Simultaneous Recovery of Vivianite and Humic Acids from Waste Activated Sludge via Ferric Trichloride Flocculation and Enzymatic Hydrolysis Co-Treatment
Abstract
1. Introduction
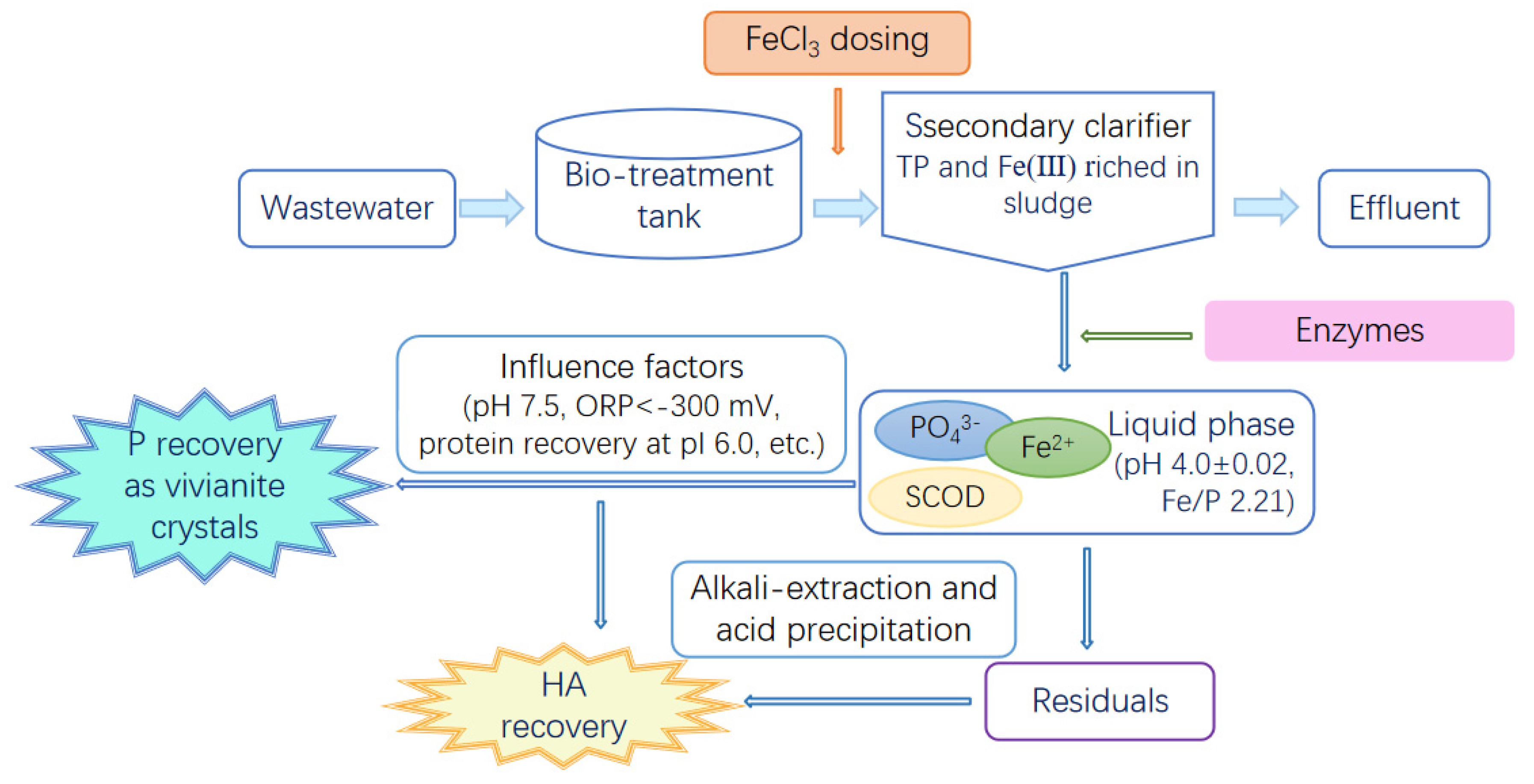
2. Materials and Methods
2.1. Reagents and Waste Activated Sludge Properties
2.2. Experimental Design and Conditions
2.3. Analysis and Statistical Methods
3. Results and Discussion
3.1. Effects of Coagulation
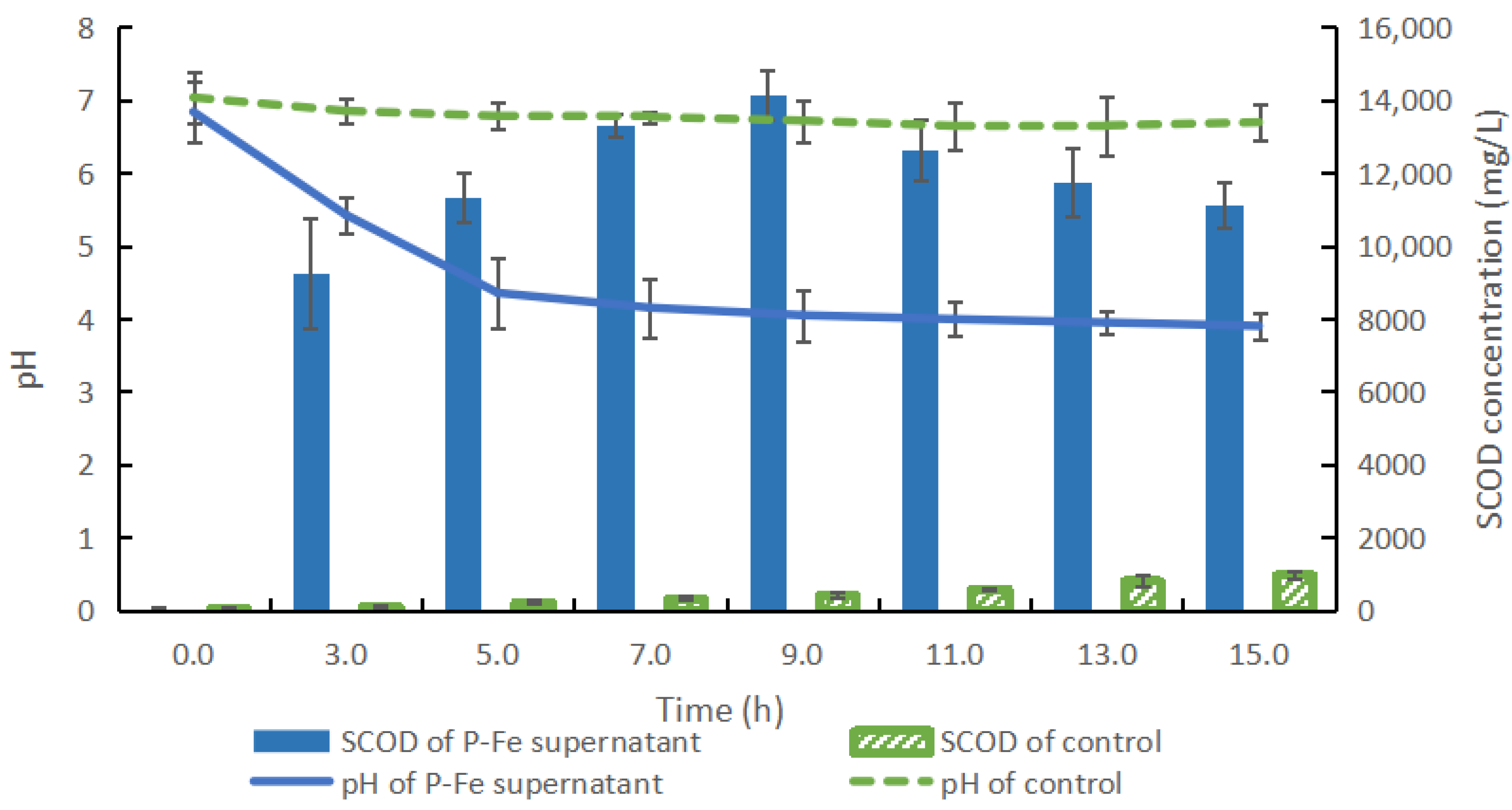
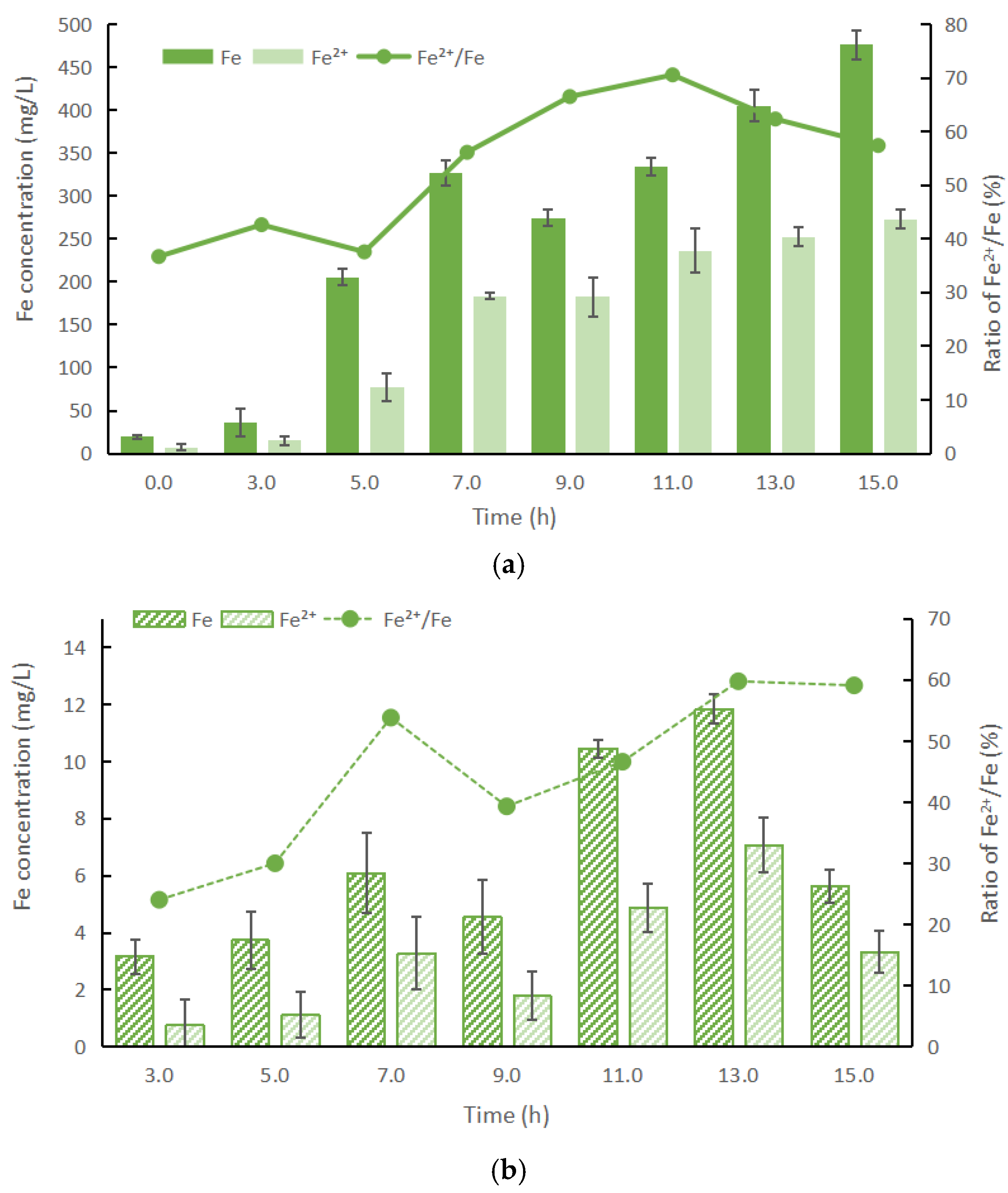
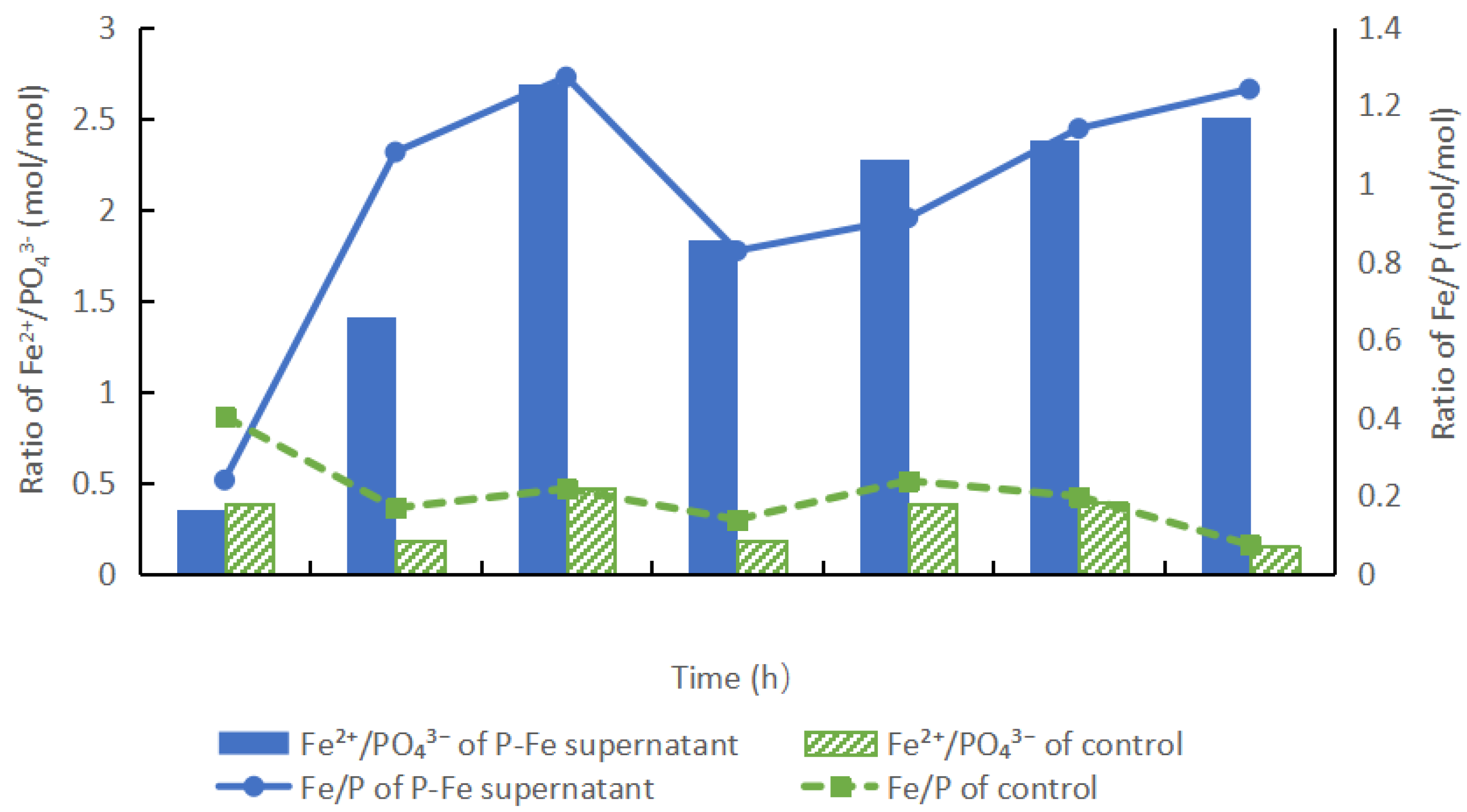
3.2. Effects of the Enzymatic Hydrolysis of P-Fe Sludge
3.2.1. PH and the Soluble Chemical Oxygen Demand Variations During the Hydrolysis Processes
3.2.2. P and Fe Concentration Changes During the Hydrolysis Processes
3.3. Vivianite Crystallization
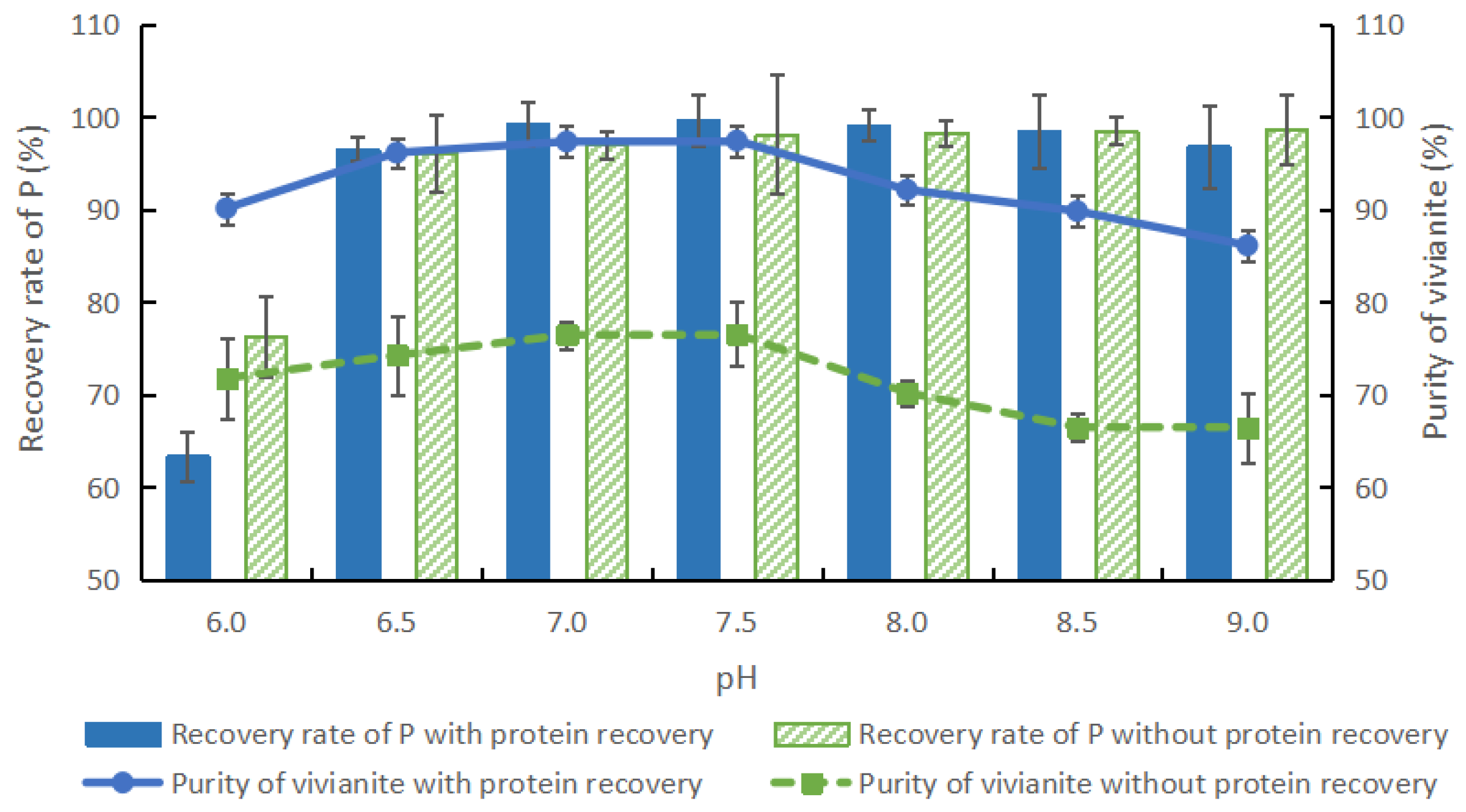
3.3.1. Effect of pH Adjustment and Protein Recovery
3.3.2. Effect of Reaction Temperatures and Time
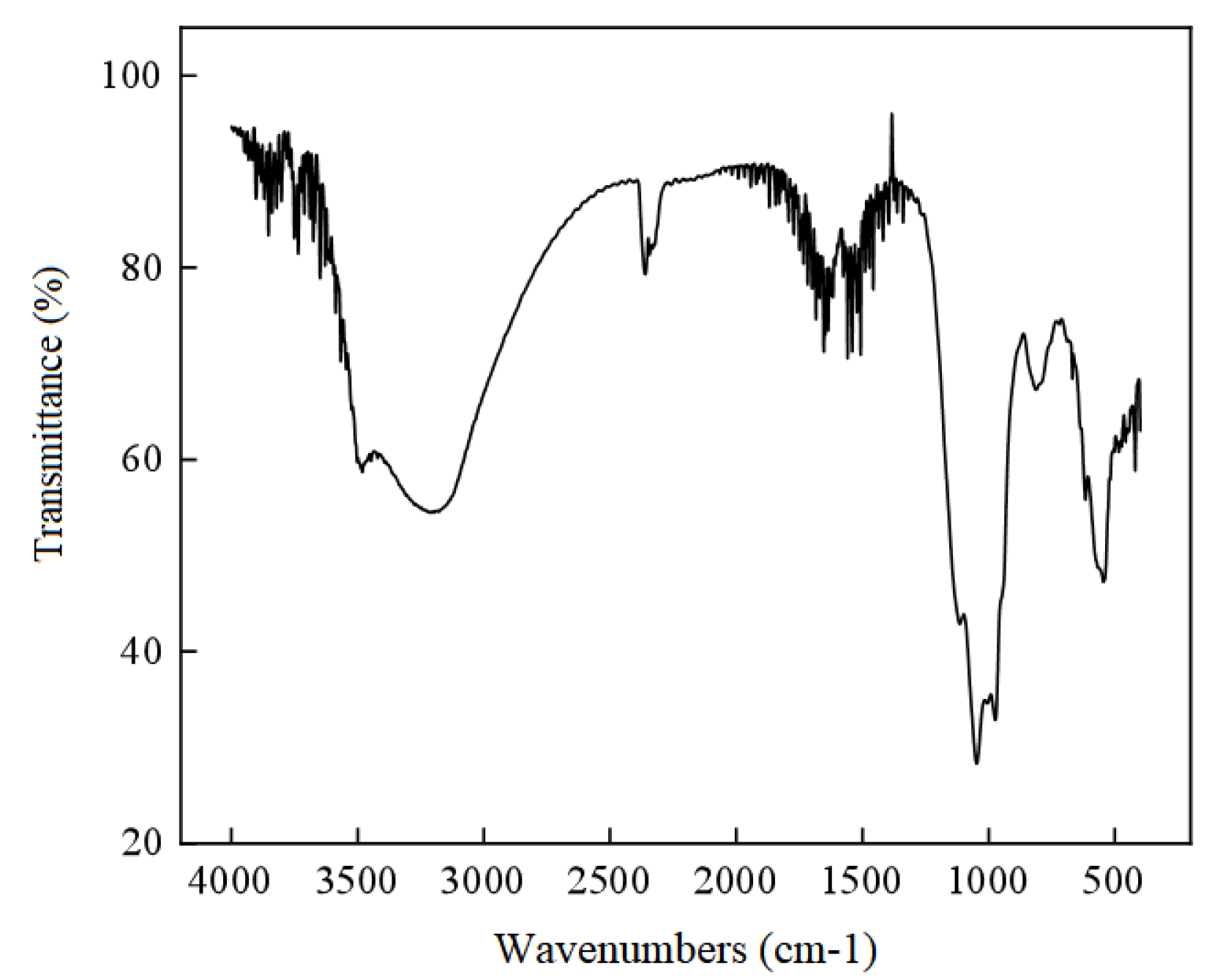
3.3.3. Spectroscopic Characterization of Vivianite
3.4. Humic Acids Characterization and Metal Sorption Affinity
3.4.1. Kinetic Characteristics of Ion Adsorption on Humic Acids
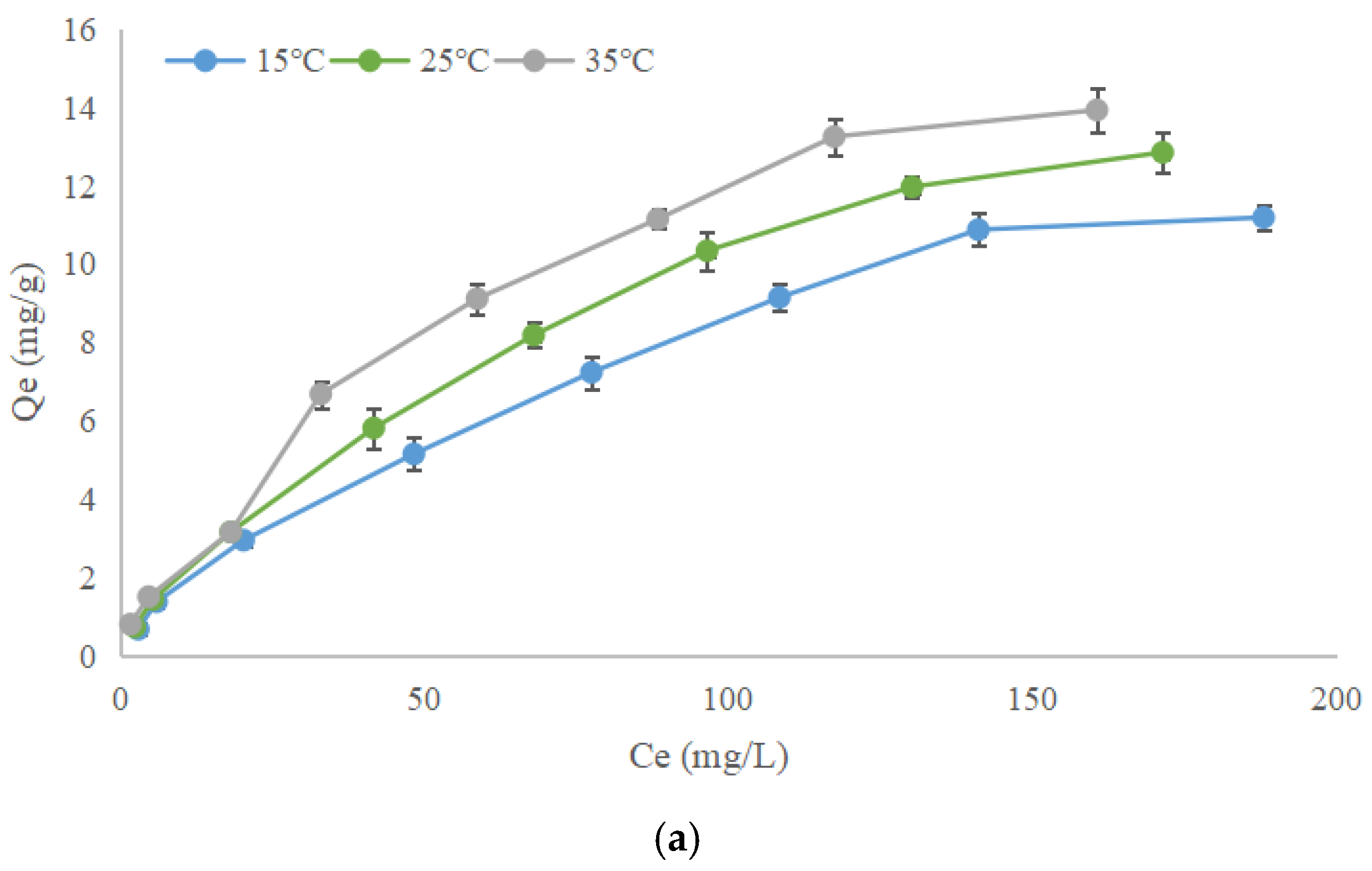
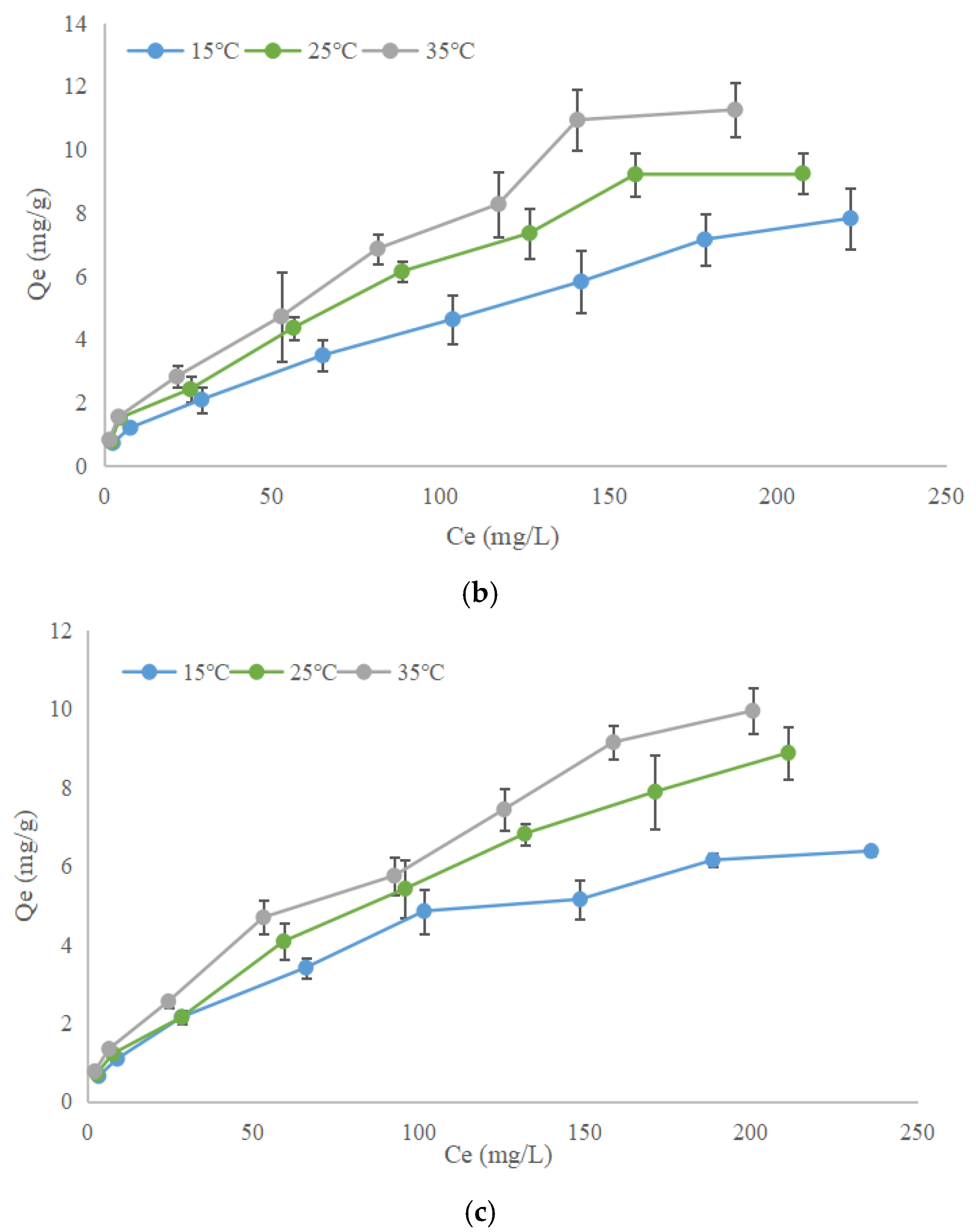
3.4.2. Adsorption Isotherm of Ions on Humic Acids
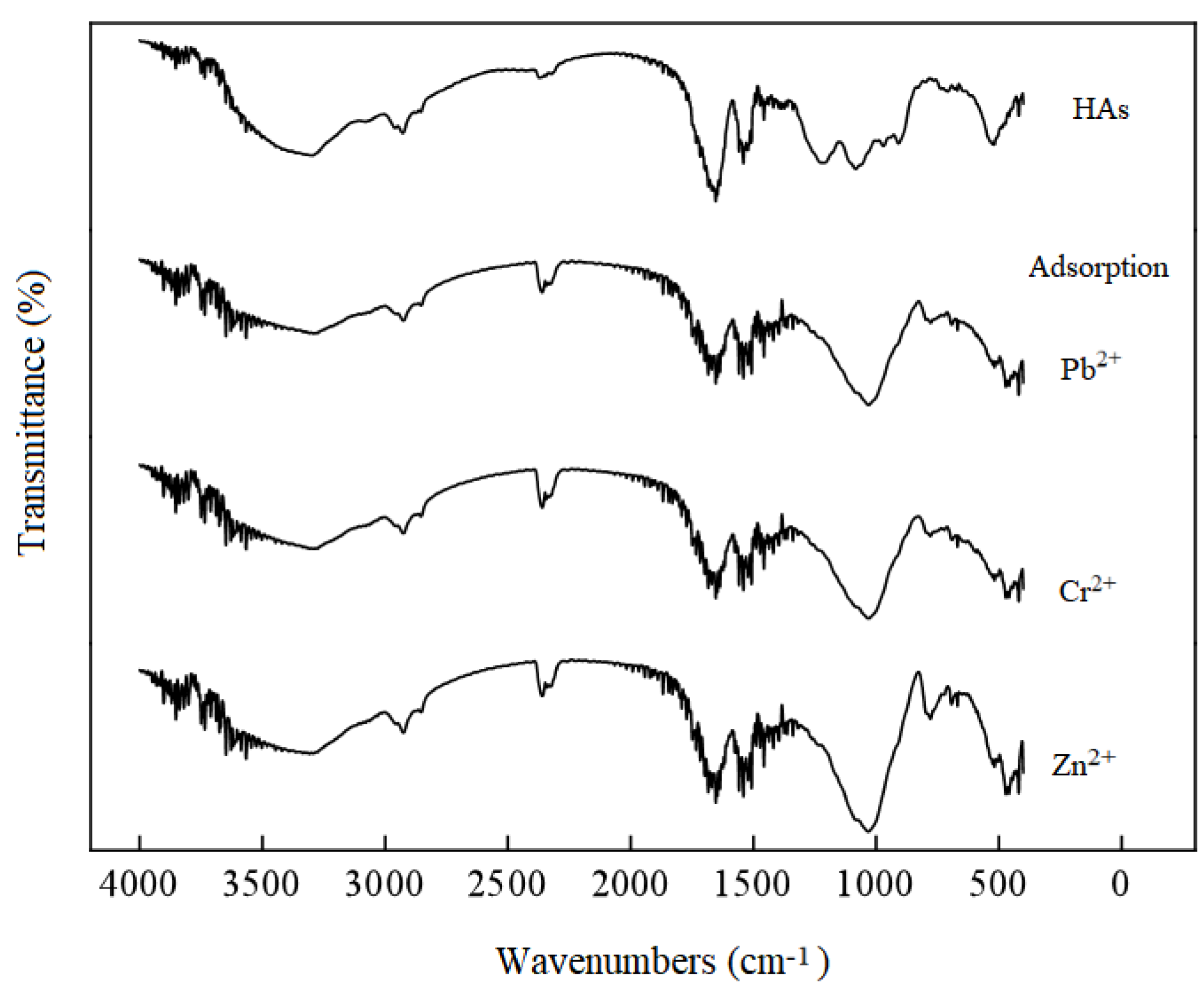
3.4.3. Characterization of Humic Acids Before and After Sorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, H.; Zhao, Y.; Qi, M.; Wang, L.; Feng, J. Phosphate recovery from wastewater via vivianite crystallization using separable ferrous modified biochar beads. Chem. Eng. J. 2024, 498, 155453. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Xu, R.; Luo, J.; Ni, B.; Fang, F. Phosphorus recovery from synthetic anaerobic fermentation supernatant via vivianite crystallization: Coupling effects of various physicochemical process parameters. Sci. Total Environ. 2023, 897, 165416. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, X.; Gao, X.; Sun, D.; Cheng, X. Compatibility of vivianite-crystallization pathway of phosphorus recovery with anaerobic digestion systems of thermally hydrolyzed sludge. Environ. Res. 2024, 260, 119640. [Google Scholar] [CrossRef] [PubMed]
- Michalska, J.; Turek-Szytow, J.; Dudło, A.; Kowalska, K.; Surmacz-Górska, J. Evaluation of the applicability of selected analytical techniques for determining the characteristics of humic substances sourced from by-products of the wastewater treatment process. Sci. Total Environ. 2023, 888, 164237. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Prot, T.; Wijdeveld, W.; Korving, L.; Dugulan, A.I.; Brück, E.; Haarala, A.; Van Loosdrecht, M.C.M. Robust magnetic vivianite recovery from digested sewage sludge: Evaluating resilience to sludge dry matter and particle size variations. Water Res. 2024, 266, 122407. [Google Scholar] [CrossRef]
- Liang, D.; Chang, J.; Wu, Y.; Wang, S.; Wang, X.; Ren, N.; Li, N. The screening of iron oxides for long-term transformation into vivianite to recover phosphorus from sewage. Water Res. 2024, 265, 122250. [Google Scholar] [CrossRef]
- Becarelli, S.; Chicca, I.; Siracusa, G.; China, S.L.; Gentini, A.; Lorenzi, R.; Munz, G.; Petroni, G.; Levin, D.B.; Gregorio, S.D. Hydrocarbonoclastic Ascomycetes to enhance co-composting of total petroleum hydrocarbon (TPH) contaminated dredged sediments and lignocellulosic matrices. New Biotechnol. 2019, 50, 27–36. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, D.; Niu, B.; Zhang, L.; Wang, X.; Zhang, S. Optimization Effects of Enzymatic Hydrolysis Pretreatment Combined with Bioaugmentation on the Aerobic Compost of Sewage Sludge. Ecol. Environ. 2021, 30, 2395–2401. (In Chinese) [Google Scholar]
- Xin, X.; He, J.; Li, L.; Qiu, W. Enzymes catalyzing pre-hydrolysis facilitated the anaerobic fermentation of waste activated sludge with acidogenic and microbiological perspectives. Bioresour. Technol. 2018, 250, 69–78. [Google Scholar] [CrossRef]
- He, J.G.; Xin, X.D.; Qiu, W.; Zhang, J.; Wen, Z.; Tang, J. Performance of the lysozyme for promoting the waste activated sludge biodegradability. Bioresour. Technol. 2014, 170, 108–114. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Li, X.; Tang, Y.; Luo, Z.; Liu, J. Enhanced Hydrolysis of Excess Sludge by External Enzymes. Environ. Sci. 2010, 31, 763–767. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Li, L.; Zhen, G.; Lu, X.; Zhang, J.; Liu, H.; Zhou, Z.; Wu, Z.; Zhang, X. Prospects for humic acids treatment and recovery in wastewater: A review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, B.; Chen, Z. Simultaneous recovery of vivianite and produce short-chain fatty acids from waste activated sludge using potassium ferrate as pre-oxidation treatment. Environ. Res. 2022, 208, 112661. [Google Scholar] [CrossRef] [PubMed]
- Amin, L.; Al-Juboori, R.A.; Lindroos, F.; Bounouba, M.; Blomberg, K.; Viveros, M.L.; Graan, M.; Azimi, S.; Lindén, J.; Mikola, A.; et al. Tracking the formation potential of vivianite within the treatment train of full-scale wastewater treatment plants. Sci. Total Environ. 2024, 912, 169520. [Google Scholar] [CrossRef] [PubMed]
- Eshun, L.E.; García-López, A.M.; Recena, R.; Coker, V.; Shaw, S.; Lloyd, J.; Delgado, A. Assessing microbially mediated vivianite as a novel phosphorus and iron fertilizer. Chem. Biol. Technol. Agric. 2024, 11, 47. [Google Scholar] [CrossRef]
- Metz, P.; Kumar, N.; Schenkeveld, W.D.C.; Obst, M.; Voegelin, A.; Mangold, S.; Kraemer, S.M. Effect of oxidation on vivianite dissolution rates and mechanism. Environ. Sci. Technol. 2024, 58, 15321–15332. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhang, Q.; Aleem, M.; Fang, F.; Xue, Z.; Cao, J. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review. Chemosphere 2019, 226, 246–258. [Google Scholar] [CrossRef]
- Saoudi, M.A.; Dabert, P.; Ponthieux, A.; Vedrenne, F.; Daumer, M. Correlation between phosphorus removal technologies and phosphorus speciation in sewage sludge: Focus on iron-based P removal technologies. Environ. Technol. 2022, 44, 2091–2103. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, J.; Loosdrecht, M.C.M. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Eshun, L.E.; Coker, V.S.; Shaw, S.; Lloyd, J.R. Strategies for optimizing biovivianite production using dissimilatory Fe(III)-reducing bacteria. Environ. Res. 2024, 242, 117667. [Google Scholar] [CrossRef]
- Knyazev, Y.V.; Mikhail, S.M.S.; Ikkert, O.P.; Semenov, S.V.; Bayukov, O.Q.; Nikolenko, A.D.; Nazmov, V.P.; Volochaev, M.N.; Dubrovskiy, A.A.; Molokeev, M.S.; et al. Microbially mediated synthesis of vivianite by Desulfosporosinus on the way to phosphorus recovery. Environ. Sci. Adv. 2024, 3, 897–911. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Gao, B.; Sillanpää, M. Phosphate substances transformation and vivianite formation in P-Fe containing sludge during the transition process of aerobic and anaerobic conditions. Bioresour. Technol. 2021, 319, 124259. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Phosphorus Recovery from Sewage or Sludge by Vivianite Crystallization; Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences: Yantai, China, 2021. (In Chinese) [Google Scholar]
- Wu, Y.; Cao, J.; Zhang, T.; Zhao, T.; Xu, R.; Zhang, Q.; Fang, F.; Luo, J. A novel approach of synchronously recovering phosphorus as vivianite and volatile fatty acids during waste activated sludge and food waste co-fermentation: Performance and mechanisms. Bioresour. Technol. 2020, 305, 123078. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhou, J.; Wang, C.; Loosdrect, M. New product of phosphorus recovery-Vivianite. Acta Sci. Circumstantiae 2018, 38, 4223–4234. (In Chinese) [Google Scholar] [CrossRef]
- Cao, J.; Wu, Y.; Zhao, J.; Jin, S.; Aleem, M.; Zhang, Q.; Fang, F.; Xue, Z.; Luo, J. Phosphorus recovery as vivianite from waste activated sludge via optimizing iron source and pH value during anaerobic fermentation. Bioresour. Technol. 2019, 293, 122088. [Google Scholar] [CrossRef]
- Wilfert, P.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C.M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef]
- Beckett, R.; Jue, Z.; Giddings, J.C. Determination of molecular weight distributions of fulvic and humic acids using flow field-flow fractionation. Environ. Sci. Technol. 1987, 21, 289–295. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Zhang, J.; Hou, Y.; Song, Y.; Lu, C.; Li, H.; Guo, J. Deciphering effects of humic acid in landfill leachate on the simultaneous nitrification, anammox and denitrification (SNAD) system from performance, electron transfer and microbial community. Sci. Total Environ. 2022, 809, 151178. [Google Scholar] [CrossRef]
- Niu, H.; Yang, H.; Tong, L. Adsorption behaviors of Au(Ⅲ) onto humic acid extracted from gold ore: Adsorptive kinetics, isotherm and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127442. [Google Scholar] [CrossRef]
- Pedroza, R.H.P.; David, C.; Lodeiro, P.; Rey-Castro, C. Interactions of humic acid with pristine poly (lactic acid) microplastics in aqueous solution. Sci. Total Environ. 2024, 908, 168366. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef]
- Li, A.; Pi, S.; Wei, W.; Chen, T.; Yang, J.; Ma, F. Adsorption behavior of tetracycline by extracellular polymeric substrates extracted from Klebsiella sp. J. Environ. Sci. Pollut. Res. Int. 2016, 23, 25084–25092. [Google Scholar] [CrossRef] [PubMed]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. State Environmental Protection Administration: Beijing, China, 2003.
- Swift, R.S. Humic Substances in Soil, Sediment, and Water; Aiken, G.R., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; John Wiley: New York, NY, USA, 1985; pp. 387–408. [Google Scholar]
- Azizian, S.; Eris, S. Chapter 6—Adsorption isotherms and kinetics. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 33, pp. 445–509. [Google Scholar] [CrossRef]
- GB 11893-89; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. State Environmental Protection Administration: Beijing, China, 1990.
- HJ 535-2009; Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry. Ministry of Ecology and Environment: Beijing, China, 2010.
- HJ/T 345-2007; Water Quality-Determination of Iron-Phenanthroline Spectrophotometry. State Environmental Protection Administration: Beijing, China, 2007.
- HJ/T 399-2007; Water Quality-Determination of the Chemical Oxygen Demand-Fast Digestion-Spectrophotometric Method. State Environmental Protection Administration: Beijing, China, 2008.
- Zhang, Y.; Yang, X.; Zhang, X.; Sun, D.; Liu, X.; Lan, R.; Zheng, M.; Loosdrecht, M.C.M.; Cheng, X. Proteins in hydrothermal carbonization liquor of sewage sludge interfere with vivianite crystallization for phosphorus recovery. Resour. Conserv. Recycl. 2024, 208, 107731. [Google Scholar] [CrossRef]
- Chang, J.; Liang, D.; Gao, Y.; Sun, Y.; Wang, X.; Ren, N.; Li, N. Nano-magnetite enhances dissimilated iron reduction to vivianite from sewage by structuring an enormous and compact electron transfer network. Water Res. 2025, 268, 122583. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, J.; Pan, Y. Vivianite crystallization method to recover phosphorus in municipal sewage enriched by biofilm method. Environ. Chem. 2022, 41, 1787–1795. (In Chinese) [Google Scholar]
- Cao, X.; Zhao, Z. Analysis of influencing factor on the component of extracellular polymeric substance(EPS). Environ. Sci. Technol. 2010, 33, 420–424. [Google Scholar]
- Yu, Y.; Lei, Z.; Yuan, T.; Jiang, Y.; Chen, N.; Feng, C.; Shimizu, K.; Zhang, Z. Simultaneous phosphorus and nitrogen recovery from anaerobically digested sludge using a hybrid system coupling hydrothermal pretreatment with MAP precipitation. Bioresour. Technol. 2017, 243, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Capitelli, F.; Chita, G.; Rosaria Ghiara, R.; Rossi, M. Crystal-chemical investigation of Fe3(PO4)2·8H2O vivianite minerals. Z. Für Krist.-Cryst. Mater. [CrossRef]
- Zhang, M.; Wang, C. Effects of Fe-Mn-modified biochar addition on anaerobic digestion of sewage sludge: Biomethane production, heavy metal speciation and performance stability. Bioresour. Technol. 2020, 313, 123695. [Google Scholar] [CrossRef]
- Fazeli, S.M.; Forghani, A.; Boguta, P.; Anoosha, M.; Owens, G. Optimal isotherm model and explanatory characteristics associated with metal ion adsorption on humic acids isolated from forest soils. J. Soils Sediments 2022, 22, 2392–2405. [Google Scholar] [CrossRef]
- Khalili, F.; Al-Banna, G. Adsorption of uranium(VI) and thorium(IV) by insolubilized humic acid from Ajloun soil-Jordan. J. Environ. Radioact. 2015, 146, 16–26. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of adsorption site energies and heterogeneous surfaces of porous materials. J. Mater. Chem. A 2019, 7, 10104–10137. [Google Scholar] [CrossRef]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction echanisms with copper ions at various pHs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of Organic Pollutants by Humic Acids: A Review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef]




| Raw Supernatant | Flocculated Supernatant | Removal Rate (%) | Standards * | |
|---|---|---|---|---|
| pH | 7.03 ± 0.07 | 6.84 ± 0.08 | - | 6–9 |
| SCOD (mg/L) | 95.5 ± 9.42 | 48.5 ± 5.89 | 49.21 ± 0.84 | 50 |
| SS (mg/L) | 18.56 ± 2.46 | 9.53 ± 1.51 | 48.70 ± 0.25 | 10 |
| TN (mg/L) | 22 ± 1.43 | 6.41 ± 1.31 | 70.86 ± 0.17 | 15 |
| TP (mg/L) | 6.54 ± 0.89 | 0.47 ± 0.19 | 92.81 ± 0.04 | 0.5 |
| PO43− (mg/L) | 5.41 ± 1.05 | 0.41 ± 0.32 | 92.42 ± 0.02 | - |
| NH4+-N (mg/L) | 4.62 ± 1.92 | 1.43 ± 0.26 | 69.01 ± 0.10 | 5 |
| Fe (mg/L) | - | 19.24 ± 3.60 | - | - |
| Fe2+ (mg/L) | - | 7.05 ± 1.31 | - | - |
| Temperature (°C) | Recovery Rate (%) | Crystal Purity (%) | Time (h) | P Recovery Rate (%) | Crystal Purity (%) |
|---|---|---|---|---|---|
| 15 | 95.16 ± 0.54 | 88.65 ± 0.66 | 0 | 95.60 ± 0.44 | 91.92 ± 0.62 |
| 20 | 96.16 ± 0.71 | 91.41 ± 0.85 | 1 | 95.99 ± 1.01 | 91.60 ± 0.47 |
| 25 | 96.85 ± 0.33 | 91.63 ± 0.54 | 2 | 96.18 ± 0.93 | 92.85 ± 0.41 |
| 30 | 96.60 ± 0.21 | 91.01 ± 0.71 | 3 | 96.13 ± 0.88 | 92.44 ± 0.80 |
| 35 | 96.10 ± 0.48 | 90.27 ± 0.52 | 4 | 95.99 ± 0.47 | 92.54 ± 1.17 |
| 40 | 95.20 ± 0.55 | 90.14 ± 0.63 | 8 | 96.28 ± 0.80 | 92.22 ± 0.94 |
| Pb2+ | Cr2+ | Zn2+ | ||
|---|---|---|---|---|
| Pseudo-first order | k1 (h−1) | −0.0255 ± 0.00 | −0.0237 ± 0.00 | −0.0172 ± 0.01 |
| b1 | 1.2759 ± 0.01 | 1.3138 ± 0.02 | 1.2804 ± 0.01 | |
| R2 | 0.8698 ± 0.01 | 0.9647 ± 0.02 | 0.9529 ± 0.03 | |
| Pseudo-second order | k2 (h−1) | 0.1424 ± 0.02 | 0.1933 ± 0.03 | 0.2161 ± 0.03 |
| b2 | 0.7221 ± 0.04 | 2.4456 ± 0.60 | 2.407 ± 0.55 | |
| R2 | 0.9996 ± 0.00 | 0.9968 ± 0.00 | 0.9866 ± 0.01 | |
| Qe (mg/g) | 6.98 ± 0.68 | 4.97 ± 0.89 | 4.85 ± 0.74 | |
| Intraparticle diffusivity | kd1 | 1.0279 ± 0.07 | 0.5519 ± 0.03 | 0.4546 ± 0.02 |
| C1 | 0.8526 ± 0.35 | 0.2276 ± 0.06 | 0.4067 ± 0.15 | |
| R12 | 0.8354 ± 0.04 | 0.9148 ± 0.04 | 0.934 ± 0.04 | |
| kd2 | 0.0151 ± 0.00 | 0.0089 ± 0.00 | 0.0296 ± 0.01 | |
| C2 | 6.5001 ± 1.01 | 4.6817 ± 1.00 | 3.9067 ± 1.08 | |
| R22 | 0.7161 ± 0.04 | 0.7491 ± 0.02 | 0.7477 ± 0.15 | |
| k | 0.1075 ± 0.01 | 0.1085 ± 0.01 | 0.1075 ± 0.02 | |
| C | 1.8311 ± 0.08 | 2.0123 ± 0.06 | 1.8311 ± 0.09 | |
| R2 | 0.6768 ± 0.05 | 0.5787 ± 0.04 | 0.6768 ± 0.05 |
| Temperature (°C) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF | 1/n | R2 | n | ||
| 15 | 0.012 | 15.970 | 0.961 | 0.366 | 0.679 | 0.992 | 1.473 | |
| Pb2+ | 25 | 0.014 | 18.210 | 0.968 | 0.432 | 0.684 | 0.996 | 1.463 |
| 35 | 0.016 | 19.420 | 0.937 | 0.548 | 0.665 | 0.988 | 1.504 | |
| Mean | 0.014 | 17.867 | 0.955 | 0.448 | 0.676 | 0.992 | 1.480 | |
| 15 | 0.013 | 9.440 | 0.887 | 0.395 | 0.539 | 0.991 | 1.857 | |
| Cr2+ | 25 | 0.016 | 11.640 | 0.887 | 0.538 | 0.537 | 0.980 | 1.863 |
| 35 | 0.016 | 14.040 | 0.846 | 0.614 | 0.550 | 0.986 | 1.819 | |
| Mean | 0.015 | 11.707 | 0.873 | 0.516 | 0.542 | 0.986 | 1.846 | |
| 15 | 0.016 | 7.870 | 0.968 | 0.326 | 0.559 | 0.996 | 1.789 | |
| Zn2+ | 25 | 0.013 | 10.920 | 0.916 | 0.358 | 0.590 | 0.992 | 1.694 |
| 35 | 0.015 | 11.880 | 0.916 | 0.458 | 0.572 | 0.995 | 1.747 | |
| Mean | 0.014 | 10.223 | 0.933 | 0.381 | 0.574 | 0.994 | 1.743 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Niu, B.; Li, Y. Simultaneous Recovery of Vivianite and Humic Acids from Waste Activated Sludge via Ferric Trichloride Flocculation and Enzymatic Hydrolysis Co-Treatment. Processes 2024, 12, 2737. https://doi.org/10.3390/pr12122737
Cheng Q, Niu B, Li Y. Simultaneous Recovery of Vivianite and Humic Acids from Waste Activated Sludge via Ferric Trichloride Flocculation and Enzymatic Hydrolysis Co-Treatment. Processes. 2024; 12(12):2737. https://doi.org/10.3390/pr12122737
Chicago/Turabian StyleCheng, Qingli, Bochao Niu, and Yuhao Li. 2024. "Simultaneous Recovery of Vivianite and Humic Acids from Waste Activated Sludge via Ferric Trichloride Flocculation and Enzymatic Hydrolysis Co-Treatment" Processes 12, no. 12: 2737. https://doi.org/10.3390/pr12122737
APA StyleCheng, Q., Niu, B., & Li, Y. (2024). Simultaneous Recovery of Vivianite and Humic Acids from Waste Activated Sludge via Ferric Trichloride Flocculation and Enzymatic Hydrolysis Co-Treatment. Processes, 12(12), 2737. https://doi.org/10.3390/pr12122737






