Molecular Dynamics of CO₂ Stripping Oil on Quartz Surfaces
Abstract
1. Introduction
2. Molecular Dynamics Simulation of Crude Oil Adsorption on the Quartz Surface
2.1. Model Establishment
2.2. Simulation of Crude Oil Component Adsorption on Quartz Surface
2.3. Analysis of the Distribution Characteristics of Crude Oil Components on Quartz Surface
3. Microscopic Mechanism of CO2 Stripping from Crude Oil
3.1. Model Establishment
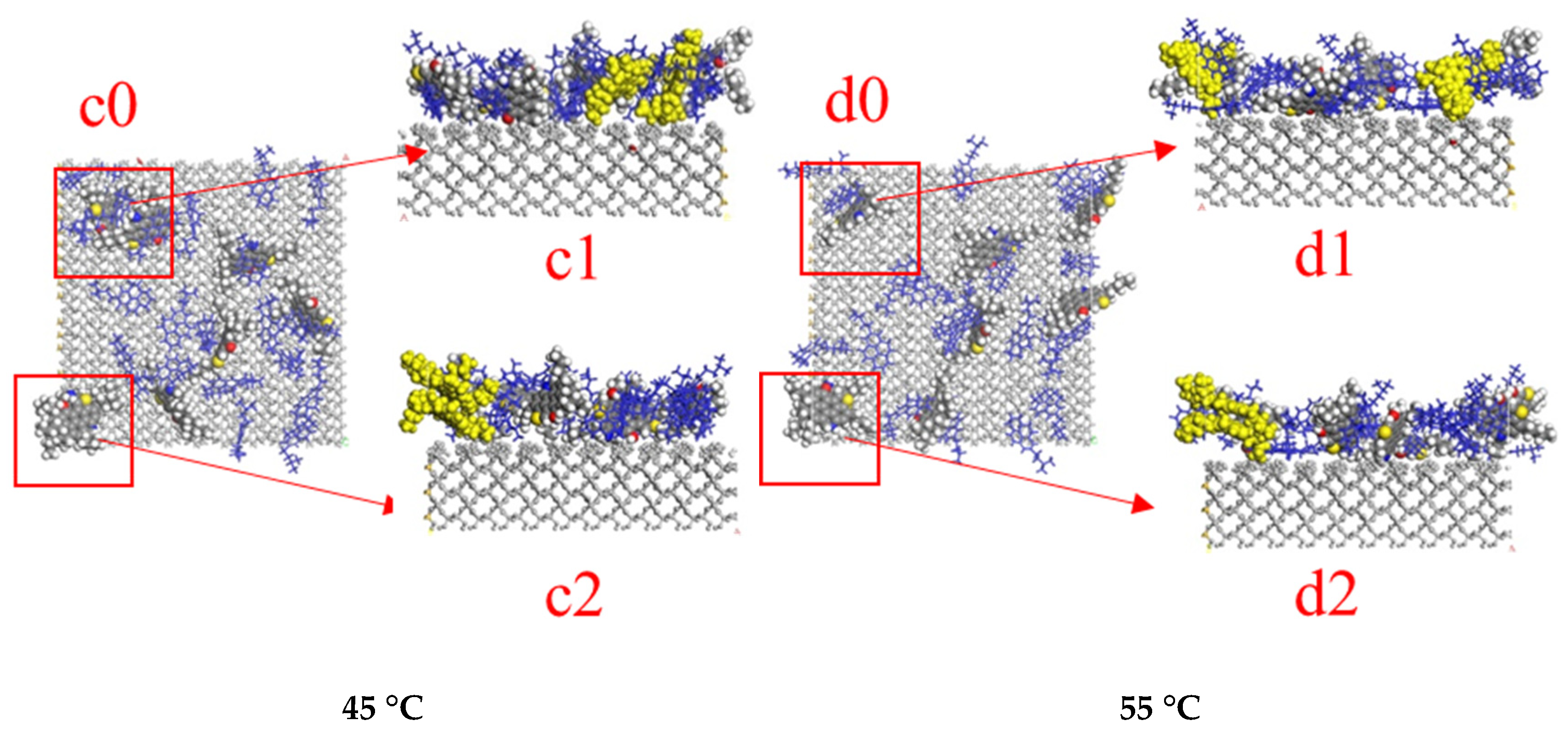
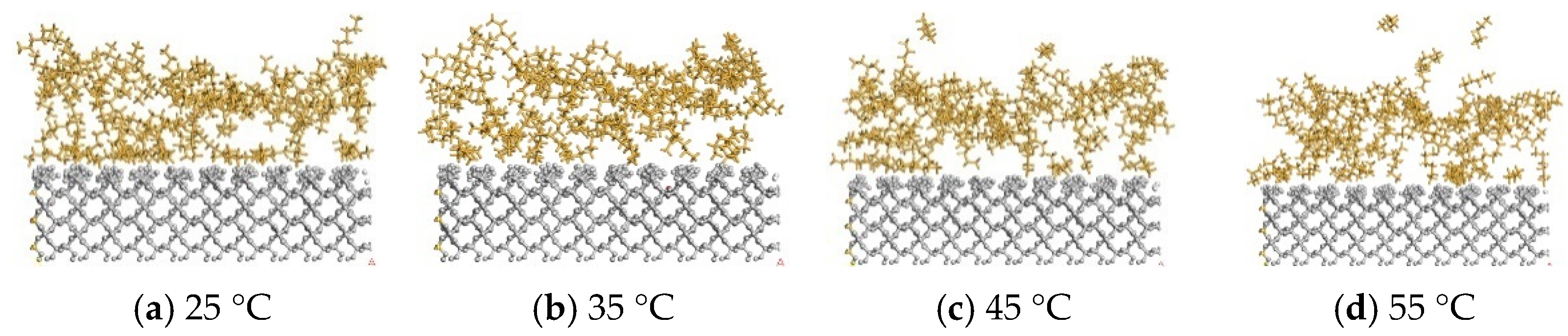
3.2. The Impact of Temperature on Oil Stripping
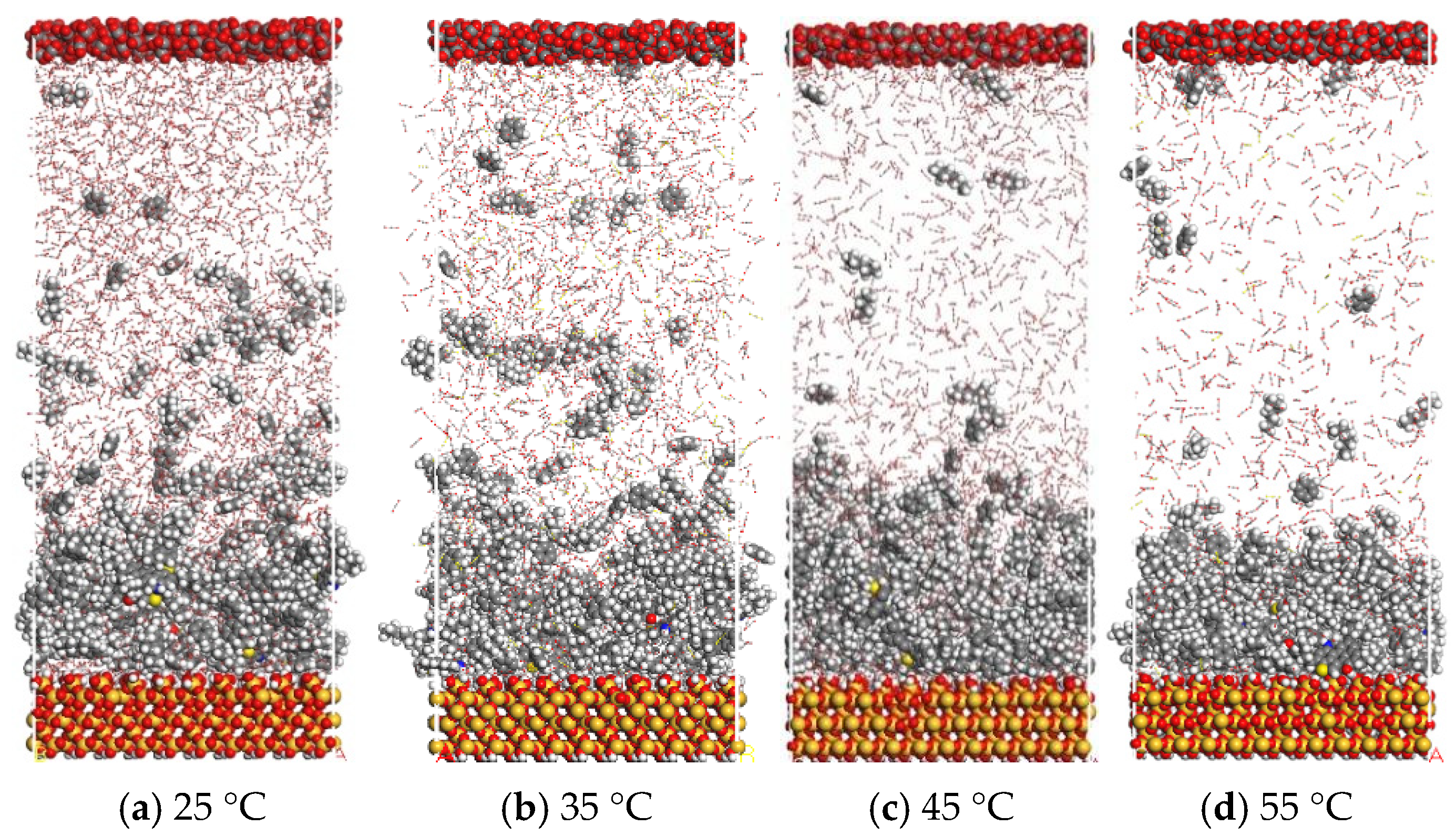
3.3. CO2 Displacement Evaluation in Crude Oil
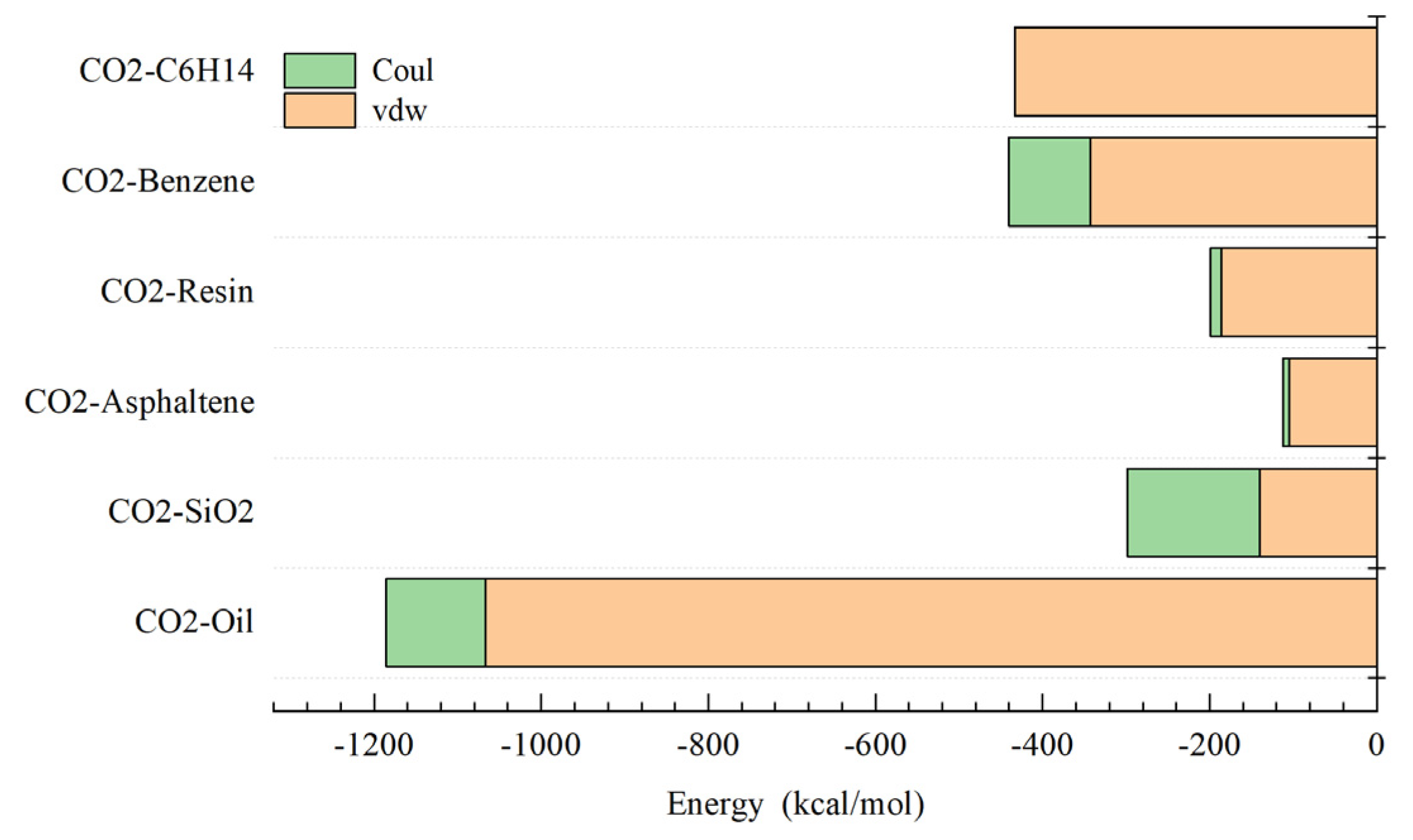
3.4. CO2 Analysis of Interactions Between Different Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, C.; Wu, S.; Yang, Z.; Pan, S.; Wang, G.; Jiang, X.; Guan, M.; Yu, C.; Yu, Z.; Shen, Y. Progress, challenge and significance of building a carbon industry system in the context of carbon neutrality strategy. Pet. Explor. Dev. 2023, 50, 210–228. [Google Scholar] [CrossRef]
- Qin, J.; Han, H.; Liu, X. Application and enlightenment of carbon dioxide flooding in the United States of America. Pet. Explor. Dev. 2015, 42, 232–240. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.H.; Jin, Y.; He, Y.F.; Chen, Z.H.; Tang, Y.; Wu, G.Y. Different reservoir types of CO2 flooding in Sinopec EOR technology development and application under “dual carbon” vision. Reserv. Eval. Dev. 2021, 11, 793–804. [Google Scholar]
- Gozalpour, F.; Ren, S.R.; Tohidi, B. CO2 EOR and storage in oil reservoir. Oil Gas Sci. Technol. 2005, 60, 537–546. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Eftekhari, A.A.; Dafnomilis, G.; Lake, L.W.; Bruining, J. On the sustainability of CO2 storage through CO2–Enhanced oil recovery. Appl. Energy 2020, 261, 114467. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Li, D.; Fu, J.; Su, Y.; Lv, Y. Experimental investigation of shale oil recovery from Qianjiang core samples by the CO2 huff-n-puff EOR method. RSC Adv. 2019, 9, 28857–28869. [Google Scholar] [CrossRef]
- Zanganeh, P.; Ayatollahi, S.; Alamdari, A.; Zolghadr, A.; Dashti, H.; Kord, S. Asphaltene deposition during CO2 injection and pressure depletion: A visual study. Energy Fuels 2012, 26, 1412–1419. [Google Scholar] [CrossRef]
- Lu, J.; Steven, H.; James, S.; Lawrence, P.; Bethany, K.; Steven, S.; Loreal, H.; Volker, H.; Nicholas, B.; José, T. Advancing CO2 enhanced oil recovery and storage in unconventional oil play—Experimental studies on Bakken shales. Appl. Energy 2017, 208, 171–183. [Google Scholar]
- Wei, J.; Zhou, X.; Zhou, J.; Li, J.; Wang, A. Experimental and simulation investigations of carbon storage associated with CO2 EOR in low-permeability reservoir. Int. J. Greenh. Gas Control 2021, 104, 103203. [Google Scholar] [CrossRef]
- Snosy, M.F.; Abu El Ela, M.; El-Banbi, A.; Sayyouh, H. Comprehensive investigation of low-salinity waterflooding in sandstone reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 2019–2034. [Google Scholar] [CrossRef]
- Zhao, Y. Laboratory experiment and field application of high pressure and high quality steam flooding. J. Pet. Sci. Eng. 2020, 189, 107016. [Google Scholar] [CrossRef]
- Kusumastuti, I.; Erfando, T.; Hidayat, F. Effects of Various Steam Flooding Injection Patterns and Steam Quality to Recovery Factor. J. Earth Energy Eng. 2019, 8, 33–39. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, J.; Yoon, H.; Shin, S. Microfluidic investigation of salinity-induced oil recovery in porous media during chemical flooding. Energy Fuels 2021, 35, 4885–4892. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Ji, W.; Li, Z. Insight into water occurrence and pore size distribution by nuclear magnetic resonance in marine shale reservoirs, southern China. Energy Fuels 2023, 37, 319–327. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Ji, W.; Wang, Q. Rock fabric of tight sandstone and its influence on irreducible water saturation in Eastern Ordos Basin. Energy Fuels 2023, 37, 3685–3696. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, Y.; Yuan, B.; Li, Z.; Zhang, Y. Imbibition behavior of oil-saturated rock: Implications for enhanced oil recovery in unconventional reservoirs. Energy Fuels 2023, 37, 13759–13768. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Xiao, H.; Zheng, T. Microscopic production characteristics of tight oil in the nanopores of different CO2-affected areas from molecular dynamics simulations. Sep. Purif. Technol. 2023, 306, 122607. [Google Scholar] [CrossRef]
- Liu, B.; Liu, W.; Pan, Z.; Yu, L.; Xie, Z.; Lv, G.; Zhao, P.; Chen, D.; Fang, W. Supercritical CO2 Breaking Through a Water Bridge and Enhancing Shale Oil Recovery: A Molecular Dynamics Simulation Study. Energy Fuels 2022, 36, 7558–7568. [Google Scholar] [CrossRef]
- Li, C.; Pu, H.; Zhong, X.; Li, Y.; Zhao, J.X. Interfacial interactions between Bakken crude oil and injected gases at reservoir temperature: A molecular dynamics simulation study. Fuel 2020, 276, 118058. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, Y.; Ma, R.; Yan, Y.; Dai, C.; Zhang, J. Oil extraction mechanism in CO2 flooding from rough surface: Molecular dynamics simulation. Appl. Surf. Sci. 2019, 494, 80–86. [Google Scholar] [CrossRef]
- Yu, T.; Li, Q.; Tan, Y.; Xu, L. Molecular dynamics simulation of CO2-N2 dissolution and stripping of oil films on pore walls based on intermolecular interaction energy. Chem. Eng. Sci. 2022, 262, 118044. [Google Scholar] [CrossRef]
- Hong, X.; Yu, H.; Xu, H.; Wang, X.; Jin, X.; Wu, H.; Wang, F. Competitive adsorption of asphaltene and n-heptane on quartz surfaces and its effect on crude oil transport through nanopores. J. Mol. Liq. 2022, 359, 119312. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Qi, C.; Li, X.; Mai, T.; Zhang, J. Mechanism of asphaltene aggregation induced by supercritical CO2: Insights from molecular dynamics simulation. RSC Adv. 2017, 7, 50786–50793. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, F.; Wang, Z.; Wang, D.; Wang, Y. Molecular simulations of oil adsorption and transport behavior in inorganic shale. J. Mol. Liq. 2020, 305, 112745. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Q.; Wang, S.; Xing, X. Oil diffusion in shale nanopores: Insight of molecular dynamics simulation. J. Mol. Liq. 2019, 290, 111183. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Sui, H.; He, L. Understanding desorption of oil fractions from mineral surfaces. Fuel 2018, 232, 257–266. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Tao, Z.; Wang, S.; Fan, J.; Feng, Q.; Xue, Q. The miscible behaviors and mechanism of CO2/CH4/C3H8/N2 and crude oil in nanoslits: A molecular dynamics simulation study. Fuel 2021, 304, 121461. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Liu, K.; Burke, N. Molecular Simulation of CO2 Solubility and Its Effect on Octane Swelling. Energy Fuels 2013, 27, 2741–2747. [Google Scholar] [CrossRef]
- Santos, M.S.; Franco, L.F.M.; Castier, M.; Economou, I.G. Molecular dynamics simulation of n-alkanes and CO2 confined by calcite nanopores. Energy Fuels 2018, 32, 1934–1941. [Google Scholar] [CrossRef]
- Peng, F.; Wang, R.; Guo, Z.; Feng, G. Molecular dynamics simulation to estimate minimum miscibility pressure for oil with pure and impure CO2. J. Phys. Commun. 2018, 2, 115028. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Sun, B.; Shen, Y.; Zhang, J.; Chen, X.; Wang, M. Molecular dynamics simulation on volume swelling of CO2–alkane system. Fuel 2015, 143, 194–201. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Gao, Y.; Zhang, Y.; Yan, Y.; Zhang, J. Enhanced oil recovery with CO2/N2 slug in low permeability reservoir: Molecular dynamics simulation. Chem. Eng. Sci. 2018, 197, 204–211. [Google Scholar] [CrossRef]
- Yu, T.; Li, Q.; Hu, H.; Tan, Y.; Xu, L. Residual oil retention and stripping mechanism of hydrophilic pore surfaces during CO2 and N2 combined gas flooding. J. Pet. Sci. Eng. 2022, 218, 110989. [Google Scholar] [CrossRef]
- Osnis, A.; Sukenik, C.N.; Major, D.T. Structure of carboxyl-acid-terminated self-assembled monolayers from molecular dynamics simulations and hybrid quantum mechanics–molecular mechanics vibrational normal-mode analysis. J. Phys. Chem. C 2012, 116, 770–782. [Google Scholar] [CrossRef]
- Holm, L.W.; Josendal, V.A. Effect of oil composition on miscible-type displacement by carbon dioxide. Soc. Pet. Eng. J. 1982, 22, 87–98. [Google Scholar] [CrossRef]
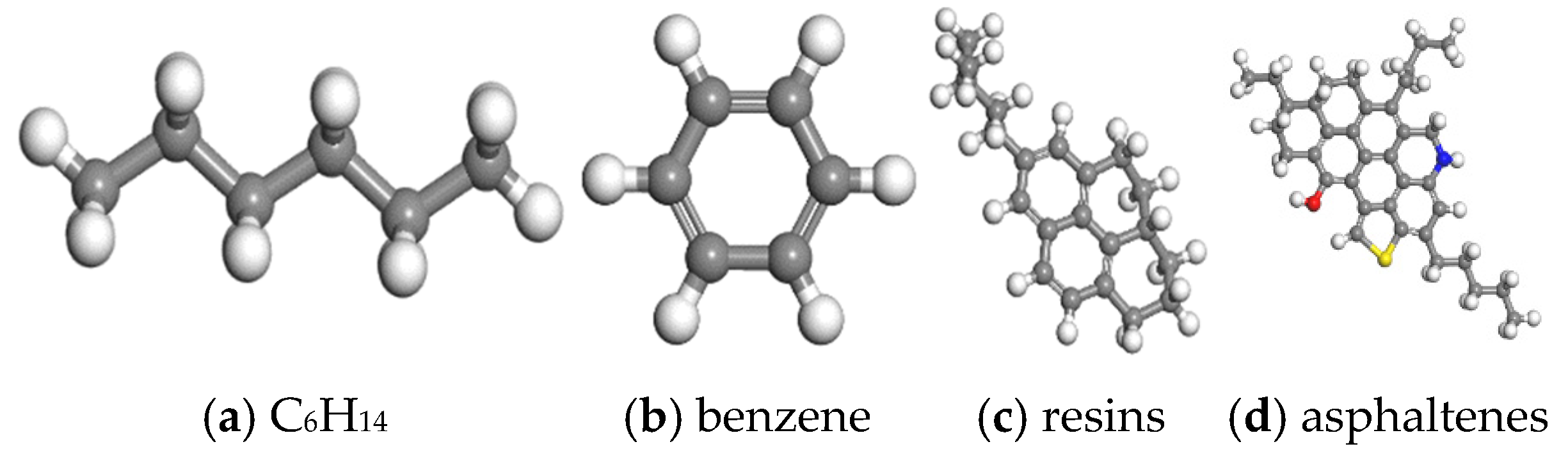







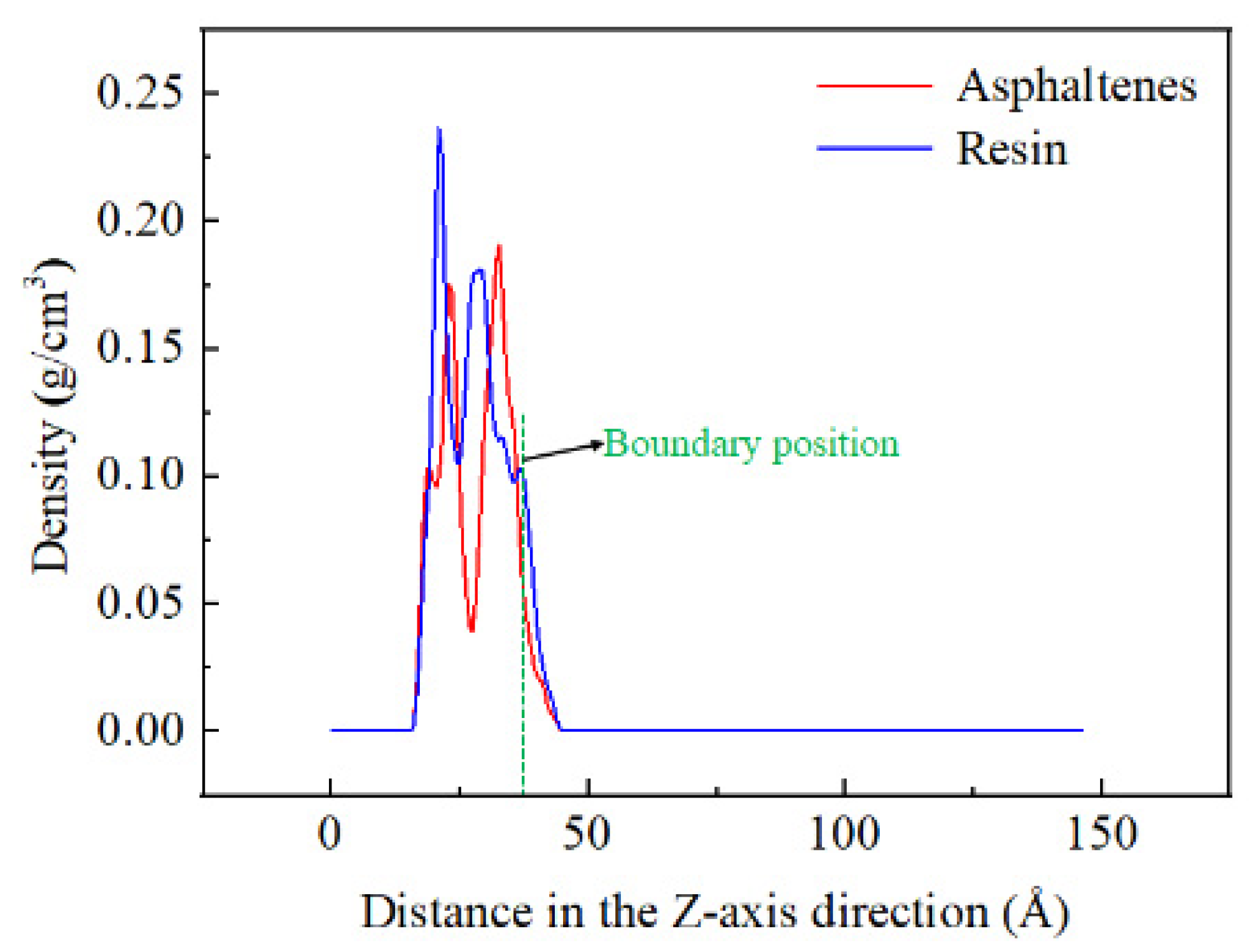
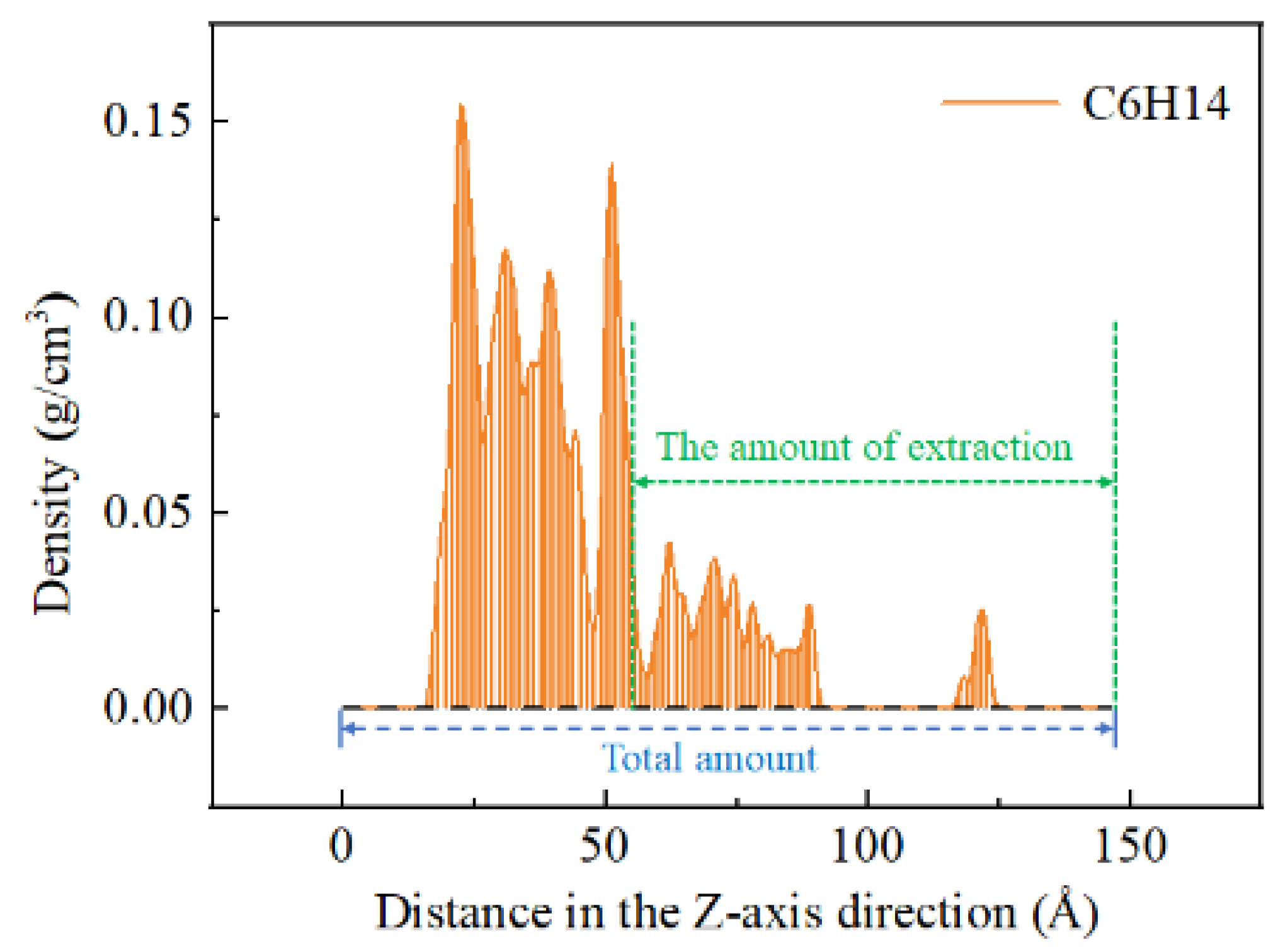
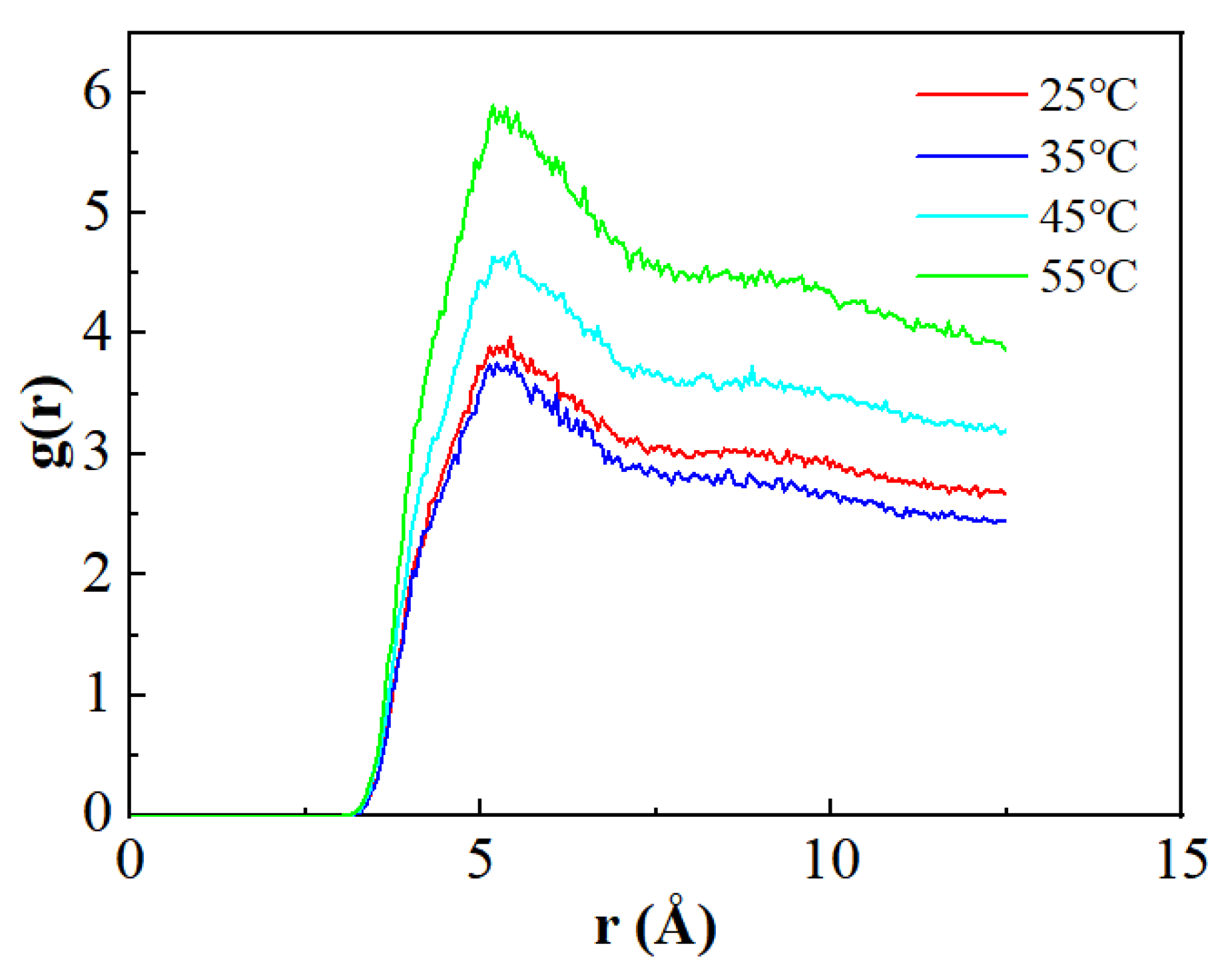
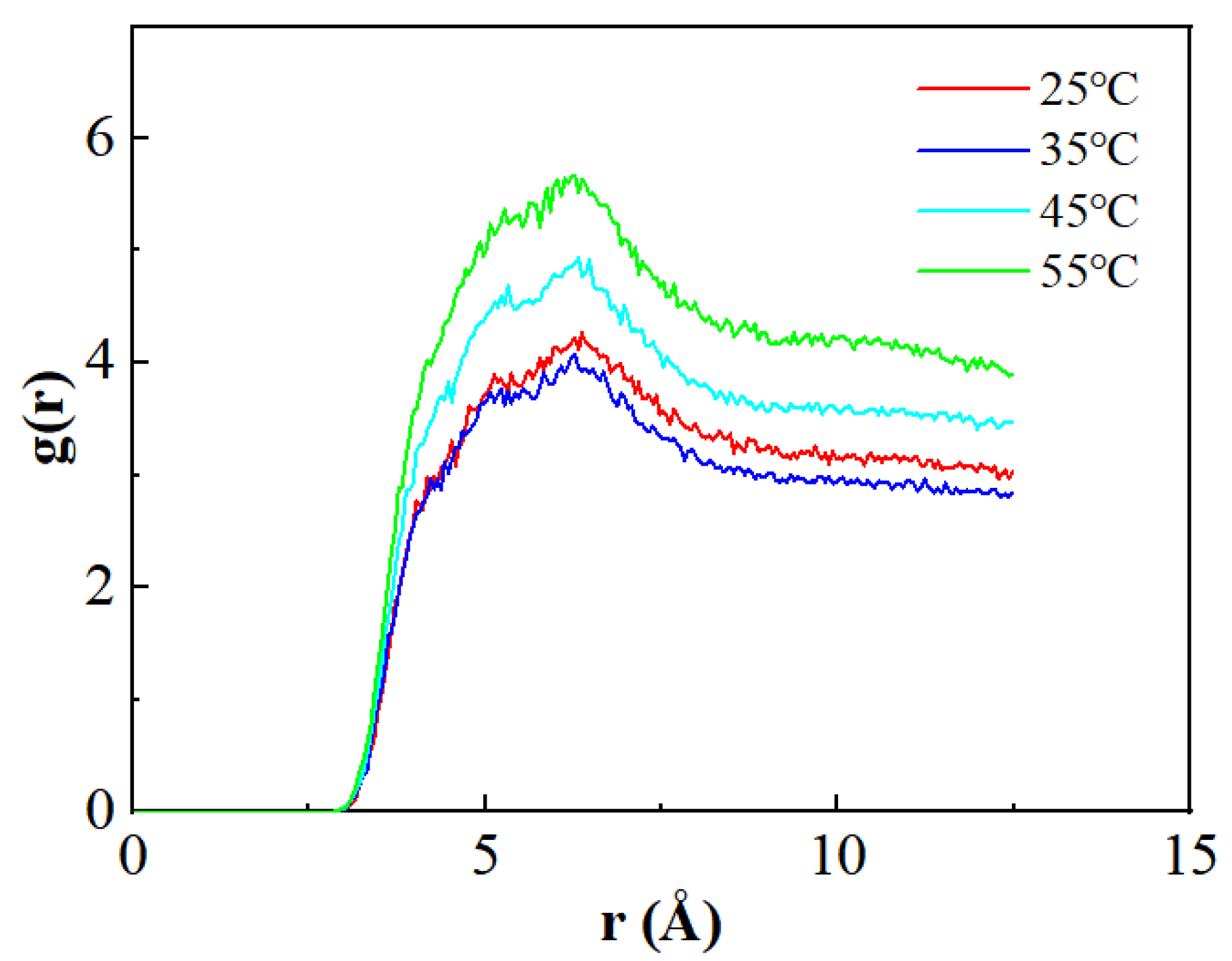

| Components | Saturated Hydrocarbon | Aromatic Hydrocarbon | Resins | Asphaltenes |
|---|---|---|---|---|
| % | 89 | 78 | 20 | 8 |
| Variable | Parameter | |||
|---|---|---|---|---|
| Temperature (°C) | 25 | 35 | 45 | 55 |
| Initial time position (Å) | 38.77 | 36.77 | 33.77 | 34.77 |
| Final time position (Å) | 45.23 | 45.17 | 49.34 | 39.04 |
| Expansion coefficient | 1.17 | 1.23 | 1.46 | 1.12 |
| Temperature (°C) | 25 | 35 | 45 | 55 |
|---|---|---|---|---|
| Extraction rate of C6H14 (%) | 36.17 | 37.87 | 15.69 | 16.16 |
| Extraction rate of Benzene (%) | 23.88 | 23.43 | 10.91 | 13.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Zhang, Y.; Xiong, H.; Tian, S.; Wang, F. Molecular Dynamics of CO₂ Stripping Oil on Quartz Surfaces. Processes 2024, 12, 2776. https://doi.org/10.3390/pr12122776
Tan Y, Zhang Y, Xiong H, Tian S, Wang F. Molecular Dynamics of CO₂ Stripping Oil on Quartz Surfaces. Processes. 2024; 12(12):2776. https://doi.org/10.3390/pr12122776
Chicago/Turabian StyleTan, Yawen, Yiqun Zhang, Hao Xiong, Shouceng Tian, and Fei Wang. 2024. "Molecular Dynamics of CO₂ Stripping Oil on Quartz Surfaces" Processes 12, no. 12: 2776. https://doi.org/10.3390/pr12122776
APA StyleTan, Y., Zhang, Y., Xiong, H., Tian, S., & Wang, F. (2024). Molecular Dynamics of CO₂ Stripping Oil on Quartz Surfaces. Processes, 12(12), 2776. https://doi.org/10.3390/pr12122776







