Investigation of Influence of High Pressure on the Design of Deep-Water Horizontal Separator and Droplet Evolution
Abstract
:1. Introduction
2. Droplet Behavior in the Separation Process and Separator Design
2.1. Behavior of Dispersed Phase Droplets During Phase Separation
2.2. Separator Design Process
3. Experimental and Numerical Setup
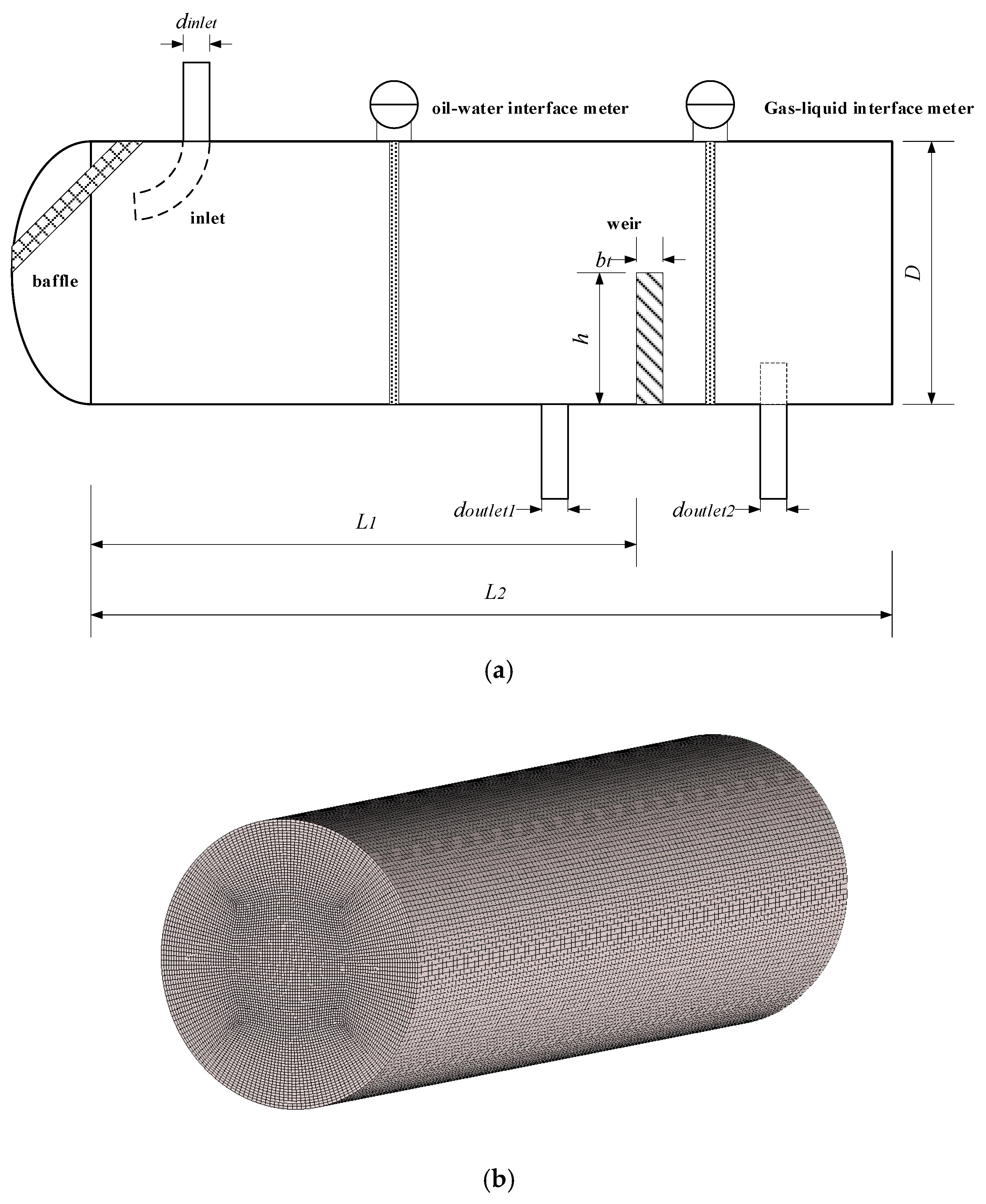
3.1. Separator
3.2. Test Facility
3.3. Separation Effect Testing
- Step 1: The samples were placed in a hot water bath for oil solubilization with the heating temperature set at 70 °C for a preheating duration of 20 min.
- Step 2: The water samples were vigorously shaken to dislodge any wall-adherent materials, ensuring a homogeneous mixture of the specimens.
- Step 3: The prepared samples were transferred into a 50 mL stoppered spectrophotometer tube.
- Step 4: Upon reaching room temperature, the samples were acidified by adding 6N hydrochloric acid to achieve a pH of less than 2. Subsequently, 5 mL of n-hexane was introduced, the mixture was vigorously shaken, and then it was left undisturbed to facilitate phase separation.
- Step 5: The stratified liquids were extracted, and their absorbance was measured according to the oil standard curve. The oil content C was then determined by referencing the absorbance value against the same standard curve.
3.4. Test Instruments
3.5. Numerical Method and Simulation Settings
4. Results and Discussion
4.1. Test Results and Separation Effect
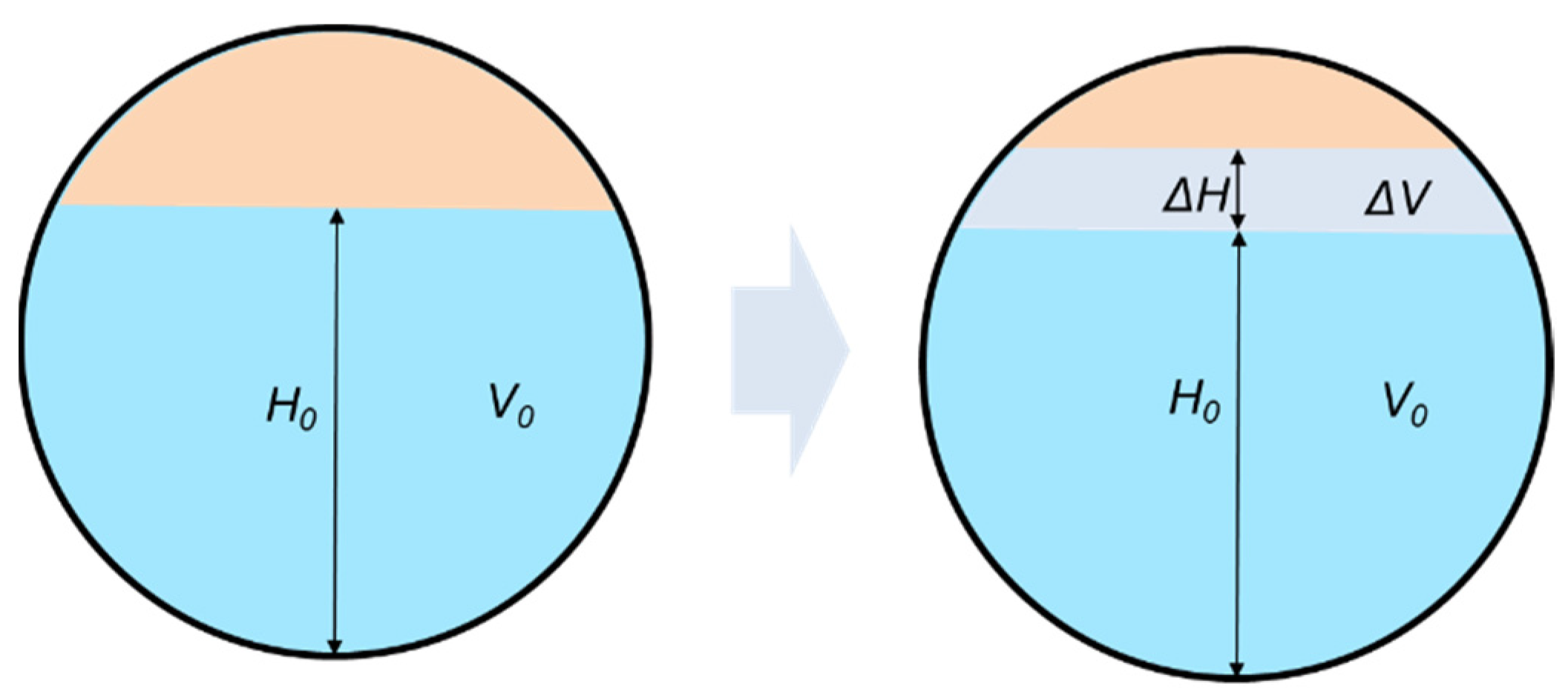
4.2. Analysis of Separation Performance
4.3. Analysis of Droplet Evolution Process and Optimization of Separator Design
5. Conclusions
- Combined with the particle size analysis of oil droplets in water, as the pressure increased, the particle size of the dispersed phase oil droplets decreased, and the characteristic particle size of the droplet group decreased. Furthermore, according to the energy analysis, the surface tension of the oil droplets decreased under high pressure, further influencing the balance between the surface energy and kinetic energy of the droplets, leading to a reduction in the droplet size.
- The internal flow within the separator cannot be described as a simple laminar flow state. The high pressure intensifies the collisions of small droplets, raising questions about the applicability of Stokes’ law. The drag coefficient of the droplet sedimentation process was inferred from the oil–water separation characteristics, and the drag coefficient for high-pressure separation was adjusted using a correction formula. The droplet settling velocities at other pressures were simulated through calculations.
- The influence of high pressure on separator design is evident in the alterations to the dispersed phase settling process and emulsion layer. Under deep-water conditions, the reduction in droplet settling velocity necessitates an increase in the design size of the corresponding separator. Additionally, the changes in the emulsion layer require the conventional layer thickness criterion to have a larger safe operating margin under high pressure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liang, J.; Zhao, Q.; Chen, J.; Zhang, J.; Yuan, Y.; Zhang, Y.; Dong, H. Characteristics of deepwater oil and gas distribution along the silk road and their controlling factors. Water 2024, 16, 240. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Wang, J.; Hou, L.; Li, H. Characteristics of oil and gas discoveries in recent 20 years and future exploration in the world. China Pet. Explor. 2018, 23, 44–53. [Google Scholar]
- Zhang, G.; Qu, H.; Zhang, F.; Chen, S. Major new discoveries of oil and gas in global deep-waters and enlightenment. Acta Pet. Sin. 2019, 40, 1–34, 55. [Google Scholar]
- Wang, Z.; Wen, Z.; He, Z.; Chen, R.; Shi, H.; Chen, X. Characteristics and enlightenment of new progress in global oil and gas exploration in recent ten years. China Pet. Explor. 2022, 27, 27–37. [Google Scholar]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Van, S.L.; Van, D.T. Selection of an optimizing inlet angle for the gas-liquid cylindrical cyclone separator on hydrokinetic behavior. J. Adv. Mar. Eng. Technol. 2020, 44, 82–87. [Google Scholar]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Jiang, W.; Hai, L.; Yang, X.; Liu, Y. Numerical study of swirl core-annular flow and factors influencing pressure drop in vertical pipelines for oi-water two-phase flow. Geoenergy Sci. Eng. 2025, 244, 213416. [Google Scholar] [CrossRef]
- Kolla, S.S.; Mohan, R.S.; Shoham, O. Analysis of gas-liquid cylindrical cyclone separator with inlet modifications using fluid–structure interaction. J. Energy Resour. Technol.—Trans. ASME 2020, 142, 042003. [Google Scholar] [CrossRef]
- Elhemmali, A.; Anwar, S.; Zhang, Y.; Shirokoff, J. A comparison of oil-water separation by gravity and electrolysis separation process. Sep. Sci. Technol. 2021, 56, 359–373. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Sun, S. Effect of temperature on oil–water separations using membranes in horizontal separators. Membranes 2022, 12, 232. [Google Scholar] [CrossRef]
- Fadaei, M.; Ameri, M.J.; Rafiei, Y.; Asghari, M.; Ghasemi, M. Experimental design and manufacturing of a smart control system for horizontal separator based on PID controller and integrated production model. J. Pet. Explor. Prod. Technol. 2024, 14, 2273–2295. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Sidiq, H.; Kader, R. Liquid carry-over control using three-phase horizontal smart separators in Khor Mor gas-condensate processing plant. J. Pet. Explor. Prod. Technol. 2024, 14, 2413–2435. [Google Scholar] [CrossRef]
- Stomquist, R.; Gustafson, S. SUBSIS-the world’s first subsea separation and injection system. World Pumps 1999, 3, 33–36. [Google Scholar] [CrossRef]
- Denney, D. Perdido Development: Subsea and Flowline Systems. J. Pet. Technol. 2010, 62, 53–55. [Google Scholar] [CrossRef]
- Arnold, K.; Stewart, M. Designing Oil and Gas Production Systems: How to size and select two-phase Separators. World Oil 1984, 199, 73. [Google Scholar]
- Arnold, K.; Stewart, M. Designing oil and gas production systems: How to size and select three-phase separators. World Oil 1984, 199, 87. [Google Scholar]
- Svrcek, W.Y.; Monnery, W.D. Design two-phase separators within the right limits. Chem. Eng. Prog. 1993, 89, 53–60. [Google Scholar]
- Monnery, W.D.; Svrcek, W.Y. Successfully specify three-phase separators. Chem. Eng. Prog. 1994, 90, 29. [Google Scholar]
- Gas Processors and Suppliers Association (GPSA). GPSA Engineering Data Book, 11th ed.; GPSA: Tulsa, OK, USA, 1998. [Google Scholar]
- Watkins, R. Sizing separators and accumulators-speed with which operators can correct upsets is included in these design considerations. Hydrocarb. Process 1967, 46, 253–261. [Google Scholar]
- Evert, O.G.; Matthew, J.R. Optimal design of two-and three-phase separators: A mathematical programming formulation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TA, USA, 3–6 October 1999. [Google Scholar]
- Ghaffarkhah, A.; Shahrabi, M.A.; Moraveji, M.K. 3D computational-fluid-dynamics modeling of horizontal three-phase separators: An approach for estimating the optimal dimensions. SPE Prod. Oper. 2018, 33, 879–895. [Google Scholar] [CrossRef]
- Laleh, A.P.; Svrcek, W.Y.; Monnery, W.D. Design and CFD studies of multiphase separators—A review. Can. J. Chem. Eng. 2012, 90, 1547–1560. [Google Scholar] [CrossRef]
- API. API SPEC 12J, Specification for Oil and Gas Separators, 8th ed.; API: Washington, DC, USA, 2008. [Google Scholar]
- Davies, S.R.; Bakke, W.; Ramberg, R.M.; Jensen, R.O. Experience to date and future opportunities for subsea processing in statoilhydro. In Proceedings of the Offshore Technology Conference, Houston, TA, USA, 3–6 May 2010. [Google Scholar]
- Capela Moraes, C.A.; da Silva, F.S.; Marins, L.P.M.; Monteiro, A.S.; de Oliveira, D.A.; Pereira, R.M.; Pereira, R.d.S.; Alves, A.; Raposo, G.M.; Orlowski, R.; et al. Marlim 3 phase subsea separation system: Subsea process design and technology qualification program. In Proceedings of the Offshore Technology Conference, Houston, TA, USA, 3–6 May 2012. [Google Scholar]
- Orlowski, R.; Euphemio, M.L.L.; Euphemio, M.L.; Andrade, C.A.; Guedes, F.; Tosta da Silva, L.C.; Pestana, R.G.; de Cerqueira, G.; Loureno, L.; Pivari, A.; et al. Marlim 3 Phase Subsea Separation System—Challenges and Solutions for the Subsea Separation Station to Cope with Process Requirements. In Proceedings of the Offshore Technology Conference, Houston, TA, USA, 3–6 May 2012. [Google Scholar]
- Shaiek, S.; Grandjean, L. Spoolsep for subsea produced water separation-experimental results. In Proceedings of the Offshore Technology Conference, Houston, TA, USA, 3–6 May 2015. [Google Scholar]
- Tadros, T. Interparticle interactions in concentrated suspensions and their bulk (Rheological) properties. Adv. Colloid Interface Sci. 2011, 168, 263–277. [Google Scholar] [CrossRef]
- Mason, T. New fundamental concepts in emulsion rheology. Curr. Opin. Colloid Interface Sci. 1999, 4, 231–238. [Google Scholar] [CrossRef]
- Pal, R. Effect of droplet size on the rheology of emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
- Mao, M.L.; Marsden, S.S. Stability of concentrated crude oil-in-water emulsions as a function of shear rate, temperature and oil concentration. J. Can. Pet. Technol. 1977, 16, PETSOC-77-02-06. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Schmiedel, P. Phase behavior of lecithin at the oil/water interface. Langmuir 1996, 12, 6443–6445. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Cheng, J.; Xie, X.; Weitz, D.A. Spontaneous formation of lipid structures at oil/water/lipid interfaces. Langmuir 2003, 19, 10281–10287. [Google Scholar] [CrossRef]
- González-Ochoa, H.; Ibarra-Bracamontes, L.; Arauz-Lara, J. Two-stage coalescence in double emulsions. Langmuir 2003, 19, 7837–7840. [Google Scholar] [CrossRef]
- Sacanna, S.; Kegel, W.K.; Philipse, A.P. Spontaneous oil-in-water emulsification induced by charge-stabilized dispersions of various inorganic colloids. Langmuir 2007, 23, 10486. [Google Scholar] [CrossRef]
- Fowkes, F.M. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. J. Phys. Chem. 1962, 66, 382. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Good, R.J. A theory for the estimation of surface and interfacial energies. I. derivation and application to interfacial tension. J. Phys. Chem. 1957, 61, 904. [Google Scholar] [CrossRef]
- Winterfeld, P.H.; Scriven, L.E.; Davis, H.T. An approximate theory of interfacial tensions of multicomponent systems: Applications to binary liquid-vapor tensions. AIChE J. 1978, 24, 1010–1014. [Google Scholar] [CrossRef]
- Guggenheim, E.A. The principle of corresponding states. J. Chem. Phys. 1945, 13, 253–261. [Google Scholar] [CrossRef]
- Queimada, A.J.; Rolo, L.I.; Caco, A.I.; Marrucho, I.M.; Stenby, E.H.; Coutinho, J.A.P. Prediction of viscosities and surface tensions of fuels using a new corresponding states model. Fuel 2006, 85, 874–877. [Google Scholar] [CrossRef]
- Kiselev, S.B.; Ely, J.F. Generalized corresponding states model for bulk and interfacial properties in pure fluids and fluid mixtures. J. Chem. Phys. 2003, 119, 8645. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C. Surface tensions of petro-diesel, canola, jatropha and soapnut biodiesel fuels at elevated temperatures and pressures. Fuel 2013, 104, 704–710. [Google Scholar] [CrossRef]
- Sano, T. Unsteady flow past a sphere at low Reynolds number. J. Fluid Mech. 1981, 112, 433–441. [Google Scholar] [CrossRef]
- Mei, R.; Adrian, R.J. Flow past a sphere with an oscillation in the free-stream and unsteady drag at finite Reynolds number. J. Fluid Mech. 1992, 237, 323–341. [Google Scholar] [CrossRef]
- Lin, Y.; Palmore, J. The effect of droplet deformation and internal circulation on drag coefficient. Phys. Rev. Fluids 2022, 7, 123602. [Google Scholar] [CrossRef]
- Feng, J. A deformable liquid drop falling through a quiescent gas at terminal velocity. J. Fluid Mech. 2010, 658, 438–462. [Google Scholar] [CrossRef]
- Harper, J.F.; Moore, D.W. The motion of a spherical liquid drop at high Reynolds number. J. Fluid Mech. 1968, 32, 367–391. [Google Scholar] [CrossRef]
- Helenbrook, B.T.; Edwards, C.F. Quasi-steady deformation and drag of uncontaminated liquid drops. Int. J. Multiph. Flow 2002, 28, 1631–1657. [Google Scholar] [CrossRef]
- Oshinowo, L.M.; Vilagines, R.G. Modeling of oil–water separation efficiency in three-phase separators: Effect of emulsion rheology and droplet size distribution. Chem. Eng. Res. Des. 2020, 159, 278–290. [Google Scholar] [CrossRef]







| Operating Pressure, P (psia) | Calculation of K |
|---|---|
| 1 ≤ P < 15 | K = 0.1821 + 0.0029P + 0.046ln(P) |
| 15 ≤ P < 40 | K = 0.35 |
| 40 ≤ P ≤ 5500 | K = 0.43 − 0.023ln(P) |
| API Gravity | Residence Time (min) |
|---|---|
| >35° | 1 |
| 20°~30° | 1~2 |
| 10°~20° | 2~4 |
| Density (kg/m3) | Viscosity (mPa·s) | Content (%) | |
|---|---|---|---|
| oil | 827.3 | 7.2 | 9.1 |
| water | 998.5 | 1.001 | 90.9 |
| Pressure (MPa) | Oil Content (mg/L) |
|---|---|
| 0.5 | 36.54 |
| 2 | 70.05 |
| 4 | 123.65 |
| 6 | 157.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhang, M.; Wang, H.; Yi, H.; Yang, M.; Hou, L.; Liu, S.; Xu, J. Investigation of Influence of High Pressure on the Design of Deep-Water Horizontal Separator and Droplet Evolution. Processes 2024, 12, 2619. https://doi.org/10.3390/pr12122619
Cui Y, Zhang M, Wang H, Yi H, Yang M, Hou L, Liu S, Xu J. Investigation of Influence of High Pressure on the Design of Deep-Water Horizontal Separator and Droplet Evolution. Processes. 2024; 12(12):2619. https://doi.org/10.3390/pr12122619
Chicago/Turabian StyleCui, Yuehong, Ming Zhang, Haiyan Wang, Hualei Yi, Meng Yang, Lintong Hou, Shuo Liu, and Jingyu Xu. 2024. "Investigation of Influence of High Pressure on the Design of Deep-Water Horizontal Separator and Droplet Evolution" Processes 12, no. 12: 2619. https://doi.org/10.3390/pr12122619
APA StyleCui, Y., Zhang, M., Wang, H., Yi, H., Yang, M., Hou, L., Liu, S., & Xu, J. (2024). Investigation of Influence of High Pressure on the Design of Deep-Water Horizontal Separator and Droplet Evolution. Processes, 12(12), 2619. https://doi.org/10.3390/pr12122619






