Evaluation Study of the Passivation Effect of Arsenic-Contaminated Farmland Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pot Experiment
2.3. Measurement Items and Methods
2.3.1. Sample Collection and Pre-Treatment
2.3.2. Soil Analyses
2.3.3. Plant Analyses
2.4. Evaluation Method
2.5. Improvement of Comprehensive Evaluation Method of Passivation Effect
2.6. Data Analysis
3. Results and Discussion
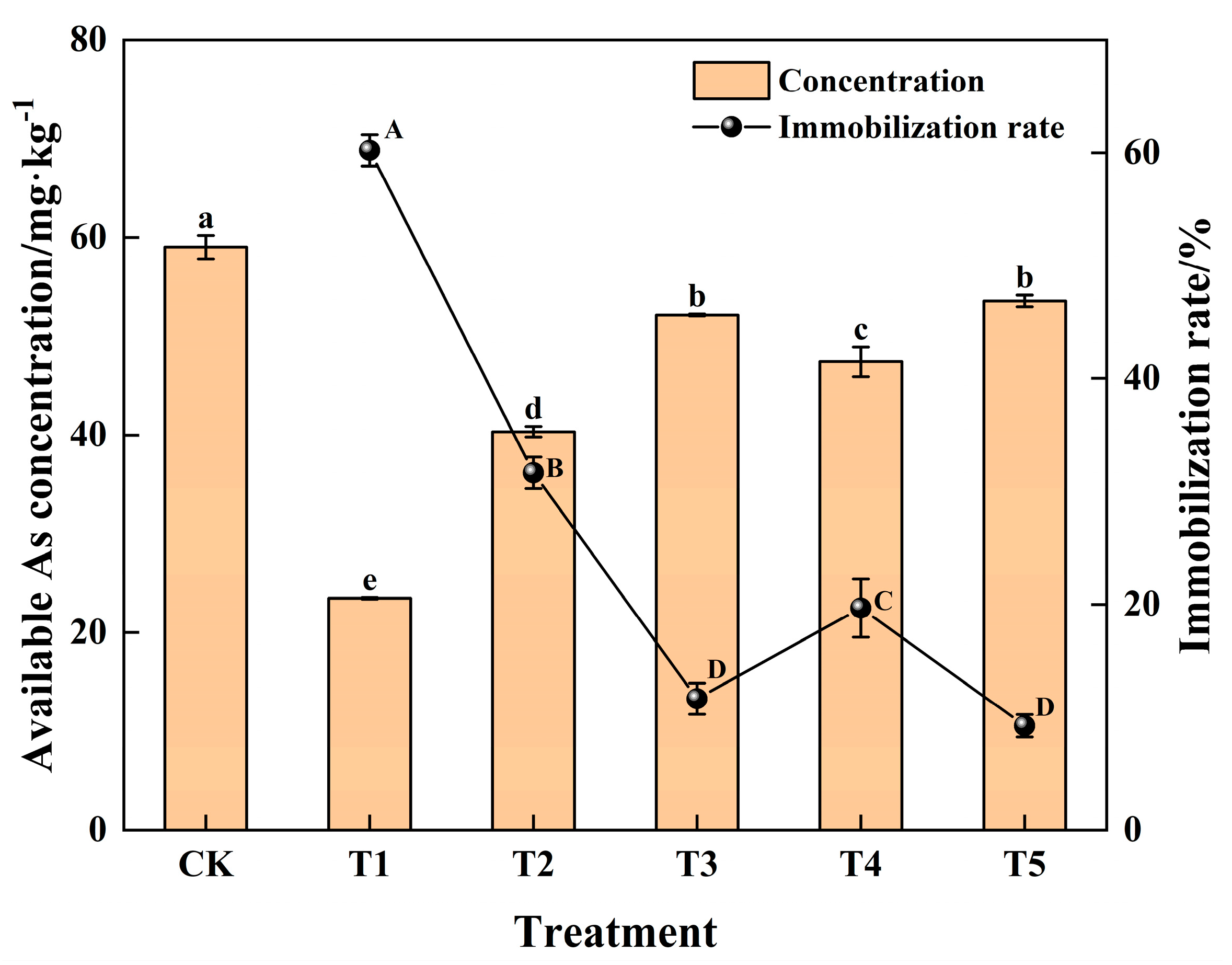
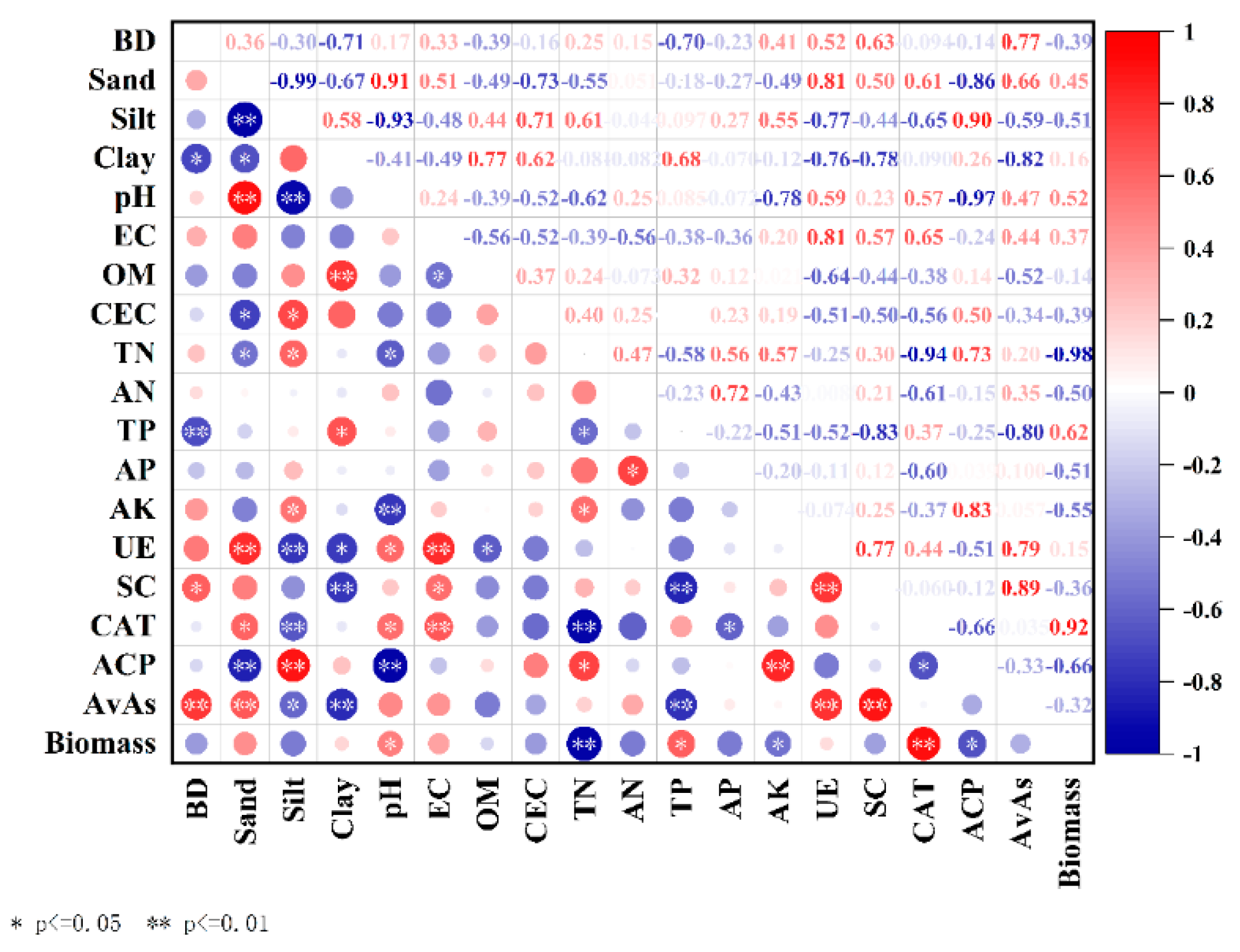
3.1. Influence of Passivated Material Application on Soil Properties
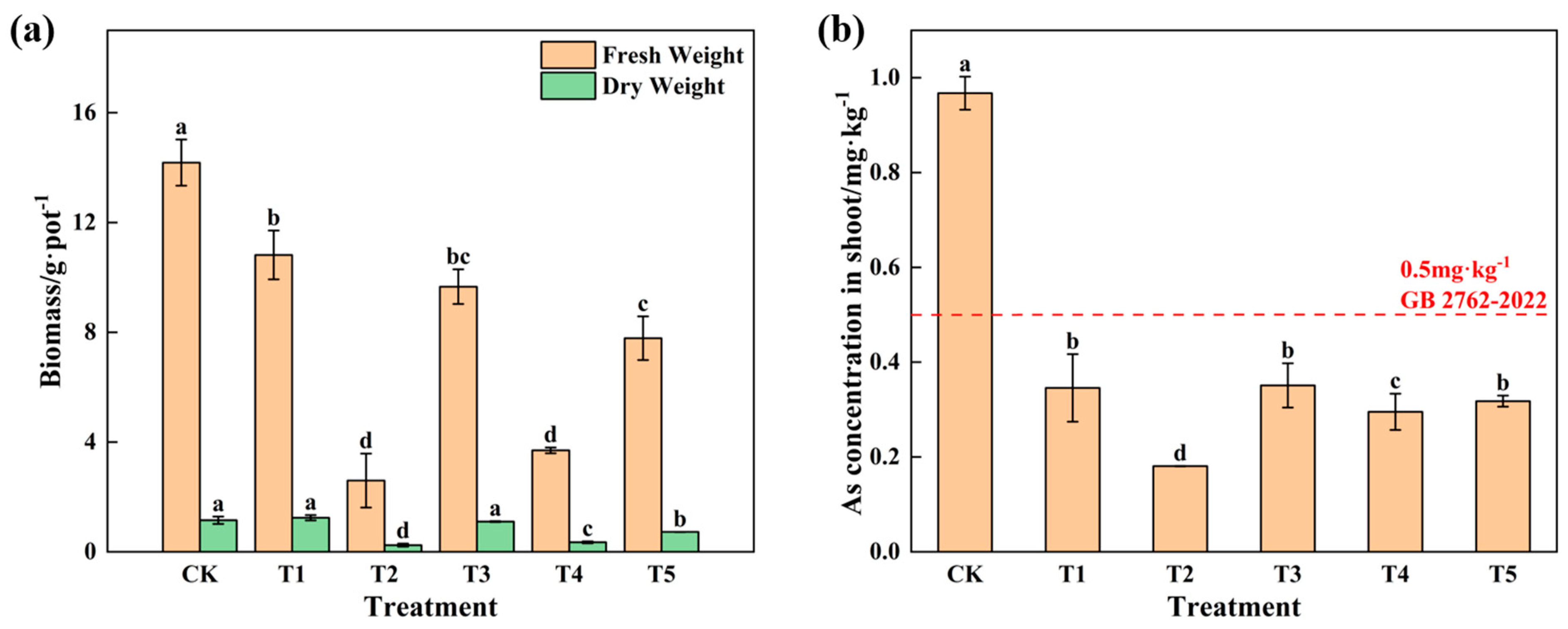
3.2. Influence of Passivated Material Application on Growth and Safety Traits of Water Spinach
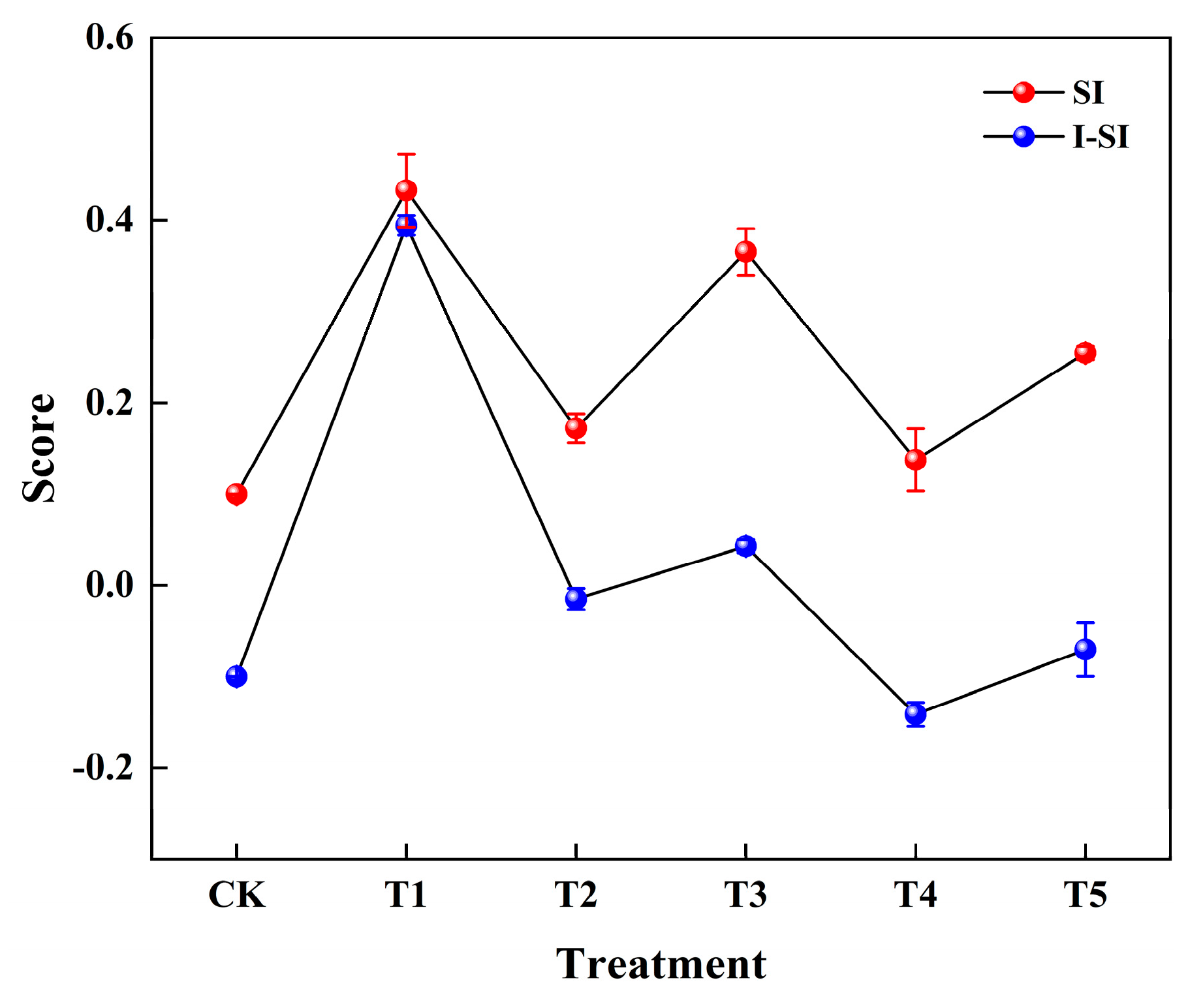
3.3. Comprehensive Evaluation of Passivation Effect
3.4. Improvement of Comprehensive Evaluation of Passivation Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Lou, Z.; Xiao, R.; Ren, Z.; Lv, X. Source analysis and source-oriented risk assessment of heavy metal pollution in agricultural soils of different cultivated land qualities. J. Clean. Prod. 2022, 341, 130942. [Google Scholar] [CrossRef]
- Huang, Y.; Miao, Q.; Kwong, R.W.M.; Zhang, D.; Fan, Y.; Zhou, M.; Yan, X.; Jia, J.; Yan, B.; Li, C. Leveraging the One Health concept for arsenic sustainability. Eco-Environ. Health 2024, 3, 392–405. [Google Scholar] [CrossRef]
- Dai, Y.; Liang, Y.; Xu, X.; Zhao, L.; Cao, X. An integrated approach for simultaneous immobilization of lead in both contaminated soil and groundwater: Laboratory test and numerical modeling. J. Hazard. Mater. 2018, 342, 107–113. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Feng, Y.-S.; Jin, F.; Zhang, L.-M.; Du, Y.-J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef]
- Zou, Q.; An, W.; Wu, C.; Li, W.; Fu, A.; Xiao, R.; Chen, H.; Xue, S. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition. Environ. Chem. Lett. 2018, 16, 615–622. [Google Scholar] [CrossRef]
- Yan, X.L.; Lin, L.Y.; Liao, X.Y.; Zhang, W.B.; Wen, Y. Arsenic stabilization by zero-valent iron, bauxite residue, and zeolite at a contaminated site planting Panax notoginseng. Chemosphere 2013, 93, 661–667. [Google Scholar] [CrossRef]
- Fresno, T.; Moreno-Jiménez, E.; Zornoza, P.; Peñalosa, J.M. Aided phytostabilisation of As- and Cu-contaminated soils using white lupin and combined iron and organic amendments. J. Environ. Manag. 2018, 205, 142–150. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zheng, X.; Cheng, W.; Shi, R.; Feng, R. Effects of foliar dressing of selenite and silicate alone or combined with different soil ameliorants on the accumulation of As and Cd and antioxidant system in Brassica campestris. Ecotoxicol. Environ. Saf. 2017, 142, 207–215. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, L.; Yang, Z.; Yang, W. Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate. J. Soils Sediments 2017, 17, 432–439. [Google Scholar]
- Liang, Y.; Min, X.; Chai, L.; Wang, M.; Liyang, W.; Pan, Q.; Okido, M. Stabilization of arsenic sludge with mechanochemically modified zero valent iron. Chemosphere 2017, 168, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vrînceanu, N.O.; Motelică, D.M.; Dumitru, M.; Calciu, I.; Tănase, V.; Preda, M. Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copșa Mică case study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Chen, F.; Mao, P.; Wang, M.; Li, Y.; Li, Y.; Xia, K.; Chen, J.; Mo, H.; Wang, F.; Li, Z.; et al. Benefit evaluation of in-situ Cd immobilization with naturally occurring minerals using an analytical hierarchy process. Chemosphere 2024, 347, 140720. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, Y.H.; Chen, Y.; Tang, M.D.; Li, Q.; Li, L.F.; Lin, X.Y.; Yin, Y.L.; Ai, S.Y. A comprehensive evaluation of remediation effects on cadmium contamination in paddy fields based on soil quality. J. Agro-Environ. Sci. 2021, 40, 1219–1228. (In Chinese) [Google Scholar]
- Zhao, Y.H.; Yang, Y.H.; Zhao, H.D.; Du, L.Y. Evaluating remediation effects of remediation agents on cadmium-contaminated paddy soil in shenyang based on principal component analysis method. Chin. J. Soil Sci. 2021, 52, 1220–1226. (In Chinese) [Google Scholar]
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Standardization Administration of China: Beijing, China, 2018.
- Li, X.; Zhang, W.J.; Wu, L.; Ren, Y.; Zhang, J.D.; Xu, M.G. Advance in indicator screening and methodologies of soil quality evaluation. Sci. Agric. Sin. 2021, 54, 3043–3056. (In Chinese) [Google Scholar]
- Lu, R. Analysis Method of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Jin, K.; Sleutel, S.; Buchan, D.; De Neve, S.; Cai, D.X.; Gabriels, D.; Jin, J.Y. Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Tillage Res. 2009, 104, 115–120. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Shiowatana, J.; McLaren, R.G.; Chanmekha, N.; Samphao, A. Fractionation of Arsenic in Soil by a Continuous-Flow Sequential Extraction Method. J. Environ. Qual. 2001, 30, 1940–1949. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Chen, C.; Peng, B.; Tan, C.; Li, H. Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 2016, 152, 196–206. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.11-2014; National Food Safety Standard Determination of Total Arsenic and Abio-Arsenic in Food. Standardization Administration of China: Beijing, China, 2014.
- Dong, M.; Huang, R.; Mao, P.; Lei, L.; Li, Y.; Li, Y.; Xia, H.; Li, Z.; Zhuang, P. Immobilization of Cadmium by Molecular Sieve and Wollastonite Is Soil pH and Organic Matter Dependent. Int. J. Environ. Res. Public Health 2021, 18, 5128. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wang, C.; Liu, M.; Zhang, D.; Li, K.; Li, N. A new method for soil health assessment based on Analytic Hierarchy Process and meta-analysis. Sci. Total Environ. 2019, 650, 2771–2777. [Google Scholar] [CrossRef]
- GB 2762-2022; National Food Safety Standard Maximum Levels of Contaminants in Foods. Standardization Administration of China: Beijing, China, 2022.
- Su, J.K.; Dong, Z.; Liu, S.Y.; Zhang, L.; Yi, X.Y. Comprehensive evaluation study on soil quality of agricultural land around Nandan mining areas. Acta Sci. Circumstantiae 2023, 43, 314–326. (In Chinese) [Google Scholar]
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. Defin. Soil Qual. A Sustain. Environ. 1994, 35, 1–12. [Google Scholar]
- Chen, H.; Zhang, W.; Yang, X.; Wang, P.; McGrath, S.P.; Zhao, F.-J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, W.; Guan, D.-X.; Teng, H.H.; Ji, J.; Ma, L.Q. Comparing CaCl2, EDTA and DGT methods to predict Cd and Ni accumulation in rice grains from contaminated soils. Environ. Pollut. 2020, 260, 114042. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhuo, X.; Guan, D.-X.; Song, Y.; Guo, C.; Ji, J. Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China. Environ. Pollut. 2020, 258, 113645. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Yang, J.; Chen, L.; Qi, J.; Zhang, L.; Zhang, J.; Huang, Q.; Zhou, T.; Zhao, Y.; et al. Roles of red mud in remediation of contaminated soil in mining areas: Mechanisms, advances and perspectives. J. Environ. Manag. 2024, 356, 120608. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Wang, X.; Li, F.; Li, B.; Liu, C.; Wang, Q.; Lei, J. Arsenic mobility and bioavailability in paddy soil under iron compound amendments at different growth stages of rice. Environ. Pollut. 2017, 224, 136–147. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.-G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Mikutta, C.; Kretzschmar, R. Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances. Environ. Sci. Technol. 2011, 45, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- El-Sayed, I.M.; Salama, W.H.; Badr, M.A. Nitrogen and spent coffee ground for enhancing nutritional, morphological, flowering and antioxidant properties of Chrysanthemum (Chrysanthemum morfolium Ramat). Plant Physiol. Biochem. 2024, 207, 108406. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Li, K.; Yan, Y.-J.; Huang, F.; Peng, D. Enhanced cadmium removal by growing Bacillus cereus RC-1 immobilized on different magnetic biochars through simultaneous adsorption and bioaccumulation. Environ. Sci. Pollut. Res. 2022, 29, 18495–18507. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cheng, G.; Jiao, K.; Shi, W.; Wang, C.; Xu, H. Mycoextraction by Clitocybe maxima combined with metal immobilization by biochar and activated carbon in an aged soil. Sci. Total Environ. 2016, 562, 732–739. [Google Scholar] [CrossRef]
- Smirnov, D.I.; Molchanova, T.V. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar] [CrossRef]
- Li, J.; Du, J.; Zhong, S.; Ci, E.; Wei, C. Changes in the profile properties and chemical weathering characteristics of cultivated soils affected by anthropic activities. Sci. Rep. 2021, 11, 20822. [Google Scholar] [CrossRef]
- Dror, I.; Yaron, B.; Berkowitz, B. The Human Impact on All Soil-Forming Factors during the Anthropocene. ACS Environ. Au 2022, 2, 11–19. [Google Scholar] [CrossRef]
| pH | OM g·kg−1 | CEC cmol·kg−1 | TN g·kg−1 | TP mg·kg−1 | TK mg·kg−1 | Clay % | Silt % | Sand % | As mg·kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| 6.41 | 17.01 | 17.17 | 1.55 | 124.95 | 307.26 | 13.46 | 40.68 | 45.86 | 256.23 |
| Indicators | CK | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| BD/g·cm−3 | 1.29 ± 0.07 a | 0.99 ± 0.02 a | 1.05 ± 0.03 a | 1.12 ± 0.04 a | 1.22 ± 0.01 a | 1.13 ± 0.03 a |
| Clay/% | 11.68 ± 0.10 ab | 12.16 ± 0.47 a | 11.23 ± 0.11 abc | 9.59 ± 0.12 c | 9.94 ± 1.11 c | 9.82 ± 0.29 c |
| Sand/% | 47.44 ± 0.46 bc | 50.48 ± 1.10 bc | 44.78 ± 0.38 c | 64.99 ± 1.88 a | 51.94 ± 1.66 b | 65.79 ± 3.72 a |
| Silt/% | 40.88 ± 0.36 ab | 37.37 ± 1.58 b | 43.99 ± 0.49 a | 25.42 ± 1.76 c | 38.12 ± 0.56 ab | 24.39 ± 3.43 c |
| pH | 5.79 ± 0.06 c | 5.72 ± 0.04 c | 5.19 ± 0.03 d | 6.27 ± 0.04 b | 5.30 ± 0.06 d | 6.78 ± 0.01 a |
| EC/μS·cm−1 | 871.67 ± 1.02 b | 47.37 ± 0.69 d | 2.18 ± 0.02 e | 1605.00 ± 2.12 a | 419.67 ± 4.99 c | 3.35 ± 0.05 e |
| OM/mg·g−1 | 13.41 ± 0.14 ab | 14.30 ± 1.99 a | 13.80 ± 1.16 ab | 11.15 ± 0.43 b | 12.70 ± 1.02 ab | 12.42 ± 1.04 ab |
| CEC/cmol·kg−1 | 16.03 ± 0.98 a | 16.90 ± 0.37 a | 17.36 ± 0.16 a | 16.25 ± 0.25 a | 16.87 ± 0.26 a | 16.63 ± 0.67 a |
| TN/g·kg−1 | 1.68 ± 0.01 c | 1.49 ± 0.03 d | 2.01 ± 0.03 a | 1.53 ± 0.01 d | 1.89 ± 0.01 b | 1.68 ± 0.05 c |
| AN/mg·kg−1 | 370.06 ± 3.42 c | 124.37 ± 1.14 e | 648.85 ± 3.04 b | 125.13 ± 1.90 e | 173.81 ± 1.90 d | 756.86 ± 4.56 a |
| TP/mg·kg−1 | 192.76 ± 2.12 a | 201.16 ± 5.20 a | 153.49 ± 3.70 b | 152.02 ± 1.77 b | 147.67 ± 3.94 b | 163.05 ± 1.30 b |
| AP/mg·kg−1 | 173.53 ± 2.53 b | 93.00 ± 2.98 d | 209.99 ± 5.06 a | 108.97 ± 5.00 d | 94.99 ± 3.28 d | 140.97 ± 9.89 c |
| AK/mg·kg−1 | 285.07 ± 5.62 d | 272.76 ± 1.79 e | 345.09 ± 0.23 b | 316.36 ± 2.83 c | 440.06 ± 3.01 a | 213.72 ± 1.76 f |
| UE/mg·(g·24 h)−1 | 0.28 ± 0.08 e | 0.85 ± 0.04 d | 0.92 ± 0.05 d | 2.17 ± 0.04 a | 1.29 ± 0.07 c | 1.53 ± 0.06 b |
| SC/mg·(g·24 h)−1 | 1.87 ± 0.10 d | 7.62 ± 0.39 c | 11.26 ± 0.16 b | 13.07 ± 0.48 a | 11.72 ± 0.33 ab | 11.62 ± 1.19 ab |
| CAT/mL·g−1 | 2.43 ± 0.10 cd | 2.86 ± 0.01 b | 1.92 ± 0.05 e | 3.12 ± 0.04 a | 2.27 ± 0.06 d | 2.49 ± 0.02 c |
| ACP/mg·kg−1 | 152.73 ± 5.57 c | 193.46 ± 1.17 b | 273.32 ± 6.99 a | 155.90 ± 5.14 c | 270.09 ± 5.96 a | 116.31 ± 7.55 d |
| Indicators | CK | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| 0.1000 | 0.0797 | −0.7801 | −0.0425 | −0.6983 | −0.3706 | |
| 0.1000 | 0.6428 | 0.8133 | 0.6373 | 0.6949 | 0.6716 | |
| SI | 0.1000 | 0.4175 | 0.1723 | 0.3654 | 0.1376 | 0.2547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Su, J.; Zhang, Z.; Huang, Y.; Wang, X.; Yi, X. Evaluation Study of the Passivation Effect of Arsenic-Contaminated Farmland Soil. Processes 2024, 12, 2802. https://doi.org/10.3390/pr12122802
Liu S, Su J, Zhang Z, Huang Y, Wang X, Yi X. Evaluation Study of the Passivation Effect of Arsenic-Contaminated Farmland Soil. Processes. 2024; 12(12):2802. https://doi.org/10.3390/pr12122802
Chicago/Turabian StyleLiu, Shuyue, Jikai Su, Zhiyu Zhang, Yajing Huang, Xiaoyao Wang, and Xiaoyun Yi. 2024. "Evaluation Study of the Passivation Effect of Arsenic-Contaminated Farmland Soil" Processes 12, no. 12: 2802. https://doi.org/10.3390/pr12122802
APA StyleLiu, S., Su, J., Zhang, Z., Huang, Y., Wang, X., & Yi, X. (2024). Evaluation Study of the Passivation Effect of Arsenic-Contaminated Farmland Soil. Processes, 12(12), 2802. https://doi.org/10.3390/pr12122802







