Synthesis of Polymeric Nanoparticles Using Fungal Biosurfactant as Stabilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Biosurfactants
2.2. Surface Tension Measurement
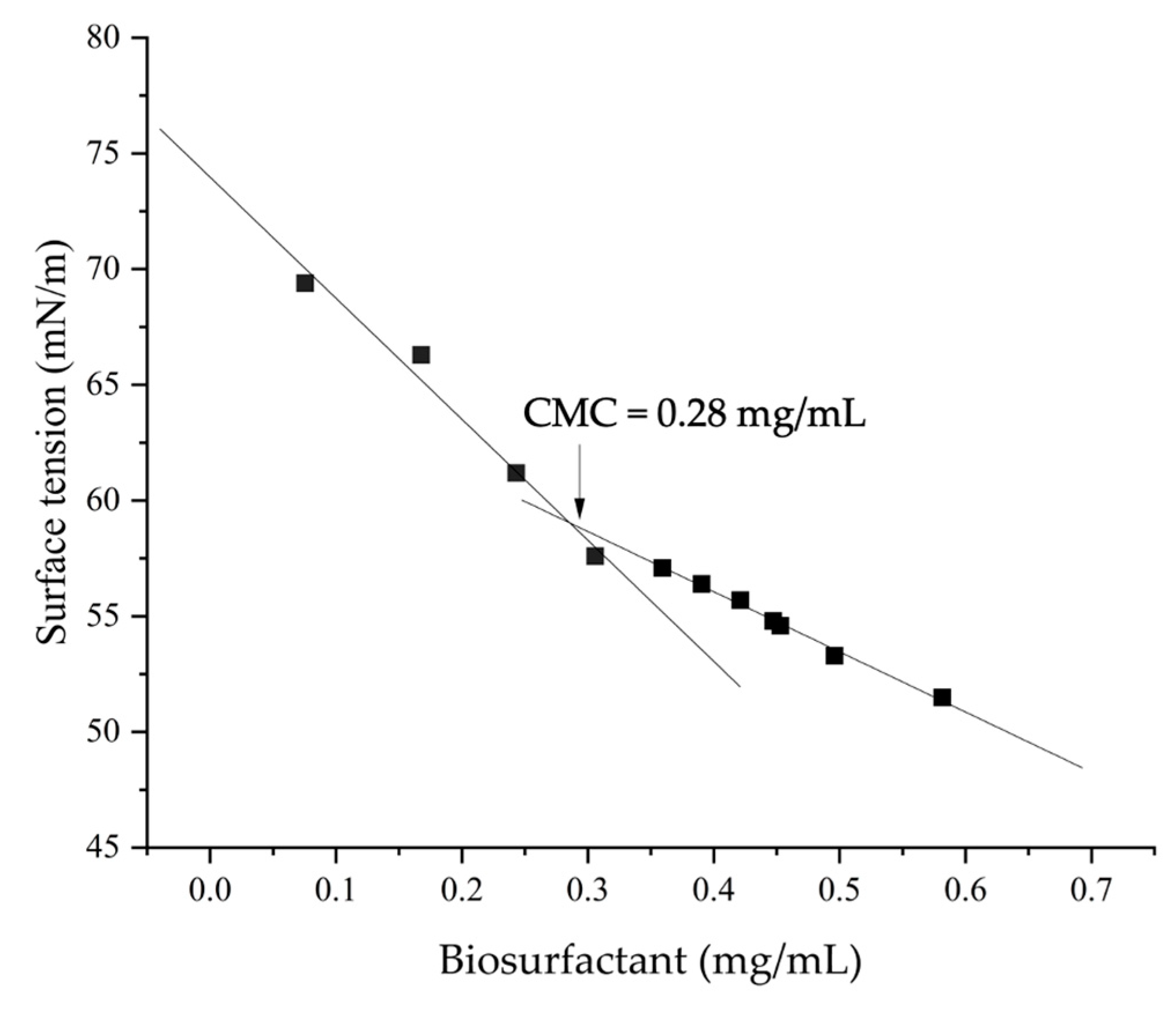
2.3. Determination of CMC
2.4. Emulsification Index
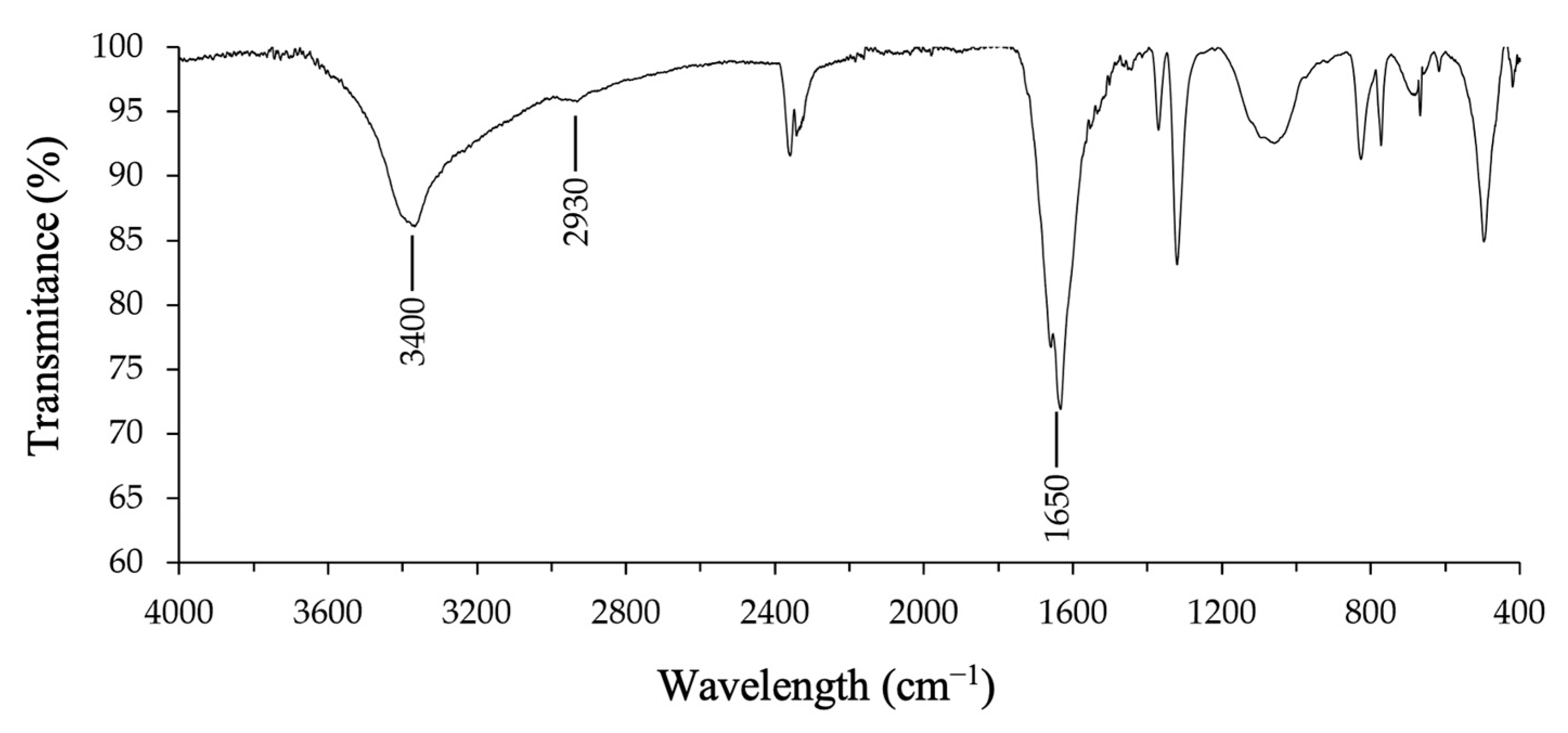
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
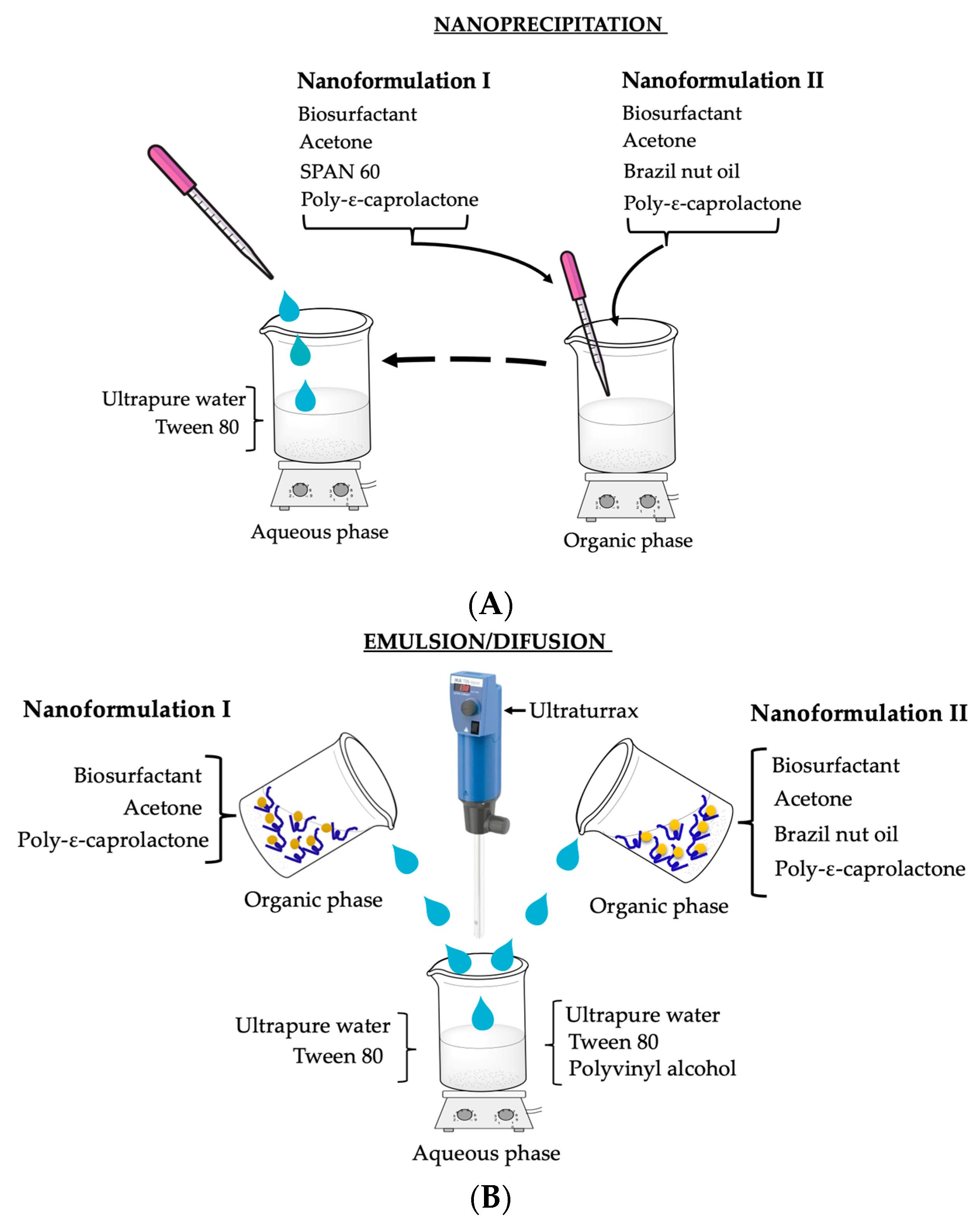
2.6. Synthesis of Polymeric Nanoparticles
2.6.1. Nanoprecipitation Method—Nanoformulation I (NPPBI) and II (NPPBII)
2.6.2. Emulsion/Diffusion—Nanoformulation I (NPEBI) and II (NPEBII)
2.7. Characterization of Polymer Nanoparticles
2.7.1. Particle Size Measurement by Dynamic Light Scattering (DLS) and Zeta Potential (ζ)
2.7.2. Scanning Electron Microscopy (SEM)
2.7.3. Transmission Electron Microscopy (TEM)
2.8. Statistical Analysis
3. Results
3.1. Production and Characterization of the Fungal Biosurfactant
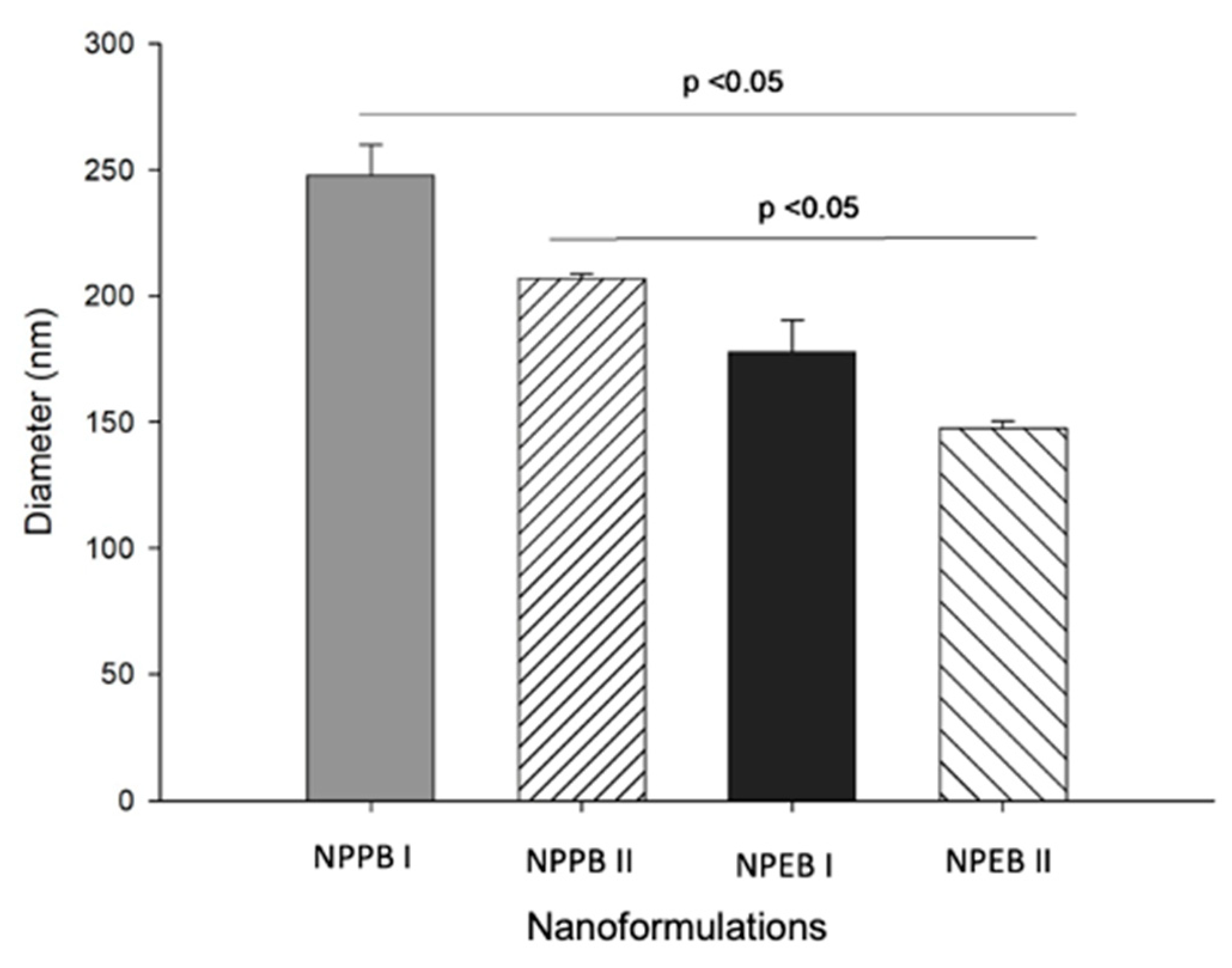
3.2. Production and Characterization of the Nanoformulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altammar, K.A.A. Review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, S.G.; Rocha, A.L.F.; de Aguiar Nunes, R.Z.; da Costa Pinto, C.; Ţălu, Ş.; da Fonseca Filho, H.D.; de Araújo Bezerra, J.; Lima, A.R.; Guimarães, F.E.G.; Campelo, P.H.; et al. Pulsatile controlled release and stability evaluation of polymeric particles containing Piper nigrum essential oil and preservatives. Materials 2022, 15, 5415. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V. Sustainable delivery systems through green nanotechnology. In Nano- and Microscale Drug Delivery Systems: Design and Fabrication; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–32. [Google Scholar] [CrossRef]
- Parupudi, A.; Mulagapati, S.H.R.; Subramony, J.A. Nanoparticle technologies: Recent state of the art and emerging opportunities. In Nanoparticle Therapeutics: Production Technologies, Types of Nanoparticles, and Regulatory Aspects; Kesharwani, P., Singh, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–46. [Google Scholar] [CrossRef]
- Jummes, B.; Sganzerla, W.G.; da Rosa, C.G.; Noronha, C.M.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antioxidant and antimicrobial poly-ε-caprolactone nanoparticles loaded with Cymbopogon martinii essential oil. Biocatal. Agric. Biotechnol. 2020, 23, 101499. [Google Scholar] [CrossRef]
- MacCraith, E.; O’Brien, F.J.; Davis, N.F. Biodegradable materials for surgical management of stress urinary incontinence: A narrative review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 153–160. [Google Scholar] [CrossRef]
- Mulinti, P.; Brooks, J.E.; Lervick, B.; Pullan, J.E.; Brooks, A.E. Strategies to improve the hemocompatibility of biodegradable biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions; Siedlecki, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 253–278. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.A.; Sudesh, K. Exploring Various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-ionic surfactants for stabilization of polymeric nanoparticles for biomedical uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. R. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K.; Verma, R.K.; Avasthi, S.; Sushma, B.Y.; Devadatha, B.; Niranjan, M.; Suwannarach, N. Current insight into traditional and modern methods in fungal diversity estimates. J. Fungi 2022, 8, 226. [Google Scholar] [CrossRef]
- Gurgel, R.S.; de Melo Pereira, D.Í.; Garcia, A.V.F.; Fernandes de Souza, A.T.; Mendes da Silva, T.; de Andrade, C.P.; Lima da Silva, W.; Nunez, C.V.; Fantin, C.; de Lima Procópio, R.E.; et al. Antimicrobial and antioxidant activities of endophytic fungi associated with Arrabidaea chica (Bignoniaceae). J. Fungi 2023, 9, 864. [Google Scholar] [CrossRef]
- Sanches, M.A.; Luzeiro, I.G.; Alves Cortez, A.C.; Simplício de Souza, É.; Albuquerque, P.M.; Chopra, H.K.; Braga de Souza, J.V. Production of biosurfactants by Ascomycetes. Int. J. Microbiol. 2021, 2021, 6669263. [Google Scholar] [CrossRef]
- Silva, A.C.S.; Santos, P.N.; Silva, T.A.L.; Andrade, R.F.S.; Campos-Takaki, G.M. Biosurfactant production by fungi as a sustainable alternative. Arq. Inst. Biol. 2018, 85, e0502017. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2018, 3, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.A.; Sitepu, I.R.; Cajka, T.; Xu, J.; Teh, H.E.; German, J.B.; Pan, Z.; Dungan, S.R.; Block, D.E.; Boundy-Mills, K.L. Extracellular fungal polyol lipids: A new class of potential high value lipids. Biotechnol. Adv. 2018, 36, 397–414. [Google Scholar] [CrossRef]
- Bae, I.; Lee, E.S.; Yoo, J.W.; Lee, S.H.; Ko, J.Y.; Kim, Y.J.; Lee, T.R.; Kim, D.; Lee, C.S. Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp. Dermatol. 2019, 28, 738–741. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Laroque, D.A.; Andrade, L.M.; Carciofi, B.A.M.; Tenório, J.A.S.; Andrade, C.J. Production of active cassava starch films; effect of adding a biosurfactant or synthetic surfactant. React. Funct. Polym. 2019, 144, 104368. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Effects of sophorolipids augmentation on the plant growth and phytoremediation of heavy metal contaminated soil. J. Clean. Prod. 2021, 280, 124406. [Google Scholar] [CrossRef]
- Chuo, S.C.; Ahmad, A.; Mohd-Setapar, S.H.; Mohamed, S.F.; Rafatullah, M. Utilization of green sophoro-lipids biosurfactant in reverse micelle extraction of antibiotics: Kinetic and mass transfer studies. J. Mol. Liq. 2019, 276, 225–232. [Google Scholar] [CrossRef]
- Silva, M.E.T.; Duvoisin, S., Jr.; Oliveira, R.L.; Banhos, E.F.; Souza, A.Q.L.; Albuquerque, P.M. Biosurfactant production of Piper hispidum endophytic fungi. J. Appl. Microbiol. 2021, 130, 561–569. [Google Scholar] [CrossRef]
- Nitschke, M.; Marangon, C.A. Microbial surfactants in nanotechnology: Recent trends and applications. Crit. Rev. Biotechnol. 2022, 42, 294–310. [Google Scholar] [CrossRef]
- De Melo Pereira, D.Í.; Gurgel, R.S.; de Souza, A.T.F.; Matias, R.R.; de Souza Falcão, L.; Chaves, F.C.M.; da Silva, G.F.; Martínez, J.G.; de Lima Procópio, R.E.; Fantin, C.; et al. Isolation and identification of pigment-producing endophytic fungi from the Amazonian Species Fridericia chica. J. Fungi 2024, 10, 77. [Google Scholar] [CrossRef]
- Castellani, A. The viability of some pathogenic fungi in sterile distilled water. J. Trop. Med. Hyg. 1939, 42, 225–226. [Google Scholar]
- Wen, G.; Cao, R.; Wan, Q.; Tan, L.; Xu, X.; Wang, J.; Huang, T. Development of fungal spore staining methods for flow cytometric quantification and their application in chlorine-based disinfection. Chemosphere 2020, 243, 125453. [Google Scholar] [CrossRef]
- Soares, A.R. Produção de Biossurfactantes Fúngicos e Aplicação No Desenvolvimento de Nanopartículas Poliméricas. Master Thesis, Amazonas State University, Manaus, Brazil, 24 June 2024. [Google Scholar]
- Monteiro, A.S.; Bonfim, M.R.Q.; Domingues, V.S.; Corrêa, A.; Siqueira, E.P.; Zani, C.L.; Santos, V.L. Identification and characterization of bioemulsifier-producing yeasts isolated from effluents of a dairy industry. Bioresour. Technol. 2010, 101, 5186–5193. [Google Scholar] [CrossRef]
- Pereira, D.D.F.; Duvoisin Junior, S.; Albuquerque, P.M. Study of biosurfactants production by Amazon fungi. J. Eng. Exact Sci. 2017, 3, 688–695. [Google Scholar]
- Makkar, R.S.; Cameotra, S.S. Biosurfactant production by a thermophilic Bacillus subtilis strain. J. Ind. Microbiol. Biotechnol. 1997, 18, 37–42. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Wongpanit, P.; Chavadej, S.; Abe, M.; Rujiravanit, R. Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum contaminated soil. Bioresource Technol. 2008, 99, 1589–1595. [Google Scholar] [CrossRef]
- Noronha, C.M.; Granada, A.F.; de Carvalho, S.M.; Lino, R.C.; Matheus, M.V.; Barreto, P.L.M. Optimization of α-tocopherol loaded nanocapsules by the nanoprecipitation method. Ind. Crops Prod. 2013, 50, 896–903. [Google Scholar] [CrossRef]
- Costa, Í.C.; Azevedo, S.G.; Sanches, E.A.; da Fonseca Filho, H.D. Characterization of polymeric nanoparticles filled with Piper nigrum essential oil by atomic force microscopy. Matéria 2021, 26, e12981. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Chittepu, O.R. Isolation and characterization of biosurfactant producing bacteria from groundnut oil cake dumping site for the control of foodborne pathogens. Grain Oil Sci. Technol. 2019, 2, 15–20. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Mnif, I.; Segovia, R.; Bouallegue, A.; Ghribi, D.; Rabanal, F. Identifcation of diferent lipopeptides isoforms produced by Bacillus mojavensis BI2 and evaluation of their surface activities for potential environmental application. J. Polym. Environ. 2023, 31, 2668–2685. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Al-Kaabi, W.J.; Al-Manhel, A.J.A.; Niamah, A.K.; Altemimi, A.B.; Al-Wafi, H.; Cacciola, F. Effects of β-glucan extracted from Saccharomyces cerevisiae on the quality of bio-yoghurts: In vitro and in vivo evaluation. J. Food Meas. Charact. 2022, 16, 3607–3617. [Google Scholar] [CrossRef]
- Abriata, J.P.; Turatti, R.C.; Luiz, M.T.; Raspantini, G.L.; Tofani, L.B.; do Amaral, R.L.F.; Swiech, K.; Marcato, P.D.; Marchetti, J.M. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater. Sci. Eng. C 2019, 96, 347–355. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater Horiz 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Khayata, N.; Abdelwahed, W.; Chehna, M.F.; Charcosset, C.; Fessi, H. Preparation of vitamin e loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Alshamsan, A. Nanoprecipitation is more efficient than emulsion solvent evaporation method to encapsulate cucurbitacin I in PLGA nanoparticles. Saudi Pharm. J. 2014, 22, 219–222. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Bouallegue, A.; Sabbah, M.; Di Pierro, P.; Salamatullah, A.M.; Bourhia, M.; Ellouz-Chaabouni, S. Properties of active levan-bitter vetch protein films for potential use in food packaging applications. ACS Omega 2023, 8, 42787–42796. [Google Scholar] [CrossRef]
- Elakkiya, V.T.; SureshKumar, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Govindarajan, M. Swift production of rhamnolipid biosurfactant, biopolymer and synthesis of biosurfactant-wrapped silver nanoparticles and its enhanced oil recovery. Saudi J. Biol. Sci. 2020, 27, 1892–1899. [Google Scholar] [CrossRef]
- Vecino, X.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M.; Moldes, A.B. Chapter Six—Nanomaterials synthesized by biosurfactants. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–301. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Synthesis of biosurfactant stabilized silver nanoparticles, characterization and their potential application for bactericidal purposes. J. Hazard. Mater. 2020, 393, 122319. [Google Scholar] [CrossRef]
- Femina, C.C.; Kamalesh, T. Advances in stabilization of metallic nanoparticle with biosurfactants- a review on current trends. Heliyon 2024, 10, e29773. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Perez-Ameneiro, M.; Vecino, X.; Pastoriza-Santos, I.; Perez-Juste, J.; Cruz, J.M.; Moldes, A.B. Biogenic synthesis of metal nanoparticles using a biosurfactant extracted from corn and their antimicrobial properties. Nanomaterials 2017, 7, 139. [Google Scholar] [CrossRef]
- Belletti, D.; Grabrucker, A.M.; Pederzoli, F.; Menrath, I.; Cappello, V.; Vandelli, M.A.; Forni, F.; Tosi, G.; Ruozi, B. Exploiting the versatility of cholesterol in nanoparticles formulation. Int. J. Pharm. 2016, 511, 331–340. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Musumeci, T.; Carbone, C.; Vicari, L.; Lauro, M.R.; Puglisi, G. Revisiting the role of sucrose in PLGA-PEG nanocarrier for potential intranasal delivery. Pharm. Dev. Technol. 2018, 23, 265–274. [Google Scholar] [CrossRef]
- Khalid, H.F.; Tehseen, B.; Sarwar, Y.; Hussain, S.Z.; Khan, W.S.; Raza, Z.A.; Bajwa, S.Z.; Kanaras, A.G.; Hussain, I.; Rehman, A. biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Abamor, E.S.; Tosyali, O.A.; Bagirova, M.; Allahverdiyev, A. Nigella sativa oil entrapped polycaprolactone nanoparticles for leishmaniasis treatment. IET Nanobiotechnol. 2018, 12, 1018–1026. [Google Scholar] [CrossRef]
- Morsy, S.M.I. Role of surfactants in nanotechnology and their applications. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 237–260. [Google Scholar]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.M.; Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Pérez, M.T.M.; Manzato, L. Biotechnological applications of nanoencapsulated essential oils: A review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Z. Naturforsch. C J. Biosci. 2020, 75, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Karam, T.K.; Ortega, S.; Ueda Nakamura, T.; Auzély-Velty, R.; Nakamura, C.V. Development of chitosan nanocapsules containing essential oil of Matricaria chamomilla L. for the treatment of cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef]
- Bezza, F.A.; Nkhalambayausi Chirwa, E.M. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere 2016, 144, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M.G. Use of nanotechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
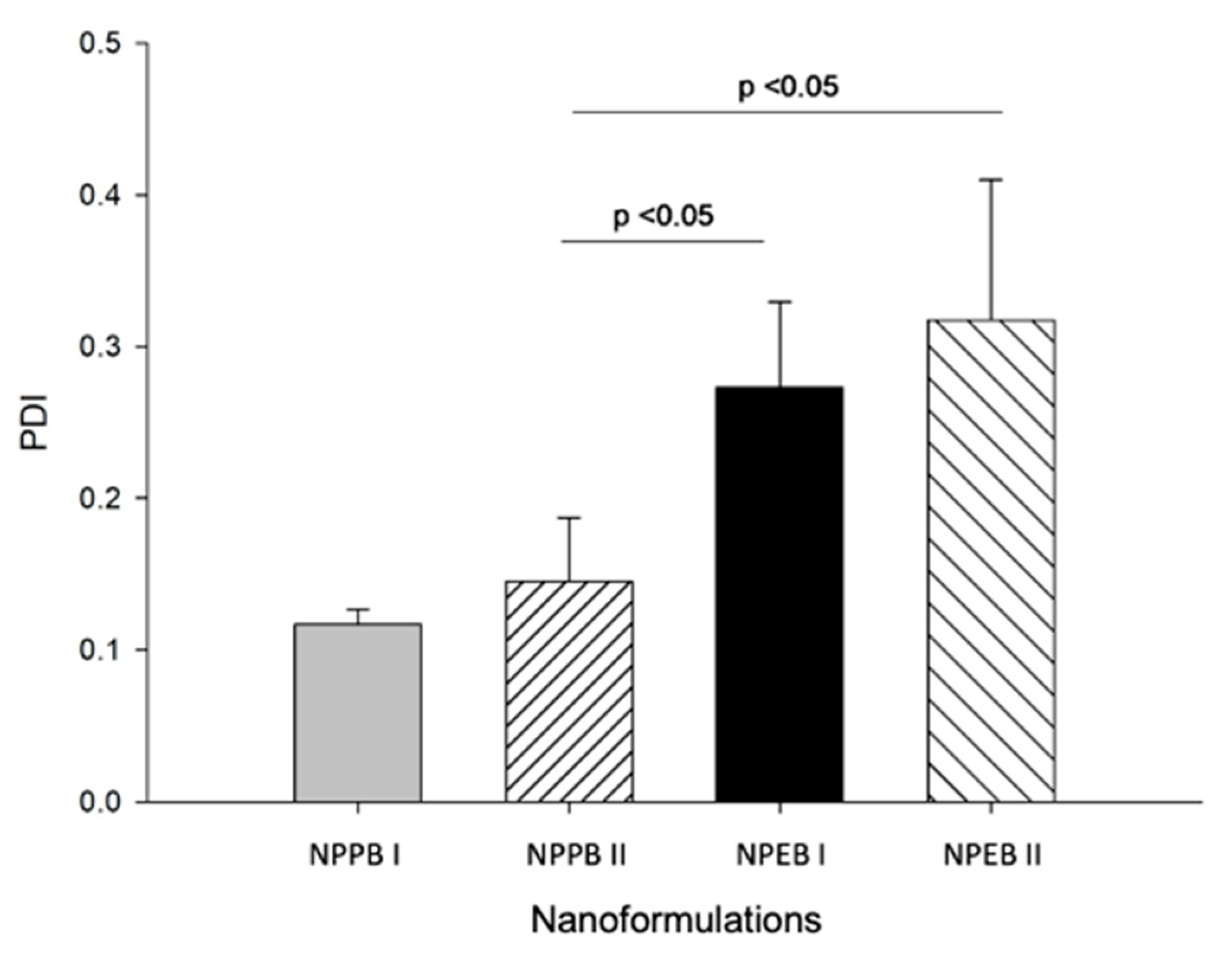


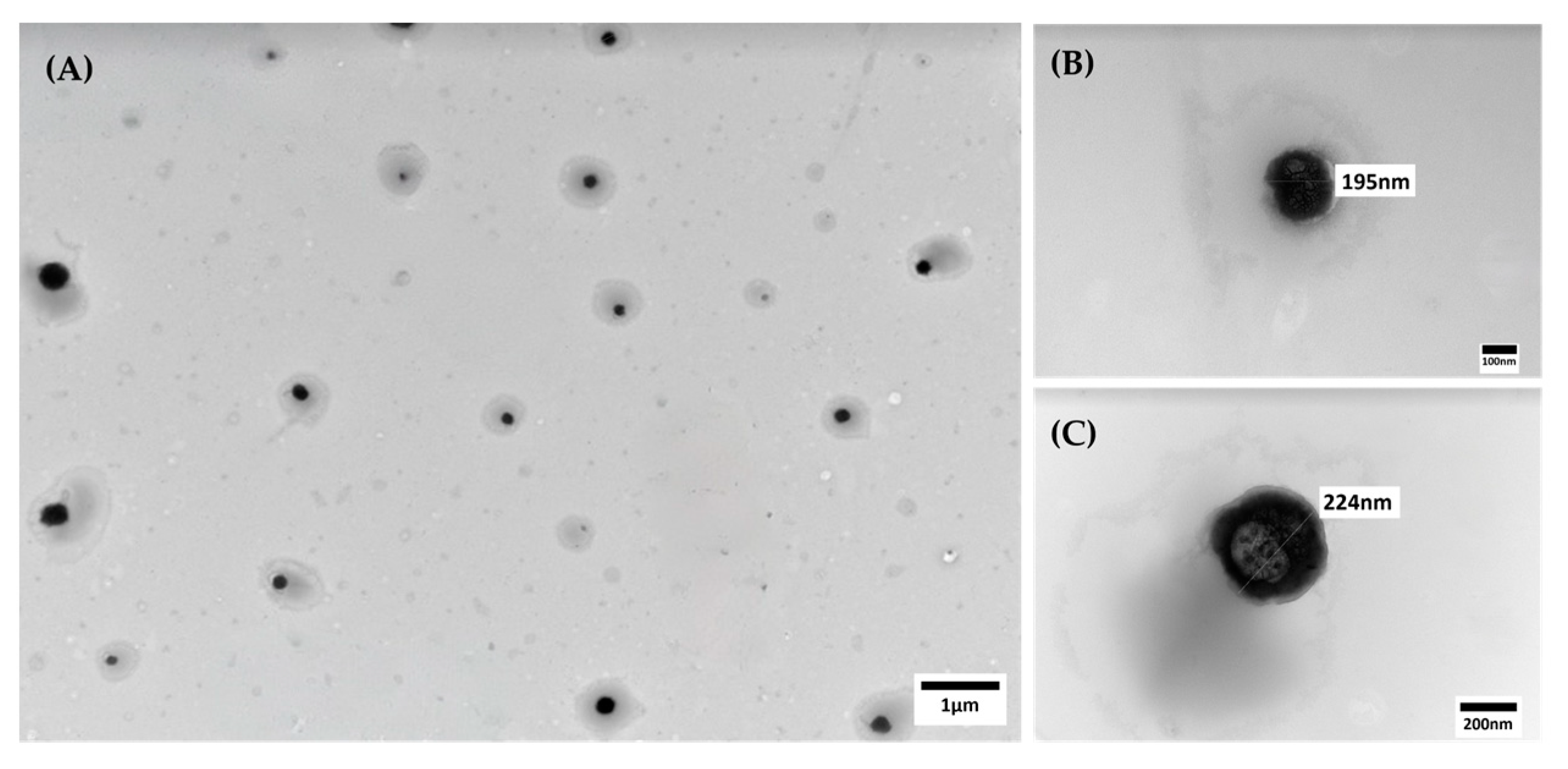
| Nanoformulation | Average Particle Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| NPPI | 237.6 ± 9.3 | 0.12 ± 0.07 | −48.50 ± 3.42 |
| NPPII | - | - | - |
| NPEI | - | - | - |
| NPEII | - | - | - |
| NPPBI | 247.8 ± 12.2 a | 0.11 ± 0.08 a,b | −45.00 ± 1.13 a |
| NPPBII | 206.9 ± 1.9 a | 0.14 ± 0.04 a | −29.10 ± 8.67 b |
| NPEBI | 177.8 ± 12.8 a,b | 0.27 ± 0.06 b | −43.39 ± 7.40 a |
| NPEBII | 147.6 ± 2.7 b | 0.32 ± 0.09 b | −21.90 ± 2.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, A.R.; Andrade, J.C.d.; Lacerda, C.D.; Azevedo, S.G.; Pérez, M.T.M.; Manzato, L.; Duvoisin Junior, S.; Albuquerque, P.M. Synthesis of Polymeric Nanoparticles Using Fungal Biosurfactant as Stabilizer. Processes 2024, 12, 2739. https://doi.org/10.3390/pr12122739
Soares AR, Andrade JCd, Lacerda CD, Azevedo SG, Pérez MTM, Manzato L, Duvoisin Junior S, Albuquerque PM. Synthesis of Polymeric Nanoparticles Using Fungal Biosurfactant as Stabilizer. Processes. 2024; 12(12):2739. https://doi.org/10.3390/pr12122739
Chicago/Turabian StyleSoares, Angélica Ribeiro, Juliano Camurça de Andrade, Caroline Dutra Lacerda, Sidney Gomes Azevedo, Maria Tereza Martins Pérez, Lizandro Manzato, Sergio Duvoisin Junior, and Patrícia Melchionna Albuquerque. 2024. "Synthesis of Polymeric Nanoparticles Using Fungal Biosurfactant as Stabilizer" Processes 12, no. 12: 2739. https://doi.org/10.3390/pr12122739
APA StyleSoares, A. R., Andrade, J. C. d., Lacerda, C. D., Azevedo, S. G., Pérez, M. T. M., Manzato, L., Duvoisin Junior, S., & Albuquerque, P. M. (2024). Synthesis of Polymeric Nanoparticles Using Fungal Biosurfactant as Stabilizer. Processes, 12(12), 2739. https://doi.org/10.3390/pr12122739








