Field Study on Washing of 4-Methoxy-2-Nitroaniline from Contaminated Site by Dye Intermediates
Abstract
1. Introduction
2. Materials and Methods
2.1. Aquifer Medium Sample Collection and Preservation
2.2. Reagent and Instrument
2.3. Method of Eluting Experiment
2.4. Determination Method
3. Results and Discussion
3.1. Alcohol Eluting Agent Eluting Experiments
3.2. Non-Ionic Surfactant Eluting Experiments
3.3. Determination of Optimal Eluting Agents and Conditions
3.4. Deionized Water Washing Experiments
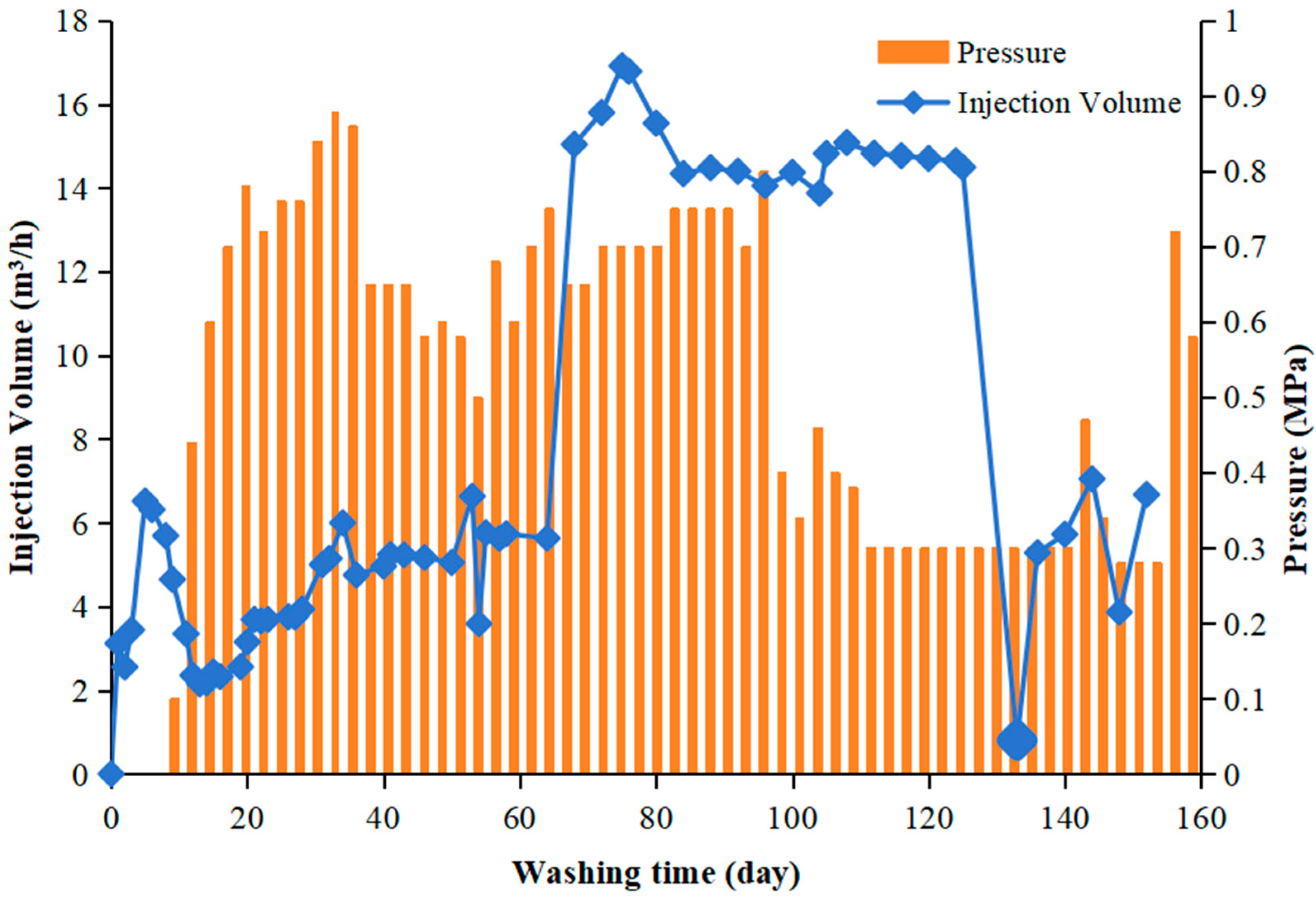
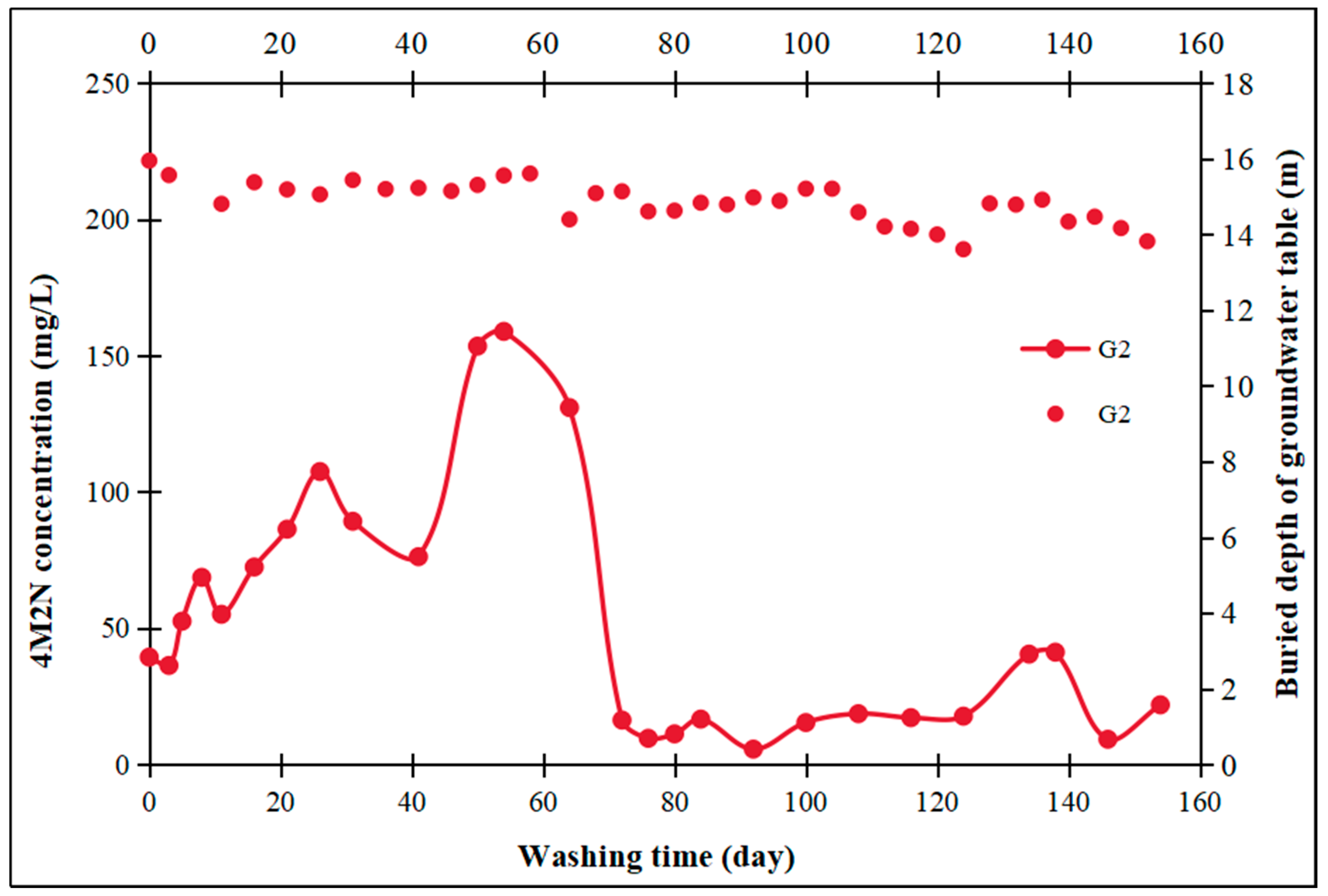
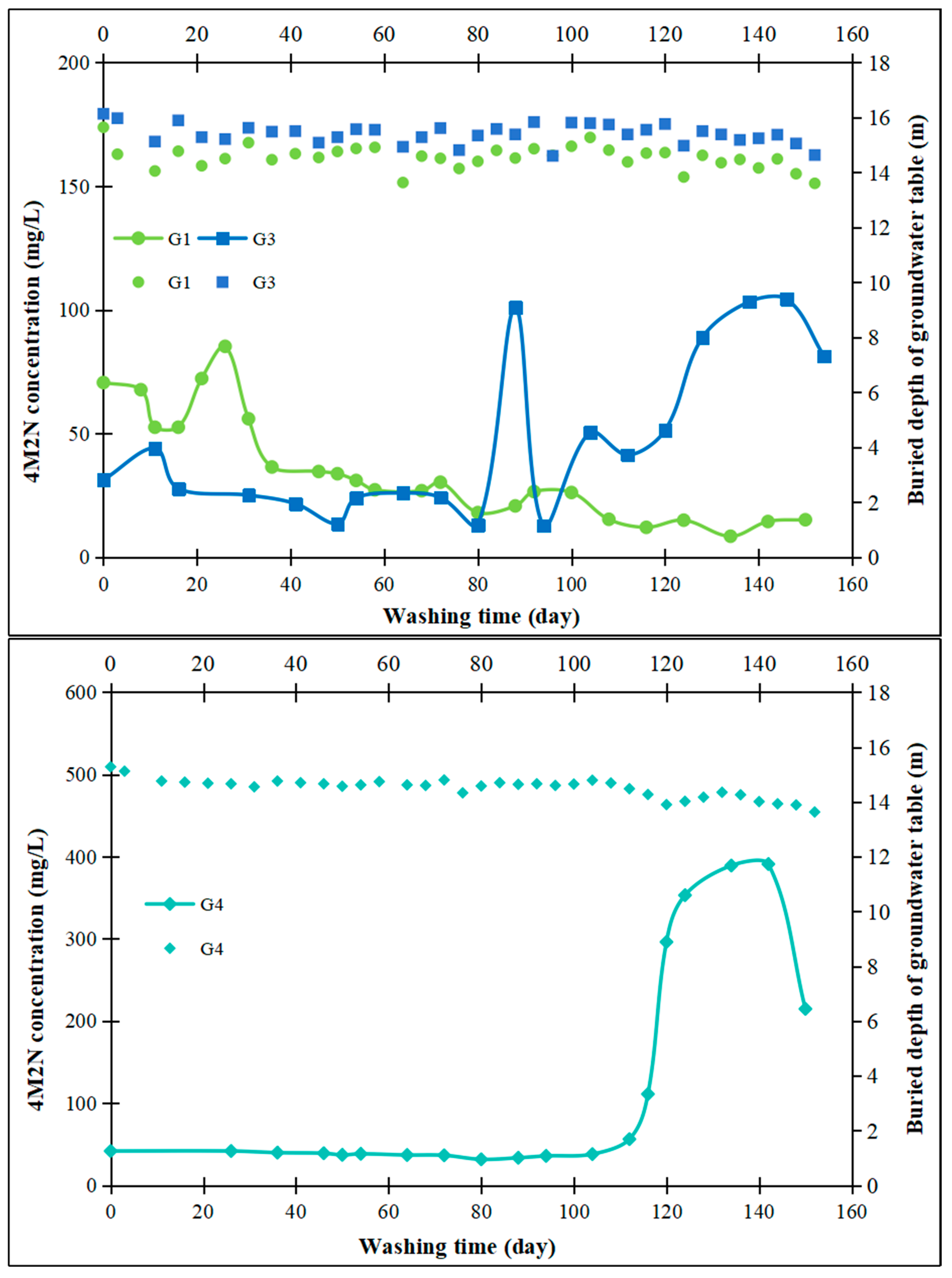
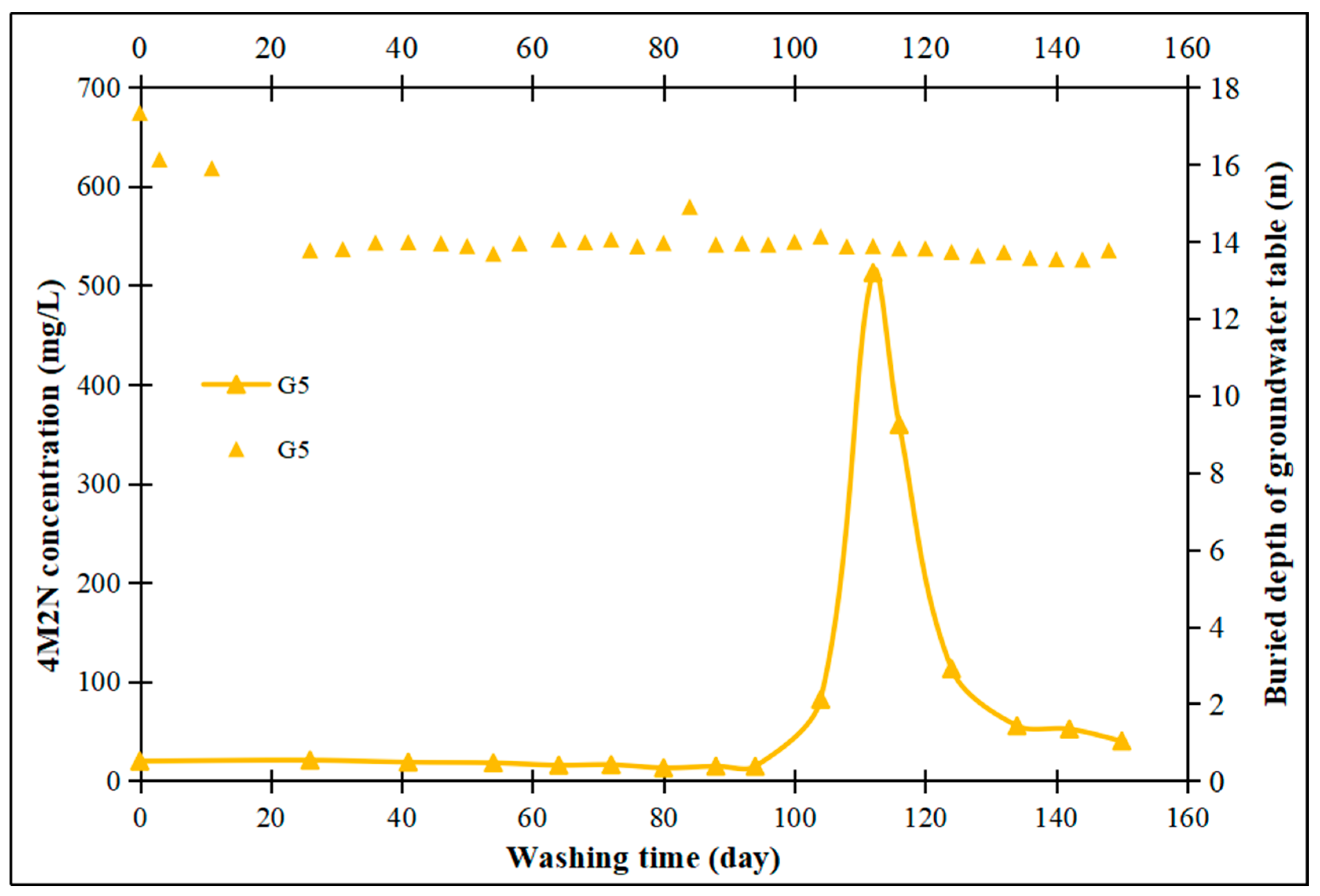
3.5. On-Site Washing Pilot Test
4. Conclusions
- (1)
- The alcohol-based eluting agents applied to leach 4-methoxy-2-nitroaniline (4M2N) from the contaminated aquifer medium showed the following effectiveness: n-propanol > ethanol > methanol. This efficacy is closely related to the polarity of the alcohol eluting agents. The eluting agents reduce the interfacial tension between contaminants and water through wetting, foaming, and emulsification effects, with the optimal concentration of alcohol needed to overcome the stable value of interfacial tension. N-propanol’s optimal conditions involve 60% concentration, a 15:1 liquid-to-solid ratio, two eluting cycles, pH 3, and a 2 h eluting duration. Notably, n-propanol exhibited the most effective eluting, achieving a 4-methoxy-2-nitroaniline concentration of 75.49 mg/kg. The liquid-to-solid ratio and number of eluting cycles emerged as the primary factors influencing the efficacy of alcohol-based eluting agents.
- (2)
- The non-ionic surfactants used to leach 4-methoxy-2-nitroaniline from the contaminated aquifer medium exhibited the following effectiveness: APG > Triton X-100 > Tween-80. The optimal concentration of APG was found to be 0.5 g/L, with a liquid-to-solid ratio of 15:1, eluting conducted four times, an elution pH of 7, and an eluting duration of 3 h. The liquid-to-solid ratio and the number of eluting cycles are the two primary factors influencing the efficacy of non-ionic surfactant eluting. The liquid-to-solid ratio directly affects the contact area and contact time between the eluting agent and the contaminants in the aquifer medium, which thus influences the effectiveness of a single eluting event, while the number of eluting cycles affects the cumulative removal of contaminants.
- (3)
- The blending of APG and Triton X-100 as an eluting agent enhances the eluting effect compared to individual non-ionic surfactants. The blended eluting agents exhibited a synergistic enhancement effect among their various components, which demonstrates greater adaptability to different remediation environments and conditions. This synergy can neutralize potential adverse effects associated with individual components, which can enhance the efficiency of contaminant elution. The optimal blending ratio of APG to Triton X-100 was determined to be 4:1.
- (4)
- n-propanol has been identified as the optimal eluting agent for removing 4-methoxy-2-nitroaniline from the aquifer medium. Optimal eluting conditions were determined as a 15:1 liquid-to-solid ratio, two eluting cycles, an elution pH of 3, 60% n-propanol concentration, and a 2 h eluting duration. Under these conditions, n-propanol exhibits significantly enhanced performance, surpassing that of the blended non-ionic surfactant agent by two times more and deionized water eluting by three times further, thereby establishing its efficacy as a superior eluting agent for 4-methoxy-2-nitroaniline removal. This is also suitable for polycyclic aromatic hydrocarbons and other nitroaniline compounds such as 4-methoxy-3-nitroaniline, etc.
- (5)
- Field pilot tests were conducted using treated groundwater washing, which revealed a significant increase in contaminant concentrations in the pumping wells and monitoring wells located downstream of the washing injection well. In contrast, in the upstream and lateral monitoring wells, the time for contaminant concentration increases was correspondingly delayed with increasing distance from the injection well. Water washing facilitates the transfer of contaminants adhering to aquifer medium particle surfaces into the aqueous solution through mechanisms such as physical washing, partial dissolution, and the promotion of contaminant migration. This approach is both cost-effective and environmentally friendly.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Gu, L.; Yuan, H.; Lou, Z.; Wang, L.; Zhang, X. Degradation pathway of the naphthalene azo dye intermediate 1-diazo-2-naphthol-4-sulfonic acid using Fenton’s reagent. Water Res. 2012, 46, 3859–3867. [Google Scholar] [CrossRef]
- Panke, S.; Wubbolts, M. Advances in biocatalytic synthesis of pharmaceutical intermediates. Curr. Opin. Chem. Biol. 2005, 9, 188–194. [Google Scholar] [CrossRef]
- Swaminathan, K.; Pachhade, K.; Sandhya, S. Decomposition of a dye intermediate, (H-acid) 1 amino-8-naphthol-3, 6 disulfonic acid in aqueous solution by ozonation. Desalination 2005, 186, 155–164. [Google Scholar] [CrossRef]
- Rosli, M.M.; Patil, P.S.; Fun, H.K.; Razak, I.A.; Dharmaprakash, S.M. 4-methoxy-2-nitroaniline. Acta Crystallogr. Sect. E 2007, 63, 1039–1040. [Google Scholar] [CrossRef]
- Hao, Y.; Su, Y. Study on the synthesis of 2-nitro-pmethoxyaniliine by method of solvent. Coal Chem. Ind. 2003, 2, 25–26. [Google Scholar]
- Si, H.; Liu, X.; He, X.; Hou, Z. Synthesis of 2-nitro-4-methoxyaniline. J. Tianjin Univ. Technol. 2009, 25, 19–21. [Google Scholar]
- Liu, D.; Dou, Y.; Gao, Y.; Zhao, S.; Zhuang, C.; Dai, G. The synthesis of the intermediate of omeprazole of N-(2-nitro-4-methoxy) phenyIamine. J. Jiangsu Norm. Univ. (Nat. Sci. Ed.) 2003, 21, 25–27. [Google Scholar]
- Khalaji, A.D.; Nikookar, M.; Das, D. Co (III), Ni (II), and Cu (II) complexes of bidentate N, O-donor Schiff base ligand derived from 4-methoxy-2-nitroaniline and salicylaldehyde: Synthesis, characterization, thermal studies and use as new precursors for metal oxides nanoparticles. J. Therm. Anal. Calorim. 2014, 115, 409–417. [Google Scholar] [CrossRef]
- Contreras, R.H.; De Kowalewski, D.G.; Facelli, J.C. The NMR analysis of the methoxy-group conformation in 4-methoxy-2-nitroaniline. J. Mol. Struct. 1982, 81, 147–149. [Google Scholar] [CrossRef]
- Gu, B.; Ji, W.; Huang, X.; Patil, P.S.; Dharmaprakash, S.M. Concentration-dependent two-photon absorption and subsequent excited-state absorption in 4-methoxy-2-nitroaniline. J. Appl. Phys. 2009, 106, 1245. [Google Scholar] [CrossRef]
- Ravikumar, C.; Joe, I.H. Electronic absorption and vibrational spectra and nonlinear optical properties of 4-methoxy-2-nitroaniline. Phys. Chem. Chem. Phys. 2010, 12, 9452–9460. [Google Scholar] [CrossRef]
- El-Mahalawy, A.M.; Wassel, A.R. Structural, optical and photoelectrical characteristics of 4-methoxy-2-nitroaniline for optoelectronic applications. Mater. Sci. Semicond. Process. 2020, 116, 105124. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, J.; Chen, J.; Chen, H.; Barnie, S. In Situ Pumping–Injection Remediation of Strong Acid–High Salt Groundwater: Displacement–Neutralization Mechanism and Influence of Pore Blocking. J. Water 2022, 14, 2720. [Google Scholar] [CrossRef]
- Sripriya, R.; Raja, K.M.; Santhosh, G.; Chandrasekaran, M.; Noel, M. The effect of structure of oil phase, surfactant and co-surfactant on the physicochemical and electrochemical properties of bicontinuous microemulsion. J. Colloid Interface Sci. 2007, 314, 712–717. [Google Scholar] [CrossRef]
- Chai, J.L.; Zhao, J.R.; Gao, Y.H.; Yang, X.D.; Wu, C.J. Studies on the phase behavior of the microemulsions formed by sodium dodecyl sulfonate, sodium dodecyl sulfate and sodium dodecyl benzene sulfonate with a novel fishlike phase diagram. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 31–35. [Google Scholar] [CrossRef]
- Zhu, M. Phase Behavior and Solubilization Properties of Middle Phase Microemulsion System Formed by Surfactant Compounding. Master’s Thesis, Shandong Normal University, Jinan, China, 2012. [Google Scholar]
- Zeng, C.; Lü, J.; Zheng, H.; Chen, X.; Huang, B. Effect of solvent on the solvolysis liquefaction of sawdust with phosphotungstic acid under supercritical condition. J. Fuel Chem. Technol. 2016, 44, 342–348. [Google Scholar]
- Yao, Z.; Lin, K.; Lu, Q.; Xu, K.; Zhang, J. Alcohol eluent for leaching and remediation of soil contaminated by polycyclic aromatic hydrocarbons. Environ. Pollut. Contr. 2017, 39, 1242–1245. [Google Scholar]
- Lu, Y.; Chen, J.; Ling, T. Removal mechanism of polychlorinated biphenyls from soil by cosolvent. Environ. Sci. 2010, 31, 205–210. [Google Scholar]
- Chen, S.; Liu, P.; Zhu, Z.; Liu, Q. Experimental study on surface tension of several alcohol aqueous solutions. J. Beijing Jiaotong Univ. 2008, 32, 112–115. [Google Scholar]
- Yang, X. The Research on the Transport Characteristics of Surfactanthydrophobic Organic Compounds Aggregate in Porous Medium. Doctoral Thesis, Hunan University, Changsha, China, 2022; p. 004709. [Google Scholar]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Tween 80 surfactant-enhanced bioremediation: Toward a solution to the soil contamination by hydrophobic organic compounds. Crit. Rev. Biotechnol. 2018, 38, 17–30. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Ruan, Y.; Guo, Q.; Liao, X.; Zhang, B. Alkyl polyglycoside: A green and efficient surfactant for enhancing heavy oil recovery at high⁃temperature and high⁃salinity condition. J. Pet. Explor. Prod. Technol. 2019, 9, 2671–2680. [Google Scholar] [CrossRef]
- Ning, D. Study and Application of Petroleum-Contaminated Soil by Ex-Situ Washing Remediation Technology. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2010. [Google Scholar]
- Haigh, S.D. A review of the interaction of surfactants with organic contaminants in soil. Sci. Total Environ. 1996, 185, 161–170. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Wang, X.; Dong, Q.; Li, X.; Dong, J. Properties of coal-based alkylbenzene sulfonate surfactants compound system. J/OL. Fine Chem. 2024, 1–14. [Google Scholar] [CrossRef]
- Ma, S. The Solubilization of Polycyclic Aromatic Hydrocarbons (PAHs) in Phospholipid Microemulsion and Its Application to the Elution and Remediation of PAHs Contaminated Soil. Master’s Thesis, Shenzhen University, Shenzhen, China, 2020. [Google Scholar]
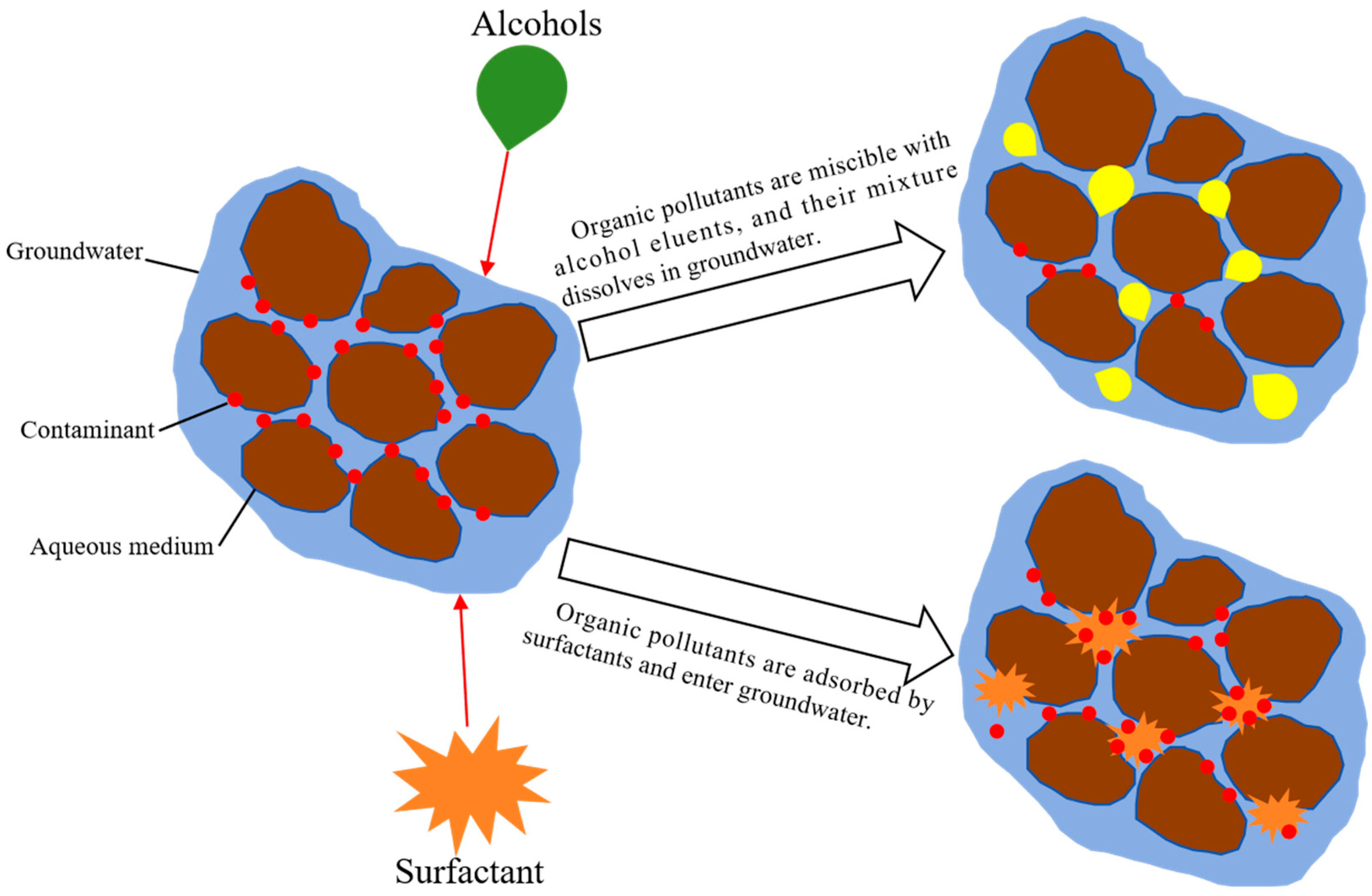


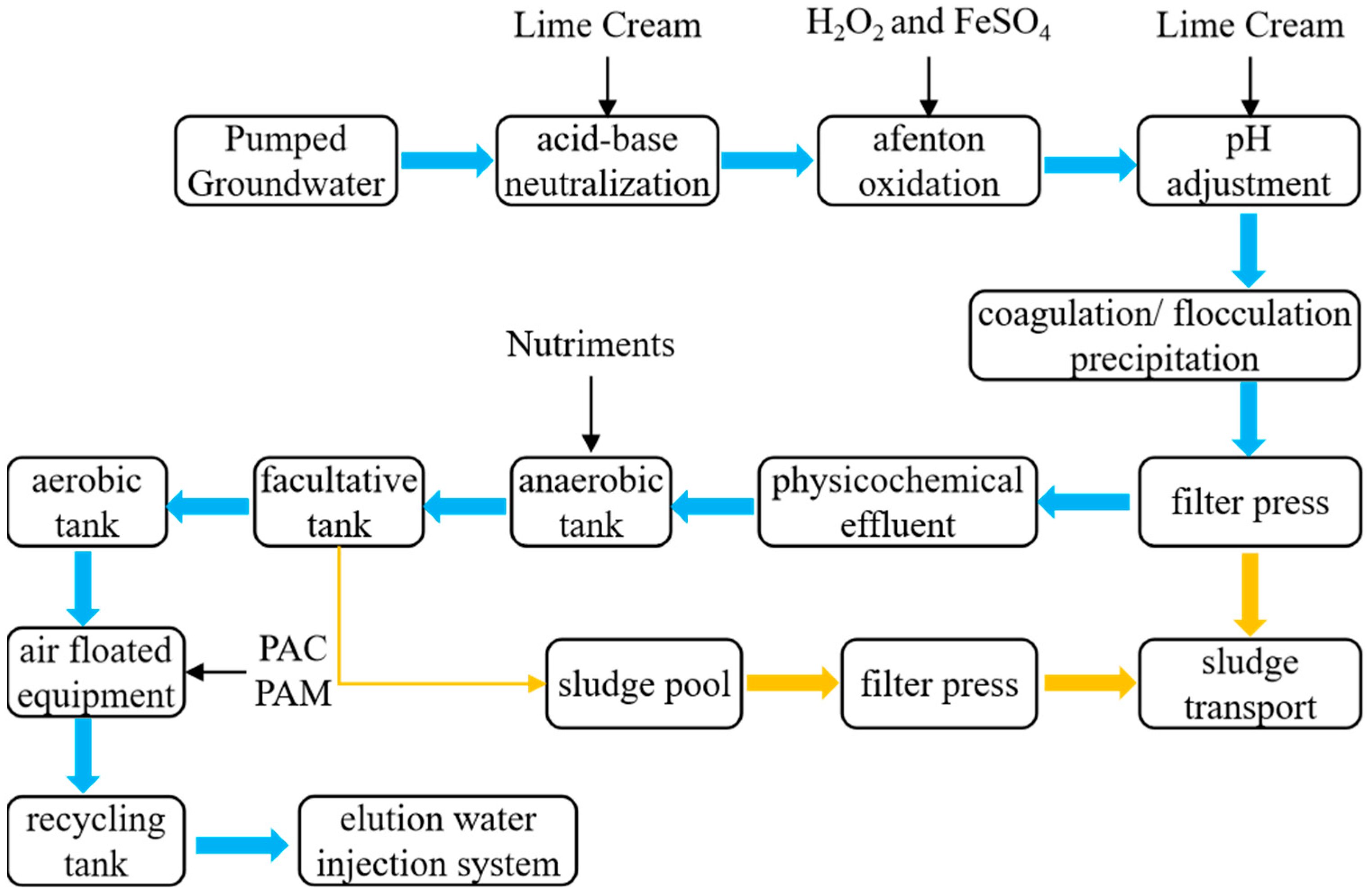


| Indicators | Conductivity | pH | Organic Matter | Moisture Content |
|---|---|---|---|---|
| Prepared Aquifer Medium Samples | 81.3 mS/m | 7.4 | 7.58 g/kg | 0.2% |
| Name | CAS | Molecular Formula | Structural Formula | Molecular Weight | Melting Point (°C) | Boiling Point (°C) | Property Description |
|---|---|---|---|---|---|---|---|
| 4-methoxy-2-nitroaniline | 97-52-9 | C7H8N2O3 | 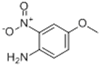 | 168.15 | 123–126 | 338 | Orange-red powder. Slightly soluble in water, soluble in ethanol and ether, and slightly soluble in benzene. |
| methanol | 67-56-1 | CH4O |  | 32.04 | −97.8 | 64.7 | Colorless transparent liquid with an irritating odor. Soluble in water and miscible with most organic solvents such as alcohols and ethers. |
| ethanol | 64-17-5 | C2H5OH |  | 46.07 | −114 | 78 | Colorless liquid with a fruity aroma. Miscible with water and most organic solvents, including ethers, chloroform, glycerol, and methanol. |
| n-propanol | 71-23-8 | C3H8O |  | 60.1 | −127 | 97 | Colorless transparent liquid with an ethanol-like odor. Miscible with water and most organic solvents such as alcohols and ethers. |
| Tween-80 | 9005-65-6 | C24H44O6 | 428.60 | Exhibits high hydrophilicity, unaffected by environmental pH. Easily soluble in water, ethanol, vegetable oils, ethyl acetate, methanol, and toluene. Insoluble in mineral oils. | |||
| APG | 141464-42-8 | C16H32O6 | 320.42 | Low surface tension, fully biodegradable in nature, non-toxic, and harmless. Resistant to strong bases, strong acids, hard water, and has strong salt tolerance. | |||
| Triton X-100 | 9002-93-1 | C34H62O11 | 646.85 | Colorless or nearly colorless transparent viscous liquid. Soluble in water, toluene, xylene, and ethanol. Insoluble in petroleum ether. |
| Level Factor | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Non-ionic surfactant concentration (g/L) | 0.5 | 1 | 2 | 5 |
| Alcohol eluent concentration (%) | 30 | 60 | 80 | 100 |
| Liquid-to-solid ratio (single, non-cumulative) | 2.5:1 | 5:1 | 10:1 | 15:1 |
| Eluent time | 0.5 h | 1 h | 2 h | 3 h |
| Eluent pH (hydrochloric acid adjustment) | 3 | 4.5 | 6 | 7 |
| Eluting cycles | 1 | 2 | 3 | 4 |
| Experiment No. | Methanol Concentration (%) | Liquid-to-Solid Ratio | Eluting Time (h) | Eluting Solution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| A1 | 30 | 2.5:1 | 0.5 | 3 | 1 | 10.79 |
| A2 | 30 | 5:1 | 1 | 4.5 | 2 | 16.14 |
| A3 | 30 | 10:1 | 2 | 6 | 3 | 22.93 |
| A4 | 30 | 15:1 | 3 | 7 | 4 | 32.46 |
| A5 | 60 | 5:1 | 0.5 | 6 | 4 | 10.91 |
| A6 | 60 | 2.5:1 | 1 | 7 | 3 | 7.92 |
| A7 | 60 | 15:1 | 2 | 3 | 2 | 51.29 |
| A8 | 60 | 10:1 | 3 | 4.5 | 1 | 8.49 |
| A9 | 80 | 10:1 | 0.5 | 7 | 2 | 32.93 |
| A10 | 80 | 15:1 | 1 | 6 | 1 | 18.86 |
| A11 | 80 | 2.5:1 | 2 | 4.5 | 4 | 22.09 |
| A12 | 80 | 5:1 | 3 | 3 | 3 | 22.57 |
| A13 | 100 | 15:1 | 0.5 | 4.5 | 3 | 31.42 |
| A14 | 100 | 10:1 | 1 | 3 | 4 | 28.29 |
| A15 | 100 | 5:1 | 2 | 7 | 1 | 16.88 |
| A16 | 100 | 2.5:1 | 3 | 6 | 2 | 10.44 |
| k1 | 20.58 | 12.81 | 21.51 | 28.24 | 13.75 | |
| k2 | 19.65 | 16.63 | 17.80 | 19.53 | 27.70 | |
| k3 | 24.11 | 23.16 | 28.30 | 15.78 | 21.21 | |
| k4 | 21.76 | 33.51 | 18.49 | 22.55 | 23.44 | |
| R | 4.46 | 20.70 | 10.49 | 12.45 | 13.95 |
| Experiment No. | Ethanol Concentration (%) | Liquid-to-Solid Ratio | Eluting Time (h) | Eluting Solution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| B1 | 30 | 2.5:1 | 0.5 | 3 | 1 | 11.49 |
| B2 | 30 | 5:1 | 1 | 4.5 | 2 | 15.79 |
| B3 | 30 | 10:1 | 2 | 6 | 3 | 29.67 |
| B4 | 30 | 15:1 | 3 | 7 | 4 | 32.65 |
| B5 | 60 | 5:1 | 0.5 | 6 | 4 | 22.89 |
| B6 | 60 | 2.5:1 | 1 | 7 | 3 | 15.66 |
| B7 | 60 | 15:1 | 2 | 3 | 2 | 26.02 |
| B8 | 60 | 10:1 | 3 | 4.5 | 1 | 14.02 |
| B9 | 80 | 10:1 | 0.5 | 7 | 2 | 32.13 |
| B10 | 80 | 15:1 | 1 | 6 | 1 | 21.67 |
| B11 | 80 | 2.5:1 | 2 | 4.5 | 4 | 27.29 |
| B12 | 80 | 5:1 | 3 | 3 | 3 | 23.15 |
| B13 | 100 | 15:1 | 0.5 | 4.5 | 3 | 69.52 |
| B14 | 100 | 10:1 | 1 | 3 | 4 | 29.77 |
| B15 | 100 | 5:1 | 2 | 7 | 1 | 16.26 |
| B16 | 100 | 2.5:1 | 3 | 6 | 2 | 19.72 |
| k1 | 22.40 | 18.54 | 34.01 | 22.61 | 15.86 | |
| k2 | 19.65 | 19.52 | 20.72 | 31.65 | 23.42 | |
| k3 | 26.06 | 26.40 | 24.81 | 23.49 | 34.50 | |
| k4 | 33.82 | 37.47 | 22.38 | 24.18 | 28.15 | |
| R | 14.17 | 18.93 | 13.28 | 9.04 | 18.64 |
| Experiment No. | n-Propanol Concentration (%) | Liquid-to-Solid Ratio | Eluting Time (h) | Eluting Solution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| C1 | 30 | 2.5:1 | 0.5 | 3 | 1 | 2.78 |
| C2 | 30 | 5:1 | 1 | 4.5 | 2 | 18.74 |
| C3 | 30 | 10:1 | 2 | 6 | 3 | 25.19 |
| C4 | 30 | 15:1 | 3 | 7 | 4 | 54.36 |
| C5 | 60 | 5:1 | 0.5 | 6 | 4 | 23.57 |
| C6 | 60 | 2.5:1 | 1 | 7 | 3 | 19.12 |
| C7 | 60 | 15:1 | 2 | 3 | 2 | 75.49 |
| C8 | 60 | 10:1 | 3 | 4.5 | 1 | 9.94 |
| C9 | 80 | 10:1 | 0.5 | 7 | 2 | 23.11 |
| C10 | 80 | 15:1 | 1 | 6 | 1 | 17.91 |
| C11 | 80 | 2.5:1 | 2 | 4.5 | 4 | 4.82 |
| C12 | 80 | 5:1 | 3 | 3 | 3 | 29.06 |
| C13 | 100 | 15:1 | 0.5 | 4.5 | 3 | 40.28 |
| C14 | 100 | 10:1 | 1 | 3 | 4 | 41.41 |
| C15 | 100 | 5:1 | 2 | 7 | 1 | 12.79 |
| C16 | 100 | 2.5:1 | 3 | 6 | 2 | 12.94 |
| k1 | 25.27 | 9.91 | 22.44 | 37.19 | 10.85 | |
| k2 | 32.03 | 21.04 | 24.30 | 18.45 | 32.57 | |
| k3 | 18.73 | 24.91 | 29.57 | 19.90 | 28.41 | |
| k4 | 26.86 | 47.01 | 26.57 | 27.34 | 31.04 | |
| R | 13.30 | 37.10 | 7.14 | 18.74 | 21.72 |
| Factors | APG Concentration (g/L) | Elution Time (h) | Liquid-to-Solid Ratio | Elution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| D1 | 0.5 | 0.5 | 2.5:1 | 3 | 1 | 7.95 |
| D2 | 0.5 | 1 | 5:1 | 4.5 | 2 | 12.20 |
| D3 | 0.5 | 2 | 10:1 | 6 | 3 | 20.70 |
| D4 | 0.5 | 3 | 15:1 | 7 | 4 | 34.80 |
| D5 | 1 | 1 | 2.5:1 | 6 | 4 | 12.80 |
| D6 | 1 | 0.5 | 5:1 | 7 | 3 | 14.25 |
| D7 | 1 | 3 | 10:1 | 3 | 2 | 15.40 |
| D8 | 1 | 2 | 15:1 | 4.5 | 1 | 16.05 |
| D9 | 2 | 2 | 2.5:1 | 7 | 2 | 7.85 |
| D10 | 2 | 3 | 5:1 | 6 | 1 | 6.65 |
| D11 | 2 | 0.5 | 10:1 | 4.5 | 4 | 24.00 |
| D12 | 2 | 1 | 15:1 | 3 | 3 | 25.65 |
| D13 | 5 | 3 | 2.5:1 | 4.5 | 3 | 9.68 |
| D14 | 5 | 2 | 5:1 | 3 | 4 | 16.00 |
| D15 | 5 | 1 | 10:1 | 7 | 1 | 11.30 |
| D16 | 5 | 0.5 | 15:1 | 6 | 2 | 19.50 |
| k1 | 18.91 | 16.43 | 9.57 | 16.25 | 10.49 | |
| k2 | 14.63 | 15.49 | 12.28 | 15.48 | 13.74 | |
| k3 | 16.04 | 15.15 | 17.85 | 14.91 | 17.57 | |
| k4 | 14.12 | 16.63 | 24.00 | 17.05 | 21.90 | |
| R | 4.79 | 1.48 | 14.43 | 2.14 | 11.41 |
| Factors | Triton X-100 Concentration (g/L) | Elution Time (h) | Liquid-to-Solid Ratio | Elution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| E1 | 0.5 | 0.5 | 2.5:1 | 3 | 1 | 9.43 |
| E2 | 0.5 | 1 | 5:1 | 4.5 | 2 | 15.60 |
| E3 | 0.5 | 2 | 10:1 | 6 | 3 | 21.00 |
| E4 | 0.5 | 3 | 15:1 | 7 | 4 | 32.40 |
| E5 | 1 | 1 | 2.5:1 | 6 | 4 | 15.50 |
| E6 | 1 | 0.5 | 5:1 | 7 | 3 | 16.65 |
| E7 | 1 | 3 | 10:1 | 3 | 2 | 17.60 |
| E8 | 1 | 2 | 15:1 | 4.5 | 1 | 15.30 |
| E9 | 2 | 2 | 2.5:1 | 7 | 2 | 11.25 |
| E10 | 2 | 3 | 5:1 | 6 | 1 | 11.30 |
| E11 | 2 | 0.5 | 10:1 | 4.5 | 4 | 24.00 |
| E12 | 2 | 1 | 15:1 | 3 | 3 | 26.10 |
| E13 | 5 | 3 | 2.5:1 | 4.5 | 3 | 11.18 |
| E14 | 5 | 2 | 5:1 | 3 | 4 | 17.80 |
| E15 | 5 | 1 | 10:1 | 7 | 1 | 12.80 |
| E16 | 5 | 0.5 | 15:1 | 6 | 2 | 22.20 |
| k1 | 19.61 | 18.07 | 11.84 | 17.73 | 12.21 | |
| k2 | 16.26 | 17.50 | 15.34 | 16.52 | 16.66 | |
| k3 | 18.16 | 16.34 | 18.85 | 17.50 | 18.73 | |
| k4 | 15.99 | 18.12 | 24.00 | 18.28 | 22.43 | |
| R | 3.61 | 1.78 | 12.16 | 1.76 | 10.22 |
| Factors | Tween-80 Concentration (g/L) | Elution Time (h) | Liquid-to-Solid Ratio | Elution pH | Eluting Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|---|
| F1 | 0.5 | 0.5 | 2.5:1 | 3 | 1 | 8.23 |
| F2 | 0.5 | 1 | 5:1 | 4.5 | 2 | 13.30 |
| F3 | 0.5 | 2 | 10:1 | 6 | 3 | 21.60 |
| F4 | 0.5 | 3 | 15:1 | 7 | 4 | 31.20 |
| F5 | 1 | 1 | 2.5:1 | 6 | 4 | 11.90 |
| F6 | 1 | 0.5 | 5:1 | 7 | 3 | 15.30 |
| F7 | 1 | 3 | 10:1 | 3 | 2 | 17.80 |
| F8 | 1 | 2 | 15:1 | 4.5 | 1 | 15.90 |
| F9 | 2 | 2 | 2.5:1 | 7 | 2 | 10.00 |
| F10 | 2 | 3 | 5:1 | 6 | 1 | 7.00 |
| F11 | 2 | 0.5 | 10:1 | 4.5 | 4 | 22.40 |
| F12 | 2 | 1 | 15:1 | 3 | 3 | 26.55 |
| F13 | 5 | 3 | 2.5:1 | 4.5 | 3 | 7.13 |
| F14 | 5 | 2 | 5:1 | 3 | 4 | 16.80 |
| F15 | 5 | 1 | 10:1 | 7 | 1 | 12.00 |
| F16 | 5 | 0.5 | 15:1 | 6 | 2 | 21.00 |
| k1 | 18.58 | 16.73 | 9.31 | 17.34 | 10.78 | |
| k2 | 15.23 | 15.94 | 13.10 | 14.68 | 15.53 | |
| k3 | 16.49 | 16.08 | 18.45 | 15.38 | 17.64 | |
| k4 | 14.23 | 15.78 | 23.66 | 17.13 | 20.58 | |
| R | 4.35 | 0.95 | 14.35 | 2.66 | 9.79 |
| No. | Eluent | Concentration | Liquid-to-Solid Ratio | pH | Elution Time | Eluting Cycles |
|---|---|---|---|---|---|---|
| A | deionized water | / | 15:1 | 7 | 3 h | 4 |
| B | deionized water | / | 15:1 | 3 | 2 h | 2 |
| C | n-propanol | 60% | 15:1 | 3 | 2 h | 2 |
| Factors | Elution Time (h) | Liquid-to-Solid Ratio | Elution pH | Washing Cycles | Elution 4-methoxy-2-nitroaniline Content (mg/kg) |
|---|---|---|---|---|---|
| G1 | 0.5 | 2.5:1 | 3 | 1 | 7.425 |
| G2 | 1 | 5:1 | 4.5 | 2 | 9.5 |
| G3 | 2 | 10:1 | 6 | 3 | 20.4 |
| G4 | 3 | 15:1 | 7 | 4 | 32.4 |
| G5 | 1 | 2.5:1 | 6 | 4 | 10.5 |
| G6 | 0.5 | 5:1 | 7 | 3 | 16.8 |
| G7 | 3 | 10:1 | 3 | 2 | 16.6 |
| G8 | 2 | 15:1 | 4.5 | 1 | 15.3 |
| G9 | 2 | 2.5:1 | 7 | 2 | 6.65 |
| G10 | 3 | 5:1 | 6 | 1 | 7.3 |
| G11 | 0.5 | 10:1 | 4.5 | 4 | 20.4 |
| G12 | 1 | 15:1 | 3 | 3 | 24.3 |
| G13 | 3 | 2.5:1 | 4.5 | 3 | 7.425 |
| G14 | 2 | 5:1 | 3 | 4 | 14.6 |
| G15 | 1 | 10:1 | 7 | 1 | 9.9 |
| G16 | 0.5 | 15:1 | 6 | 2 | 19.2 |
| k1 | 15.96 | 8.00 | 15.73 | 9.98 | |
| k2 | 13.55 | 12.05 | 13.16 | 12.99 | |
| k3 | 14.24 | 16.83 | 14.35 | 17.23 | |
| k4 | 15.93 | 22.80 | 16.44 | 19.48 | |
| R | 2.41 | 14.80 | 3.28 | 9.49 |
| Type | Number | Distance from an Injection Well | Screening Location |
|---|---|---|---|
| Washing injection well | GZ | / | 8m above the bottom |
| Monitoring well | G1 | 10.9 m | Full sieve |
| G2 | 9 m | Full sieve | |
| G3 | 14 m | Full sieve | |
| G4 | 18 m | Full sieve | |
| G5 | 26.2 m | 15 m above the bottom | |
| Extraction well | DC1 | 17 m | 3 m above the bottom |
| DC2 | 18.1 m | 3 m above the bottom | |
| DC3 | 32.4 m | 3 m above the bottom | |
| DC4 | 16.3 m | 3 m above the bottom | |
| DC5 | 30 m | 3 m above the bottom |
| Index | pH | ORP (mV) | CON (μs/cm) | DO (mg/L) | Sulfate (mg/L) | CODCr (mg/L) | 4-chloroaniline (µg/L) | 4-methoxy-2-nitroaniline (µg/L) | 4-methoxy-3-nitroaniline (µg/L) | 3-chloroaniline (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Injection Water (Average Value) | 6.98 | 75 | 6048 | 6.68 | 4500 | 24 | 10 | 28.6 | 292 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lao, K.; Chen, C.; Zhu, H.; Yang, Y.; Chen, H.; Pang, H. Field Study on Washing of 4-Methoxy-2-Nitroaniline from Contaminated Site by Dye Intermediates. Processes 2024, 12, 2801. https://doi.org/10.3390/pr12122801
Wang Z, Lao K, Chen C, Zhu H, Yang Y, Chen H, Pang H. Field Study on Washing of 4-Methoxy-2-Nitroaniline from Contaminated Site by Dye Intermediates. Processes. 2024; 12(12):2801. https://doi.org/10.3390/pr12122801
Chicago/Turabian StyleWang, Zhili, Kangwen Lao, Chen Chen, Hong Zhu, Yanfei Yang, Honghan Chen, and Hao Pang. 2024. "Field Study on Washing of 4-Methoxy-2-Nitroaniline from Contaminated Site by Dye Intermediates" Processes 12, no. 12: 2801. https://doi.org/10.3390/pr12122801
APA StyleWang, Z., Lao, K., Chen, C., Zhu, H., Yang, Y., Chen, H., & Pang, H. (2024). Field Study on Washing of 4-Methoxy-2-Nitroaniline from Contaminated Site by Dye Intermediates. Processes, 12(12), 2801. https://doi.org/10.3390/pr12122801






