (FTIR) spectrometer. The structure of the fluid loss additive ADAM-J was analyzed via infrared spectroscopy, and the structure of the synthetic product was verified. Because the fluid loss additive ADAM-J synthesized in this study is organic, the infrared characteristic absorption peaks of organic compounds are in the range of 4000–400 cm−1 wave numbers [16]. So, the spectra were recorded in the range of 4000–400 cm−1.
- (2)
- Nuclear magnetic resonance hydrogen spectrum analysis of fluid loss additive ADAM-J
The presence of various groups in the polymer fluid loss additive was analyzed via H NMR, and the structure of the synthesized product was verified. The purified polymer powder was characterized by using a German BRUKER ADVANCE 400M superconducting nuclear magnetic resonance spectrometer (BRUKER, Billerica, MA, USA). D2O was used as the dissolved medium in this experiment.
- (3)
- Thermogravimetric analysis of fluid loss additive ADAM-J
Thermogravimetric analysis (TG) was performed on the polymer, using the German Netzsch thermogravimetric analyzer (Netzsch, Selb, Germany) to determine the temperature resistance of the synthetic fluid loss additive ADAM-J. The experimental conditions were as follows: heating rate was 10 °C·min−1, carrier gas was N2, carrier gas velocity was 10 mL·min−1, and the test range was 70–600 °C.
- (4)
- Determination of molecular weight of fluid loss additive ADAM-J
In order to determine the molecular weight of the fluid loss additive, gel permeation chromatography was used to characterize the relative molecular weight.
2.2.4. Evaluation Method for Salt Resistance of Fluid Loss Additive ADAM-J
In order to ensure the stability of the fluid loss additive of cementing slurry, a salt sensitivity evaluation of the synthetic fluid loss additive is needed [17,18,19]. For this design of different brine concentrations for a cement slurry preparation and fluid loss test, the cement slurry formula is 70 g Jiahua Class G oil well cement + 30 g metakaolin + 9 g sodium silicate + 44 g water + 2 g fluid loss additive ADAM-J + 0.5 g defoamer. The brine concentration of water for the slurry preparation is 0%, 6%, 12%, 18%, and 27%, respectively.
2.2.5. Analysis Method of Action Mechanism of Fluid Loss Additive ADAM-J
- (1)
- SEM analysis method
In order to observe the difference between the cement filter cake formed by the base slurry and the filter cake formed by the addition of ADAM-J, the filter cakes obtained from the two formulations were dried and analyzed by using an FEI Nova Nano 450 field emission scanning electron microscope (FEI, Hillsboro, OR, USA). The mode of SEM is SE.
- (2)
- Method for Zeta potential analysis of filter cake
In order to determine whether the fluid loss additive was adsorbed on the surface of cement particles [20,21,22], the Zeta potential of the filter cake without fluid loss additive and with fluid loss additive was tested by using a Malvern Zetasizer 2000 Zeta instrument (Malvern Panalytical, Malvern, UK).
The filter cake, which was formed via the fluid loss experiment of base slurry, and the cement slurry of base slurry + fluid loss additive ADAM-J were ground into powder. After dispersing the powder with water, a Malvern Zetasizer 2000 Zeta instrument was used for a potential analysis.
- (3)
- Method for viscosity analysis of aqueous solution
In this paper, an NDJ-9S digital viscometer was used to test the viscosity of the fluid loss additives JSJ-3 and ADAM-J.
The test formula was freshwater solution (176 g water + 8 g fluid loss additive) and subsaturation saline solution (176 g water + 8 g fluid loss additive + 38.6 g industrial sodium chloride).
- (4)
- Infrared analysis
A Thermo Scientific Nicolet iS10 Fourier-transform infrared (FTIR) spectrometer (Thermo Scientific, Waltham, MA, USA) was used to characterize the structure of the white powder. The structure of the fluid loss additive ADAM-J was analyzed according to the IR spectrum. The spectra were recorded in the range of 4000–400 cm−1.
- (5)
- Nuclear magnetic resonance hydrogen spectrum analysis
The purpose of this section is to verify the structure of the synthetic product by analyzing the presence of various groups of polymer fluid loss additives via a nuclear magnetic resonance hydrogen spectrum analysis. The purified polymer powder was characterized by the German BRUKER ADVANCE 400M NMR spectrometer (BRUKER, Billerica, MA, USA) with superconducting magnet. In this experiment, D2O was used as the dissolved medium.
- (6)
- Thermogravimetric analysis
In order to determine the temperature resistance of fluid loss additive ADAM-J, the polymer was analyzed by using the German NETZSCH TG209F3 thermogravimetric analyzer (NETZSCH, Selb, Germany) for thermogravimetric analysis (TG). The experimental conditions are as follows: heating rate is 10 °C min−1, carrier gas is N2, carrier gas velocity is 10 mL·min−1, and test temperature range is 70–600 °C.
3. Results and Discussion
3.1. Molecular Structure Design
3.1.1. Molecular Structure Principle of Anti-Salt Fluid Loss Additive
The main mechanism of polymeric fluid loss additive: the polymer molecules absorb on the surface of the cement particles by the adsorption end of the molecular chain, and free ends of polymer molecular chains can form cemented reticular colloidal aggregates of colloidal aggregation through mutual crosslinking, bound free fluid [23], under a certain pressure difference, between the cement particles form thin impervious bed, stop free water in the slurry penetrating into formation.
In order to make the fluid loss additive have good salt resistance, the following two methods can be considered when polymerizing and selecting monomers. Method 1, introduce a large number of anionic groups [24,25]. The cations in the salt water change the morphology of polymer molecular chains and weaken the adsorption of anionic groups on the surface of cement particles [26,27], and the effect of fluid loss control becomes worse. Therefore, the introduction of a large number of anionic groups (sulfonic group and carboxyl group) can mitigate the adverse effects of cations. Method 2, introduce a rigid monomer [28]. In terms of the structure of the polymer molecular chain, the introduction of a sulfonic group, benzene ring, or ring structure of pyrrolidone can increase the rigidity of the polymer [29] and inhibit the curl and contraction of the polymer molecular chain, thus enhancing the stability of copolymer.
3.1.2. Selection of Synthetic Monomers
In this paper, it is necessary to synthesize a kind of salt-resistant and temperature-resistant fluid loss additive, which needs to be optimized from hydrophilic monomers, salt-resistant monomers, and adsorbent monomers.
- (1)
- Among adsorbent monomers, acrylamide and N,N-dimethyl acrylamide have good adsorption because the hydrogen atom on N of N,N-dimethyl acrylamide is replaced by two methyl groups; the steric hindering of methyl group leads to the amide group having good stability under acidic or alkaline conditions.
- (2)
- Among salt-resistance monomers, 2-propylene acyl amino-2-methyl propane sulfonic acid, sodium styrene sulfonic acid, and N-vinyl pyrrolidone have large side effects, but since 2-acryloy-2-methylpropyl sulfonic acid is low in cost, it was preferred as the salt-resistant monomer.
- (3)
- Among hydrophilic monomers, itaconic acid is not considered because of its strong retarding property [30]. Not only does maleic anhydride have strong hydrophilicity [31], but also its ring structure can enhance the rigidity of the polymer molecular chain and improve the temperature resistance of the polymer molecular chain [32,33], so maleic anhydride was selected as the adsorbent monomer.
In this paper, considering the cost and synthesis process, four monomers—AMPS, DMAA, MA, and AM—were selected for tetrad copolymerization.
According to the design of the molecular structure, we planned to use a quaternary copolymer to synthesize the anti-salt additive. The expected molecular structure diagram is shown in Figure 1.
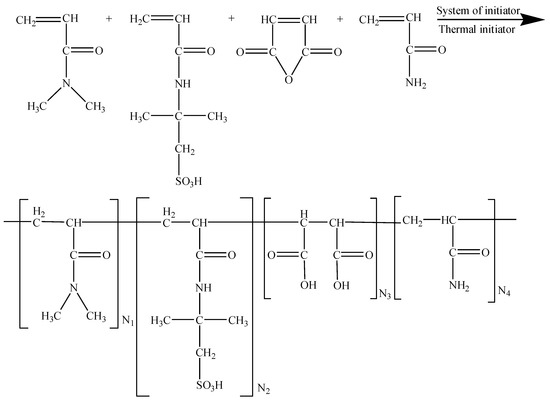
Figure 1.
Expected structure of AMPS/DMAA/MA/AM quaternary copolymer.
3.2. Single Factor Experiment
3.2.1. Optimization of Monomer Ratio
Four monomers in different molar ratio conditions were used, and according to the improved synthesis steps for the preparation of fluid loss additive, the slurry system formula used in the fluid loss experiment was 70 g Jiahua Class G oil well cement + 30 g metakaolin + 9 g sodium silicate + 44 g water (18% concentration of brine) + 3 g fluid loss additive ADAM-J + 0.5 g defoamer. The experimental conditions were as follows: 90 °C, 6.9 MPa, and 0.2% initiator.
The synthetic products with different monomer ratios were tested, and the test results are shown in Table 3.

Table 3.
Fluid loss effect tests of different monomer ratios.
It can be seen from Table 3 that, keeping other conditions unchanged, the polymers synthesized by 15 groups with different monomer ratios have great differences in fluid loss ability in 18% brine cement slurry. Maleic anhydride can significantly enhance the fluid loss reduction performance of ADAM-J.
When the monomer ratio of AMPS:DMAA:AM:MA is 6:1:4:0.5, 6:3:2:0.3, and 6:2:3:0.5, the polymer formed has the best effect of fluid loss control, with fluid loss of 30 mL·(30 min)−1, 34 mL·(30 min)−1, and 32 mL·(30 min)−1, respectively. Considering that AM is an industrial-grade material in the experiment (low price) and DMAA (high purity and high price) has a high cost, the combination ratio with the lowest mole ratio of DMAA was selected as the optimal monomer ratio, which lays a foundation for realizing the industrialization with high performance and low cost in the future. The monomer ratio of 6:1:4:0.5 was selected as the monomer ratio in subsequent tests.
3.2.2. Effect of Reaction Temperature on Fluid Loss
In the process of polymerization, the reaction temperature directly affects the speed of polymerization and the degree of reaction, which also affects the degree of polymerization of reaction products [17]. The effect of the reaction temperature on the fluid loss performance of the polymerization product was evaluated.
Keeping other conditions unchanged and changing the reaction temperature of the polymer, the experimental temperature was set as 45 °C, 50 °C, 55 °C, 60 °C, and 65 °C. The polymerization products were dried and crushed, and the fluid loss test was carried out. The cement slurry formula was 70 g Jiahua Class G oil well cement +30 g metakaolin+ 9 g sodium silicate + 44 g water (18% brine) + 3 g fluid loss additive ADAM-J + 0.5 g defoamer. The experimental conditions were 90 °C and 6.9 MPa, and the test results are shown in Figure 2.
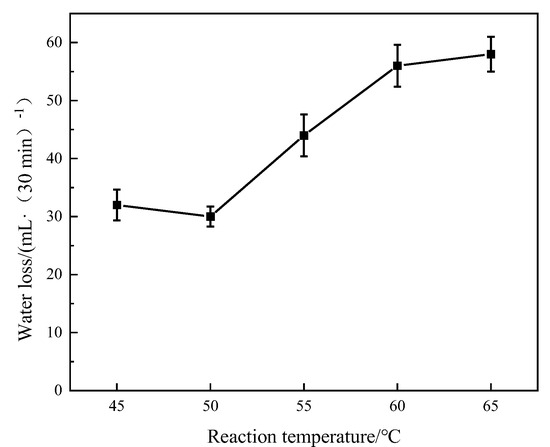
Figure 2.
Effect of polymer reaction temperature on fluid loss.
As can be seen from Figure 2, a water loss test was carried out under the condition of 18% brine concentration. With the increase in the reaction temperature, the fluid loss of the polymer gradually increased. When the synthesis temperature is 50 °C, the fluid loss is the lowest. When the temperature rises above 55 °C, the fluid loss increases gradually. It is concluded that the rate of free radical formation induced by the initiator is slow when the reaction temperature is low, resulting in a decrease in the number of molecular chains in the whole system, while the total number of monomers remains unchanged. The number of monomers polymerized on each molecular chain increases, the length of each molecular chain increases, and the fluid loss effect increases. It also shows that the polymer formed at the lower temperature has a better fluid loss effect, and the polymer at the high temperature has a poor fluid loss effect. Therefore, the optimal temperature to determine the polymer reaction is 50 °C.
3.2.3. Effect of Initiator Dosage on Fluid Loss
In the process of polymerization, the dosage of the initiator directly affects the molecular weight of the polymerization product [34]. When the dosage of the initiator is too low, it is difficult to initiate the polymerization of the monomer. When the dosage of the initiator is too high, the molecular weight of the polymerization product is low, and it is difficult to have a good fluid loss control effect. To this end, it is necessary to determine the appropriate initiator dosage.
With a monomer AMPS:DMAA:AM:MA ratio of 6:1:4:0.5, a reaction temperature of 50 °C, the initiator dosage changed (0.12%, 0.14%, 0.16%, 0.18%, 0.20%), and other reaction conditions remaining unchanged, the fluid loss of the polymerization products was tested after drying and grinding, and the cement slurry formula was 70 g Jiahua Class G oil well cement +30 g metakaolin + 9 g sodium silicate + 44 g water (18% brine) + 3 g fluid loss additive ADAM-J + 0.5 g defoamer. The experimental conditions were 90 °C and 6.9 MPa, and the test results are shown in Figure 3.
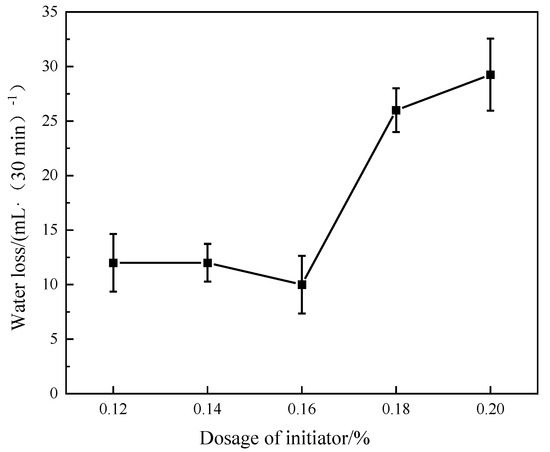
Figure 3.
Effect of initiator dosage on fluid loss.
As can be seen from Figure 3, with the dosage of the initiator (potassium persulfate and sodium bisulfite), the fluid loss of the cement slurry shows a trend of a slight decrease at first and then a rise. The fluid loss can be controlled within 10–12 mL·(30 min)−1 under the condition of 3% dosage of fluid loss additive when the initiator dosage is 0.12–0.16%. The change in fluid loss showed a slight decreasing trend. However, with the increase in the initiator dosage, the fluid loss gradually increased. The reason for this is that, as the initiator dosage increases, the number of free radicals produced in the polymerization systems increases [35]. Competitive bonding of free radicals to amide groups is formed, reducing the number of polymerizable monomers in the molecular chain of the synthesized product [36]. So, the polymer molecular weight is decreased, thus reducing the slurry filtrate’s viscosity. This eventually leads to an increase in the fluid loss of the cement slurry. Considering the cost of the initiator addition and fluid loss of the cement slurry, the optimal initiator addition was determined to be 0.16%.
3.2.4. Effect of pH of Polymerization System on Fluid Loss Effect
In the process of polymerization, the pH of the solution will affect the fluid loss effect of the polymerization product. Therefore, the influence of the pH value of the polymerization system on the fluid loss effect of the polymerization product is studied in this section.
The monomer ratio was controlled as AMPS:DMAA:AM:MA = 6:1:4:0.5, the reaction temperature was 50 °C, the initiator dosage was 0.16%, the monomer mass fraction was 20%, and the reaction time was 5 h. Under the same conditions, the pH value of the polymerization system was changed, and sodium hydroxide was used to adjust the pH value to 3, 5, 7, 9, and 11, respectively. Polymerization took place under different pH conditions. The polymerization product was dried and crushed, and then the fluid loss test of cement slurry was carried out. The test results are shown in Figure 4. The analysis shows that the fluid loss of the fluid loss additive is the lowest when the pH is 7, and the fluid loss effect of the polymerization product becomes worse when the pH is too high or too low, so the optimal pH of the polymer fluid loss additive is 7.
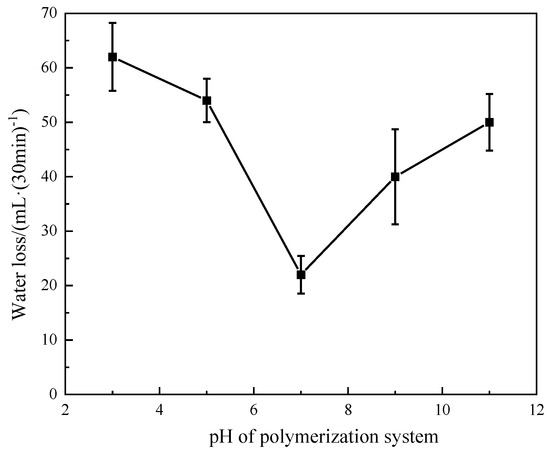
Figure 4.
Effect of pH of polymerization system on fluid loss effect of polymerization products.
To sum up, the optimal synthesis conditions of the fluid loss additive were determined by changing the monomer ratio, reaction temperature, initiator dosage, monomer mass fraction, and other factors: AMPS: DMAA: AM: MA molar ratio was 6:1:4:0.5, monomer mass fraction was 20%, pH value was 7, reaction temperature was 50 °C, initiator dosage was 0.16% (potassium persulfate:sodium bisulfite is 1:1), reaction time was 5 h, the synthesized fluid loss additive used was ADAM-J, and the fluid loss additive used in subsequent experiments was the quaternized copolymer ADAM-J synthesized in this paper unless otherwise specified.
3.3. Characterization Analysis of ADAM-J
In this paper, the fluid loss additive ADAM-J was characterized via an infrared spectrum analysis, hydrogen nuclear magnetic resonance spectroscopy, and a thermogravimetric analysis. The molecular structure and monomer polymerization of the fluid loss additive were studied by conducting an infrared spectrum analysis and hydrogen nuclear magnetic resonance spectroscopy. The purpose of a thermogravimetric analysis is to study the temperature resistance of synthetic products.
In the polymer characterization experiment, in order to eliminate the interference of incomplete reacted monomers in the polymerization products on the test results, the polymerization products should be purified first, and the specific operation steps are as follows. A small amount of the reacted polymer solution was put into a dialysis membrane with a retained molecular weight of 25,000 and then stirred and dialyzed in distilled water for 3 days to remove the small amounts of unreacted monomers in the product. The purified polymer was put into a glass surface dish for freeze-drying, and white polymer powder was obtained after water removal.
3.3.1. Infrared Analysis of Fluid Loss Additive ADAM-J
The structure of the synthetic product was verified, as shown in Figure 5.
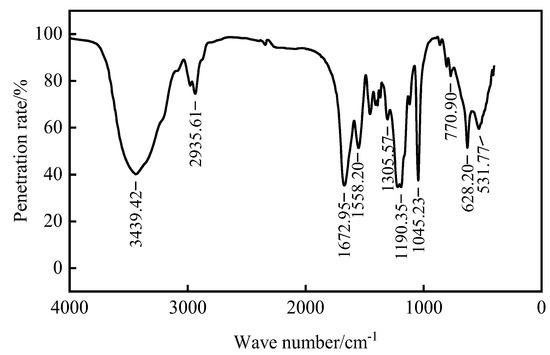
Figure 5.
Infrared spectrogram of four copolymer fluid loss additives.
As can be seen from Figure 5, there is a stretching vibration absorption peak of NH2- for the primary amide at 3439 cm−1, and there is a stretching vibration absorption peak of CH2− at 2935 cm−1. At 1672 cm−1, there is a stretching vibration absorption peak of C=O in the amide group. The stretching vibration absorption peak of COO- exists at 1558 cm−1. The stretching vibration absorption peak of SO2− exists at 1045 cm−1. However, there is no characteristic absorption peak of C=C in the range of 1635–1620 cm−1, indicating that there is no small molecule containing C=C in ADAM-J. It can be seen that all monomers were polymerized, and the product obtained is the target product.
3.3.2. Nuclear Magnetic Resonance Hydrogen Spectrum Analysis of Fluid Loss Additive ADAM-J
The measured hydrogen NMR spectra are shown in Figure 6.
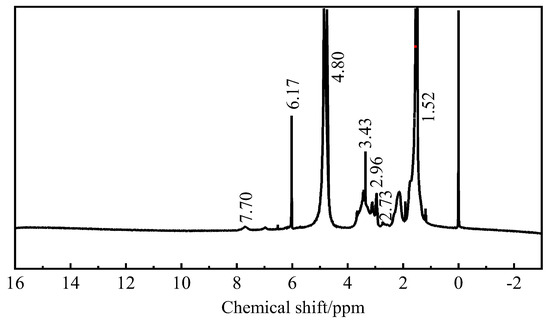
Figure 6.
Nuclear magnetic resonance hydrogen spectrum of four copolymer fluid loss additive.
Figure 6 shows that δ = 4.80 ppm is the absorption peak of solvent-heavy water. δ = 7.70 ppm is the characteristic proton peak of the -NH- group in AMPS. δ = 1.52 ppm is the characteristic proton absorption peak of the -CH3 group in AMPS. Between 3.00 and 3.95 ppm are characteristic proton absorption peaks of the -CH group on the main chain of copolymerization. δ = 6.17 ppm is the N-H multiple proton absorption peak of primary amide in AM, and δ = 2.73 ppm is characteristic proton absorption peak of the -CH3 in DMAA. The characteristic proton absorption peak of the -CH group in MA is at δ = 2.96 ppm.
Combined with the functional group information in the infrared spectrum analysis of the copolymer, the synthetic fluid loss additive ADAM-J is a quaternary copolymer.
3.3.3. Thermogravimetric Analysis of Fluid Loss Additive ADAM-J
The experimental results are shown in Figure 7.
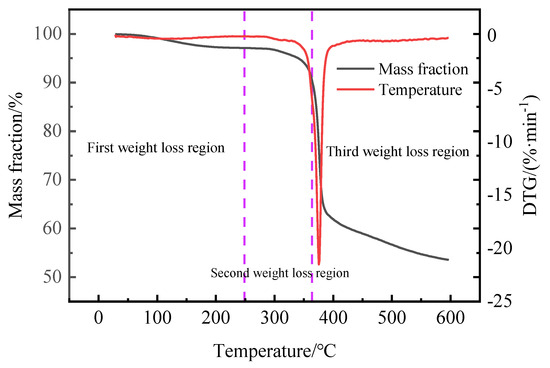
Figure 7.
Thermogravimetric analysis of synthetic fluid loss additive.
As can be seen from Figure 7, the polymer weight loss curve shows three weight loss zones between 70 and 600 °C. In the temperature range of 70–248.6 °C, the mass loss of the fluid loss additive is 2.91%. The mass loss at this stage comes mainly from the evaporation of physically adsorbed water. In the temperature range of 248.6–363.9 °C, the mass loss was 9.56%. The mass loss at this stage is due to the removal of structural water. In the temperature range of 363.9–426.7 °C, the mass loss was 40%. The mass loss comes from the thermal decomposition of unstable oxygen-containing functional groups (C=O, COOH, etc.) in the fluid loss additive [37,38]. The decomposition temperature of the quaternary copolymer was more than 360 °C, indicating that the fluid loss additive ADAM-J had good temperature resistance. The analysis shows that the introduction of rigid sulfonic acid and amide groups into the polymer chain improves the rigidity of the molecular chain, thus improving the temperature resistance of the polymer [39].
3.3.4. Determination of Molecular Weight of Fluid Loss Additive ADAM-J
In order to determine the molecular weight of ADAM-J, gel permeation chromatography was used in this paper to characterize the relative molecular weight, and the results are shown in Table 4.

Table 4.
Molecular weight and dispersion coefficient of fluid loss additive ADAM-J.
As can be seen from Table 4, the weight average molecular weight of the fluid loss additive ADAM-J is 1,182,950, the number average molecular weight is 201,831, and the dispersion coefficient is 5.86. ADAM-J has a wide molecular weight distribution, which is conducive to the adsorption of different sizes of fluid loss additive molecules on the surface of cement particles. The better the adsorption, the better the fluid loss effect of the fluid loss additive ADAM-J.
3.4. Evaluation of Fluid Loss Additive
Although the evaluation of the fluid loss additive in the development process of the previous section was carried out under 18% salt concentration, it is not known whether the synthetic fluid loss additive was sensitive to the salt concentration. In order to ensure the stability of the fluid loss performance of the cement slurry in the salt rock cap layer, a salt sensitivity evaluation of the synthetic fluid loss additive is needed [40,41]. For this design of different salt concentrations of cement slurry preparation and fluid loss test, the cement slurry formula was 70 g Jiahua Class G oil well cement +30 g metakaolin+ 9 g sodium silicate + 44 g water + 2 g fluid loss additive ADAM-J + 0.5 g defoamer. The prepared cement paste is shown in Figure 8. The salt concentration of water for slurry preparation is 0%, 6%, 12%, 18%, and 27%, respectively. The test results of the fluid loss and compressive strength of set cement are shown in Table 5. The experimental conditions are 90 °C and 6.9 MPa.

Figure 8.
Cement–metakaolin paste.

Table 5.
Evaluation of salt resistance of cement slurry system.
As can be seen from Table 5, with the increase in the brine concentration, the compressive strength of the cement stone decreases from 31.36 MPa to about 22.60 MPa, which still meets the standard of the 24 h compressive strength of cement stone being more than 14 MPa. The fluid loss of freshwater cement slurry can be controlled at 12 mL·(30 min)−1, and 24 mL·(30 min)−1 in semi-saturated brine. The dosage of the fluid loss additive can be reduced if the fluid loss requirement is not strict.
3.5. Analysis of Action Mechanism of Fluid Loss Additive ADAM-J
In this section, the mechanism of the fluid loss additive ADAM-J was analyzed by means of the SEM analysis, Zeta potential analysis, viscosity change, and molecular weight determination of polymer aqueous solution in fresh water and saturated salt water.
3.5.1. SEM Analysis
In order to observe the difference between the cement filter cake formed by the base slurry and the filter cake formed by the addition of ADAM-J, which was determined via a fluid loss experiment, the filter cakes obtained by the two formulas were dried, respectively, and analyzed by using an FEI Nova Nano 450 field emission scanning electron microscope (SEM). The analysis results are shown in Figure 9 and Figure 10.
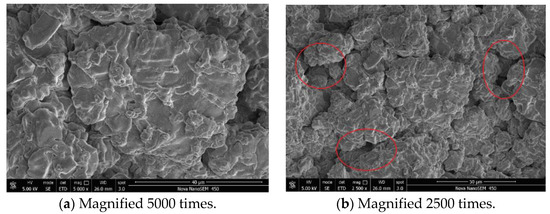
Figure 9.
SEM (SE) of filter cake formation from base slurry.
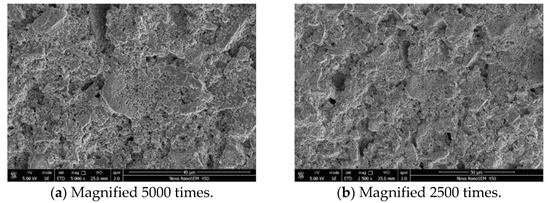
Figure 10.
SEM (SE) of filter cake formed by adding fluid loss additive ADAM-J to base slurry.
It can be seen from Figure 9 and Figure 10 that the surface compactness of the cement filter cake is obviously changed after the addition of the fluid loss additive ADAM-J into cement. In the pure cement, the cement slurry filter cake has obvious connected pores, which provide channels for the fluid loss of the cement slurry. Under the effect of the experimental pressure difference, the free water in the cement slurry passes through the connected channels quickly, and the gas penetration phenomenon occurs quickly. However, the filter cake formed by adding the fluid loss additive has a dense structure (as shown in Figure 10), and there are basically no obvious connected holes. According to the analysis, as the negatively charged copolymer molecular chain was wrapped over the surface of cement particles, the long chain structure of the fluid loss additive was deformed under the action of pressure, filling part of the holes and connected channels of filter cake, making it more difficult for free water to pass through mud cake, so as to achieve the purpose of fluid loss [42,43,44,45].
3.5.2. Zeta Potential Analysis of Filter Cake
In order to determine whether the fluid loss additive is adsorbed on the surface of cement particles, the Zeta potential of the filter cakes without fluid loss additive and with fluid loss additive was tested by using a Malvern Zetasizer 2000 Zeta instrument.
The filter cake formed by the cement slurry of base slurry and base slurry with fluid loss additive ADAM-J was ground into powder through a fluid loss experiment. After using water dispersion, the potential analysis was carried out by using the Malvern Zetasizer 2000 Zeta instrument. The experimental results are shown in Table 6.

Table 6.
Zeta potential of filter cake of base slurry and base slurry + ADAM-J.
It can be seen from Table 6 that the Zeta potential value of pure cement slurry is positive, and the Zeta potential of the system is electronegative when a fluid loss additive is added to cement slurry. The analysis shows that the fluid loss additive ADAM-J itself has negative functional groups (sulfonic acid group and carboxylic acid group), and pure cement slurry is positively charged, so the sulfonic acid group and carboxylic acid group will be adsorbed on the surface of cement particles [46,47], and these negatively charged copolymer molecules make cement particles with a strong negative charge. On the other hand, due to the presence of non-ionic groups (such as amides) on the polymer molecular chain, the free water in cement slurry is bound by strong adsorption, so as to achieve a good fluid loss control effect.
3.5.3. Viscosity Analysis of Aqueous Solution
In this paper, an NDJ-9S digital viscometer was used to test the viscosity of the fluid loss additives JSJ-3 and ADAM-J.
The test formula was freshwater solution (176 g water + 8 g fluid loss additive) and subsaturation saline solution (176 g water + 8 g fluid loss additive + 38.6 g industrial sodium chloride), and the test results are shown in Table 4.
As can be seen from Table 7, the fluid loss additives JSJ-3 and ADAM-J can improve the liquid viscosity of cement slurry in freshwater slurry, but in subsaturation saline, the viscosity of aqueous solution with the fluid loss additive JSJ-3 decreases from 35.23 mPa·s to 12.89 mPa·s. The viscosity of the aqueous solution decreased by 63.41%, while the viscosity of the aqueous solution with ADAM-J decreased by 27.20%, from 51.10 mPa·s to 37.20 mPa·s. Moreover, it still maintains good viscosity characteristics, indicating that the fluid loss additive ADAM-J can maintain high viscosity in subsaturation saline and increase the flow resistance of filtrate, thus showing a good fluid loss effect in brine.

Table 7.
Viscosity variation in aqueous solutions in fresh water and subsaturation saline with different fluid loss additives.
4. Conclusions
Through research and analysis, the following conclusions are obtained:
- (1)
- The optimal synthesis conditions of the fluid loss additive were determined by changing the monomer ratio, reaction temperature, initiator dosage, monomer mass fraction, and other factors: AMPS:DMAA:AM:MA molar ratio was 6:1:4:0.5; monomer mass fraction was 20%; pH value was 7; reaction temperature was 50 °C; initiator dosage was 0.16% (potassium persulfate: sodium bisulfite is 1:1); reaction time was 5 h; and the synthesized fluid loss additive used was ADAM-J, which has excellent fluid loss performance and salt resistance.
- (2)
- With the increase in the brine concentration, the compressive strength of cement stone decreases from 31.36 MPa to about 22.60 MPa, which still meets the standard 24 h compressive strength of cement stone of more than 14 MPa. The fluid loss of freshwater cement slurry can be controlled at 12 mL·(30 min)−1, and 24 mL·(30 min)−1 in semi-saturated brine.
- (3)
- Through the scanning electron microscopy, Zeta potential analysis, viscosity change in aqueous solution, and polymer molecular weight analysis of the action mechanism of ADAM-J, we can see that the main role includes the following three aspects: (a) After adding the fluid loss additive ADAM-J, the set cement structure became more compact compared with the pure set cement due to the negatively charged copolymer molecular chains wrapped over the cement particles’ surface, deformation under the action of pressure, and better process of filling the cement hydration hole and connecting channel, making the cement filter cake’s structure more compact compared with the pure cement filter cake, thus achieving the effect of fluid loss. (b) The sulfonic acid group and carboxylic acid group of the fluid loss additive ADAM-J will adsorb on the surface of cement particles. These negatively charged copolymer molecules give the surface of cement particles strong negative electricity. The hydrophilic groups on the polymer molecular chain bind free water, so as to achieve a good fluid loss effect. (c) ADAM has a high molecular weight, which can still ensure the viscosity of cement slurry filtrate of 37.20 mPa·s in subsaturation brine, greatly increasing the flow resistance of filtrate to achieve the effect of fluid loss.
Author Contributions
Methodology, X.L., S.Z. and H.L.; Validation, H.L.; Formal analysis, H.Y.; Resources, S.Z.; Writing—original draft, X.L. and H.Z.; Writing—review & editing, H.Y. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Young Scientists Fund of the National Natural Science Foundation of China grant number 51804332 and National Natural Science Foundation of China (General Program) grant number 51974355.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Xiaojiang Li and Shiming Zhou were employed by the SINOPEC Research Institute of Petroleum Engineering Co., Ltd. Author Junfeng Zhao was employed by the SINOPEC Matrix Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kupwade-Patil, K.; Chin, S.H.; Johnston, M.L.; Maragh, J.; Masic, A.; Büyüköztürk, O. Particle Size Effect of Volcanic Ash towards Developing Engineered Portland Cements. J. Mater. Civ. Eng. 2018, 30, 14. [Google Scholar] [CrossRef]
- Kocak, Y. Effects of metakaolin on the hydration development of Portland-composite cement. J. Build. Eng. 2020, 31, 9. [Google Scholar] [CrossRef]
- Petre, I.; Amzica, F.; Ilie, G.; Draganoaia, C. High Performance Portland Metakaolin Cement. Rev. Rom. Mat. 2008, 38, 255–259. [Google Scholar]
- Huang, G.D.; Li, Y.Q.; Zhang, Y.T.; Zhu, J.L.; Li, D.W.; Wang, B. Effect of Sodium Hydroxide, Liquid Sodium Silicate, Calcium Hydroxide, and Slag on the Mechanical Properties and Mineral Crystal Structure Evolution of Polymer Materials. Crystals 2021, 11, 1586. [Google Scholar] [CrossRef]
- Ahdaya, M.; Imqam, A. Investigating geopolymer cement performance in presence of water based drilling fluid. J. Pet. Sci. Eng. 2019, 176, 934–942. [Google Scholar] [CrossRef]
- Liu, H.J.; Bu, Y.H.; Sanjayan, J.G.; Nazari, A.; Shen, Z.H. The application of coated superabsorbent polymer in well cement for plugging the microcrack. Constr. Build. Mater. 2016, 104, 72–84. [Google Scholar] [CrossRef]
- Velayati, A.; Tokhmechi, B.; Soltanian, H.; Kazemzadeh, E. Cement slurry optimization and assessment of additives according to a proposed plan. J. Nat. Gas Sci. Eng. 2015, 23, 165–170. [Google Scholar] [CrossRef]
- Hao, Y. Research and application of anti-leakage drilling fluid. Bulg. Chem. Commun. 2016, 48, 215–221. [Google Scholar]
- Tang, X.; Yuan, B.; Yang, Y.G.; Xie, Y.Q. Preparation and performance of AMPS/AA/DMAA/SA copolymer as a filtrate reducer for oil well cementing. J. Appl. Polym. Sci. 2016, 133, 9. [Google Scholar] [CrossRef]
- Guo, J.T.; Lu, H.C.; Liu, S.Q.; Jin, J.Z.; Yu, Y.J. The novel fluid loss additive HTF-200C for oil field cementing. Petroleum Explor. Dev. 2012, 39, 385–390. [Google Scholar] [CrossRef]
- Yang, Y.P.; Li, M.; Zhang, W.; Jiang, B.L.; Xu, W. Synthesis and performance study of amphoteric ion fluid loss additive SSS/AM/FA/DMDAAC. J. Polym. Res. 2023, 30, 14. [Google Scholar] [CrossRef]
- Cao, L.; Liu, C.; Tian, H.Y.; Jia, D.D.; Wang, D.J.; Xu, Y.; Guo, J.T. Adsorption interaction between cement hydrates minerals with fluid loss additive investigated by fluorescence technique. Constr. Build. Mater. 2019, 223, 1106–1111. [Google Scholar] [CrossRef]
- Cai, Q.; Xie, Z.H.; Jiang, X.Y.; Liu, Y.; Yan, H.T. Synthesis of Maleic Anhydride-Acrylamide Copolymer and Study of Its Adsorption Properties by ICP-AES. Spectrosc. Spectr. Anal. 2012, 32, 1946–1949. [Google Scholar] [CrossRef]
- Chang, X.F.; Sun, J.S.; Zhang, F.; Lv, K.H.; Zhou, X.Y.; Wang, J.T.; Zhao, J.W. A novel zwitterionic quaternary copolymer as a fluid-loss additive for water-based drilling fluids. In Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; Taylor and Francis: Abingdon, UK, 2020; p. 14. [Google Scholar] [CrossRef]
- GB/T 19139-2012; Testing of Well Cements. National Standards of People’s Republic of China: Beijing, China, 2012.
- Feng, G.J.; Wang, Y.; Li, P.; Guo, T.T. Comparison of mid-infrared regular transmittance measurement. In Proceedings of the International Conference on Measurement, Instrumentation and Automation (ICMIA 2012), Guangzhou, China, 15–16 September 2012; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012. [Google Scholar]
- Li, M.; Xiao, W.Y.; Zhang, H.; Yu, Y.J.; Liu, Z.S.; Xie, D.B. An effective salt-tolerant fluid loss additive-suitable for high temperature oil well cement. J. Dispersion Sci. Technol. 2021, 42, 730–741. [Google Scholar] [CrossRef]
- Sun, J.S.; Chang, X.F.; Lv, K.H.; Wang, J.T.; Zhang, F.; Jin, J.F.; Zhou, X.Y.; Dai, Z.W. Environmentally friendly and salt-responsive polymer brush based on lignin nanoparticle as fluid-loss additive in water-based drilling fluids. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 621, 15. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiao, W.F.; Liu, X.X.; Dong, L.T. A new type of fluid loss additive P1301 for oil field cementing. In Proceedings of the 3rd International Conference on Chemical, Metallurgical Engineering (ICCMME 2013), Zhuhai, China, 10–11 December 2013; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012. [Google Scholar]
- Bardhan, A.; Vats, S.; Prajapati, D.K.; Halari, D.; Sharma, S.; Saxena, A. Utilization of mesoporous nano-silica as high-temperature water-based drilling fluids additive: Insights into the fluid loss reduction and shale stabilization potential. Geoenergy Sci. Eng. 2024, 232, 14. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.S.; Lv, K.H.; Ji, Y.X.; Liu, J.P.; Huang, X.B.; Bai, Y.R.; Wang, J.T.; Jin, J.F.; Shi, S.L. Temperature- and Salt-Resistant Micro-Crosslinked Polyampholyte Gel as Fluid-Loss Additive for Water-Based Drilling Fluids. Gels 2022, 8, 289. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.W.; Li, X.Q.; Dai, X.D.; Zhang, Z.X.; Wang, D.X.; Wang, Y.; Jiang, S.Y. Poly(ionic liquids) based on β-cyclodextrin as fluid loss additive in water-based drilling fluids. J. Mol. Liq. 2022, 350, 11. [Google Scholar] [CrossRef]
- Plank, J.; Brandl, A.; Zhai, Y.N.; Franke, A. Adsorption behavior and effectiveness of poly(N,N-dimethylacrylamide-co-Ca 2-acrylamido-2-methylpropanesulfonate) as cement fluid loss additive in the presence of acetone-formaldehyde-sulfite dispersant. J. Appl. Polym. Sci. 2006, 102, 4341–4347. [Google Scholar] [CrossRef]
- Bu, Y.H.; Liu, H.J.; Nazari, A.; He, Y.J.; Song, W.Y. Amphoteric ion polymer as fluid loss additive for phosphoaluminate cement in the presence of sodium hexametaphosphate. J. Nat. Gas Sci. Eng. 2016, 31, 474–480. [Google Scholar] [CrossRef]
- Fan, M.L.; Wang, L.; Li, J.; He, P.; Lai, X.J.; Gao, J.H.; Liu, G.R.; Wen, X. Preparation of supramolecular viscoelastic polymers with shear, temperature, and salt resistance/sensitivity by amphiphilic functional monomer modification. Polym. Test 2022, 116, 12. [Google Scholar] [CrossRef]
- He, Y.; Shu, X.; Wang, X.M.; Yang, Y.; Liu, J.P.; Ran, Q.P. Effects of polycarboxylates with different adsorption groups on the rheological properties of cement paste. J. Dispersion Sci. Technol. 2020, 41, 873–883. [Google Scholar] [CrossRef]
- Ran, Q.P.; Somasundaran, P.; Miao, C.W.; Liu, J.P.; Wu, S.S.; Shen, J. Adsorption Mechanism of Comb Polymer Dispersants at the Cement/Water Interface. J. Dispersion Sci. Technol. 2010, 31, 790–798. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, L. Effect of molecular flexibility on the rheological and filtration properties of synthetic polymers used as fluid loss additives in water-based drilling fluid. RSC Adv. 2019, 9, 8608–8619. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Sun, J.S.; Wang, R.; Yang, J.; Qu, Y.Z.; Wang, J.; Huang, H.J.; Cheng, R.C.; Gao, S.F.; Ren, H. Preparation of a salt-responsive Gemini viscoelastic surfactant for application to solids-free drilling fluids. J. Appl. Polym. Sci. 2023, 140, 15. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.F.; Bai, W.; Ma, C.; Xiong, C.D. Effect of Polymerization Temperature on Polymerization Degree and Structure of Calcium Polyphosphate. J. Inorg. Mater. 2012, 27, 174–178. [Google Scholar] [CrossRef]
- Chen, X.R.; Liu, Z.G.; Fu, X.Y.; Xie, X.M.; Wang, T.; Rong, Z.J.; Nong, Y.C.; Lu, Z.R. Enhanced adsorption and properties of TPEG-type superplasticizers modified by maleic anhydride: A perspective from molecular architecture and conformation. Mater. Struct. 2024, 57, 17. [Google Scholar] [CrossRef]
- Gilbert, E.; Morales, G.; Spontón, M.; Estenoz, D. Design of thermosetting polymeric systems based on benzoxazines modified with maleic anhydride. J. Appl. Polym. Sci. 2018, 135, 13. [Google Scholar] [CrossRef]
- Zou, G.; Fang, K.; He, P.S.; Zhang, Y.Z.; Wu, D.C. Static and dynamic behavior of poly (styrene-maleic anhydride) monolayer. Acta Chim. Sin. 2003, 61, 1246–1250. [Google Scholar]
- Galhardo, E.; Machado, P.M.B.; Lona, L.M.F. Living free radical polymerization using cyclic trifunctional initiator. J. Appl. Polym. Sci. 2012, 124, 3900–3904. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Kim, H.C.; Kim, J.S.; Chang, Y.W.; Kim, D.H. Novel itaconic acid-based superabsorbent polymer with improved gel strength and salt resistance using 2-acrylamido-2-methyl-1-propanesulfonic acid. Polym. Adv. Technol. 2022, 33, 392–399. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, F.S.; Chen, S.N.; Long, W.J. Synthesis and Weak Hydrogelling Properties of a Salt Resistance Copolymer Based on Fumaric Acid Sludge and Its Application in Oil Well Drilling Fluids. Gels 2022, 8, 251. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, M.; Sun, Y.J.; Guo, C.; Zhang, Z.H.; Fang, L.W. Preparation and performance of oil well cement fluid loss additive ANAFM. J. Dispersion Sci. Technol. 2023, 44, 2200–2209. [Google Scholar] [CrossRef]
- Xuan, Y.; Jiang, G.C.; Li, Y.Y. Nanographite Oxide as Ultrastrong Fluid-Loss-Control Additive in Water-Based Drilling Fluids. J. Dispersion Sci. Technol. 2014, 35, 1386–1392. [Google Scholar] [CrossRef]
- Cao, L.; Guo, J.T.; Tian, J.H.; Xu, Y.; Hu, M.M.; Guo, C.; Wang, M.Y.; Fan, J.J. Synthesis, characterization and working mechanism of a novel sustained-release-type fluid loss additive for seawater cement slurry. J. Colloid Interface Sci. 2018, 524, 434–444. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.S.; Lv, K.H.; Ji, Y.X.; Ji, J.T.; Liu, J.P. Nano-Modified Polymer Gels as Temperature- and Salt-Resistant Fluid-Loss Additive for Water-Based Drilling Fluids. Gels 2022, 8, 547. [Google Scholar] [CrossRef]
- Wei, Z.J.; Wang, M.S.; Li, Y.; An, Y.H.; Li, K.J.; Bo, K.; Guo, M.Y. Sodium alginate as an eco-friendly rheology modifier and salt-tolerant fluid loss additive in water-based drilling fluids. RSC Adv. 2022, 12, 29852–29864. [Google Scholar] [CrossRef]
- Kök, M.V.; Bal, B. Effects of silica nanoparticles on the performance of water-based drilling fluids. J. Pet. Sci. Eng. 2019, 180, 605–614. [Google Scholar] [CrossRef]
- Mao, H.; Wang, W.J.; Ma, Y.L.; Huang, Y. Synthesis, characterization and properties of an anionic polymer for water-based drilling fluid as an anti-high temperature and anti-salt contamination fluid loss control additive. Polym. Bull. 2021, 78, 2483–2503. [Google Scholar] [CrossRef]
- Nooripoor, V.; Nazemi, R.; Hashemi, A. Employing Nano-sized Additives as Filtration Control Agent in Water-based Drilling Fluids: Study on Barium Sulfate, Bentonite, Surface-modified Bentonite, Titanium Oxide, and Silicon Oxide. In Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; Taylor and Francis: Abingdon, UK, 2020; p. 17. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.J.; Qiu, S.X.; Liu, J.T.; Ou, Z.T.; Li, X.G.; Ji, F.; Zhang, L.; Liu, S.S.; Yang, L.L.; et al. Application of a Core-Shell Structure Nano Filtration Control Additive in Salt-Resistant Clay-Free Water-Based Drilling Fluid. Polymers 2023, 15, 4331. [Google Scholar] [CrossRef]
- Chen, D.; Guo, J.T.; Xu, Y.; Hu, M.M.; Li, P.P.; Jin, J.Z.; Yu, Y.J. Adsorption behavior and mechanism of a copolymer used as fluid loss additive in oil well cement. Constr. Build. Mater. 2019, 198, 650–661. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure-property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).