Inactivation of Aspergillus Species and Degradation of Aflatoxins in Water Using Photocatalysis and Titanium Dioxide
Abstract
1. Introduction
1.1. Photocatalysis Combined with TiO2 Is Used for the Degradation of AF
1.2. Advantages of the Use of TiO2 as a Photocatalyst for AF Degradation
1.3. Recent Findings on the Use of Photocatalysis and TiO2 to Degrade AF in Different Matrices
1.4. Using Photocatalysis and TiO2 to Eliminate Aspergillus sp. And AF in Water
1.4.1. TiO2 Nanoparticles
1.4.2. Activated Carbon and TiO2
1.4.3. Biochar and TiO2
1.4.4. Composite and TiO2
1.4.5. Metals and TiO2
| Aflatoxin Type|Sample Type | TiO2-Based Photocatalyst | Percent of Degradation|Time | Light Source | Reference |
|---|---|---|---|---|
| AFB1|Aqueous solution | 20 mL of AFB1 solution at a concentration of 1 μg/mL and 6 mg of AC/TiO2 irradiated with UV–vis light | 95%|120 min at pH 7 | High-pressure mercury lamp (130 w, 350–450 nm wavelengths) | [27] |
| AFB1|Aqueous solution | TiO2/UiO-67 under visible light, with 10 mg photocatalyst and 100 mL of an AFB1 aqueous solution at a concentration of 0.5 mg/mL | 98.9%|80 min | 300 W high-pressure xenon lamp with a UV-cutoff filter | [42] |
| AFB1|Aqueous solution | 6 mg of 4% SDB-6-K-9@TiO2 in a 100 µg/mL AFB1 solution, pH 7, under simulated sunlight | 95%|180 min | Simulated sunlight (300–1000 nm) | [41] |
| Aqueous suspension of A. flavus spores | 25 mg of Ag/TiO2 powder suspended in 50 mL of spore suspension containing 106 CFU/mL | >90%|15 min | 300 W Xenon lamp (PLS SXE300, Beijing Perfectlight Inc., Beijing, China) with a visible light filter (λ > 420 nm) | [33] |
| Aqueous suspension of A. niger spores | Pd-C-TiO2 was tested under visible light using an A. niger suspension containing 105 spores/mL and a catalyst concentration of 1%. | 100% fungal mortality|96 h, pH 3.97 in a batch-type reactor | 7.25 mW cm−2; 9 tubes of T5 fluorescent lamps (FL8D-EX; OA lighting Kanjin Supply Co., Ltd., Taoyuan City, Taiwan) with a wavelength-cutting filter underneath the lamps at 0.5 cm were used. Radiation wavelength below 410 nm entirely cutting | [39] |
| Aqueous cultures of S. aureus and E. coli|Aqueous suspension of A. flavus spores | A concentration of 1 g/L of TiO2 nanoparticles was applied to strain suspensions containing 1.5 × 108 UFC/mL under UV light | Decrease in culture population from 1.5 × 108 to less than 50 UFC/mL within 60 min | UV lamp (details not specified) | [40] |
1.5. Comparison of the Performance of TiO2 Photocatalyst and Other Methods for the Degradation of Aflatoxins in Water
2. Final Considerations
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kechukwu, V.O.; Adelusi, O.A.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem. 2024, 436, 137775. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ghazali, F.M.; Mahyudi, N.A.; Samsudin, N.I.P. Biocontrol of aflatoxins using non-aflatoxigenic Aspergillus flavus: A literature review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of Aflatoxins Induces Severe Hepatotoxicity Through Multiple Mechanisms. Front. Pharmacol 2021, 11, 605823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Herrera-Balandrano, D.D.; Shi, X.C.; Chen, X.; Liu, F.Q.; Laborda, P. Occurrence of aflatoxins in water and decontamination strategies: A review. Water Res. 2023, 232, 119703. [Google Scholar] [CrossRef] [PubMed]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Liu, Y.; Bian, K. Structures of reaction products and degradation pathways of aflatoxin b1 by ultrasound treatment. Toxins 2019, 11, 526. [Google Scholar] [CrossRef]
- Pal, M.; Lema, A.G.; Ejeta, D.I.; Gowda, L. Global Public Health and Economic Concern due to Aflatoxins. Glob. J. Res. Med. Sci. 2021, 1, 5–8. Available online: https://gjrpublication.com/wp-content/uploads/2022/01/GJRMS120102.pdf (accessed on 7 November 2024).
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- The Commission of the European Communities. COMMISSION REGULATION (EC) No 1881/2006: Maximum Levels for Certain Contaminants in Foodstuffs. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 20 September 2024).
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Gbashi, S.; Edwin Madala, N.; De Saeger, S.; De Boevre, M.; Adekoya, I.; Ayodeji Adebo, O.; Berka Njobeh, P. The socio-economic impact of mycotoxin contamination in Africa. In Mycotoxins—Impact and Management Strategies; IntechOpen: London, UK, 2018. [Google Scholar]
- Saha Turna, N.; Comstock, S.S.; Gangur, V.; Wu, F. Effects of aflatoxin on the immune system: Evidence from human and mammalian animal research. Crit. Rev. Food Sci. Nutr. 2023, 64, 9955–9973. [Google Scholar] [CrossRef]
- Wangikar, P.B.; Dwivedi, P.; Sinha, N.; Sharma, A.K.; Telang, A.G. Effects of aflatoxin B1 on embryo fetal development in rabbits. Food Chem. Toxicol. 2005, 43, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, A.; Hashmi, M.F.; Sbar, E. Aflatoxin Toxicity; NIH: Bethesda, MD, USA, 2020. [Google Scholar]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The Toxic Effects of Aflatoxin B1 and Aflatoxin M1 on Kidney through Regulating L-Proline and Downstream Apoptosis. Biomed. Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Patras, A.; Pendyala, B.; Vergne, M.J.; Bansode, R.R. Performance of a UV-A LED system for degradation of aflatoxins B1 and M1 in pure water: Kinetics and cytotoxicity study. Sci. Rep. 2020, 10, 13473. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, F.; Xue, X.; Wang, Z.; Fan, B.; Ha, Y. Structure elucidation and toxicity analyses of the radiolytic products of aflatoxin B 1 in methanol-water solution. J. Hazard Mater. 2011, 192, 1192–1202. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Wang, Y.; Chen, Z. Structure elucidation and toxicity analyses of the degradation products of aflatoxin B1 by aqueous ozone. Food Control 2013, 31, 331–336. [Google Scholar] [CrossRef]
- Liu, R.; Wang, R.; Lu, J.; Chang, M.; Jin, Q.; Du, Z.; Wang, S.; Li, Q.; Wang, X. Degradation of AFB1 in aqueous medium by electron beam irradiation: Kinetics, pathway and toxicology. Food Control 2016, 66, 151–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, Y.; Guan, E.; Bian, K. Degradation of aflatoxin B1 by water-assisted microwave irradiation: Kinetics, products, and pathways. LWT 2021, 152, 112310. [Google Scholar] [CrossRef]
- Møller, C.O.d.A.; Freire, L.; Rosim, R.E.; Margalho, L.P.; Balthazar, C.F.; Franco, L.T.; Sant’Ana, A.; Corassin, C.H.; Rattray, F.P.; F.d.Oliveira, C.A. Effect of Lactic Acid Bacteria Strains on the Growth and Aflatoxin Production Potential of Aspergillus parasiticus, and Their Ability to Bind Aflatoxin B1, Ochratoxin A, and Zearalenone in vitro. Front. Microbiol. 2021, 12, 655386. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226. [Google Scholar] [CrossRef]
- Aiko, V.; Edamana, P.; Mehta, A. Decomposition and detoxification of aflatoxin B1 by lactic acid. J. Sci. Food Agric. 2016, 96, 1959–1966. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials. 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Sinar, M.S.I.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Asikin Mijan, N.; Tan, Y.H.; Mansir, N. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst. Food Control 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Gonçalves, G.; Podlogar, M. The potential of rGO@ TiO2 photocatalyst for the degradation of organic pollutants in water. Sustainability 2022, 14, 12703. [Google Scholar] [CrossRef]
- Nur, A.S.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Kanakaraju, D.; anak Kutiang, F.D.; Lim, Y.C.; Goh, P.S. Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization. Appl. Mater. Today 2022, 27, 101500. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Santos, P.R.M.; Silva, C.I.Q.; Azenha, M.A. Latest developments on TiO2-based photocatalysis: A special focus on selectivity and hollowness for enhanced photonic efficiency. Appl. Catal. A Gen. 2021, 623, 118243. [Google Scholar] [CrossRef]
- Yang, D.; Wei, H.; Yang, X.; Cheng, L.; Zhang, Q.; Li, P.; Mao, J. Efficient Inhibition of Aspergillus flavus to Reduce Aflatoxin Contamination on Peanuts over Ag-Loaded Titanium Dioxide. Toxins 2023, 15, 216. [Google Scholar] [CrossRef]
- Alluqmani, S.M.; Loulou, M.; Ouerfelli, J.; Alshahrie, A.; Salah, N. Elaboration of TiO2/carbon of oil fly ash nanocomposite as an eco-friendly photocatalytic thin-film material. Ceram. Int. 2021, 47, 13544–13551. [Google Scholar] [CrossRef]
- Xu, C.; Ye, S.; Cui, X.; Song, X.; Xie, X. Modelling photocatalytic detoxification of aflatoxin B1 in peanut oil on TiO2 layer in a closed-loop reactor. Biosyst. Eng. 2019, 180, 87–95. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Reduction of aflatoxin B1 by magnetic graphene oxide/TiO2 nanocomposite and its effect on quality of corn oil. Food Chem. 2021, 343, 128521. [Google Scholar] [CrossRef] [PubMed]
- Magzoub, R.A.M.; Yassin, A.A.A.; Abdel-Rahim, A.M.; Gubartallah, E.A.; Miskam, M.; Saad, B.; Sabar, S. Photocatalytic detoxification of aflatoxins in Sudanese peanut oil using immobilized titanium dioxide. Food Control 2019, 95, 206–214. [Google Scholar] [CrossRef]
- Xu, C.; Ye, S.; Cui, X.; Zhang, Q.; Liang, Y. Detoxification of aflatoxin B1 in peanut oil by iodine doped supported TiO2 Thin film under ultraviolet light irradiation. Curr. Nanosci. 2019, 15, 188–196. [Google Scholar] [CrossRef]
- Huang, S.M.; Weng, C.H.; Tzeng, J.H.; Huang, Y.Z.; Anotai, J.; Yen, L.T.; Chang, C.J.; Lin, Y.T. Kinetic study and performance comparison of TiO2-mediated visible-light-responsive photocatalysts for the inactivation of Aspergillus niger. Sci. Total Environ. 2019, 692, 975–983. [Google Scholar] [CrossRef]
- Babaei, E.; Dehnad, A.; Hajizadeh, N.; Valizadeh, H.; Reihani, S.F.S. A study on Inhibitory Effects of Titanium Dioxide Nanoparticles and its Photocatalytic Type on Staphylococcus aureus, Escherichia coli and Aspergillus flavus. Appl. Food Biotechnol. 2016, 3, 115–123. [Google Scholar]
- Zhang, J.; Ying, Z.; Li, H.; Liu, X.; Ma, D.; Yu, H. Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins 2024, 16, 429. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Guo, W.; Wu, Z.; Yin, Y.; Li, Z. Enhanced photocatalytic activity of TiO2/UiO-67 under visible-light for aflatoxin B1 degradation. RSC Adv. 2022, 12, 6676–6682. [Google Scholar] [CrossRef]
- Barata, C.; Alanon, P.; Gutierrez-Alonso, S.; Riva, M.C.; Fernández, C.; Tarazona, J.V. A Daphnia magna feeding bioassay as a cost effective and ecological relevant sublethal toxicity test for environmental risk assessment of toxic effluents. Sci. Total Environ. 2008, 405, 78–86. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Li, X.; Huang, X. A critical review on chemical analysis of heavy metal complexes in water/wastewater and the mechanism of treatment methods. Chem. Eng. J. 2022, 429, 131688. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hsu, Y.H. Effects of reaction temperature on the photocatalytic activity of TiO2 with Pd and Cu cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; Meunier, C.; Henriet, O.; Mahillon, J.; Suarez-Ojeda, M.E.; Del Moro, G.; De Sanctis, M.; Di Iaconi, C.; Weissbrodt, D.G. An integrative review of granular sludge for the biological removal of nutrients and recalcitrant organic matter from wastewater. Chem. Eng. J. 2018, 336, 489–502. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, S.; Zhuang, L.; Hu, B.; Wang, S.; Chen, J.; Wang, X. Application of biochar-based photocatalysts for adsorption-(photo) degradation/reduction of environmental contaminants: Mechanism, challenges and perspective. Biochar 2022, 4, 45. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A critical review on metal-organic frameworks and their composites as advanced materials for adsorption and photocatalytic degradation of emerging organic pollutants from wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
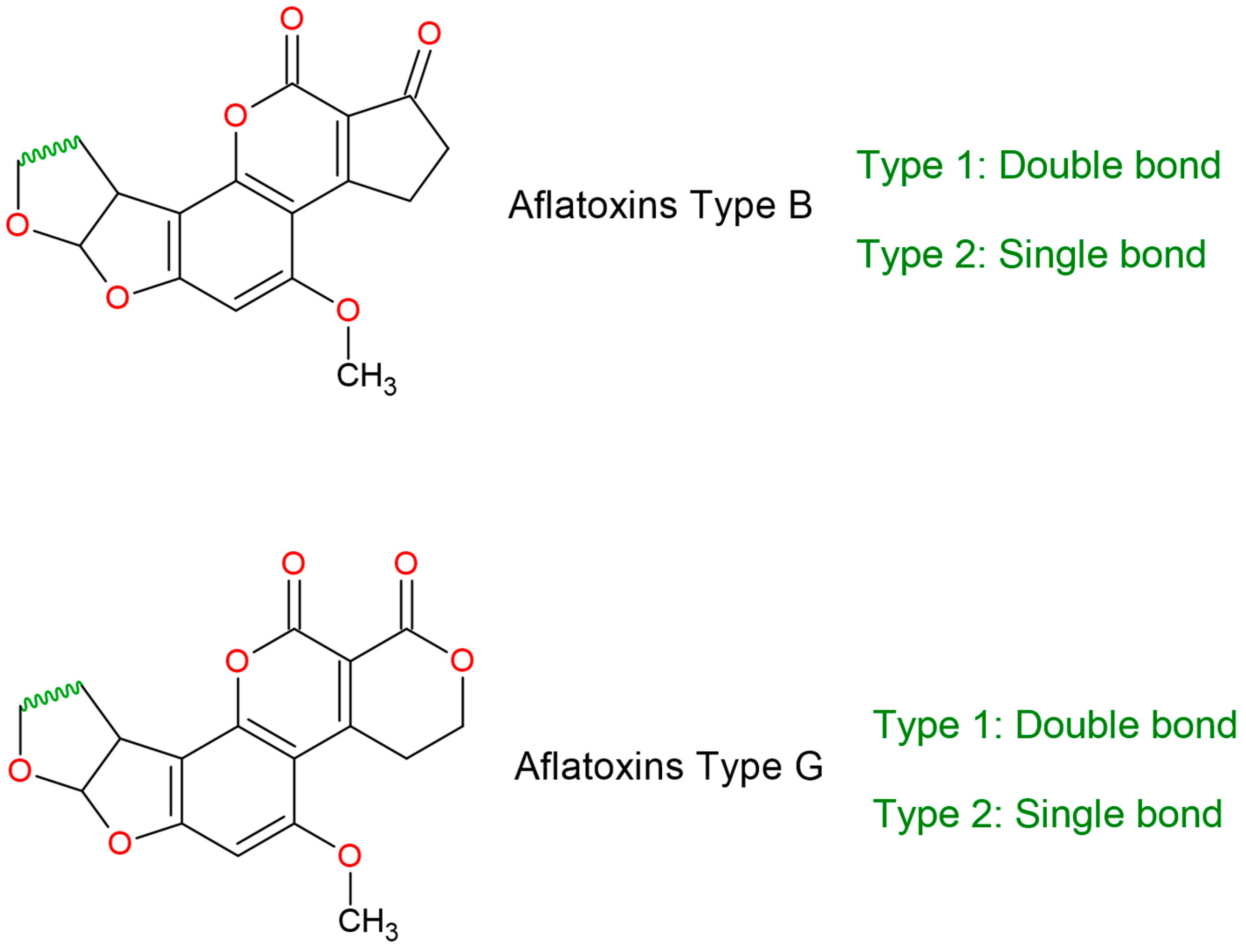
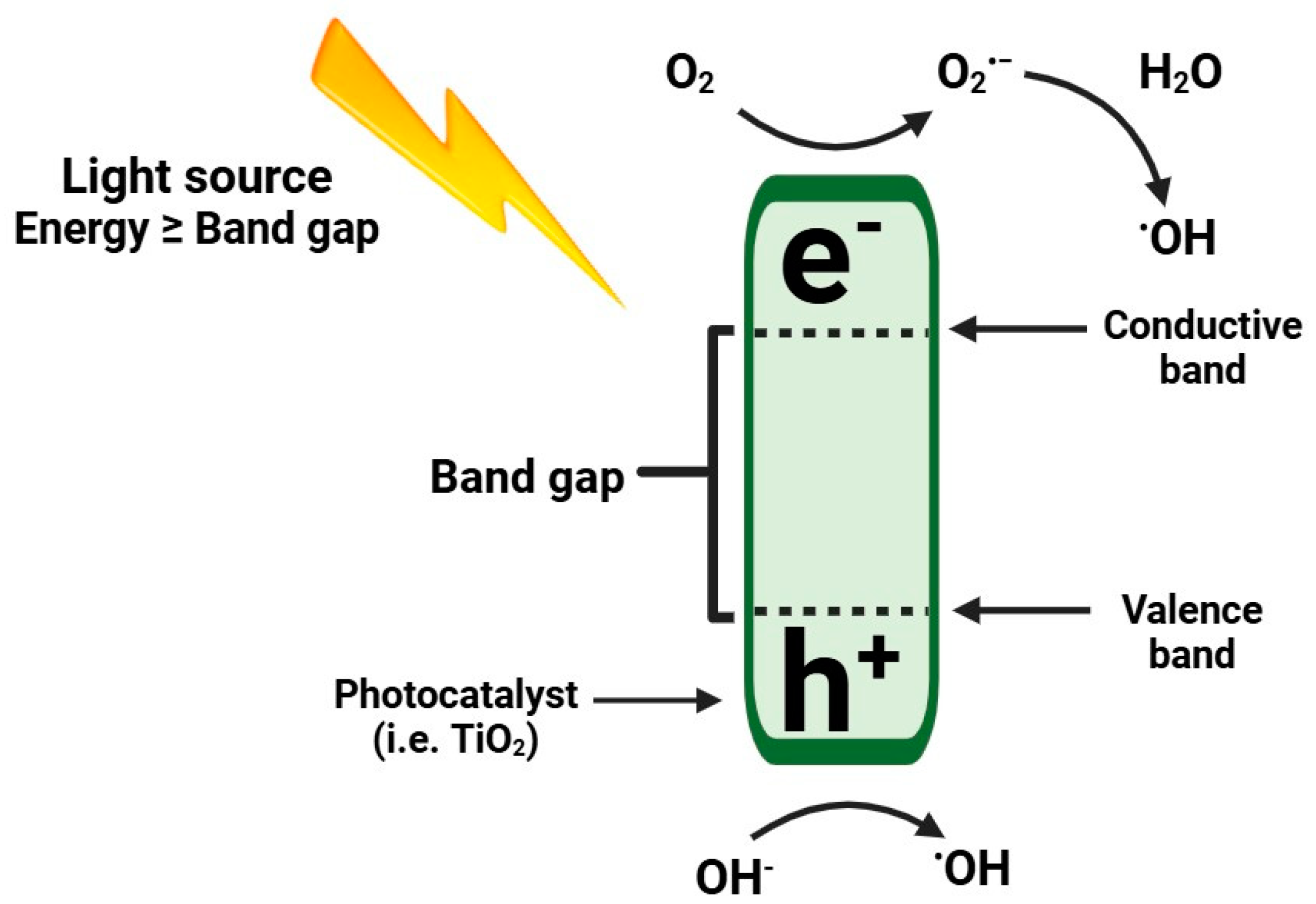
| Aflatoxin Type | Economic and Health Effects | Reference |
|---|---|---|
| AF in general | In Africa, the annual cost exceeds USD 750 million, while food exporters in the European Union incur about USD 670 million each year. | [11] |
| AF in general | Aflatoxin concerns cost US maize farmers USD 160 million annually. | [7] |
| AF in general | Climate change-related AF contamination could cost the US corn industry USD 52.1 million to USD 1.68 billion annually. | [10] |
| AFB1 | Consuming 20–120 μg/kg of AFB1 per day for one to three weeks can cause acute toxicity and may be lethal. | [12] |
| AFB1 | Teratogenic effects were observed in rabbits when administrated AFB1 orally during gestation days 6–18 at a dose of 100 μg/kg. | [13] |
| AFB1 | Regardless of the dose, AF increases cancer risk by intercalating into DNA and alkylating bases through an epoxide moiety, leading to p53 gene mutations. | [14] |
| AF in general | The liver is the primary organ affected by AF, which are metabolized into highly toxic forms that cause liver damage through various mechanisms. | [3] |
| AFB1, AFM1 | Renal damage is caused by AFB1 (500 μg/kg), AFM1 (3500 μg/kg), and the combination of AFB1 with AFM1 through the activation of oxidative stress. | [15] |
| AF in general | Aflatoxicosis may occur if AF consumption reaches 1 mg/kg or higher. | [12] |
| AF in general | Immune alterations can occur when consumption exceeds 0.9068 pmol mg−1, leading to changes in the innate immune system. | [12] |
| Method|Aflatoxin Type|Sample Type | Working Conditions | Percentage of Degradation| Elimination|Detoxification|Toxicity Assays | Reference |
|---|---|---|---|
| UV-A LED irradiation (physical)|AFB1 and AFM1|Aqueous solution | 365 nm, 4 °C, 1200 mJ/cm2, 156 s, initial concentrations of 1 µg/mL and 2 µg/mL for AFB1 and AFM1 | 70%|Assays conducted with HepG2 cells|No significant cytotoxicity | [16] |
| Co60 gamma ray (physical) |AFB1|Ethanol–water solution | Initial concentration at 20 mg/L, with a dose rate of 0.31 Gy s−1, measured at room temperature | Toxicity analyses of radiolytic products were based on the quantitative structure–activity relationship. A double bond equivalent (DBE) of 70% for the radiolytic products was lower than that of AFB1. | [17] |
| Ozonolysis (physical)|AFB1 | Ozone solutions at a concentration of 20 mg/L, maintained at temperature range of 19.5–20.5 °C | Toxicity analysis conducted by DBE revealed low values, specifically between 6.5 and 12.5. | [18] |
| Electron beam (physical)|AFB1 |Aqueous solution | 5 MeV, 2 kGy/s, 4 °C, and an initial concentration of 5 ppm | 100%|In assays conducted with HepG-2 cells, there was an observed decrease in cell viability of 13%. | [19] |
| Ultrasound (physical)|AFB1|Aqueous solution | 20 kHz, 6.6 W/cm3, 25 °C, 80 min, and an initial concentration of 10 mg/L | 85.1%|Toxicity analysis through an index of hydrogen deficiency (IHD) indicated that 75% of the AFB1 reaction products had values lower than 11.5. | [6] |
| Water-assisted microwave irradiation (WMI) (physical)|AFB1|Aqueous solution | 500 W, 140 °C, 35 min, and an initial concentration of 0.1 mg/L | 74%|Toxicity was determined by DBE, with values ranging from 6.5 to 11.5 considered low. | [20] |
| Degradation with lactic acid bacteria (LAB) Levilactobacillus spp. 2QB383 (biological)|AFB1|Potassium phosphate buffer and Aspergillus parasiticus NRRL 2999 cultures | Heat-killed LAB cultures were prepared by heating at 100 °C for 1 h. A 25 µL inoculum of each micro-organism was added to 200 µL yeast extract sucrose broth, resulting in a final volume of 250 µL. This setup achieved a final concentration of 5.0 log10 CFU/mL for the LAB and/or 5.0 log10 spores/mL for the fungus. The cultures were maintained under static conditions in the dark for a duration of 7 days. | 100% inhibition of AFB1 production | [21] |
| Ozone as an oxidizing agent (chemical)|AFB1 and AFG1|Aqueous solution | AFB1 and AFG1 had initial concentrations of 2 ppb and 10 ppb, respectively. The temperature was 308.15 K, and an ozone concentration of 40 ppm caused the greatest degradation in both aflatoxins. | 100 %|No toxicity assays were conducted. | [22] |
| Lactic acid–heat (chemical–physical)|AFB1|Aqueous solution | 80 °C, 120 min, pH 3–4, and an initial concentration of 1 µg/mL | 85%|Toxicity assays were conducted using HeLa cells, revealing a decrease in toxicity of the degradation products ranging from 1.2 to 15.4% | [23] |
| Plant extract from Ocimum basilicum|AFB1 and AFB2|Aqueous solution | 30 °C, 72 h, pH 8. Initial concentrations of AFB1 and AFB2 were 100 and 50 µg/L, respectively. | 90.4 and 88.6% for AFB1 and AFB2, respectively|Growth inhibition of aflatoxigenic isolates was recorded at 82–87% for AFB1 and AFB2, respectively|Toxicity bioassays using brine shrimps (Artemia salina) indicated a mortality rate of only 9.2–22.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Núñez-Salas, R.E.; Rodríguez-Delgado, M.M.; Villarreal-Chiu, J.F. Inactivation of Aspergillus Species and Degradation of Aflatoxins in Water Using Photocatalysis and Titanium Dioxide. Processes 2024, 12, 2673. https://doi.org/10.3390/pr12122673
Quintanilla-Villanueva GE, Luna-Moreno D, Núñez-Salas RE, Rodríguez-Delgado MM, Villarreal-Chiu JF. Inactivation of Aspergillus Species and Degradation of Aflatoxins in Water Using Photocatalysis and Titanium Dioxide. Processes. 2024; 12(12):2673. https://doi.org/10.3390/pr12122673
Chicago/Turabian StyleQuintanilla-Villanueva, Gabriela Elizabeth, Donato Luna-Moreno, Raisa Estefanía Núñez-Salas, Melissa Marlene Rodríguez-Delgado, and Juan Francisco Villarreal-Chiu. 2024. "Inactivation of Aspergillus Species and Degradation of Aflatoxins in Water Using Photocatalysis and Titanium Dioxide" Processes 12, no. 12: 2673. https://doi.org/10.3390/pr12122673
APA StyleQuintanilla-Villanueva, G. E., Luna-Moreno, D., Núñez-Salas, R. E., Rodríguez-Delgado, M. M., & Villarreal-Chiu, J. F. (2024). Inactivation of Aspergillus Species and Degradation of Aflatoxins in Water Using Photocatalysis and Titanium Dioxide. Processes, 12(12), 2673. https://doi.org/10.3390/pr12122673







