Numerical Simulation of Mercury Conversion During Pulverized Coal Combustion
Abstract
1. Introduction
2. Modeling and Methods
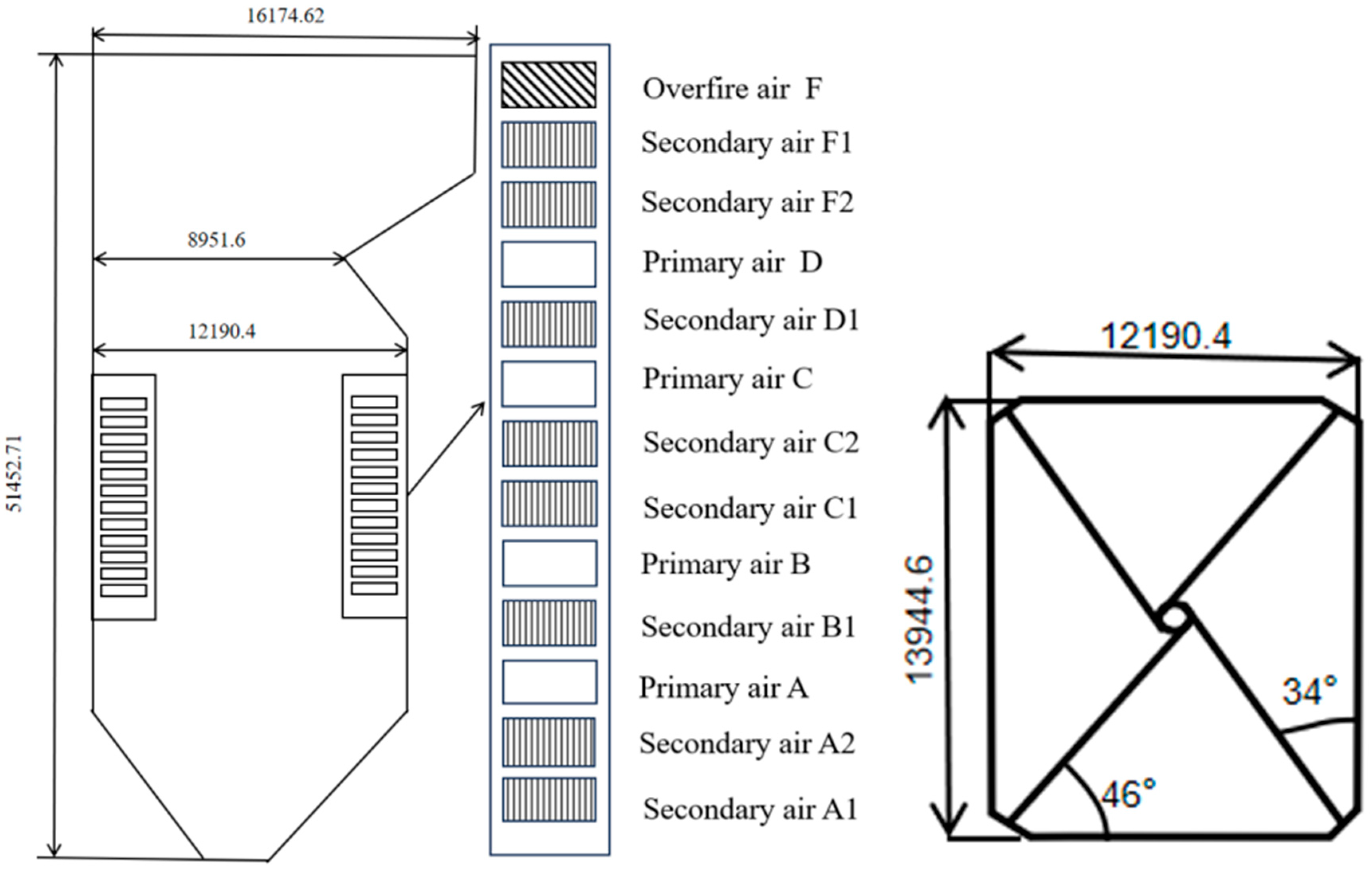
2.1. Boiler Introduction and Numerical Grid Generation
2.2. Boundary Conditions and Model Selection
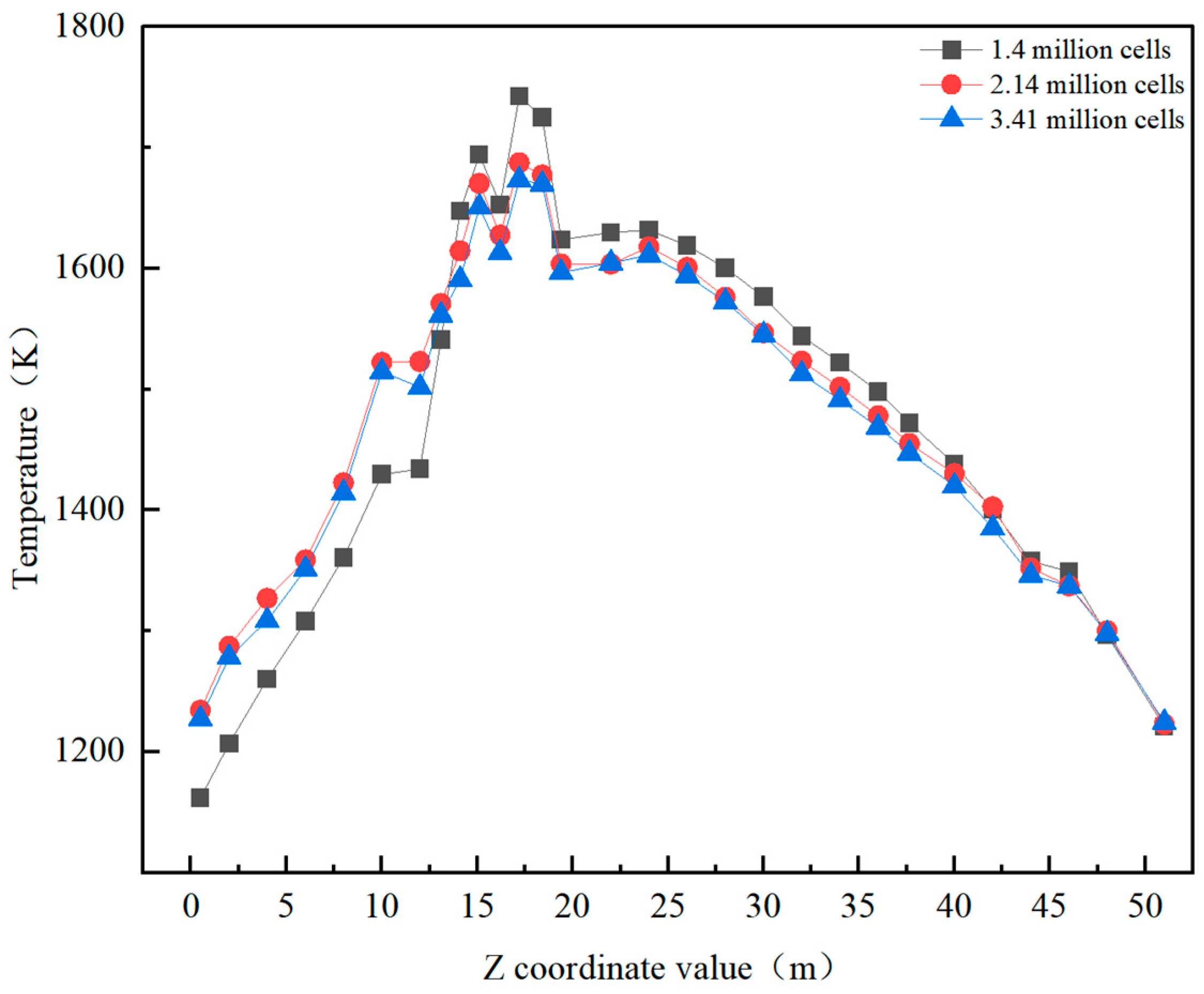
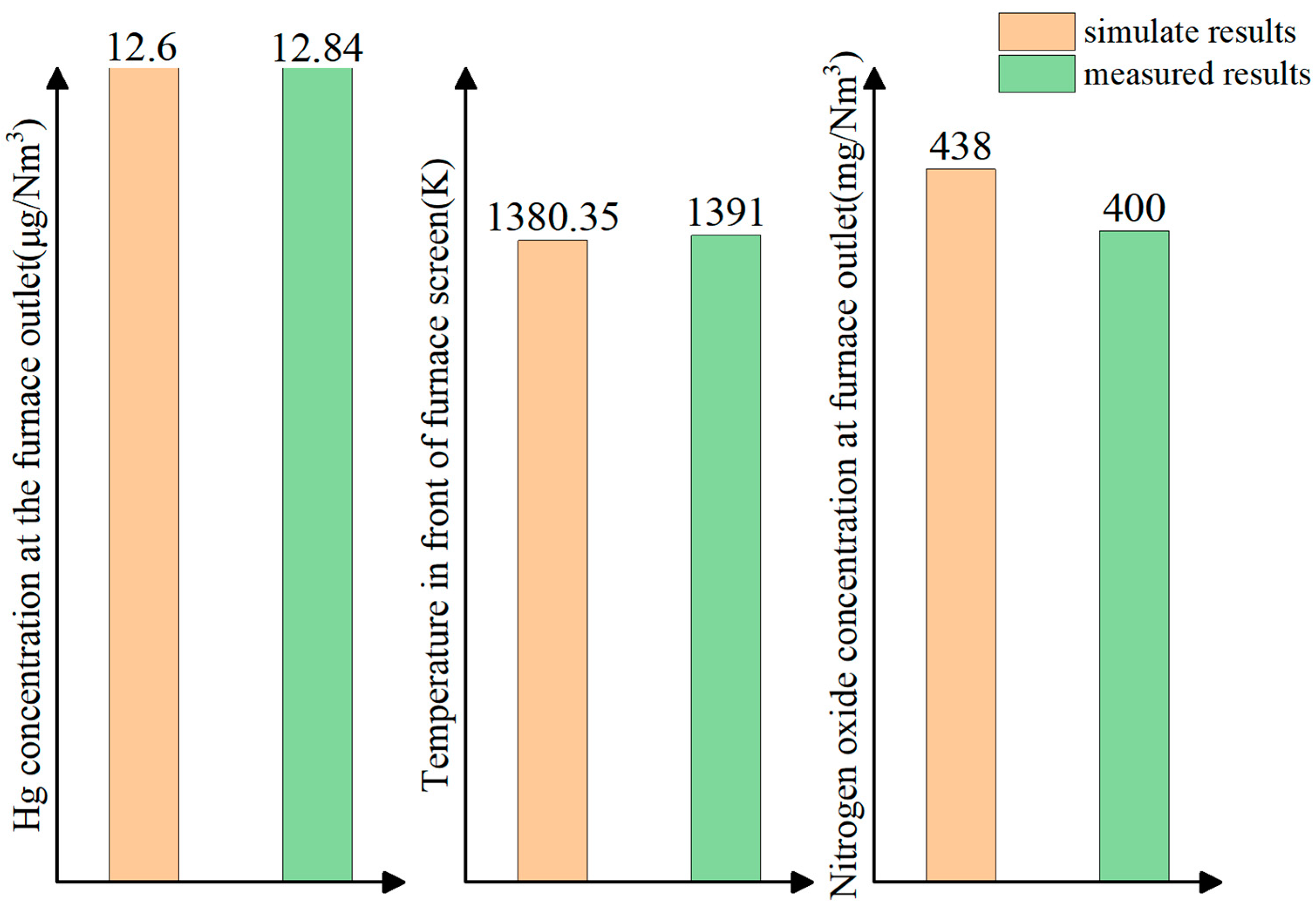
2.3. Model Validation
3. Results and Discussion
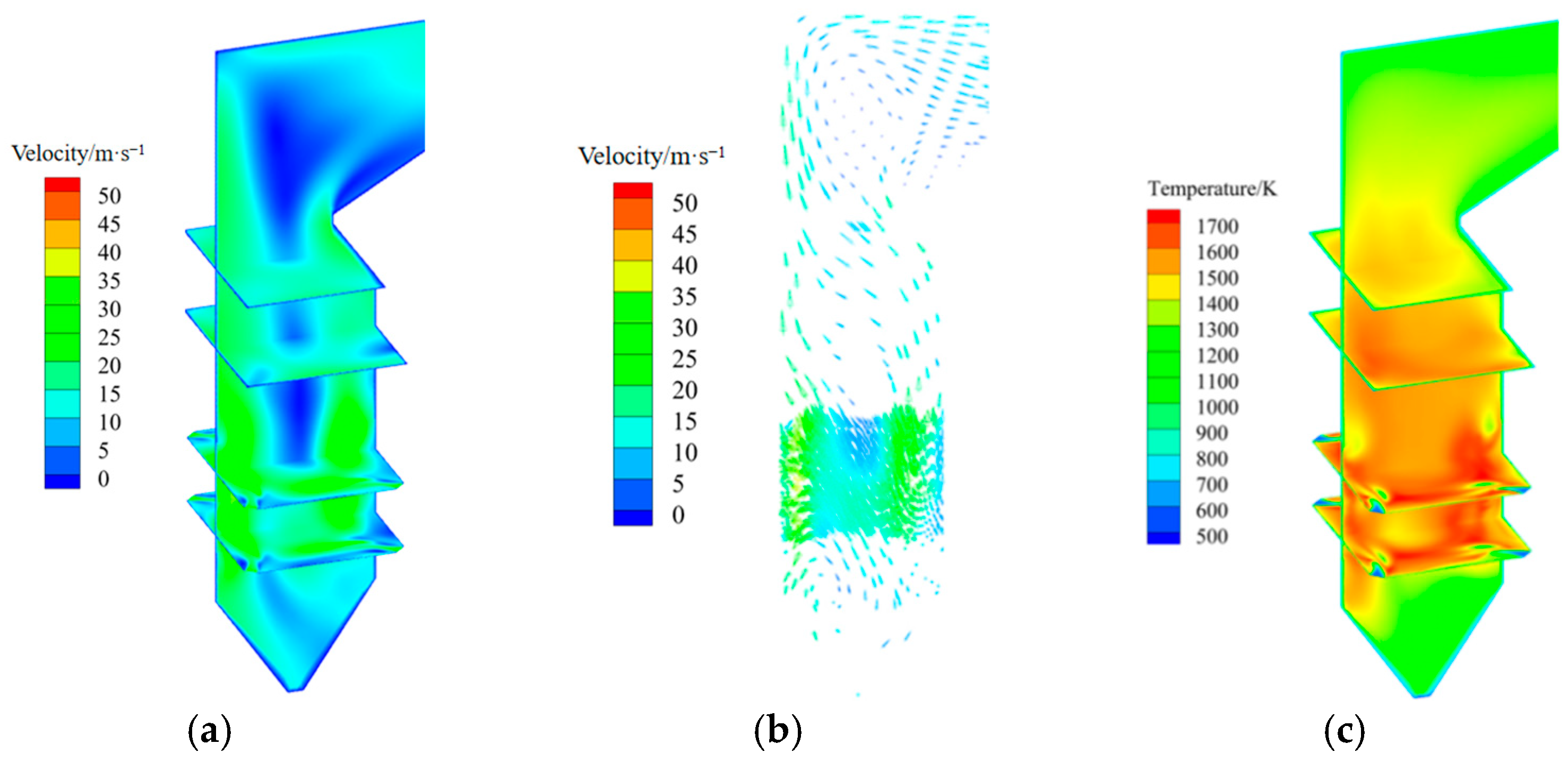
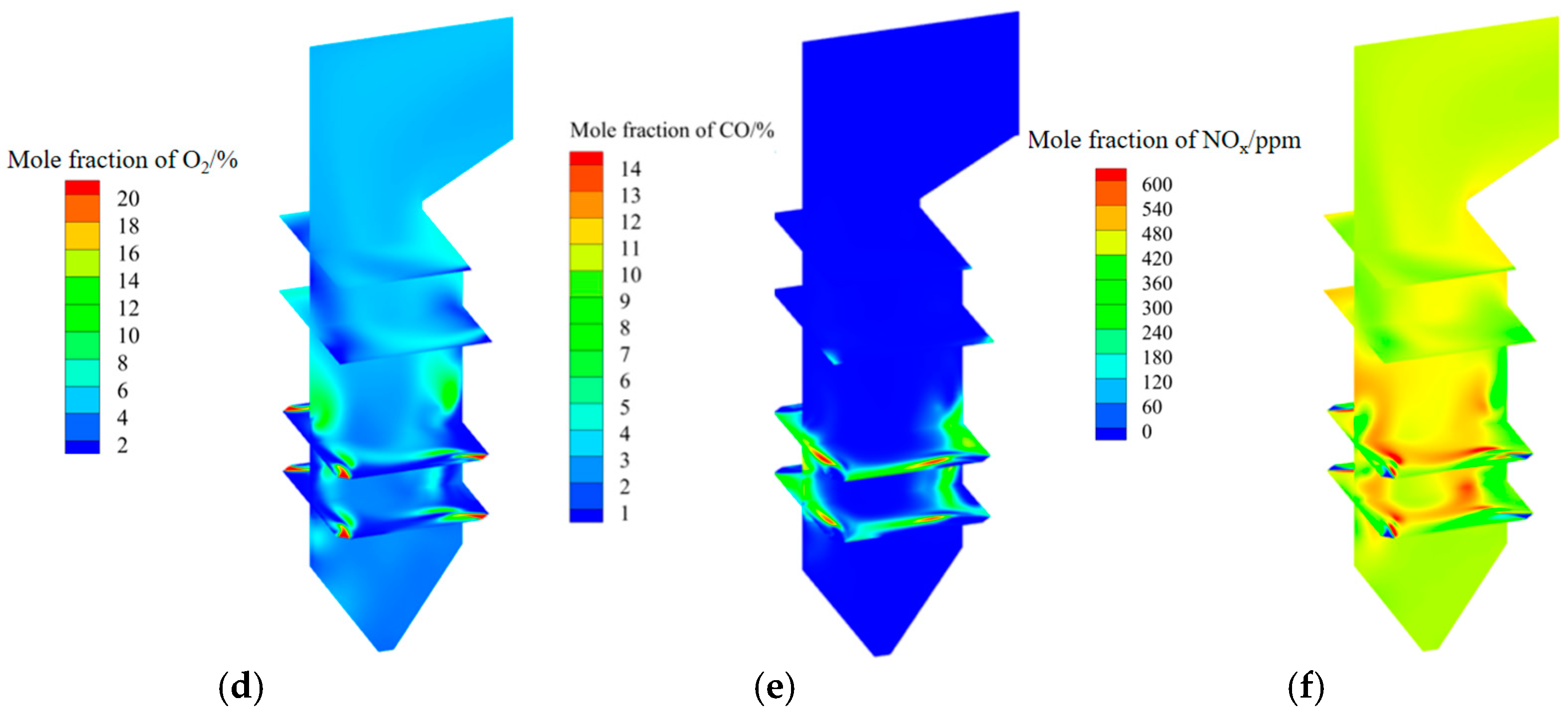
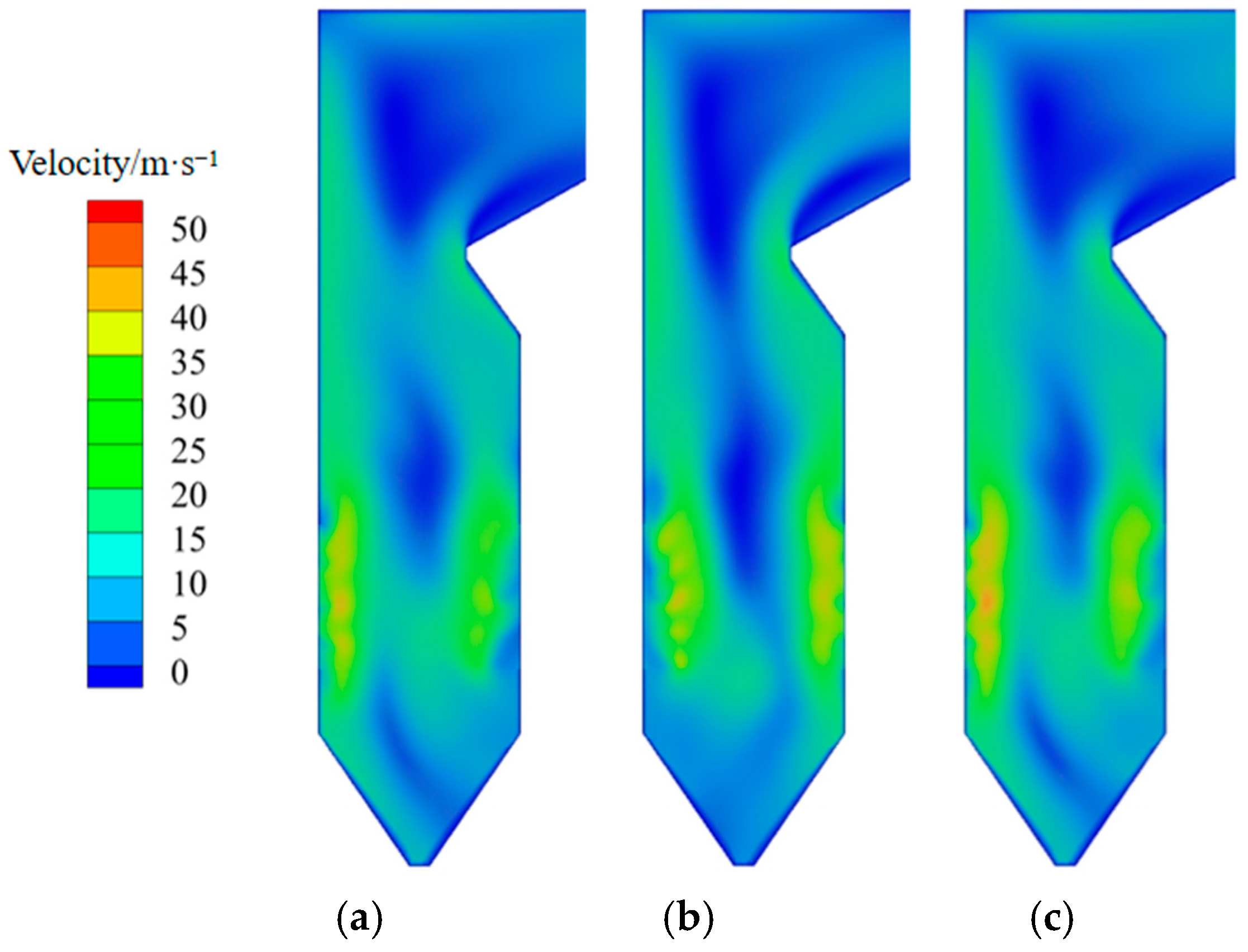
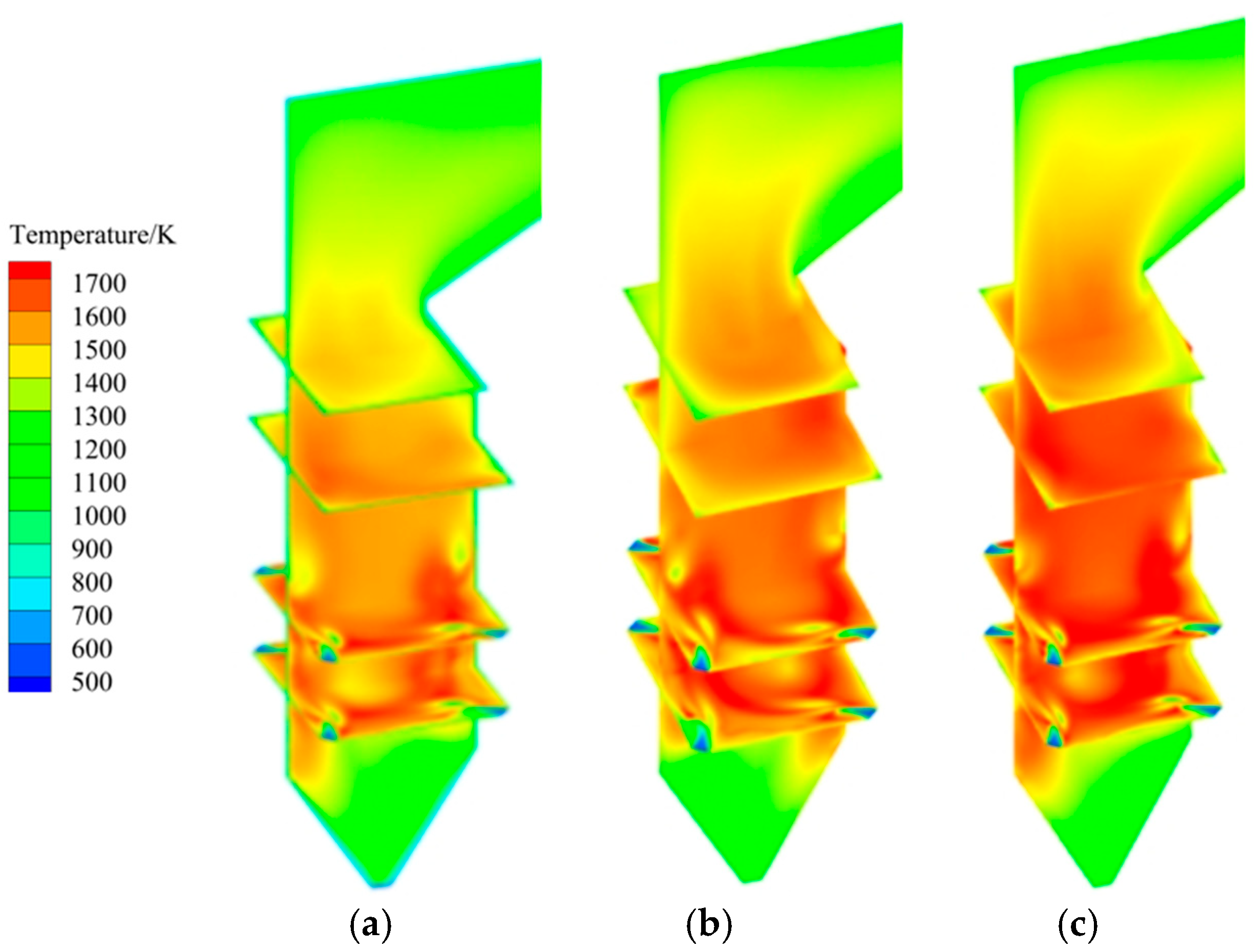
3.1. Analysis of Original Operating Conditions
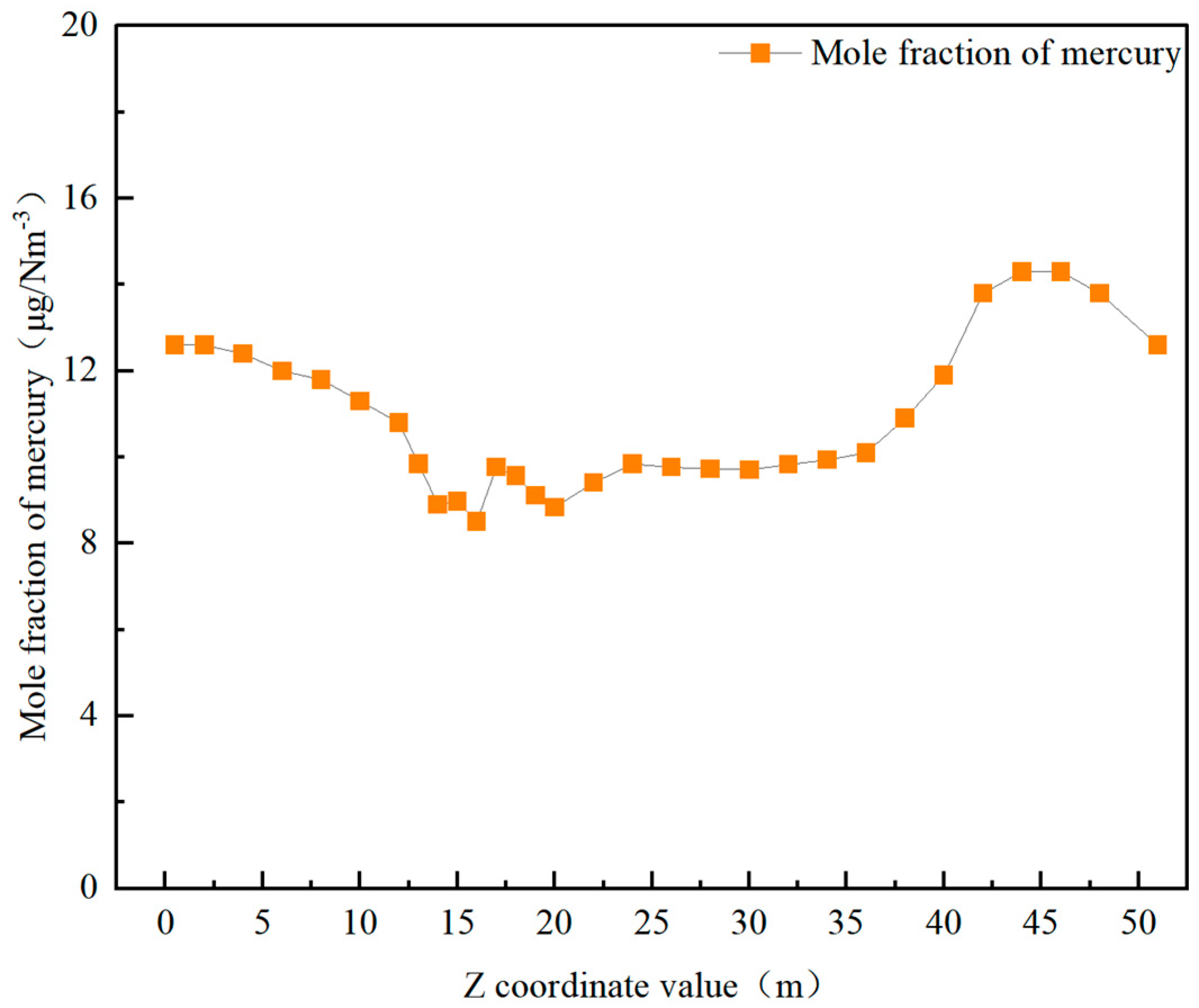
3.2. Mercury Distribution Under Original Operating Conditions
3.3. Mercury Distribution Under Various Operating Conditions
3.3.1. The Influence of Excess Air Coefficient on Mercury Distribution
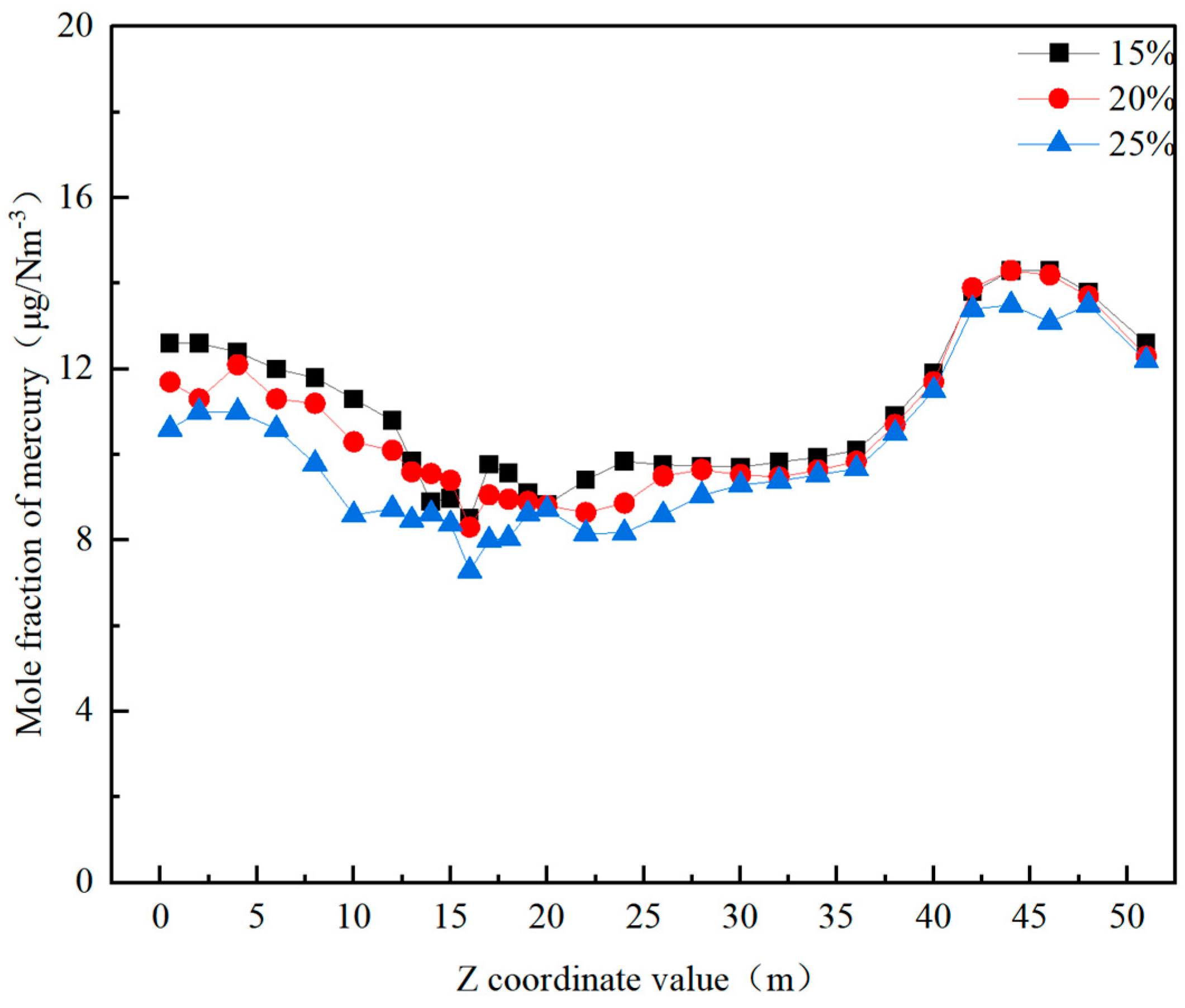
3.3.2. The Effect of the Overfire Air Ratio on Mercury Distribution
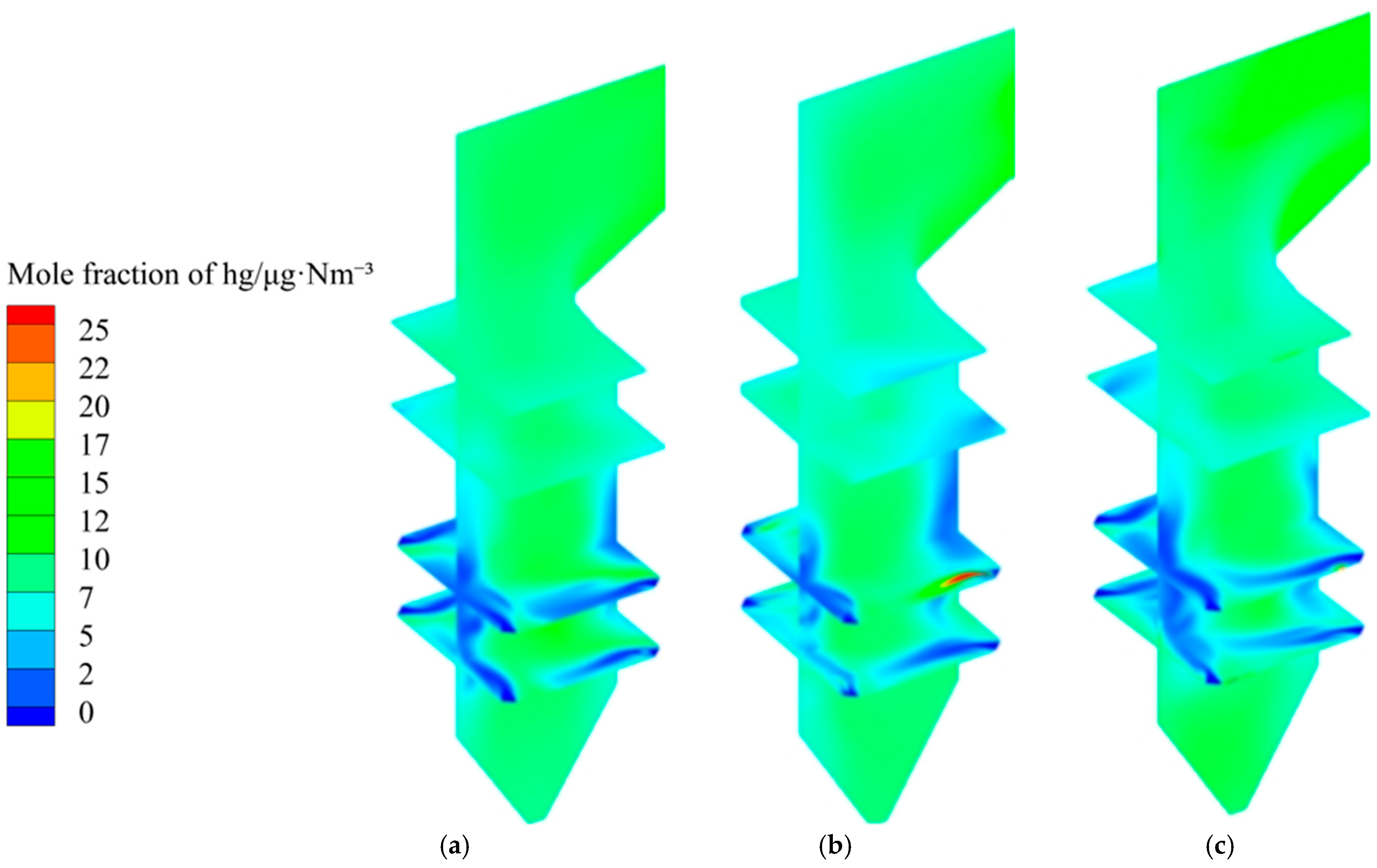
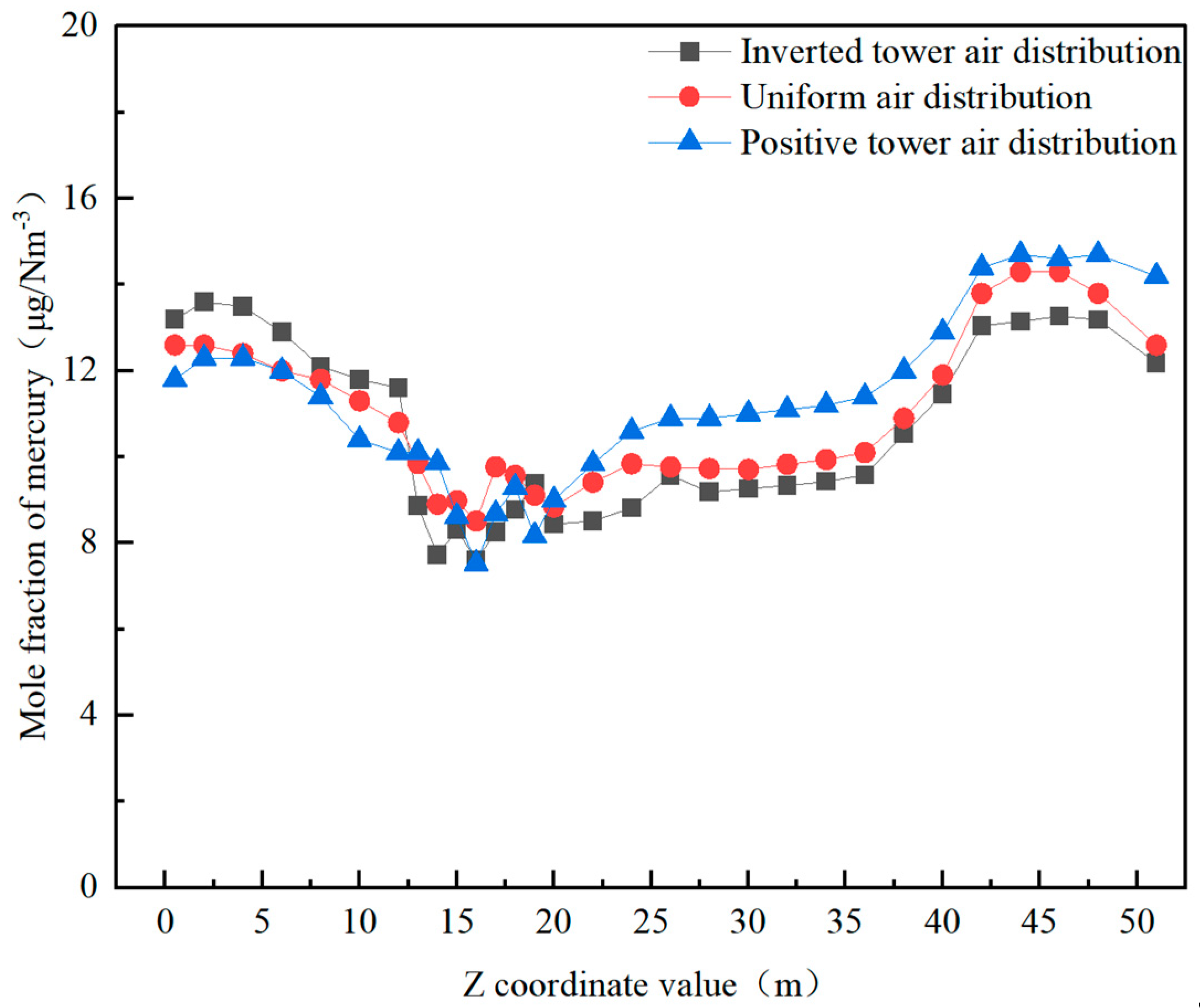
3.3.3. The Effect of Air Distribution Mode on Mercury Distribution
4. Conclusions
- (1)
- The volume fraction of elemental mercury in the cold ash hopper area decreases as the excess air coefficient increases. This can be attributed to several factors. Existing studies have shown that oxygen can oxidize elemental mercury [40]. An increase in the excess air coefficient promotes the oxidation reaction during combustion, making elemental mercury more easily oxidized to its oxidized form, thereby reducing its content. In the burner area, a decrease in the excess air coefficient leads to the incomplete combustion of pulverized coal. Concurrently, a reduction in oxygen availability limits the oxidation of elemental mercury. Consequently, the content of elemental mercury in the burner area remains relatively unchanged. In the burnout and horizontal flue areas, the content of elemental mercury increases as the excess air coefficient decreases. When the excess air coefficient is reduced from 1.21 to 1.16 and 1.11, the content of elemental mercury in the flue gas at the furnace outlet increases by 16.7% and 25.3%, respectively.
- (2)
- Reducing the burnout air ratio from 25% to 15% leads to an increase in the content of elemental mercury in the cold ash hopper area. This occurs because a reduction in the burnout air ratio lowers the temperature and combustion efficiency in the burner, burnout, and horizontal flue areas, slowing the oxidation rate of mercury and leading to a relatively higher content of elemental mercury. Numerous studies have confirmed the critical role of the combustion temperature and efficiency in mercury oxidation [41]. When the burnout air ratio increases from 15% to 20% and 25%, the content of elemental mercury in the flue gas at the furnace outlet decreases by 5.4% and 7.6%, respectively.
- (3)
- Under different air distribution modes, the content of elemental mercury in the cold ash hopper area is lower with the positive pagoda air distribution method. In contrast, it is relatively higher with the equal and inverted pagoda air distribution methods. The content of elemental mercury in the burner area shows minimal differences among the three air distribution methods. Adopting the inverted pagoda air distribution method in the burnout and horizontal flue areas can reduce the content of elemental mercury. This may be because the inverted pagoda air distribution method better promotes the oxidation reaction during the later stages of combustion, reducing the content of elemental mercury. The content of elemental mercury in the flue gas at the furnace outlet decreases by 7.9%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernández-Miranda, N.; Rodríguez, E.; Lopez-Anton, M.A.; García, R.; Martínez-Tarazona, M.R. A new approach for retaining mercury in energy generation processes: Regenerable carbonaceous sorbents. Energies 2017, 10, 1311. [Google Scholar] [CrossRef]
- Lv, Q.; Cai, M.; Wang, C.A.; He, Y.; Che, D.F. Investigation on elemental mercury removal by cerium modified semi-coke. J. Energy Inst. 2020, 93, 666–678. [Google Scholar] [CrossRef]
- Dvornikov, A.G. Forms of mercury in Donets Basin Coals. Geol. Zh. 1981, 41, 96–101. [Google Scholar]
- Zhao, F.H. Experimental Study on the Distribution and Occurrence Mechanism of Harmful Trace Elements in Coal and Leaching of Combustion Products. Ph.D. Thesis, Beijing Graduate School of China University of Mining and Technology, Beijing, China, 1997. [Google Scholar]
- Luttrell, G.H.; Kohmuench, J.N.; Yoon, R.-H. An evaluation of coal preparation technologies for controlling trace element emissions. Fuel Process. Technol. 2000, 65–66, 407–422. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Chou, C. Abundance and modes of occurrence of mercury in some low-sulfur coals from China. Int. J. Coal Geol. 2008, 73, 19–26. [Google Scholar] [CrossRef]
- Wu, J.; Xie, W.; Tan, J.; Liu, L. Understanding the sources of mercury release from coal: A combined experimental and molecular simulation study. J. Hazard. Mater. 2023, 460, 132429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Teng, Y.; Zhang, K.; Peng, H.; Cheng, F.; Yoshikawa, K. Mercury migration behavior from flue gas to fly ashes in a commercial coal-fired CFB power plant. Energies 2020, 13, 1040. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion. Prog. Energ. Combust. 2020, 79, 100844. [Google Scholar] [CrossRef]
- Contreras, M.L.; Ganesh, N.; Rodilla, I.; Bahillo, A. Assess of biomass co-firing under oxy-fuel conditions on Hg speciation and ash deposit formation. Fuel 2018, 215, 395–405. [Google Scholar] [CrossRef]
- Jiang, J.K.; Hao, J.M.; Wu, Y.; Streets, D.G.; Duan, L.; Tian, H.Z. Preliminary establishment of mercury emission inventory from coal combustion in China. Environ. Sci. 2005, 26, 34–39. [Google Scholar]
- Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol. 2000, 65, 289–310. [Google Scholar] [CrossRef]
- Senior, C.L.; Sarofim, A.F.; Zeng, T.; Helble, J.J.; Mamani-Paco, R. Gas-phase transformations of mercury in coal-fired power plants. Fuel Process. Technol. 2000, 63, 197–213. [Google Scholar] [CrossRef]
- Zheng, Y.; Jensen, A.D.; Windelin, C.; Jensen, F. Review of technologies for mercury removal from flue gas from cement production processes. Prog. Energy Combust. Sci. 2012, 38, 599–629. [Google Scholar] [CrossRef]
- Liu, H.X.; Chen, Z.M.; Guo, G.F.; Yin, C.M.; Mei, L.L. Review on generation, testing, emission and treatment technologies of Hg in coal-fired flue gas. In Proceedings of the 18th China Electrostatic Precipitator Conference, Nanjing, China, 1 October 2019; pp. 666–673. [Google Scholar]
- Wu, X.Q.; Wang, J.X. Analysis of migration and transformation characteristics of mercury in coal-fired power plants. Guangdong Chem. Ind. 2018, 45, 85–87. [Google Scholar]
- Zhou, J.S.; Luo ZW Ren, J.L.; Cen, K.F. Study on mercury emission during coal pyrolysis or combustion. Combust. Sci. Technol. 2002, 5, 103–108. [Google Scholar] [CrossRef]
- Jin, F. Analysis and Numerical Simulation of Mercury Conversion During Coal Combustion. Master’s Thesis, North China Electric Power University, Beijing, China, 2016. [Google Scholar]
- Zhang, H.R. Numerical Simulation of Emission, Migration and Adsorption Purification of Mercury Pollutants in Coal Combustion Process. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2012. [Google Scholar]
- Zhu, Y.Q.; Zhou, J.S.; He, S.; Cen, K.F. Numerical simulation of mercury emission during pulverized coal combustion. J. Zhejiang Univ. Eng. Ed. 2009, 43, 942–947. [Google Scholar]
- Lyu, Q.; Wang, C.; Liu, X.; Che, D. Numerical Study on the Homogeneous Reactions of Mercury in a 600 MW Coal-Fired Utility Boiler. Energies 2022, 15, 446. [Google Scholar] [CrossRef]
- Muhammad, S.; Li, Y.; Yang, H.; Jin, L.; Li, D.; Hu, H. Removal of elemental mercury from coal combustion flue gas by sodium halides impregnated red mud. J. Fuel Chem. Technol. 2024, 53, 1–17. [Google Scholar]
- Gao, Z.; Chen, F.; Wang, J.; Wang, T.; Zhang, Y. Study on Mercury Emissions and Ash Characteristics of 600 MW Brown Coal-Fired Unit. Power Gener. Technol. 2023, 44, 543. [Google Scholar]
- Triwibowo, D.; Purnomo, D.; Ningrum, R.S.; Triastuti, T.; Afandi, A.Y.; Kusumaningrum, W.B.; Pramasari, D.A.; Kurniawan, Y.D.; Widyaningrum, B.A. Effect of modified boiler ash from sugarcane bagasse for mercury adsorption: Physical and chemical properties. AIP Conf. Proc. 2024, 2973, 020009. [Google Scholar]
- Hrdlička, J.; Pilař, L.; Vodička, M.; Skopec, P.; Jeníková, J.; Dvořák, M. Alternative sorbents for mercury capture in flue gas from lignite combustion. Appl. Therm. Eng. 2024, 236, 121875. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Q.; Li, D.; Liu, H.; Che, D. CFD investigation on combustion and NOx emission characteristics in a 600 MW wall-fired boiler under high temperature and strong reducing atmosphere. Appl. Therm. Eng. 2017, 126, 407–418. [Google Scholar] [CrossRef]
- Che, D. Boilers-Theory, Design and Operation, 1st ed.; Xi’an Jiaotong University Press: Xi’an, China, 2008; p. 245. [Google Scholar]
- Fang, Q. Numerical Simulation Methods and Applications of Combustion; China Electric Power Press: Beijing, China, 2017. [Google Scholar]
- Zhao, J.X. Numerical Simulation of Combustion; Science Press: Beijing, China, 2002. [Google Scholar]
- Ma, L.; Fang, Q.; Yin, C.; Wang, H.; Zhang, C.; Chen, G. A novel corner-fired boiler system of improved efficiencyand coal flexibility and reduced NOx emissions. Appl. Energy 2019, 238, 453–465. [Google Scholar] [CrossRef]
- Hurt, R.; Sun, J.K.; Lunden, M. A kinetic model of carbon burnout in pulverized coal combustion. Combust. Flame 1998, 113, 181–197. [Google Scholar] [CrossRef]
- Yao, G.; Han, X.; Liu, Z.; Tang, H.; Zhou, Y.; Wang, Z. Low-NOx study of a 600 MW tangentially fired boiler based on pulverized coal preheating method. Case Stud. Therm. Eng. 2023, 48, 103156. [Google Scholar] [CrossRef]
- Gu, W.; Zheng, Z.; Zhao, N.; Wang, X.; Cheng, Z. Combustion characteristics of a 660 MW tangentially fired pulverized coal boiler considering different loads, burner combinations and horizontal deflection angles. Case Stud. Therm. Eng. 2024, 64, 105520. [Google Scholar] [CrossRef]
- Vuthaluru, R.; Vuthaluru, H.B. Modelling of a wall fired furnace for different operating conditions using FLUENT. Fuel Process. Technol. 2006, 87, 633–639. [Google Scholar] [CrossRef]
- Haas, J.; Tamura, M.; Weber, R. Characterization of coal blends for pulverized fuel combustion. Fuel 2001, 80, 1317–1323. [Google Scholar] [CrossRef]
- Vasquez, S.A.; Ivanov, V.A. A Phase Coupled Method for Solving Multiphase Problems on Unstructured Meshes. In Proceedings of the ASME 2000 Fluids Engineering Division Summer Meeting, Boston, MA, USA, 11–15 June 2000. [Google Scholar]
- Hill, S.C.; Smoot, L.D. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energ. Combust. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, C.G.; Xu, M. Characterization of mercury emissions from a coal-fired power plant. Energy Fuels 2007, 21, 898–902. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yi, G.; Li, N.; Che, D. Effects of air staging conditions on the combustion and NOx emission characteristics in a 600 MW wall fired utility boiler using lean coal. Energ. Fuel. 2013, 27, 5831–5840. [Google Scholar] [CrossRef]
- Preciado, I.; Young, T.; Silcox, G. Mercury oxidation by halogens under air-and oxygen-fired conditions. Energy Fuels 2014, 28, 1255–1261. [Google Scholar] [CrossRef]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90, 4–20. [Google Scholar] [CrossRef]







| Parameter | MCR | Control Load |
|---|---|---|
| Superheater steam flow rate | 1110 T/h | 721.5 T/h |
| Superheater outlet steam pressure | 174 bar | 174 bar |
| Superheater outlet steam temperature | 540 °C | 540 °C |
| Reheater steam flow rate | 878.8 T/h | 592 T/h |
| Reheater inlet steam temperature | 328 °C | 298 °C |
| Reheater inlet steam pressure | 37.8 bar | 25.1 bar |
| Reheater outlet steam temperature | 540 °C | 540 °C |
| Reheater outlet steam pressure | 35.6 bar | 23.7 bar |
| Economizer inlet feedwater temperature | 296 °C | 268 °C |
| Economizer inlet feedwater pressure | 196.6 bar | 180.7 bar |
| Component | Symbol | Actual Coal Quality |
|---|---|---|
| Total moisture | 13.4% | |
| Ash | 14.65% | |
| Volatile matter | 26.37% | |
| Carbon | 58.96% | |
| Hydrogen | 3.41% | |
| Nitrogen | 0.82% | |
| Sulfur | 0.37% | |
| Oxygen | 8.39% | |
| Low calorific value | 22,440 kJ/kg |
| Item | Value |
|---|---|
| Primary air velocity | 24.09 m/s |
| Secondary air velocity | 45.49 m/s |
| Overfire air velocity | 34.63 m/s |
| Primary air temperature | 607 K |
| Secondary air temperature | 612 K |
| Coal feed rate | 127.4 t/h |
| Total air flow rate | 1190 t/h |
| Excess air coefficient | 1.21 |
| Elementary Reaction | Pre-Exponential Factor, A | Temperature Coefficient, β | Activation Energy, E (cal/mol) |
|---|---|---|---|
| Hg + Cl + M = HgCl + M [18,35] | 2.40 × 108 | 1.4 | −14,400 |
| Hg + Cl2 = HgCl + Cl [18,35] | 1.39 × 1014 | 0.0 | 34,000 |
| HgCl + Cl2 = HgCl2 + Cl [18,35] | 1.39 × 1014 | 0 | 1000 |
| Hg + HOCl = HgCl + OH [18,35] | 4.27 × 1013 | 0 | 19,000 |
| Hg + HCl = HgCl + H [18,35] | 4.94 × 1014 | 0 | 79,300 |
| HgCl + HCl = HgCl2 + H [18,35] | 4.94 × 1014 | 0 | 21,500 |
| Cl + Cl + M = Cl2 + M [18,35] | 14.4 | 0 | −1800 |
| H + Cl + M = HCl + M [18,35] | 17 | 0 | 0 |
| HCl + H = H2 + Cl [18,35] | 13.36 | 0 | 3500 |
| H + Cl2 = HCl + Cl [18,35] | 13.93 | 0 | 1200 |
| O + HCl = OH + Cl [18,35] | 3.53 | 2.87 | 3510 |
| O + Cl2 = ClO + Cl [18,35] | 12.79 | 0 | 3585 |
| O + ClO = Cl + O2 [18,35] | 13.2 | 0 | −193 |
| Cl + HO2 = HCl + O2 [18,35] | 13.03 | 0 | 894 |
| Cl + HO2 = OH + ClO [18,35] | 13.39 | 0 | −388 |
| ClO + H2 = HOCl + H [18,35] | 11.78 | 0 | 14,100 |
| H + HOCl = HCl + OH [18,35] | 13.98 | 0 | 7620 |
| Cl + HOCl = HCl + ClO [18,35] | 12.26 | 0 | 258 |
| Cl2 + OH = Cl + HOCl [35] | 12.1 | 0 | 1810 |
| O + HOCl = OH + ClO [35] | 12.78 | 0 | 4372 |
| HOCl + M = OH + Cl + M [18,35] | 10.25 | −3 | 56,720 |
| Hg + ClO2 = HgO + ClO [35] | 1.87 × 107 | 0 | 51,270 |
| Hg + O3 = HgO + O2 [35] | 7.02 × 1014 | 0 | 42,190 |
| Hg + N2O = HgO + N2 [35] | 5.08 × 1010 | 0 | 59,810 |
| HgO + HCl = HgCl + OH [35] | 9.63 × 104 | 0 | 8920 |
| HgO + HOCl = HgCl + HO2 [35] | 4.11 × 1013 | 0 | 60,470 |
| Hg + ClO = HgO + Cl [35] | 1.38 × 1012 | 0 | 8320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Yan, B. Numerical Simulation of Mercury Conversion During Pulverized Coal Combustion. Processes 2024, 12, 2910. https://doi.org/10.3390/pr12122910
Ma J, Yan B. Numerical Simulation of Mercury Conversion During Pulverized Coal Combustion. Processes. 2024; 12(12):2910. https://doi.org/10.3390/pr12122910
Chicago/Turabian StyleMa, Jiuli, and Beibei Yan. 2024. "Numerical Simulation of Mercury Conversion During Pulverized Coal Combustion" Processes 12, no. 12: 2910. https://doi.org/10.3390/pr12122910
APA StyleMa, J., & Yan, B. (2024). Numerical Simulation of Mercury Conversion During Pulverized Coal Combustion. Processes, 12(12), 2910. https://doi.org/10.3390/pr12122910






