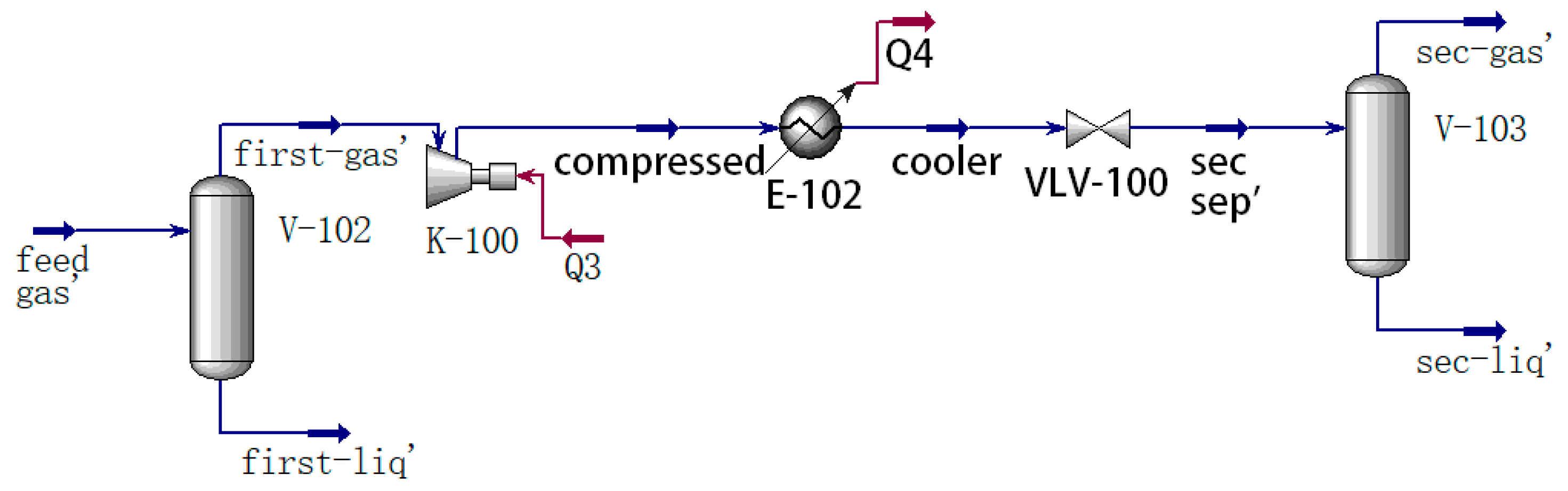
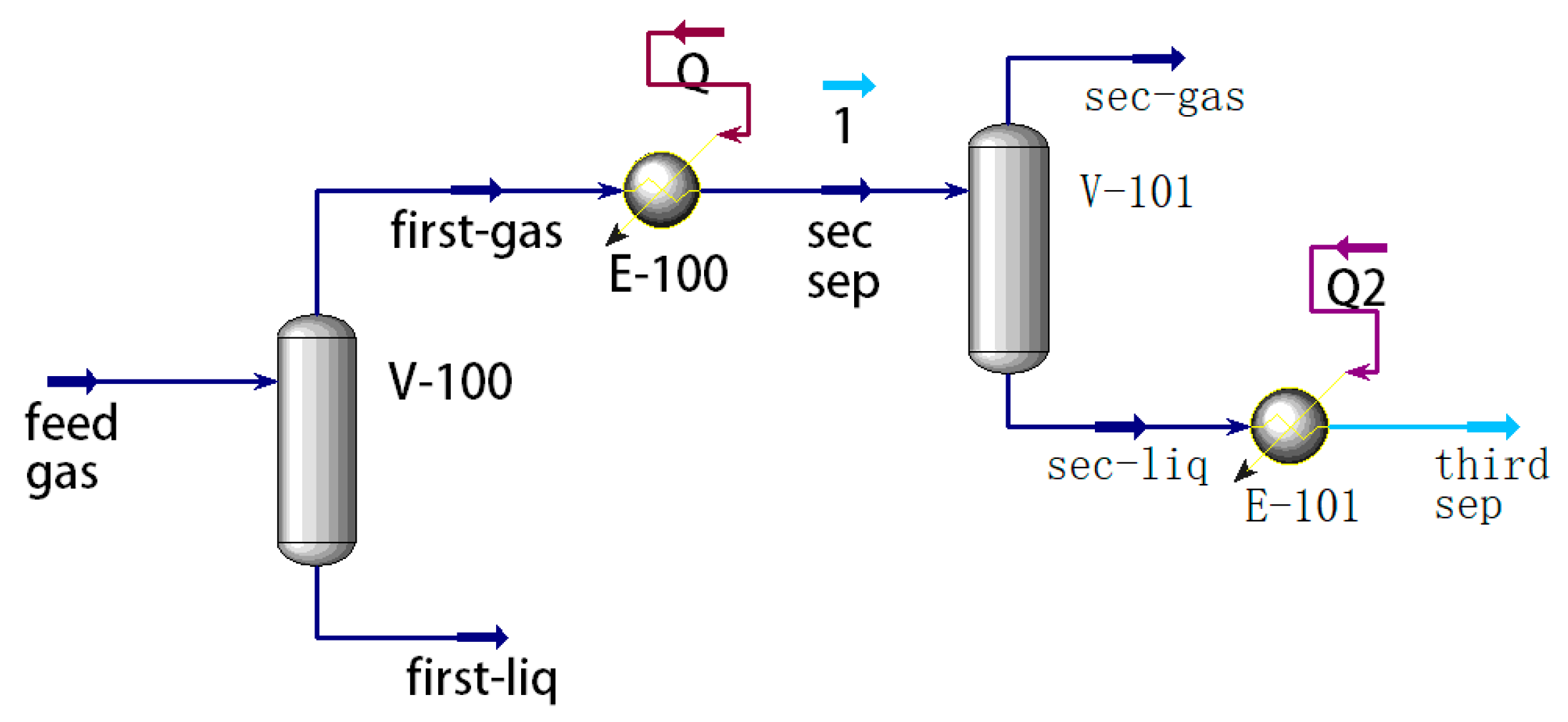
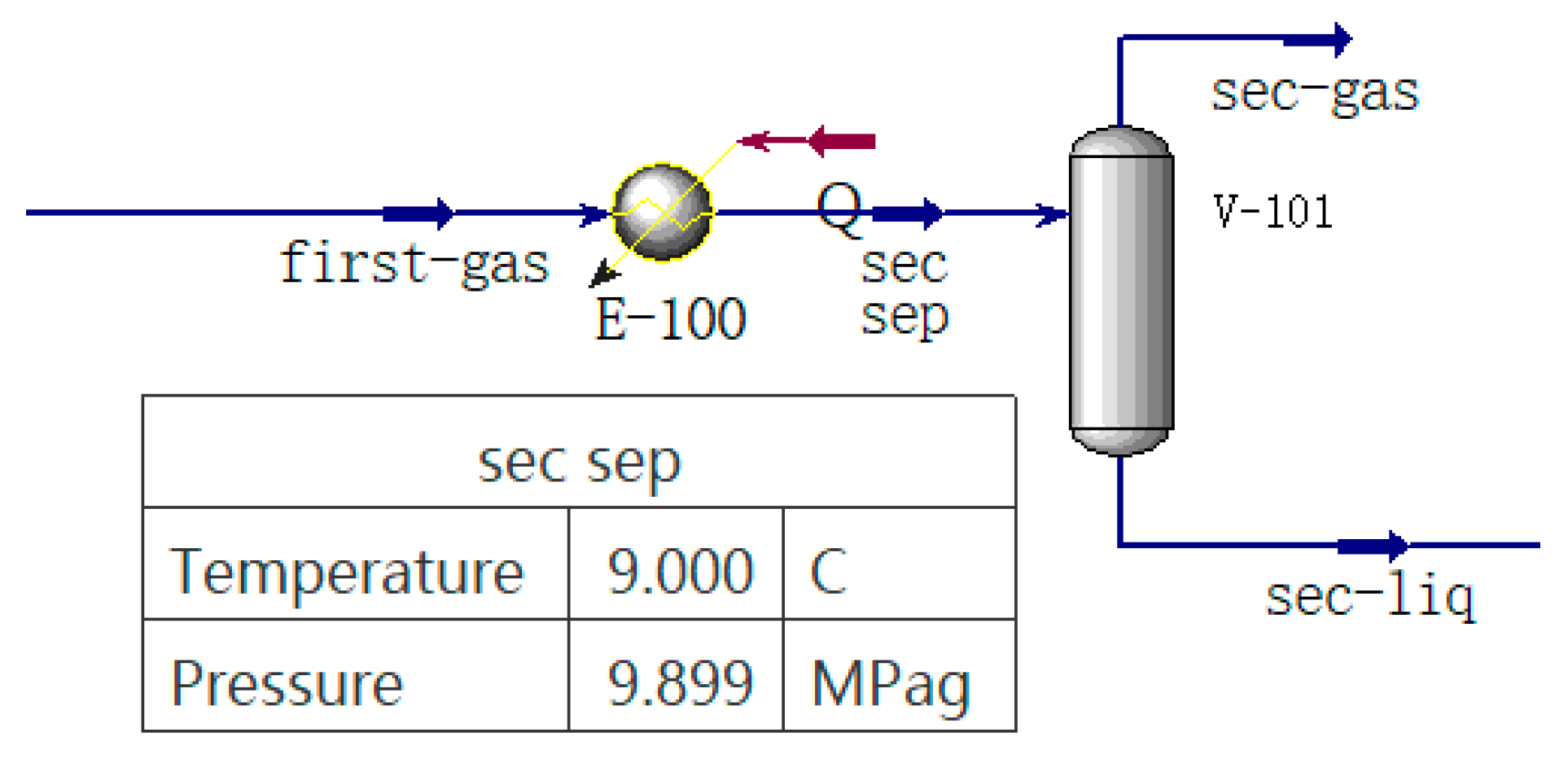
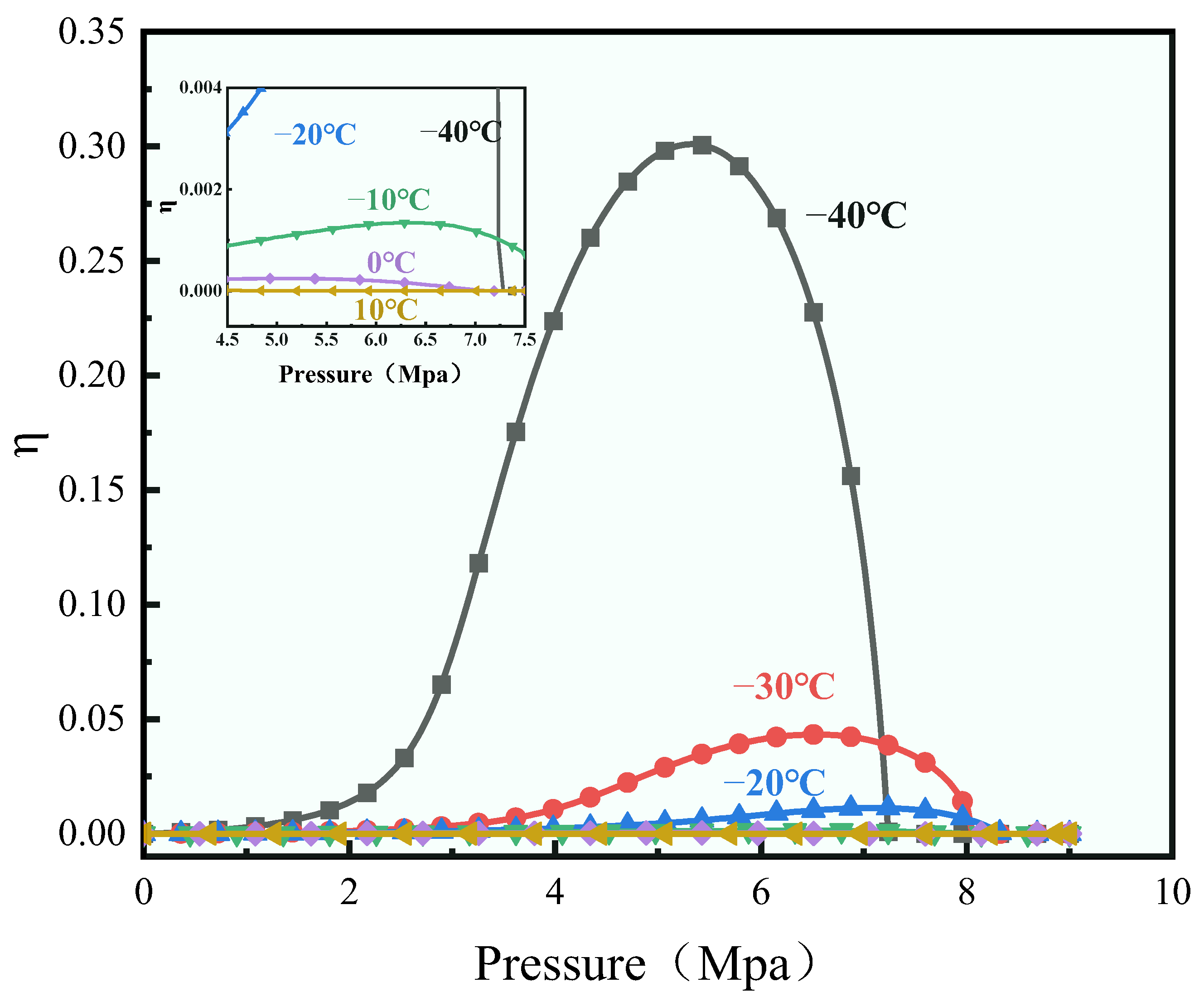
1. Introduction
CO
2 is a potential threat to human survival and development [
1]. Carbon capture, utilization and storage technology achieve the goal of carbon neutrality by reducing CO
2 in the atmosphere, which is of great significance for the construction of green energy systems. Therefore, it is necessary to focus on the development of efficient methods conducive to CCUS technology for the continuous development of existing decarbonization technologies [
2,
3]. In the middle and late stages of oil and gas field development, due to the large amount of CO
2 produced in the process of natural gas mining and processing, the natural gas extracted has higher CO
2 [
4]. In the mining and processing of offshore platforms, the increase in CO
2 content in mining may greatly affect the total power consumption in the mining and processing process [
5]. Moreover, the produced gas has more CO
2, which will aggravate the corrosion of the pipeline, and because the injection of CO
2 into the oil and gas field can improve the recovery rate of the oil and gas field and can increase the production of CH
4 by changing the temperature and pressure conditions [
6], which also increases the content of CO
2 in the produced gas. It can be seen that it is not economical and reliable to transport the extracted natural gas pipeline to land for deacidification and decarbonization, so it is also very necessary to complete the decarbonization process on the offshore platform [
7]. In order to realize the separation and reinjection of CO
2, the cost of technology and energy consumption is relatively expensive, and it may occupy most of the area of offshore production equipment [
8]. At present, the commonly used decarburization processes are low-temperature separation technology, solvent absorption method, membrane separation method and pressure swing adsorption method [
9,
10]. The advantages and disadvantages of the different approaches are shown in
Table 1 below. The oil and gas produced in some deep-water offshore oilfields have high concentrations of CO
2. While treating natural gas, a high concentration of CO
2 needs to be treated. The low-temperature separation process is suitable for the case of high CO
2 content and reinjection, but the equipment investment cost is relatively high, and the energy consumption is relatively high. As a relatively mature decarburization technology, physical and chemical solvent absorption methods, such as the alcohol amine method, also have the disadvantage of high energy consumption. Membrane separation technology separates CH
4 and CO
2 through the pores of the membrane, but it has the problem of hydrocarbon loss and frequent replacement of the membrane and has a more complex natural gas purification membrane separation device, so it is not applicable to offshore platforms [
11,
12,
13,
14]. The pressure swing adsorption process usually requires more adsorption towers for high-purity CO
2 and high hydrocarbon recovery. The adsorption film of different materials has different physical and chemical properties, so the material selection of the adsorption film can also determine the CO
2 removal performance. Through the continuous distribution of concentration as a parameter to build a mathematical model, combined with the boundary conditions to obtain the theoretical results, the corresponding concentration distribution, different simulation software to study the decarbonization process model, and can perform better simulation and prediction [
15]. It has large equipment investment and space occupation and is not suitable for offshore oil and gas separation [
16]. The study of CO
2 desorption by microwave heating using microporous granular activated carbon has a faster desorption rate than conventional heating desorption, but it is not suitable for high CO
2 content and high-pressure oil production on offshore platforms [
17]. Radiofrequency heated reactor systems, including post-combustion carbon capture, are more efficient and have shorter desorption times than conventionally heated benchtop reactors, but the high energy consumption and cost investment are also reasons why they are not suitable for this situation [
18]. Therefore, for the decarburization process of offshore natural gas, the separation of system phase characteristics is used to enter people’s eyes, and the multi-component mixture is separated into different phases under different temperature and pressure conditions, and then different phases are separated to achieve the initial decarburization of offshore natural gas.
The characteristics of gas–liquid equilibrium are used for separation and recovery in practical engineering. For example, in the process of floating production, storage and transportation in Brazil, the content of CO
2 may be as high as 40% [
19]. Therefore, the HISEP seabed efficient separation technology proposed by Brazil, such as a high-pressure dense phase seabed separator, separates it into at least two phases. One is a liquid rich in hydrocarbons, and the other is a dense supercritical phase rich in carbon dioxide, which can make CO
2 reinjection without reaching the ground [
20,
21], for example, for the separation of components in a liquid homogeneous system. The difference in phase characteristics of each component is usually used for separation. For example, the crude oil stabilization process is used to recycle the associated gas in the production of crude oil. In the process of crude oil production, the crude oil is initially separated. After flashing, pressurization and cooling, the components undergo phase change; the non-condensable gas is removed from the raw material gas tank, and the liquid hydrocarbon is removed from the light hydrocarbon recovery device [
22,
23]. Ahad Ghaemi et al. [
15] numerically simulated the process of carbon dioxide absorption and capture. By increasing the axial position through the column, the temperature of the liquid phase and the concentration of CO
2 in the liquid phase were reduced. Lin et al. [
24] compared the positive pressure flash distillation and fractionation separation process of crude oil and used HYSYS to simulate and calculate the changes of C
4 and below components with temperature and pressure during the stabilization of crude oil by PR equation. The light hydrocarbon recovery process also uses the difference in phase characteristics of the components to separate. Yang Wanyu et al. [
25] separated lightly by the expansion refrigeration method and established a phase equilibrium model. The HYSYS was used to calculate the phase equilibrium characteristics of the CO
2-CH
4 system at different temperatures under a certain recovery pressure and to predict the CO
2 freezing temperature at the top of the demethanizer. Lu et al. [
26] further analyzed the influence of temperature and pressure on the light hydrocarbon recovery process based on the composition of feedstock gas. Through the sensitivity analysis of the light hydrocarbon process parameters, it is considered that the C
2 recovery rate is mainly affected by the content of the C
2 component.
Many scholars have also studied the phase characteristics of CO
2-containing natural gas. Most of the studies on phase equilibrium can be calculated using software data and models, and it has been proved that these calculation models include most of the systems, including the CO
2-CH
4 system. Zhu Likai [
27] studied the phase characteristics of the CH
4-CO
2 system in the process of low-temperature separation and modeled the phase equilibrium characteristics and situation of the system. In order to develop high-purity CO
2, Xu et al. [
28] studied the N
2-CH
4-CO
2 ternary system and obtained the phase equilibrium characteristics in the near-critical region. Sterner et al. [
29] also studied the phase equilibrium characteristics of the CH
4-CO
2 system at low temperatures and obtained the isobaric temperature composition diagram. Matcalfe et al. [
30] established a mathematical model to study the effect of phase equilibrium on CO
2 displacement in the process of CO
2-enhanced oil recovery by numerical simulation. Ali et al. [
31] established an artificial neural network to predict the low-temperature phase characteristics of the gas–liquid–solid equilibrium of the CH
4-CO
2 binary system. In order to obtain the relevant thermophysical properties and phase characteristics of multi-component mixtures with higher accuracy, some scholars have improved and selected the equation of state of the CO
2-alkane system and obtained a model with higher calculation accuracy. Tsivintzelis et al. [
32] established accurate thermodynamic models for pure gaseous, liquid and supercritical CO
2 characteristics and mixtures of CO
2 and hydrocarbons. They used the CPA equation of state to study and obtained good phase equilibrium characteristics. Abdolbaghi [
33] studied the vapor–liquid equilibrium of the binary system of n-alkanes and CO
2. The vapor–liquid equilibrium of the system was predicted by combining the software calculation model with the thermodynamic model, and reliable results were obtained. The error is lower than the conclusion of the PR equation and the WS mixing rule. Wu [
34] studied the binary mixture of carbon dioxide and fluoroethane. The temperature and pressure of the mixture measured by the gas–liquid equilibrium system were analyzed using static constant temperature. The collected gas–liquid phase components were analyzed by gas chromatograph. The gas–liquid equilibrium data were fitted by the PR equation and WS mixing rule. Gui [
35] studied the phase equilibrium data of CO
2 and hydrocarbons under reservoir conditions through experimental analysis and tested the thermodynamic models under different temperatures, pressures and compositions by these data. PR and improved quartic equation of state were combined with different mixing rules to calculate, and a thermodynamic model for characterizing the phase equilibrium of multi-component systems was proposed. Abunahman et al. [
36] used cubic and CPA equations of state to accurately predict the thermodynamic properties of light oil containing high concentrations of CO
2.
Through the above scholars’ research on phase equilibrium separation, it is found that it is very necessary to study the phase equilibrium of multi-component systems containing CO2 and for offshore natural gas decarburization. It is necessary to consider the problems of equipment investment, operation management and space occupation at the same time. The research on phase equilibrium separation of multi-component mixed systems is also a common preliminary separation method. In this paper, through the study of phase characteristics of a CO2 mixed system, the optimal equation of state suitable for simulation is selected, and the phase equilibrium separation of a multi-component complex mixed system is studied.