1. Introduction
With the rapid increase in the production of municipal solid waste (MSW) [
1,
2], there is an urgent need for efficient, cost-effective, and environmentally friendly waste treatment methods. Thermochemical waste-to-energy processes, such as MSW incineration, have emerged as the most widely adopted approach [
3,
4]. In China, the grate furnace, accounting for over 70% of the installed capacity, is the predominant device used for MSW combustion [
5]. During MSW combustion, samples undergo three main stages: drying, pyrolysis, and char combustion [
6]. Among them, pyrolysis is the initial chemical reaction that the materials undergo, and accounts for the primary weight loss throughout the combustion process. Conducting experimental and modeling studies on MSW pyrolysis has two important significances. On one hand, relevant research helps to better describe and understand the chemical reaction mechanisms during the pyrolysis stage of the MSW combustion process, laying the groundwork for establishing a comprehensive MSW combustion model [
7]. On the other hand, such research can assist in optimizing the structural design of industrial-scale MSW combustion reactors and adjusting operational conditions [
8]. However, there are some issues that need to be addressed in both the experimental and modeling aspects of MSW pyrolysis research.
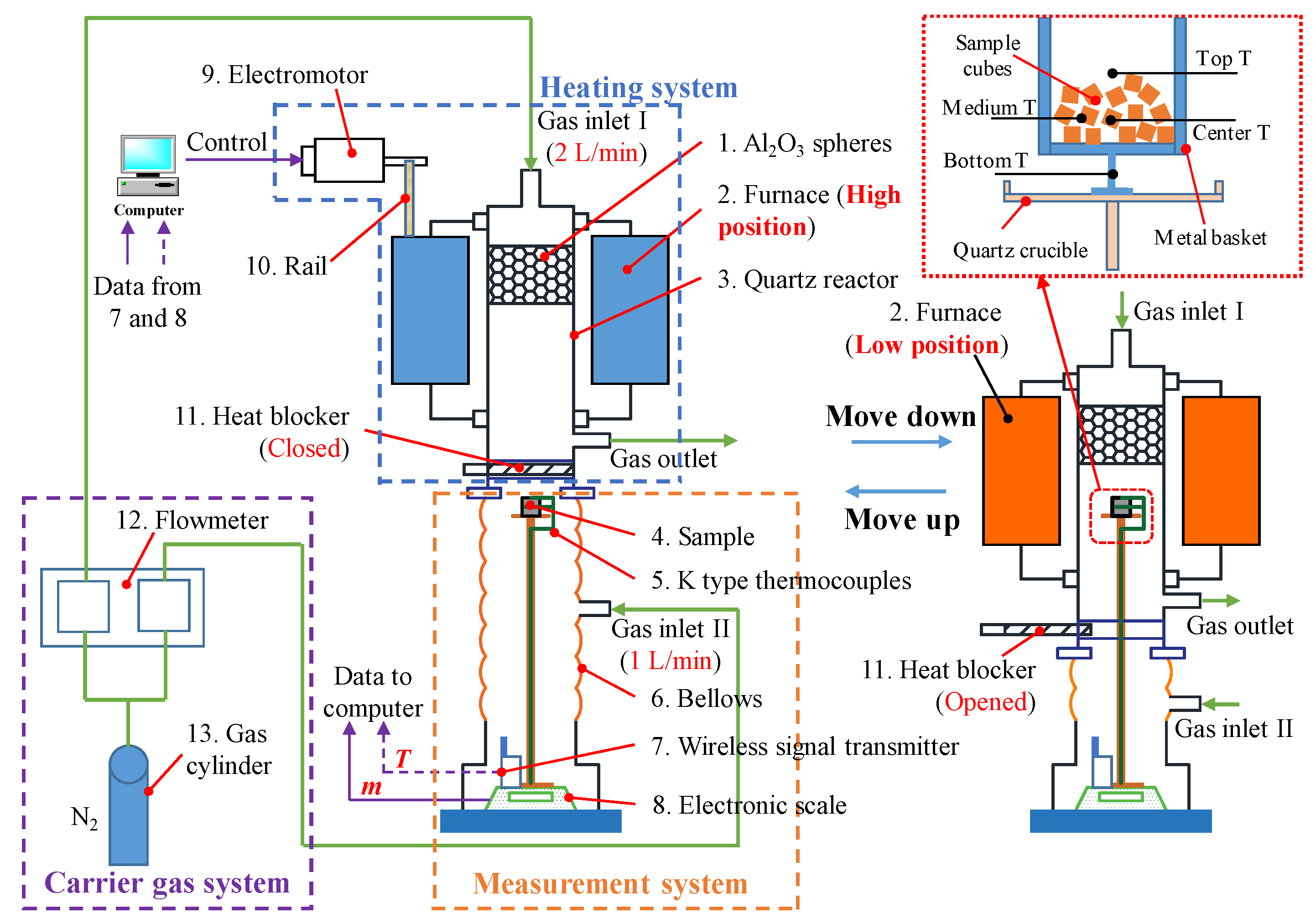
The pyrolysis characteristics of MSW in a grate furnace during the incineration process can be summarized as follows: (a) The sample size is relatively large, typically ranging from 5 to 100 mm [
9], which impacts both heat transfer and gas diffusion during the pyrolysis and combustion processes [
10]. (b) The heating rate of the samples is fast, with a maximum heating rate exceeding 500 K/min, due to the swift introduction of samples into the high-temperature zone [
11]. Previous studies have shown that varying heating rates can result in differences in pyrolysis kinetics and reaction pathways [
12,
13]. Additionally, two important signals—sample weight and temperature—should ideally be measured directly during experiments, as they are crucial for determining kinetic parameters [
14]. In conclusion, the key aspects of experimental research on MSW pyrolysis focus on obtaining reliable thermogravimetric data under conditions that closely resemble those found in a grate furnace. However, most experimental apparatus currently available do not meet these requirements. Common devices used to study MSW pyrolysis include thermogravimetric analyzers (TGAs) [
13,
15,
16,
17], macro TGAs [
18,
19,
20], drop tube furnaces [
21,
22], and fluidized bed reactors [
23]. A TGA [
13,
15,
16,
17] is a commonly applied device to conduct MSW pyrolysis studies due to its high level of automation and measurement resolution. The maximum heating rate of a conventional TGA is generally below 120 K/min [
15,
16]. Some studies have mentioned that certain advanced TGAs can achieve heating rates as high as 500 K/min [
13,
17]. However, such commercial TGAs are only capable of conducting experiments for samples smaller than 6 mm. In addition, the sample temperature is usually regarded to be the same as a furnace temperature [
24], which is not as reliable as the direct measurement by thermocouples. A macro TGA [
18,
19] is designed to accommodate larger samples, which functions similar to that of a TGA. Therefore, the drawbacks of TGAs, including the slow heating rate and inability to directly measure the sample temperature, still remain. A drop tube furnace [
21,
22] and fluidized bed reactor [
23] can perform fast heating pyrolysis experiments and record sample weight. However, these two devices also cannot directly obtain the sample temperature during pyrolysis. In order to conduct pyrolysis experiments with conditions similar to a grate furnace and record comprehensive and reliable thermogravimetric data, a newly built fast heating macro TGA (referred to as the Macro TGA) was applied. Due to its distinctive design, this device can perform pyrolysis experiments on centimeter-scale MSW samples at a fast heating rate, while measuring the on-line sample weight and temperature directly.
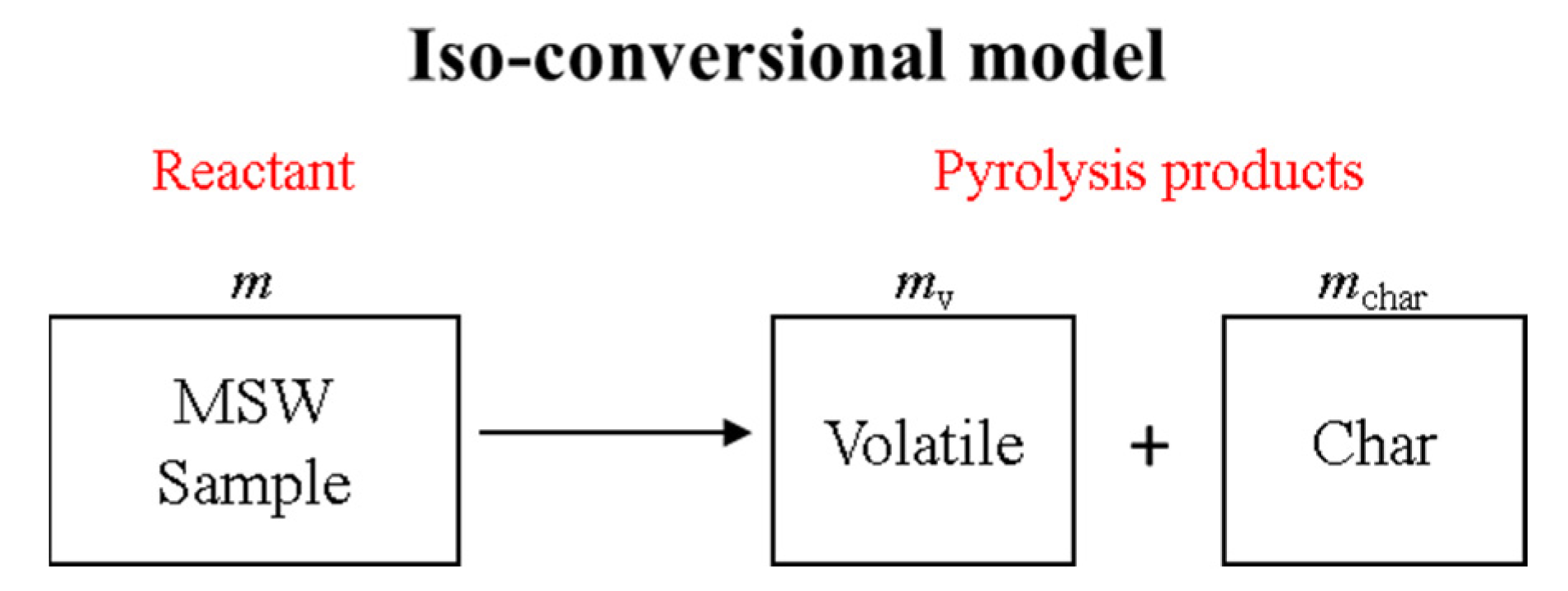
The most commonly used kinetic models for describing and predicting the characteristics of MSW pyrolysis are the iso-conversional model (ICM) and the distributed activation energy model (DAEM) [
25,
26,
27]. The ICM assumes that the MSW sample undergoes a single-step reaction during pyrolysis, with kinetic parameters such as the pre-exponential factor
A and activation energy
E varying as the conversion
X increases [
28,
29,
30,
31,
32,
33]. There are two main advantages of the ICM compared with other methods. Firstly, the ICM is a model-free method so that its calculation process to obtain kinetic parameters is concise, with no necessity to consider the pyrolysis mechanisms [
34]. Secondly, under most conditions, the prediction accuracy of the ICM is relatively accurate based on its principles. Nonetheless, the International Confederation for Thermal Analysis and Calorimetry (ICTAC) has highlighted that the ICM may not be appropriate for modeling pyrolysis in specific biomass and plastic samples with complex mechanisms [
35]. These samples can display characteristics such as parallel reactions, multi-step processes [
36], or catalytic effects. Furthermore, while the ICM typically offers reliable predictions, it falls short in providing a thorough description of MSW pyrolysis, particularly regarding the parallel reaction characteristics.
The DAEM posits that the sample consists of several characteristic components (CCs), with pyrolysis involving the parallel reactions of these CCs. During the pyrolysis process, each CC has its own weight fraction and kinetic parameters, undergoing a single-step reaction. Unlike the ICM, the parameters for each CC remain constant throughout pyrolysis. To determine the activation energy distribution, common approaches include both model-fitting and model-free methods. In model-fitting methods [
26,
37], the pyrolysis mechanism and kinetic parameters must first be established. Utilizing these parameters and mechanisms, the pyrolysis conversion and rate can then be calculated under various conditions. Optimization techniques, such as genetic algorithms (GA), can be employed to minimize errors in the kinetic parameter predictions. However, model-fitting methods often require significant computational resources. Conversely, the model-free method in DAEM typically segments the sample into a finite number of CCs with equal weight fractions [
38]. Generally, results obtained from methods in the ICM at each conversion value can inform the initial values of kinetic parameters for each CC [
38]. Nevertheless, the assumption that all CCs undergo single-step reactions limits the DAEM’s applicability for certain MSW samples, such as PET [
36].
In this study, several common issues encountered in the experimental and modeling research on MSW pyrolysis were addressed. A macro TGA was employed to investigate the pyrolysis characteristics of two representative MSW materials: cedar and polyethylene (PE). This device overcomes the limitations found in conventional equipment, such as the inability to conduct fast heating pyrolysis experiments and the lack of direct sample temperature measurements. Pyrolysis experiments were performed under both slow (10 K/min) and fast heating conditions (700, 800, and 900 °C). A newly developed discrete distributed activation energy model (DDAEM), in conjunction with a traditional ICM and DAEM, was utilized to analyze and predict pyrolysis characteristics under fast heating rates. The DDAEM, which is built upon the traditional DAEM, accommodates both single- and multi-step reaction pathways for each CC. As a result, the DDAEM offers improved predictions for the pyrolysis conversion and rate of PE compared to both the ICM and DAEM under fast heating conditions. Furthermore, the DDAEM facilitates a more thorough analysis of the factors contributing to the lower accuracy of the ICM and DAEM when predicting the pyrolysis of complex MSW samples.
5. Conclusions
This study employed the Macro TGA to perform pyrolysis experiments on cedar and PE under both slow (10 K/min) and fast (700, 800, and 900 °C) heating rates. A newly developed DDAEM, along with the traditional ICM and DAEM, was employed to predict pyrolysis characteristics under fast heating rates.
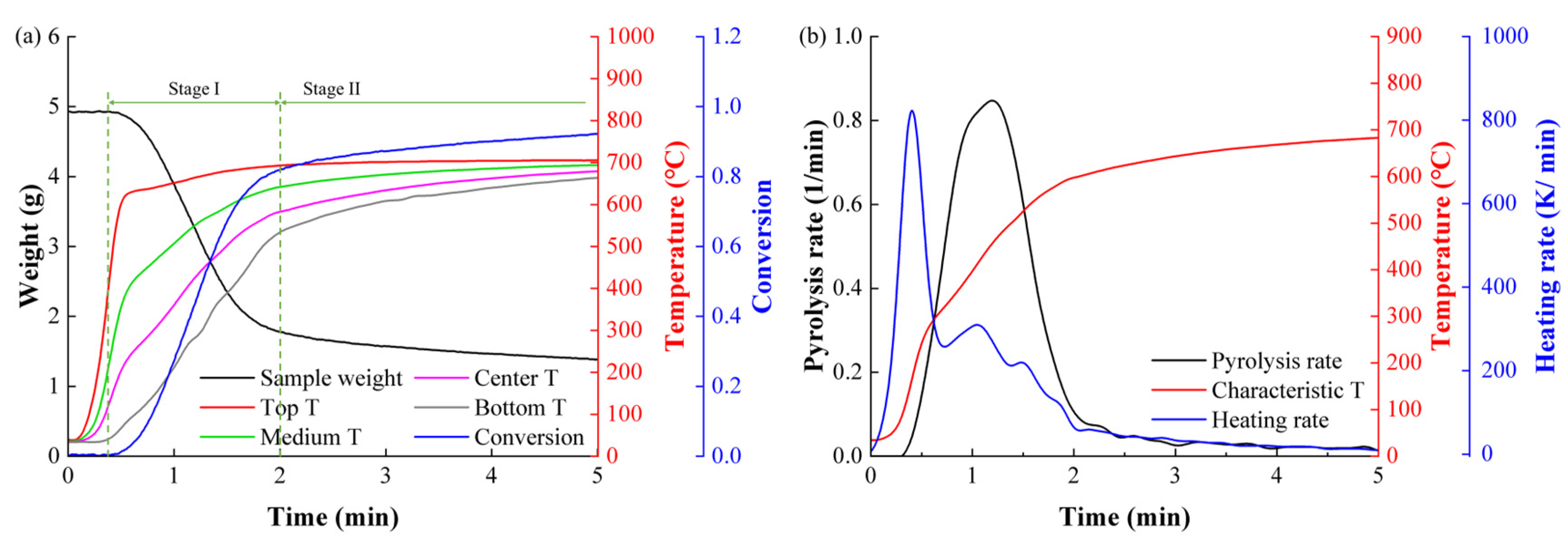
For cedar, the pyrolysis rate curve exhibited distinct shoulder-peak characteristics under slow heating, while only a single peak was observed under fast heating. At a fast heating rate of 700 °C, the pyrolysis rate for cedar increased from 0.685 to 0.847 min−1 as the temperature rose over 100 °C, from 351 to 455 °C. This phenomenon indicates that all CCs underwent single-step reactions. The average activation energies calculated from the ICM and DDAEM were 66.37 and 48.08 kJ/mol, respectively. Both the ICM and DDAEM provide accurate predictions of pyrolysis conversion and rate; however, the prediction accuracy of the DAEM is considerably lower.
The pyrolysis rate curve for PE displayed two prominent peaks under fast heating, whereas only one peak was evident under slow heating, highlighting a significant difference from cedar. At a fast heating rate of 700 °C, the rate increased from 0.217 to 1.008 min−1 with a smaller temperature rise from 630 to 656 °C. Therefore, some CCs in PE are considered to undergo multi-step pyrolysis reactions. The DDAEM demonstrated significantly higher predictive accuracy than the ICM and DAEM. The DDAEM showed that, as PE conversion increased from 0.25 to 0.65, the average activation energy of 58.32 kJ/mol calculated by ICM represented the final step reaction rather than the rate-limiting step.