Abstract
Garambullo fruit (Myrtillocactus geometrizans) is a rich source of phytochemical compounds that exhibit antioxidant, anti-hyperglycemic, and anti-inflammatory activities, helping to prevent diseases associated with oxidative stress. The objective of this study was to evaluate phenolic compound (PC), betalain (BL), betaxanthin (BX), and betacyanin (BC) contents, and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory) in microencapsulated garambullo extract during in vitro gastrointestinal digestion and storage. Microencapsulation was performed using spray drying. Arabic Gum (GA, 10% in feed solution) and soy protein isolate (SPI, 7% in feed solution) were used as wall materials. After in vitro digestion, the microcapsules (GA, SPI) exhibited higher bioaccessibility (p ≤ 0.05) of PC, BL, BX, and BC, and higher antioxidant activity (AA), compared to the non-encapsulated extract. Both microcapsules showed bioaccessibility in anti-hyperglycemic activity: α-amylase (GA: 90.58%, SPI: 84.73%), α-glucosidase (GA: 76.93%, SPI: 68.17%), and Dipeptidyl peptidase-4 (DPP-4) (GA: 52.81%, SPI: 53.03%); and in anti-inflammatory activity: cyclooxygenase-1 (COX-1) (GA: 78.14%, SPI: 77.90%) and cyclooxygenase-2 (COX-2) (GA: 82.77%, SPI: 84.99%). During storage, both microcapsules showed a similar trend with a significant decrease (p ≤ 0.05) in PC (GA: 39.29%, SPI: 39.34%), BL (GA: 21.17%, SPI: 21.62%), BX (GA: 23.89%, SPI: 23.45%), BC (GA: 19.55%, SPI: 19.84%), and AA (GA: 41.59%, SPI: 42.51%) after 60 days at 30 °C. Both microcapsules retained anti-hyperglycemic activity evaluated by the inhibitory activity of α-amylase (GA: 68.84%, SPI: 70.18%), α-glucosidase (GA: 59.93%, SPI: 58.69%), and DPP-4 (GA: 52.81%, SPI: 53.01%), and anti-inflammatory activity evaluated by the inhibitory activity of COX-1 (GA: 82.18%, SPI: 82.81%) and COX-2 (GA: 81.11%, SPI: 81.08%). Microencapsulation protected the phytochemical compounds and in vitro biological activities by allowing controlled release during in vitro digestion compared to the non-encapsulated extract. However, after 60 days storage at 30 °C, 60% of PC and AA, 80% of BL, BX, and BC, and 20–45% of the anti-hyperglycemic and anti-inflammatory activity were lost.
1. Introduction
The Cactaceae family is native to the American continent and comprises around 1400 species, of which Mexico harbors almost half (669 species), with 518 of these species being endemic to the country [1]. Myrtillocactus geometrizans, commonly known as garambullo, is one of these endemic species. The edible fruit of this species is a round-ovoid berry with an average diameter of 1.5 cm, which is purple in color when ripe. The thin skin encases a gelatinous pulp that contains numerous small black seeds [2].
Garambullo is underexploited and rarely commercialized due to its short shelf life, as it is highly perishable. Nevertheless, the fruit is an important source of health-promoting phytochemical compounds, including vitamin C, phenolic compounds (gallic acid, ellagic acid, caffeic acid, vanillin, quercetin, tannins, rutin, anthocyanins, and flavonols), and betalains. These compounds are known for their antioxidant properties, which help prevent diseases linked to oxidative stress; furthermore, these compounds also have anti-hyperglycemic and anti-inflammatory activity [3,4].
Extracted phytochemical compounds are sensitive to environmental conditions (humidity, temperature, light, and oxygen), the presence or absence of metals, enzymes (gastrointestinal digestion process), and storage conditions [5,6]. Therefore, it is crucial to employ protection mechanisms that preserve phytochemical compounds until their absorption in the intestine, ensuring they exert beneficial effects on human health.
Microencapsulation is a process that protects phytochemical compounds from unfavorable conditions and allows for controlled release of these compounds. Among the various microencapsulation techniques, spray drying is considered low-cost compared to other drying methods; it is a reliable technique and is widely used in food, pharmaceutical, and cosmetic industries [6]. Additionally, this drying method has been used to preserve the stability and antioxidant activity of phytochemical compounds of different sources [7].
To date, no studies have been found that analyze phenolic compound (PC), betalain (BL), betaxanthin (BX), and betacyanin (BC) contents, as well as in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory) in microencapsulated garambullo extract during the in vitro gastrointestinal digestion process and storage.
Previous studies on garambullo have typically focused on fresh fruit and juice, analyzing phytochemical compounds [5,8,9], biological activities (antioxidant, hypoglycemic, and anti-inflammatory) [3,4], and stability of phytochemical compounds during in vitro digestion [4,10]. Other studies have explored the microencapsulation of betalains obtained from garambullo fruit by spray drying [6], and antioxidant capacity and bioactive compound preservation post-drying (freeze-drying and Instant Controlled Pressure Drop) [11].
The studies have demonstrated that the phytochemical compounds of garambullo exhibit biological activity (antioxidant, anti-hyperglycemic, and anti-inflammatory). However, these biological properties have not been analyzed in microencapsulated garambullo extract after in vitro gastrointestinal digestion or during storage.
Based on the above, the objective of this study was to evaluate PC, BL, BX, BC, and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory) in microencapsulated garambullo extract during in vitro gastrointestinal digestion and storage.
The present research work analyzes, for the first time, the content of phytochemical compounds and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory) in microencapsulated garambullo extract during in vitro digestion and storage. The authors hope that the present work contributes to a better understanding and use of the garambullo fruit in the food industry as an ingredient to produce functional foods and, thereby, to obtain the benefits of the compounds present in this fruit.
2. Materials and Methods
2.1. Chemical and Reagents
All reagents were analytical grade. ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid), α-Amylase (EC 3.2.1.1), α-Glucosidase (EC 3.2.1.21), pepsin from porcine gastric mucosa (EC 3.4.23.1), pancreatin from porcine pancreas (EC 232.468.9), bile extract porcine (EC 232.369.0), Folin–Ciocalteu Reagent, gallic acid, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), and DPP4 inhibitor Screening Assay Kit were acquired from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Acarbose was obtained from Bayer AG (Leverkusen, Germany). COX Colorimetric Inhibitor Screening Assay Kit was obtained from Cayman Chemicals, Inc. (Ann Arbor, MI, USA). Gum arabic was acquired from Agricurama S.P.R. de R.L. de C.V. (Mexico City, Mexico) and soy protein isolate was obtained from Condimentos Naturales Tres Villas, S.A. de C.V. (Mexico City, Mexico).
2.2. Samples
The fruits of M. geometrizans were obtained from the Municipality of Valle de Santiago of the State of Guanajuato, Mexico (20°23′34″ N, 101°11′29″ W, 1720 m above sea level) in July 2023. The fruits were harvested at full ripeness, with no visible physical damage, pathogens, or insect-related harm. The selected fruits were washed with distilled water to remove soil and any foreign matter.
2.3. Storage and Freezing
Cleaned fruits were placed in vacuum-sealed polyethylene bags (Selovac, 200B, São Paulo, SP, Brazil) and stored in a freezer (Mabe, CHM11BPS0, Mexico City, Mexico) at −20 °C until analysis. This procedure was carried out on the same day the fruits were collected.
2.4. Extraction of Phytochemical Compounds
The fruits were thawed at room temperature (25 °C ± 1 °C) and placed on a metal tray for drying at 60 °C (Blue Lindberg V0914A, Thermo Scientific, Waltham, MA, USA) for 24 h. The dried fruits were then ground in a food processor (Magic Bullet, MBR-1101, Los Angeles, CA, USA) until a fine powder was obtained. This powder was sieved in a No. 60-mesh sieve (250 μm) to homogenize the particle size.
For the extraction of phytochemical compounds, garambullo powder was mixed with a 30% ethanol–water solution (v/v) in a 1:10 ratio and incubated at 30 °C (Blue Lindberg V0914A, Thermo Scientific, Waltham, MA, USA) for 30 min with constant stirring at 100 rpm. The supernatant was filtered using Whatman No. 4 paper and collected. A second extraction was performed under the same conditions. The two extracts were combined, centrifuged at 15,000× g (Cole-Parmer, DT1305044, CO, Eaton Socon, UK) for 15 min, and filtered again (Whatman No. 4). The filtrate was concentrated in a rotary evaporator (Büchi I-300 Pro, Flawil, Switzerland) at 55 rpm, 40 °C, and 77 mBar until a concentration of around 2 mg GAE/mL was obtained. The concentrated extract was used to determine PC, BL, BX, BC, and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory).
2.5. Analytical Methods
2.5.1. Quantification of PC
PC was analyzed using the methodology of Singleton and Rosi [12]. Absorbance was obtained at 750 nm (Jenway 6320D, Staffordshire, UK). The results are expressed as mg of gallic acid equivalents (GAE) per 100 g of dry weight (mg GAE/100 g dw).
2.5.2. Quantification of BL, BX, and BC
BL was determined by the method of MoBhammer et al. [13]. Absorbance was obtained (Jenway 6320D, Staffordshire, UK) at 480 nm for betaxanthins (BX) and 538 nm for betacyanin (BC). The results are expressed as mg of indicaxanthin equivalents (IE) per 100 g of dry weight (mg IE/100 g dw) for BX, and in mg of betanin equivalents per 100 g of dry weight (mg BE/100 g dw) for BC. BL was obtained by adding BX and BC content.
2.5.3. In Vitro Biological Activities
Antioxidant Activity (AA)
ABTS assay was assessed according to Brand-Williams et al. [14]. The absorbance was measured at 734 nm (Jenway 6320D, Staffordshire, UK). The results are expressed as µmol of Trolox equivalents (TE) per 100 g of dry weight (µmol TE/100 g dw).
Anti-Hyperglycemic Activity
Anti-hyperglycemic activity was determined by the inhibition of α-amylase, α-glucosidase, and Dipeptidyl peptidase-4 (DPP-4).
Inhibition of α-amylase (%) was performed by the method of Adisakwattana et al. [15]. The absorbance was measured at 540 nm (Jenway 6320D, Staffordshire, UK). Acarbose was used as a reference inhibitor (1 mg/mL).
Inhibition of α-glucosidase (%) was determined by the method of Bhatia et al. [16]. The absorbance was measured at 405 nm using a microplate reader (Varioskan Lux, Thermo Scientific, Waltham, MA, USA). Acarbose was used as a reference inhibitor (1 mg/mL).
Inhibition of DPP-4 (%) was assessed with the DPP4 inhibitor Screening Assay Kit (MAK203). The absorbance was measured at λex = 360 and λem = 460 nm using a microplate reader (Varioskan Lux, Thermo Scientific, Waltham, MA, USA). The kit used sitagliptin as a reference inhibitor.
Anti-Inflammatory Activity
Anti-inflammatory activity was determined by the inhibition of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). The inhibition of COX-1 (%) and COX-2 (%) was determined with a COX Colorimetric Inhibitor Screening Assay Kit. The absorbance was measured at 590 nm using a microplate reader (Varioskan Lux, Thermo Scientific, Waltham, MA, USA).
2.6. Microencapsulation
The concentrated extract was dried using a semi-pilot spray dryer (GEA-NIRO Atomizer, Mobile Minor, Copenhagen, Denmark), which was equipped with a two-fluid spraying nozzle and a cyclone separator to collect the powder. The air spraying pressure was 0.5 kgf/cm2. Microencapsulation was performed using the optimal conditions reported by Roa-Tort et al. [17]: 170 °C inlet air temperature (Ti) and 80 °C outlet temperature (T0). Two wall materials were selected (Gum arabic, GA 10%, and soy protein isolate, SPI 7%, in the feed solution) to determine which material provides greater protection to the phytochemical compounds during the in vitro digestion process and storage.
2.7. Characterization of the Microcapsules
2.7.1. Moisture Content and Water Activity (aw)
Moisture content was measured using the thermobalance method (OHAUS MB200, Montville, NJ, USA). Water activity (aw) was measured using an AquaLab Cx-2 (Decagon, Pullman, WA, USA).
2.7.2. Retention Efficiency
The retention efficiency percentage (%RE) was calculated using Equation (1) by relating the quantity of phytochemical compounds in the microcapsules to the quantity of these in the solution prior to drying (extract + wall material). For these determinations, 1 mL of the feed solution or reconstituted microcapsules (to the same concentration as the feed solution) was made up to 4 mL of a methanol:ethanol solution (1:1) and stirred with a vortex (MX-S, DLAB, Beijing, China) for 1 min (to precipitate the wall material), and centrifuged for 10 min at 3500 rpm. The supernatant was used for the analysis [18].
2.7.3. Particle Size Distribution
Particle size distribution and particle size were obtained with a laser diffraction particle size analyzer (IM 026 2006 series, Malvern, UK) with a 100 mm lens. Hexane was used as a dispersant to obtain particle size distribution, equivalent spherical diameter (D[4,3]), and the Sauter diameter (D[3,2]).
2.7.4. Morphology of Microcapsules
This analysis was obtained with a scanning electron microscope (SEM) (JSM-5800LV, Jeol, Peabody, MA, USA) operating at 5 kV and 1000× magnification.
2.8. Simulated Gastrointestinal Conditions
The bioaccessibility of PC, BL, BX, BC, and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory) was determined according to Brodkorb et al. [19] using the INFOGEST methodology (static in vitro simulation of gastrointestinal digestion). The INFOGEST protocol simulates digestion in the oral, gastric, and small intestine phases. The oral phase was carried out at 37 °C and pH 7, with 100 rpm stirring for 2 min, using α-amylase (75 U/mL). The next phase (gastric) was carried out at 37 °C and pH 2, with 100 rpm stirring for 120 min, using pepsin from porcine gastric mucosa (2000 U/mL). The final phase (intestinal) was carried out at 37 °C and pH 7, with 100 rpm stirring for 120 min, using pancreatin from porcine pancreas (100 U/mL) and porcine bile extract (160 mM). At the end of each phase (oral, gastric, and intestinal), aliquots were collected and centrifuged (Cole-Parmer, DT1305044, CO, UK) at 1796× g for 10 min. The supernatant was used to determine PC, BL, BX, BC, and in vitro biological activities (antioxidant, anti-hyperglycemic, and anti-inflammatory).
The effect of simulated gastrointestinal conditions on PC, BL, BX, BC, and in vitro biological activities was calculated using Equation (2) (recovery percentage, %R) and Equation (3) (bioaccessibility percentage, %B), which relates the content of PC, BL, BX, BC, or in vitro biological activities released at the end of oral and gastric phases (%R) and intestinal phase (%B) to the content before digestion.
2.9. Phytochemical Compounds and In Vitro Biological Activities During Storage
The microcapsules (GA and SPI) were placed in amber jars and stored at 30 °C (Blue Lindberg V0914A, Thermo Scientific, Waltham, MA, USA) for 60 days. Every 15 days, PC, BL, BX, BC, and AA were quantified [20]. Anti-hyperglycemic and anti-inflammatory activities were quantified at the beginning (day 0) and the end of storage (day 60).
2.10. Statistical Analysis
All analyses were performed in triplicate. The results were statistically analyzed using Minitab 19 software (Minitab, LLC., State College, PA, USA). Descriptive statistics (mean and standard deviation) were applied, along with one-way analysis of variance (ANOVA) and Tukey’s test for mean comparisons (significance level of 5%).
3. Results and Discussion
3.1. Phytochemical Compounds and In Vitro Biological Activities of Garambullo Extract
Table 1 shows that PC had the highest content (1423.81 ± 1.68 mg GAE/100 g dw), followed by BL (94.06 ± 1.51 mg/100 g dw), which is composed of BX (38.89 ± 1.39 mg IE/100 g dw) and BC (55.18 ± 1.02 mg BE/100 g dw). The contents of PC, BL, BX, and BC were higher than those reported by other authors [4,6]. The above may be attributed to factors such as the extraction process or fruit characteristics (ripeness and harvest season), agronomic practices, among others [6].

Table 1.
Phytochemical compounds and in vitro biological activities of garambullo extract.
In garambullo, PC has been associated mainly with AA [2]; therefore, the high PC content is related to the high AA (287,649.69 ± 11.47 μmol TE/100 g dw) obtained in the garambullo extract.
The garambullo extract exhibited anti-hyperglycemic activity (Table 1) by inhibiting the enzymes α-amylase (1.22 ± 0.04%), α-glucosidase (3.81 ± 0.15%), and DPP-4 (23.21 ± 0.61%). This indicates that the garambullo extract primarily inhibits the DPP-4 enzyme, by approximately 6 times more than α-glucosidase and 16 times more than α-amylase.
The anti-hyperglycemic activity of the garambullo extract has been previously studied [3,4], reporting low to moderate inhibition of α-amylase (6–15%) and high inhibition of α-glucosidase (30%). However, in the present study, the inhibition of both enzymes was comparatively low compared with the reports of these authors.
The anti-hyperglycemic activity of the garambullo extract can be attributed to phenolic compounds such as quercetin and betalains. It has been reported that the inhibition mechanism of α-amylase may be due to the disruption of the enzyme’s disulfide bridges by polyphenols [21].
On the other hand, the inhibition mechanism of α-glucosidase has been reported to be due to the binding of phenolic compounds such as flavonoids to the enzyme’s active site. However, some phenolic compounds compete with each other or bind to other enzyme sites, leading to low hydrophobic interaction, resulting in a low inhibitory response [22].
To date, the inhibition of DPP-4 in garambullo extract has not been reported; however, inhibition of this enzyme has been studied in other fruits [23], plants [24], and seeds [25]. The DPP-4 enzyme blocks incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are released from cells in response to the presence of nutrients. Therefore, inhibiting DPP-4 is important as it leads to higher levels of GLP-1 and GIP, facilitating glucose regulation by stimulating insulin secretion [26]. The results obtained in this work show that the extract primarily inhibits the DPP-4 enzyme, compared with α-glucosidase and α-amylase; therefore, this could be the main anti-hyperglycemic mechanism of the garambullo extract.
The garambullo extract exhibited anti-inflammatory activity (Table 1) by inhibiting the COX-1 (56.65 ± 0.25%) and COX-2 (59.02 ± 0.98%) enzymes. COX-2 inhibition is necessary to exert any anti-inflammatory activity, while COX-1 inhibition is associated with side effects, particularly gastrointestinal, renal, and anti-platelet aggregation effects of conventional non-steroidal anti-inflammatory drugs (NSAIDs) [27].
Phytochemical compounds (polyphenols, betalains) have been widely studied in various in vivo and in vitro investigations, demonstrating that they can reduce inflammatory agents such as tumor necrosis factor-α (TNF-α), cytokines (IL-6, 8, 1β), and cyclooxygenases (COX-1, COX-2). One of the main mechanisms is the reduction of reactive oxygen species (ROS) that attack cells and stimulate the production of growth factors that neutralize inflammatory agents [28,29].
3.2. Microencapsulation by Spray Drying with GA and SPI
Microencapsulation by spray drying had a yield of 86.26% and 36.81% with GA and SPI, respectively. Shofinita and Langrish [30] mentioned that a yield above 60% is considered efficient drying. For the GA solutions, a twin fluid spraying nozzle was used. On the other hand, due to the high viscosity of the protein isolate solution, for the SPI solutions a centrifugal disk was used as the spray system, resulting in a lower yield (36.81%) compared to GA (86.26%). Jansen-Alves et al. [31] reported that proteins form films that quickly become sticky, causing the solution to stick to the walls of the drying chamber, with the powder adhering to the equipment.
3.2.1. Moisture Content and Water Activity (aw) of Microcapsules
The moisture content and water activity of the GA microcapsules were 2.09 ± 0.14% and 0.23, respectively, while the SPI microcapsules had values of 2.63 ± 0.18% and 0.18, respectively. The moisture content obtained (GA: 2.09 ± 0.14%, SPI: 2.63 ± 0.18%) is typical for spray-dried products. Moisture values of 1–6% are desirable to preserve the stability of the microcapsules during storage [32]. The obtained aw values (GA: 0.23, SPI: 0.18) are below the minimum water activity (aw ≤ 0.6) required for the growth and proliferation of pathogenic microorganisms [6]. Therefore, the microcapsules obtained are stable against microbial deterioration.
3.2.2. Content and Retention (%) of Phytochemical Compounds of Microcapsules
The SPI microcapsules presented a slightly higher content (p ≤ 0.05) of phytochemical compounds (phenolic compounds, betaxanthins, and betacyanins) compared to the GA microcapsules (Figure 1a–d). This could be due to the lower wall percentage in SPI microcapsules (7%) compared to GA (10%), leading to a higher phytochemical compound content for the same mass of microcapsules due to a lower dilution effect.
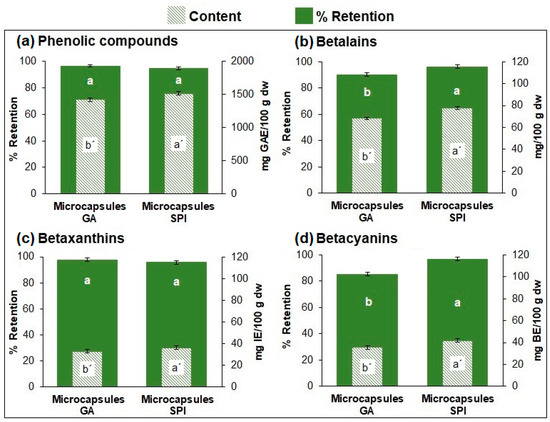
Figure 1.
Content and retention (%) of (a) phenolic compounds, (b) betalains, (c) betaxanthins, and (d) betacyanins of microcapsules. Results are expressed as mean ± standard deviation (n = 3). Different lowercase letters indicate significant difference (p ≤ 0.05) between samples. GA: Arabic Gum. SPI: soy protein isolate.
Regarding the retention of phytochemical compounds (Figure 1a–d), the results indicate that most (≥90%) of the phytochemical compounds were retained in the microcapsules with both wall materials. The statistical analysis showed differences between both capsules for betalains and betacyanins, but not for phenolic compounds and betaxanthins.
3.2.3. Retention (%) and Content of Antioxidant Activity and Inhibition (%) of In Vitro Biological Activities of Microcapsules
The SPI microcapsules exhibited a higher content (p ≤ 0.05) of AA (377,289.46 μmol TE/100 g dw) compared to the GA microcapsules (316,853.26 μmol TE/100 g dw) (Figure 2a). This is due to the higher phytochemical compound content (phenolic compounds, betaxanthins, and betacyanins) in SPI microcapsules compared to GA microcapsules (Figure 1a–d). Consequently, the higher content of phytochemical compounds in SPI microcapsules resulted in higher AA. On the other hand, both microencapsulates retained AA above 90% (GA: 92.03%, SPI: 92.57%) with no significant difference (p ≤ 0.05) (Figure 2a).
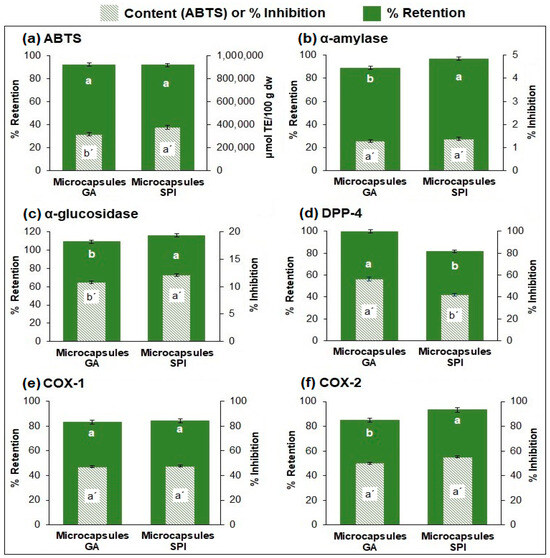
Figure 2.
Retention (%) and content of AA, and inhibition of in vitro biological activities of microcapsules. (a) Antioxidant activity, (b–d) anti-hyperglycemic activity (α-amylase, α-glucosidase, DPP-4), (e,f) anti-inflammatory activity (COX-1 and COX-2). Results are expressed as mean ± standard deviation (n = 3). Different lowercase letters indicate significant difference (p ≤ 0.05) between samples. GA: Arabic gum. SPI: soy protein isolate.
Regarding anti-hyperglycemic activity (Figure 2b–d), both microcapsules primarily inhibited the DPP-4 enzyme (GA: 56.24%, SPI: 42.23%), followed by α-glucosidase (GA: 10.82%, SPI: 12.18%), and finally α-amylase (GA: 1.28%, SPI: 1.38%). The inhibition retention for all three enzymes was above 80%. For α-amylase and α-glucosidase, retention was statistically higher for SPI microcapsules, while for DPP-4, retention was statistically higher for GA microcapsules.
Concerning anti-inflammatory activity (Figure 2e,f), both microcapsules inhibited COX-1 (GA: 47.14%, SPI: 47.71%) and COX-2 (GA: 50.05%, SPI: 55.06%) enzymes with no significant difference. The retention of the inhibition of both enzymes was over 85%, showing for COX-2 a slightly higher retention (statistically difference) for SPI microcapsules.
3.2.4. Particle Size Distribution of Microcapsules
Table 2 shows that microcapsules with SPI had a significantly larger (p ≤ 0.05) Sauter diameter (D[3,2]) and equivalent sphere diameter (D[4,3]) than the microcapsules with GA. This could be because the percentage of solids used in the solution before drying was 7% for SPI, while for GA it was 10%.

Table 2.
Particle size distribution of microcapsules.
The span value represents the width of the particle size distribution, and there is a direct relationship between this value and particle size dispersion; the lower the span value, the greater the uniformity of size distribution [33]. In Table 2, although the span value is significantly higher (p ≤ 0.05) in SPI microcapsules, the dispersion is quite similar to the GA size distribution (Figure 3).
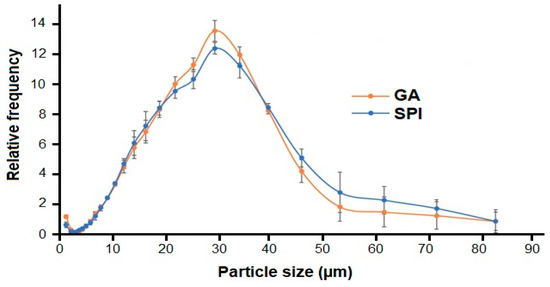
Figure 3.
Particle size distribution of microcapsules. GA: Arabic gum. SPI: soy protein isolate.
The GA and SPI particles exhibited homogeneous and monomodal distribution (Figure 3). The size of GA and SPI microcapsules ranged from 1.21 μm to 83.65 μm, with most particles concentrated in the 25 to 35 μm range.
3.2.5. Morphology of Microcapsules
Microcapsules with GA and SPI exhibited shrunken spherical shapes with wrinkled surfaces and concavities (Figure 4), but shrinkage was more pronounced in SPI microcapsules due to both the nature of the wall material (a protein) and the fact that a smaller amount of wall material was used compared to microcapsules with GA (7% compared with 10% respectively); although GA microcapsules also present wrinkled surfaces and concavities, they tend to be more spherical.
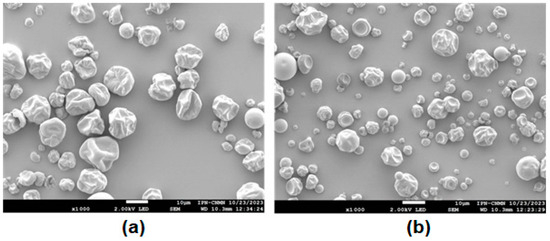
Figure 4.
Micrographs of microcapsules: (a) GA and (b) SPI at 1000×. GA: Arabic gum. SPI: soy protein isolate.
As can be seen in Figure 4, in general, due to the higher viscosity of the solution, SPI microcapsules are on average larger than GA microcapsules, which is consistent with the results in Table 2. The shapes of particles with both GA and SPI are similar to those reported by Ramirez-Damián et al. [34] who encapsulated red banana peel extract.
No microparticles with cracks or ruptures were observed, indicating that the phytochemical compounds were adequately protected. The morphological irregularities or wrinkles are attributed to the rate of water evaporation occurring during spray drying. It has been reported that the higher the drying temperature (faster evaporation), the smoother and more defined the surfaces; conversely if the temperature is not high, the particles shrink during the moisture evaporation process [35].
Similar results were obtained by Rocha et al. [36], who observed wrinkles and non-homogeneous sphericity in microcapsules of jabuticaba, jussara, and blueberry extracts, which were associated with factors such as wall material moisture, solid content, and drying temperature.
3.2.6. Bioaccessibility of Phytochemical Compounds and In Vitro Biological Activities Under Simulated Gastrointestinal Conditions
Gastrointestinal simulation was conducted with the concentrated extract (with no wall material) and the microcapsules (GA, SPI). Figure 5a–e shows the percentage of recovery (oral and gastric phases) and the percentage of bioaccessibility (intestinal phase) of the phytochemical compounds and AA in the concentrated extract and the microcapsules.
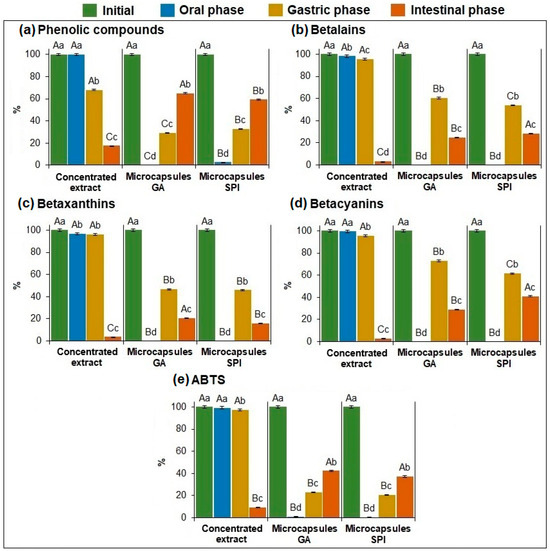
Figure 5.
Recovery (%) and bioaccessibility (%) of: (a) phenolic compounds, (b) betalains, (c) betaxanthins, (d) betacyanins, and (e) antioxidant activity of the concentrated extract and microcapsules. Data represent mean ± standard deviation (n = 3). Different capital letters indicate significant differences (p ≤ 0.05) in the same group (initial content, oral, gastric, and intestinal phases). Different lowercase letters indicate significant differences (p ≤ 0.05) in the same sample. GA: Arabic gum. SPI: soy protein isolate.
Regarding phenolic compounds (Figure 5a), for the concentrated extract, the recovery percentage in the oral phase is the same as in the original one, indicating that there was no loss (no statistical difference) in phenolic compounds in this phase. For both microcapsules (GA and SPI), the recovery percentage during the oral phase was very low (GA: 0.002%, SPI: 2.72%), indicating that the phenolic compounds have not been released due to the protection provided by the wall materials.
In the gastric phase, the concentrated extract showed a significantly (p ≤ 0.05) lower recovery percentage (68.02%) compared to the oral phase (100%) because the extract was in direct contact with the gastric medium, degrading part of the phenolic compounds. In contrast, in both microcapsules, the recovery percentage was significantly (p ≤ 0.05) higher (GA: 29.07%, SPI: 32.49%) than in the oral phase (GA: 0.002%, SPI: 2.72%). This may be due to the interaction with the digestive enzymes during the gastric phase, which allows the partial release of phenolic compounds.
In the intestinal phase, the concentrated extract exhibited low bioaccessibility (17.59%), indicating the loss/degradation of phenolic compounds due to their instability in high-pH conditions [37]. On the other hand, microcapsules, having the protection of wall materials had significantly (p ≤ 0.05) higher bioaccessibility (GA: 64.94%, SPI: 59.15%) compared to the concentrated extract (17.59%). This indicates that both wall materials protected the phenolic compounds during digestion, making them available for potential absorption. Figure 5a shows a slight but statistically higher bioaccessibility for GA than SPI microcapsules.
Regarding betalains (Figure 5b), betaxanthins (Figure 5c), and betacyanins (Figure 5d), for the concentrated extract it is shown that there was no loss of these compounds in the oral and gastric phase, due to the stability of these compounds at low pH [4], but bioaccessibility was very low (BT: 3.27%, BX: 3.69%, BC: 2.97%), indicating the degradation of these compounds in the concentrated extract which have no protection with the wall material.
On the other hand, in both microcapsules, as happened with phenolic compounds, the recovery percentage during the oral phase was very low for GA (BT: 0.04%, BX: 0.04%, BC: 0.04%) and SPI (BT: 0.04%, BX: 0.05%, BC: 0.04%), indicating that BT, BX, and BC have not been released due to the protection provided by the wall materials. Conversely, during the gastric phase, both microcapsules showed a significant (p ≤ 0.05) increase in recovery percentage of betalains (GA: 60.41%, SPI: 53.82%), betaxanthins (GA: 46.59%, SPI: 45.93%), and betacyanins (GA: 73.03%, SPI: 61.46%), indicating that around 50% of the encapsulated material was released during the gastric phase. There was a significant decrease (p ≤ 0.05) in the three compounds from the gastric phase to the intestinal phase, showing bioaccessibility for betalains (GA: 25.03%, SPI: 28.59%), betaxanthins (GA: 20.64%, SPI: 15.73%), and betacyanins (GA: 29.03%, SPI: 41.04%), which were considerably higher than those obtained in the concentrated extract, making them available for potential absorption. This indicates again that both wall materials gave protection to these compounds during digestion.
Regarding antioxidant activity in the concentrated extract and both microcapsules (Figure 5e), the same trend described for phenolic compounds (Figure 5a) was observed. This is because, in garambullo, the antioxidant activity is primarily due to phenolic compounds [2]. GA and SPI microcapsules had low recovery percentages in the oral (GA: 0.98%, SPI: 0.62%) and gastric phases (GA: 23.18%, SPI: 20.46%), indicating that the antioxidant compounds were not fully released. However, in the intestinal phase, both microcapsules showed significantly (p ≤ 0.05) higher bioaccessibility (GA: 42.32%, SPI: 37.08%) compared to the concentrated extract (9.23%). This indicates that the encapsulated extract with both wall materials retained the antioxidant activity of the phytochemical compounds compared to the concentrated extract.
Bioaccessibility of anti-hyperglycemic activity (α-amylase, α-glucosidase, DPP-4) and anti-inflammatory activity (COX-1, COX-2) in the concentrated extract and microcapsules (GA, SPI) are shown in Figure 6a,b. In vitro bioaccessibility of both biological activities was determined only at the end of the digestion process, referring to the initial inhibitory capacity before digestion.
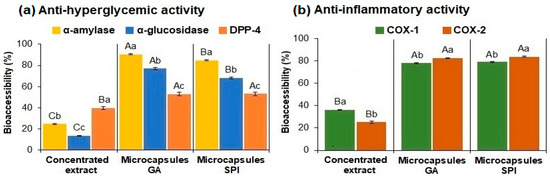
Figure 6.
Bioaccessibility of: (a) anti-hyperglycemic activity (α-amylase, α-glucosidase, DPP-4) and (b) anti-inflammatory activity (COX-1, COX-2) of the concentrated extract and microcapsules. Data represent mean ± standard deviation (n = 3). Different capital letters indicate significant differences (p ≤ 0.05) in the same enzyme between samples. Different lowercase letters indicate significant differences (p ≤ 0.05) in the same sample. GA: Arabic gum. SPI: soy protein isolate.
Regarding the inhibitory effect on α-amylase (Figure 6a), the GA microcapsules exhibited 90.58% bioaccessibility (equivalent to 1.16% inhibition), while the SPI microcapsules exhibited 84.73% (1.17% inhibition). In contrast, the concentrated extract showed significantly (p ≤ 0.05) lower bioaccessibility (24.45%) (0.25% inhibition). The wall materials provided protection from the conditions present during digestion, resulting in a higher percentage of bioaccessibility in the microcapsules compared to the concentrated extract.
Regarding α-glucosidase (Figure 6a), both the concentrated extract and the microcapsules followed the same trend as α-amylase. GA microcapsules showed 76.93% bioaccessibility (8.76% inhibition), while the SPI microcapsules exhibited 68.17% (8.27% inhibition). In contrast, the concentrated extract showed significantly (p ≤ 0.05) lower bioaccessibility (13.41%) (0.53% inhibition).
Regarding DPP-4 (Figure 6a), GA microcapsules exhibited 52.81% bioaccessibility (29.71% inhibition) and the SPI microcapsules 53.03% (27.39% inhibition); there was no significant difference (p ≤ 0.05) between these values. The concentrated extract showed significantly (p ≤ 0.05) lower bioaccessibility (40.15%) (11.69% inhibition).
These results indicate that the wall materials used provided protection against the conditions present during digestion, resulting in higher bioaccessibility in the microcapsules compared to the concentrated extract (p ≤ 0.05), thus allowing the wall materials to retain anti-hyperglycemic activity.
Regarding the anti-inflammatory activity (Figure 6b), for COX-1, the microcapsules with GA exhibited 78.14% bioaccessibility (36.83% inhibition), while the microcapsules with SPI showed 77.90% bioaccessibility (40.55% inhibition), with no significant difference (p ≤ 0.05) between the values. Conversely, the concentrated extract showed significantly (p ≤ 0.05) lower bioaccessibility (36.21%) (20.51% inhibition). The COX-2 displayed the same trend as that observed for COX-1. The microcapsules with GA exhibited 82.77% bioaccessibility (41.43% inhibition), and the microcapsules with SPI demonstrated 84.99% (42.89% inhibition), with no significant difference (p ≤ 0.05). The concentrated extract presented significantly (p ≤ 0.05) lower bioaccessibility (25.19%) (14.86% inhibition).
Digestion is a complex process involving enzymes and changes in pH; however, the results previously shown indicate that the microencapsulation process provided protection to the phytochemical compounds and in vitro biological activities, compared to the concentrated extract, by allowing a controlled release during the in vitro digestion process.
3.2.7. Retention (%) of Phytochemical Compounds and In Vitro Biological Activities During Storage
Figure 7a,b show retention (%) of phenolic compounds, betalains, betaxanthins, betacyanins, and antioxidant activity during the storage of microcapsules (GA, SPI) at 30 °C.
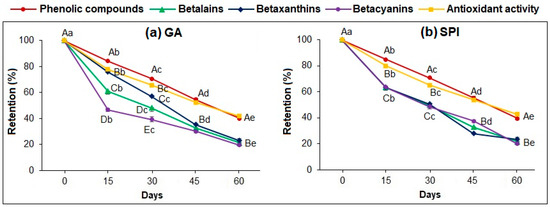
Figure 7.
Retention (%) of: phenolic compounds, betaxanthins, betacyanins and antioxidant activity of microcapsules during storage at 30 °C: (a) GA and (b) SPI. Data represent mean ± standard deviation (n = 3). Different capital letters indicate significant differences (p ≤ 0.05) between phytochemical compounds. Different lowercase letters indicate significant differences (p ≤ 0.05) in the same phytochemical compound. GA: Arabic gum. SPI: soy protein isolate.
Both microcapsules exhibited a decreasing trend (p ≤ 0.05) for all phytochemicals and antioxidant activity analyzed, obtaining at the end of the storage time (60 days) the following retention: PC (GA: 39.29%, SPI: 39.34%); BL (GA: 21.17%, SPI: 21.62%); BX (GA: 23.89%, SPI: 23.45%); BC (GA: 19.55%, SPI: 19.84%), and AA (GA: 41.59%, SPI: 42.51%).
For both microcapsules, there was no significant difference (p ≤ 0.05) in the retention percentage between PC and AA; however, there was a significant difference (p ≤ 0.05) in the retention percentages of BL, BX, and BC during storage time, but at the end of the 60 days, there was no significant difference in any of the compounds analyzed. This indicates again that antioxidant activity is attributed to the phenolic compounds, in agreement with the report of Ramírez-Rodríguez et al. [2].
Retention of a phytochemical compound in the microcapsule during storage is influenced by the concentration gradient and the attractive forces between them, such as hydrogen bonding, Van der Waals forces, cross-linking degree, and crystallinity. Additionally, the release or retention of a compound is controlled by the solubility and permeability of the core material in the protective wall [6]. Aldana et al. [38] reported that the stability of the microcapsules is determined by whether its transition temperature is higher than the storage temperature; thus, the core material is released by diffusion at a rate that increases with temperature.
Retention (%) of anti-hyperglycemic and anti-inflammatory activity (Table 3) was determined only at the end (day 60) of storage, referring to the initial inhibitory capacity at the beginning of storage (day 0).

Table 3.
Retention (%) of anti-hyperglycemic activity (α-amylase, α-glucosidase, DPP-4) and anti-inflammatory activity (COX-1, COX-2) at the end of storage (day 60) at 30 °C in microcapsules.
At the end of the storage period (day 60), both microcapsules exhibited retention of anti-hyperglycemic activity: α-amylase (GA: 68.84%, SPI: 70.18%), α-glucosidase (GA: 59.93%, SPI: 58.69%), DPP-4 (GA: 52.81%, SPI: 53.01%), and anti-inflammatory activity: COX-1 (GA: 82.18%, SPI: 82.81%), COX-2 (GA: 81.11%, SPI: 81.08%).
Regarding anti-hyperglycemic activity, it is noteworthy that although the retention of the inhibition percentage in both microcapsules was lower for DPP-4 than for α-glucosidase and α-amylase, the remaining inhibitory activity of DPP-4 (GA: 29.70% inhibition; SPI: 22.39% inhibition) was 5 times larger than for α-glucosidase (GA: 6.16% inhibition, SPI: 6.54% inhibition) and 20 times larger than for α-amylase (GA and SPI: 0.95% inhibition).
For anti-inflammatory activity remaining at the end of storage, there was no significant difference (p ≤ 0.05) between COX-1 (GA: 38.74% inhibition, SPI: 38.68% inhibition) and COX-2 (GA: 44.11% inhibition, SPI: 45.59% inhibition) for both microcapsules, indicating that the wall materials used enabled the retention of anti-inflammatory activity during storage.
4. Conclusions
Garambullo is a good source of phytochemical compounds (polyphenols, betaxanthins, and betacyanins) with antioxidant, anti-hyperglycemic, and anti-inflammatory activities. Therefore, it was of utmost importance to protect these extracted compounds and their biological activities by microencapsulation using GA and SPI as wall materials. The microcapsules obtained provided protection to the phytochemical compounds and preserved antioxidant, anti-hyperglycemic, and anti-inflammatory activities during in vitro digestion by allowing their controlled release, as compared with the concentrated extract. However, after 60 days of storage at 30 °C, 60% of PC and AA, 80% of BL, BX, and BC, and 20–45% of the anti-hyperglycemic and anti-inflammatory activities were lost. Therefore, more research is needed, with the aim of increasing the wall material content to try to improve the retention of these compounds and their biological activity during storage at different temperatures.
Author Contributions
Conceptualization: I.R.-A., O.G.M.-M. and G.O.-R.; Methodology: I.R.-A., O.G.M.-M., G.O.-R., T.G.-V. and O.A.R.-M.; Formal Analysis: O.G.M.-M., G.O.-R., T.G.-V. and O.A.R.-M.; Investigation: I.R.-A.; Resources: O.G.M.-M. and G.O.-R.; Writing—Original Draft Preparation: O.G.M.-M.; Writing—Review and Editing: O.G.M.-M. and G.O.-R.; Visualization: O.G.M.-M. and G.O.-R.; Supervision: O.G.M.-M. and G.O.-R.; Project Administration: O.G.M.-M. and G.O.-R.; Funding Acquisition: O.G.M.-M. and G.O.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Authors thank to Instituto Politécnico Nacional for the financial support through grant SIP-20220180, 20230301, 20240041. I.R.-A. wishes to express his gratitude to Consejo Nacional de Humanidades, Ciencias y Tecnología (CONAHCYT) for the scholarship provided with number 1206193.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Authors wish to thank to Consejo Nacional de Humanidades, Ciencias y Tecnología (CONAHCYT) and Secretaría de Investigación y Posgrado-Instituto Politécnico Nacional (SIP-IPN).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SEMARNAT (Secretaría de Agricultura y Desarrollo Rural). El Garambullo una Oportunidad Para Prosperar. Available online: https://www.gob.mx/agricultura/articulos/el-garambullo-una-oportunidad-para-prosperar (accessed on 1 September 2024).
- Ramírez-Rodríguez, Y.; Martínez Huélamo, M.; Pedraza Cheverri, J.; Ramírez, V.; Martínez Tagueña, N.; Tujillo, J. Ethnobotanical, nutritional and medicinal properties of Mexican drylands Cactaceae Fruits: Recent findings and research opportunities. Food Chem. 2020, 312, 126073–126086. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; Martínez-Samayoa, P.; Ramos-Gómez, M.; Guzmán, H.; Salgado, L.M. Antidiabetic and renal protective properties of berrycactus fruit (Myrtillocactus geometrizans). J. Med. Food. 2015, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.; Cano, M.P. In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087–128098. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Hernández, M.G.; Guevara-Lara, F.; Reynoso-Camacho, R.; Guzmán-Maldonado, S.H. Effects of madurity stage and storage on cactus Berry (Myrtillocactus geometrizans) phenolics, vitamin C, betalains and their antioxidant properties. Food Chem. 2011, 129, 1744–1750. [Google Scholar] [CrossRef]
- Gómez-Espinoza, D.; Ríos-Fuentes, B.; Aguirre-Mancilla, C.L.; Villaseñor-Ortega, F.; Pérez-Pérez, M.C.I. Microencapsulation of betalains obtained from garambullo fruit (Myrtillocactus geometrizans) by spray drying. Rev. Mex. Ing. Quim. 2024, 23, 24247–24258. [Google Scholar] [CrossRef]
- Rios-Aguirre, S.; Gil-Garzón, M.A. Microencapsulation of Bioactive Compounds in Diverse Matrices by Spray Drying: A Literature Review. TecnoLógicas 2021, 24, e1836–e1846. [Google Scholar] [CrossRef]
- García-Barrera, F.A.; Reynoso, C.R.; González de Mejía, E. Stability of betalains extracted from garambullo (Myrtillocactus geometrizans). Food Sci. Technol. Int. 1998, 4, 115–120. [Google Scholar] [CrossRef]
- Vázquez-Cruz, M.A.; Jiménez-García, S.N.; Torres-Pacheco, I.; Guzmán-Maldonado, S.H.; Guevara-González, R.G.; Miranda-López, R. Effect of maturity stage and storage on flavor compounds and sensory description of berrycactus (Myrtillocactus geometrizans). J. Food Sci. 2012, 77, C366–C373. [Google Scholar]
- Sánchez-Recillas, E.; Campos-Vega, R.; Pérez-Ramírez, I.F.; Luzardo-Ocampo, I.; Cuéllar-Núñez, M.L.; Vergara-Castañeda, H.A. Garambullo (Myrtillocactus geometrizans): Effect of in vitro gastrointestinal digestion on the bioaccessibility and antioxidant capacity of phytochemicals. Food Funct. 2022, 13, 4699–4713. [Google Scholar] [CrossRef]
- Santiago-Mora, P.D.; Cardador-Martínez, A.; Téllez-Pérez, C.; Montejano-Gaitan, J.G.; Martin del Campo, S.T. In-vitro antioxidant capacity and bioactive compounds preservation post-drying on berrycactus (Myrtillocactus geometrizans). J. Food Res. 2017, 6, 121–133. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic and reagents. AJEV 1965, 16, 144–158. [Google Scholar] [CrossRef]
- MoBhammer, M.R.; Stinzing, F.C.; Carle, R. Colour studies on fruit juice blends from Opuntia and Hylocereus cacti and betalain-containing model solution derived therefrom. Food Res. Int. 2005, 38, 975–981. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical methods to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement Altern. Med. 2019, 19, 74. [Google Scholar] [CrossRef]
- Roa-Tort, A.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Ramos-Monroy, O.A. Extraction and microencapsulation of phytochemical compounds from mango peel (Mangifera indica L.) var. “Kent” and assessment of bioaccessibility through in vitro digestion. Processes 2024, 12, 154. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Recio, I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef]
- Oboh, H.A.; Obayiuwana, O.A.; Aihie, E.O.; Iyayi, J.I.; Udoh, E.J. Beetroot (Beta vulgaris) juice inhibits key carbohydrate metabolizing enzymes associated with type II diabetes. Nig. J. Basic Appl. Sci. 2020, 28, 1–6. [Google Scholar]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Miyuki, K.; Yamane, T.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Identification and characterization of a dipeptidyl peptidase IV inhibitor from aronia juice. BBRC 2015, 465, 433–436. [Google Scholar]
- Chakrabarti, R.; Bhavtaran, S.; Narendra, P.; Varghese, N.; Vanchhawng, L.; Mohamed, H.; Thirumurugan, K. Dipeptidyl Peptidase- IV Inhibitory Activity of Berberis aristata. J. Nat. Prod. 2011, 4, 158–163. [Google Scholar]
- Malik, A.; Aziz, G.M.; Adhiah, A.H. The Potential of some Plant Extracts as Radical Scavengers and Dipeptidyl Peptidase-4 Inhibitors. Baghdad Sci. J. 2019, 16, 0162. [Google Scholar]
- Kazeem, M.; Bankole, H.; Ogunrinola, O.; Wusu, A.; Kappo, A. Functional foods with dipeptidyl peptidase-4 inhibitory potential and management of type 2 diabetes: A review. Food Front. 2021, 2, 153–162. [Google Scholar] [CrossRef]
- Salazar, E.; Orellana, A.; Pimentel, E. Inhibidores específicos de Cox-2: Riesgo para el paciente Cardiópata? Acta Odontol. Venez. 2004, 42, 58–59. [Google Scholar]
- Allegra, M.; Tesoriere, L.; Livrea, M.A. Betalain inhibits the myeloperoxidase/nitrite-induced oxidation of human low-density lipoproteins. Free Rad. Res. 2007, 41, 335–341. [Google Scholar] [CrossRef]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from cactus pear fruit exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef]
- Shofinita, D.; Langrish, T.A.G. Spray drying of orange peel extracts: Yield, total phenolic content and economic evaluation. J. Food Eng. 2014, 130, 31–42. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Fernandes, K.F.; Crizel-Cardozo, M.M.; Krumreich, F.D.; Borges, C.D.; Zambiazi, R.C. Microencapsulation of Propolis in Protein Matrix Using Spray Drying for Application in Food Systems. Food Bioprocess Technol. 2018, 11, 1422–1436. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, Y.S. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020, 269, 109744–109754. [Google Scholar] [CrossRef]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Damián, M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Téllez-Medina, D.I.; Ramos-Monroy, O.A. Microencapsulation of red banana peel extract and bioaccessibility assessment by in vitro digestion. Processes 2022, 10, 768. [Google Scholar] [CrossRef]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.; Calderas, F.; González-Laredo, R.; Rocha-Guzmán, N.; Ochoa-Martínez, L.; Bernand-Bernand, M. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT-Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Rocha, J.; de Barros, F.; Perrone, T.; Viana, K.; Tavares, G.; Stephani, R.; Stringheta, P. Microencapsulation by atomization of the mixture of phenolic extracts. Powder Technol. 2019, 343, 317–325. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885–125898. [Google Scholar] [CrossRef]
- Aldana, A.S.; Sandoval, E.R.; Aponte, A.A. Encapsulación de aditivos para la industria de alimentos. Ing. Comp. 2024, 5, 73–83. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).