1. Introduction
Tailings are the primary solid waste generated during ore beneficiation and are often stored in tailing ponds. When exposed to the natural environment, tailings interact with air and precipitation, resulting in complex ion dissolution processes [
1,
2]. The leaching of heavy metal ions poses significant risks to both environmental and human health. Tailings are characterized by large quantities, fine particle sizes, and the presence of various adsorbed heavy metals and contaminants on their surfaces [
3]. Open-pit storage of these tailings not only occupies a substantial amount of land but also poses serious environmental risks to the surrounding ecosystem. On one hand, fine tailing particles can easily be dispersed as dust under the influence of wind, and once airborne, these dust particles can be transported over long distances by atmospheric turbulence, contributing to atmospheric pollution. On the other hand, tailings are prone to the leaching and release of heavy metals due to the flushing and infiltration of rainwater, leading to the transportation of contaminants via surface runoff and leachate into nearby surface and groundwater systems [
3]. Such processes can result in the contamination of downstream farmlands and water bodies [
4,
5,
6]. In the lead–zinc tailing pond located in northern Guangxi, China (Latitude: 24.2293° N, Longitude: 109.3641° E), heavy metals in downstream agricultural soils have been reported to exceed national safety standards (Pb: 1000 mg/kg, Zn: 1000 mg/kg, Cd: 20 mg/kg), posing potential risks to local agriculture and water systems [
7]. This elevated concentration, in the range of 1150–1350 mg/kg for Pb, 1300–1800 mg/kg for Zn, and 5–15 mg/kg for Cd, combined with the high bioavailability and strong mobility of these heavy metals, results in persistent and recurrent contamination of the local environment, making it a significant environmental concern [
8]. Hence, understanding the leaching mechanisms and influencing factors of heavy metals in tailings is essential for developing effective environmental management strategies.
Acid mine drainage (AMD) from sulfide-rich tailings is a widespread environmental hazard that dramatically increases the release of heavy metals, particularly in areas that are vulnerable to acid rain, such as South China [
9,
10,
11]. Acid rain, characterized by sulfuric and nitric acids, typically exhibits low pH values ranging from 2.0 to 4.0 in this region, creating conditions that enhance the mobility of heavy metals. However, the behavior of heavy metals in low-sulfur tailings with high acid neutralization capacity remains largely unknown. Unlike sulfide-rich tailings, which are prone to generating AMD and releasing toxic metals under acidic conditions, the tailings in northern Guangxi are rich in limestone, imparting a high acid neutralization capacity that theoretically mitigates heavy metal leaching [
12,
13]. Low-sulfide tailings, like those studied here, account for approximately 15–20% of the total tailings produced in China, primarily found in regions with significant limestone deposits. Nevertheless, even with this buffering capacity, ion exchange reactions between hydrogen ions (H
+) and heavy metal cations (e.g., Pb
2+, Zn
2+, and Cd
2+) can still occur under acidic conditions, as acid rain can temporarily overwhelm the acid neutralization capacity (ANC) of the tailings. This process facilitates the leaching of heavy metals, increasing their environmental risks [
14]. As a result, investigating the leaching characteristics of low-sulfur tailings under varying acidic conditions becomes crucial for assessing their potential environmental risks.
To comprehensively evaluate these behaviors, it is necessary to consider multiple factors that influence the release of heavy metals from tailings. The release of toxic metals from tailings is influenced by various factors, including pH, particle size, weathering degree, metal concentration, bioavailability, geochemical composition, bacterial activity, and vegetation growth. Numerous studies have demonstrated that acid rain can enhance the mobility of heavy metals in soil ecosystems due to ion exchange reactions between heavy metals and hydrogen ions, which are dominant under acidic conditions. An acidic environment facilitates the release or dissolution of metal ions, leading to elevated concentrations of these ions in both soil and water bodies. For example, the displacement of cations such as Pb, Cd, and Zn by H
+ reduces the cation exchange capacity (CEC) of the soil and enhances the leaching potential of these metals [
15]. Moreover, particle size plays a significant role in leaching behavior, with fine particles exhibiting greater reactivity and leachability due to their larger specific surface area. Likewise, the degree of weathering also influences metal mobility, as prolonged exposure to environmental conditions can alter the fraction distribution and bioavailability of metals, thereby affecting their leaching behavior. Considering these influential factors, further research is needed to elucidate the complex interactions governing heavy metal leaching in tailings.
The ANC of tailings is also a critical factor that influences the release of heavy metals under acidic conditions, primarily driven by the presence of carbonate minerals (e.g., calcite and dolomite), as well as calcium and magnesium oxides and hydroxides, which react with hydrogen ions to neutralize acidity. Tailings with high ANC are expected to buffer acid inputs more effectively, thus reducing the potential for acid generation and metal leaching. Conversely, tailings with low ANC may generate low pH conditions, leading to enhanced metal extraction and mobilization. Therefore, understanding the role of ANC in controlling metal leaching is essential for effective environmental management and pollution control. While static methods like acid-base accounting (ABA) and net acid generation (NAG) tests have greatly advanced the prediction of acid generation potential in mine waste materials, research systematically addressing the correlation between ANC and metal release characteristics in low-sulfur tailings remains limited [
16,
17].
Despite the high acid neutralization capacity of low-sulfur tailings, the persistent heavy metal contamination observed in downstream farmlands indicates that these tailings can still pose significant environmental risks under acid rain conditions [
18]. The lead–zinc tailings in northern Guangxi, China, are located in a typical karst region rich in carbonate minerals, which significantly contribute to their high acid neutralization capacity. Mining activities in this region have been ongoing since the 1950s, with a small flotation plant established near the mining pit in 1958 to process ores. Previous studies have reported that the agricultural soils downstream of this lead–zinc tailing pond exhibit severe heavy metal contamination (e.g., Pb and Zn), with concentrations far exceeding national safety standards, even after 40 years of cultivation. Furthermore, soil organic carbon remains at low levels, indicating a long-term adverse impact on soil quality [
19,
20]. Low organic carbon levels reduce the soil’s capacity to retain heavy metals, thereby increasing the potential for metal discharge and environmental risks. These findings suggest that the presence of carbonate-rich minerals in the tailings may not be sufficient to mitigate heavy metal release under certain environmental conditions, necessitating further research on leaching mechanisms. However, studies focusing on the release behavior of heavy metals in tailings with high acid neutralization capacity are still limited. Therefore, this study aims to investigate the leaching behavior of heavy metals in these tailings under different pH conditions, particle sizes, and vertical profile depths, providing valuable insights for the comprehensive utilization and management of tailing ponds. Thus, exploring the relationship between ANC, acid rain pH, and heavy metal release in these tailings is crucial for effective pollution control and management.
This research is designed to (1) assess the potential for acid formation in Pb/Zn mine tailings from Guangxi through the application of ABA and NAG techniques, (2) analyze the distribution of heavy metal speciation in different layers of the tailing pond, (3) investigate the influence of ANC on the leaching behavior of heavy metals under varying simulated acid rain (SAR) pH, particle size, and weathering degree conditions, and (4) assess the leaching characteristics of heavy metals in the disturbed layer of the tailing pond. The findings of this study are expected to provide valuable insights for scientists, engineers, environmental regulators, and policymakers to better understand the release behavior of heavy metals in low-sulfur tailings and the associated environmental risks, ultimately aiding in the development of effective environmental management strategies for low-sulfur tailings in karst regions.
2. Materials and Methods
2.1. Sample Preparation
To provide a clearer understanding of the sampling locations, a map (
Figure 1) illustrating the specific sites within the lead–zinc tailing pond in northern Guangxi is included. Nine surface tailing samples were collected from a lead–zinc tailing pond in northern Guangxi, China, using a stratified sampling strategy based on a “cross” distribution map to cover various locations within the pond area. The sampling depth profile ranged from 0 to 30 cm, which was divided into three distinct layers: surface layer (0–10 cm), middle layer (10–20 cm), and deep layer (20–30 cm). This sampling design was adopted to investigate the variations in weathering degrees and heavy metal concentrations (Pb, Zn, and Cd) across different depths of the tailing deposits.
The tailing samples were collected from the disturbed layer (0–30 cm depth) below the surface of the tailing pond. This layer is frequently exposed to external disturbances, such as wind erosion, surface runoff, and rainfall infiltration, which can alter the physicochemical properties of the tailings and influence the distribution and migration of heavy metals. It also serves as the primary zone where interactions between tailings, plants, and microorganisms occur during remediation and reclamation processes. Understanding the properties and behavior of this layer is therefore crucial for evaluating both the environmental risks associated with tailings and for designing effective remediation strategies.
The collected samples were first air-dried at ambient temperature in a greenhouse to minimize moisture content without affecting the chemical characteristics of the samples. Once dried, at a low temperature (≤40 °C) to prevent chemical changes such as oxidation of metal sulfides, the samples were sieved sequentially through 40-mesh, 100-mesh, and 200-mesh sieves. The sieving process was performed gently to avoid generating heat or excessive mechanical force that could alter the particle characteristics, ensuring that the nominal chemical properties and grain size distribution were maintained. The processed samples were then stored in sealed plastic bags for subsequent leaching experiments.
Prior to conducting the leaching tests, the basic physicochemical properties of the tailing samples were thoroughly characterized. The analyses included measurements of the original pH and electrical conductivity of the tailings, mineral composition using X-ray diffraction (XRD), and elemental composition using X-ray fluorescence (XRF). In addition, the total heavy metal content was determined, and the chemical partition of heavy metals in the tailings was analyzed to understand their distribution patterns in different fractions. These preliminary analyses provided a comprehensive understanding of the tailing materials, laying a foundation for evaluating the leaching behavior of heavy metals under simulated acid rain conditions.
2.2. Analysis of Basic Physicochemical Properties of the Tailings
The pH of the tailings was measured using a standard soil pH determination method. Specifically, the tailings were mixed with deionized water (purity ≥ 18.2 MΩ·cm, initial pH ~6.5, conductivity < 1 µS/cm) at a ratio of 1:2.5, stirred for 2 min, and left to settle for 30 min before measuring the pH with a pH meter. To determine the total heavy metal content, 0.1 g of the tailing sample was digested using a mixed acid solution consisting of 4 mL concentrated nitric acid, 2 mL concentrated hydrochloric acid, and 1 mL hydrofluoric acid. The digestion was conducted over 24 h, followed by microwave digestion. The heavy metal concentrations were then measured using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Perkin-Elmer Optima 7000DV, Waltham, MA, USA).
The elemental composition of the tailings was analyzed using X-ray fluorescence spectrometry (XRF), and the mineralogical composition was characterized using X-ray diffraction analysis (XRD, X’Pert PRO, PANalytical, Almelo, The Netherlands). Electrical conductivity (EC) was measured using a conductivity meter (DDS-801, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China).
2.3. Acid Production Capacity Analysis of the Tailings
The acid production capacity of the tailings was evaluated using the acid-base accounting (ABA) method in the static leaching experiment, which included indicators such as acid neutralization capacity (ANC), net acid generation (NAG), net acid producing potential (NAPP), and maximum potential acidity (MPA) [
21].
The ANC of the tailing samples was determined by mixing 1 g of the bulk tailings with 25 mL of 0.2 M HCl in a 100 mL beaker [
21]. After the reaction and cooling, the excess acid was titrated with 0.2 M NaOH to calculate the ANC value based on the corresponding formula. Subsequently, NAPP was calculated using the following equation, where MPA represents the maximum potential acidity:
MPA was determined by multiplying the pyritic sulfur content (%) of the sample, measured using an elemental analyzer, by a conversion factor of 30.6. This conversion factor is based on stoichiometric calculations, assuming complete oxidation of pyritic sulfur to sulfuric acid, with negligible uncertainty. While pyritic sulfur is the dominant contributor to MPA in these tailings, other sulfur-bearing minerals such as chalcopyrite may contribute locally. The measurement uncertainty for pyritic sulfur and ANC is estimated to be ±0.5% and ±5%, respectively, reflecting the limitations of the analytical methods.
The pyritic sulfur content was measured using an elemental analyzer. NAG was determined by mixing 2.5 g of the pulverized tailing sample with 250 mL of 15% H
2O
2 in a 500 mL conical flask. The mixture was left to react in a fume hood for 24 h and then boiled for 1 h. After cooling to ambient temperature, the final NAG-pH was measured, and the solution acidity was determined by titration with 0.1 M NaOH to reach pH 7. Both ANC and NAG values were expressed in terms of kg H
2SO
4/t [
22].
2.4. Heavy Metal Speciation in Tailings
The modified BCR sequential extraction method was used to analyze the speciation of heavy metals in the tailings, aiming to determine their mobility and potential environmental hazards [
23]. The detailed experimental procedures are shown in
Table 1 below:
2.5. Static Leaching Experiments of Tailings
2.5.1. Effect of Simulated Acid Rain pH on Heavy Metal Leaching from Tailings
Simulated acid rain was prepared based on the typical composition of acid rain observed in Guangxi over the years, using a sulfuric acid/nitric acid molar ratio of 4:1 [
24]. The pH of the simulated acid rain was adjusted to 2.0, 3.0, 4.0, and 5.0 using ultrapure water in the laboratory, which resulted in lower ionic strength compared to natural rainwater. This lower ionic strength may enhance metal mobility in the laboratory compared to field conditions, where rainwater typically contains additional dissolved ions. Then, 4 g of tailing sample sieved through a 100-mesh sieve was mixed with 20 mL of the prepared acid rain solutions of different pH values and gently stirred for 2 min to ensure uniform contact, followed by a settling period without further stirring to replicate static leaching conditions. The 100-mesh fraction was chosen to represent common particle sizes in weathered tailings, while the 1:5 solid/liquid ratio ensured adequate interaction and reproducibility in laboratory conditions. The pH and electrical conductivity (EC) of the leachates were measured at 0.5 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h, and 96 h intervals. After each measurement, the leachate was filtered through filter paper, diluted to a final volume of 25 mL, and the concentrations of heavy metals (Pb, Zn, and Cd) in the solution were determined using an inductively coupled plasma optical emission spectrometer (ICP-OES).
2.5.2. Effect of Tailing Particle Size on Heavy Metal Ion Leaching
To evaluate the effect of particle size on heavy metal leaching, 4 g of tailing samples with different particle sizes (40 mesh, 100 mesh, 200 mesh) were added to 20 mL of simulated acid rain at pH 3.0. While similar trends may be expected at other low pH values (e.g., pH 2.0), the overall leaching rates and particle size effects could vary at higher pH levels, where metal solubility and mobility are reduced. The pH and EC of the leachates were measured at 0.5 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h, and 96 h intervals. After each time point, the leachate was filtered through filter paper and diluted to a final volume of 25 mL, and the concentrations of Pb, Zn, and Cd in the solution were analyzed using ICP-OES.
2.5.3. Effect of Simulated Acid Rain on Heavy Metal Leaching from Tailings in the Disturbed Layer at Different Depths
Tailing samples from different depths (0 cm, 15 cm, 30 cm) were collected and sieved through a 100-mesh sieve. The estimated water content was in the range 10–20% in surface layers and 5–10% in deeper layers, while the local tailings’ permeability is generally reported to be low-to-moderate (10−6 to 10−4 cm/s), based on previous studies of similar tailings. For each depth, 4 g of the sample was mixed with 20 mL of simulated acid rain at pH 3.0. The pH and EC of the leachates were recorded at 0.5 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h, and 96 h intervals. After each measurement, the leachate was filtered and diluted to a final volume of 25 mL, and the concentrations of Pb, Zn, and Cd were measured using ICP-OES.
3. Results and Discussion
3.1. Characterization of Tailing Samples
3.1.1. Mineralogy and Chemical Composition
The elemental composition of the tailing samples was determined using X-ray fluorescence (XRF), as presented in
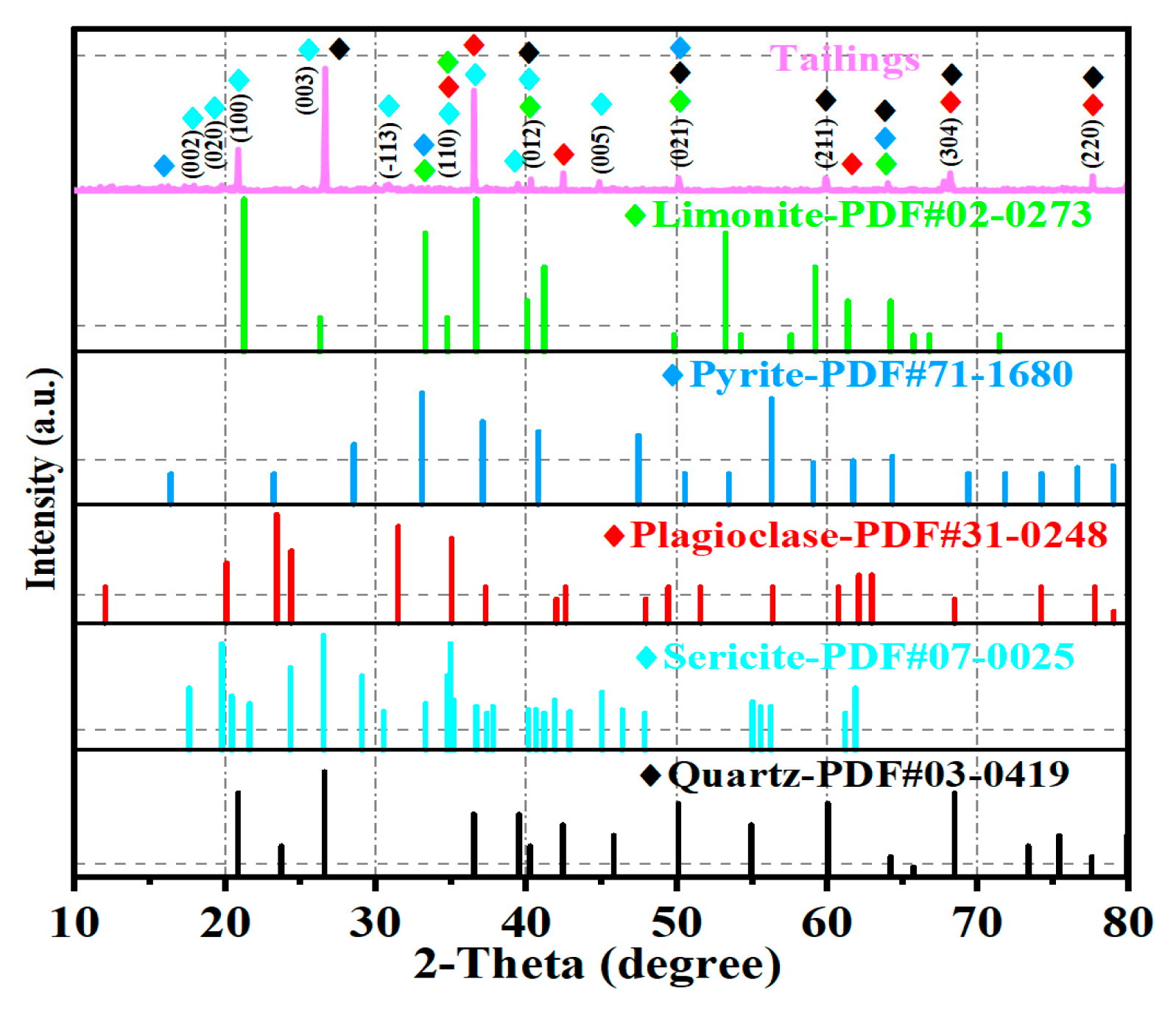
Table 2. The samples were homogenized by grinding to a fine powder (≤200 mesh) prior to analysis and correspond to depths of 0 cm, 15 cm, and 30 cm, respectively. The results indicate that the primary constituents of the Pb/Zn tailings are quartz and calcium–aluminum–iron compounds. X-ray diffraction (XRD) analysis further revealed that the major mineral phases include quartz, sericite, pyrite, limonite, and plagioclase (
Figure 2). The high calcium content, potentially derived from plagioclase, may contribute to the tailings’ acid neutralization capacity [
25]. These minerals may correspond with the presence of calcium identified in the XRF analysis, reinforcing their potential role in neutralizing acidity and influencing metal release and retention. The measured pH values of the tailings ranged from 6.54 to 6.95, indicating a slightly acidic nature. This pH range is notably higher than that of typical non-ferrous metal tailings, likely due to the low sulfur content (0.37%), which resulted in a reduced acid generation potential [
26].
3.1.2. Heavy Metal Concentrations and Environmental Implications
As presented in
Table 3, the concentrations of Pb, Zn, and Cd in the tailings exceed the limits established by the Standard for Pollution Control on the Storage and Disposal Site for General Industrial Solid Wastes [
27].
The XRD analysis revealed an absence of detectable mineral phases associated with Pb-Zn-bearing minerals (
Figure 2).
This is likely due to the low content of these metals, making them indistinguishable from the dominant mineral phases. The presence of pyrite, despite its relatively low abundance, suggests a limited but notable acid generation potential. The low sulfur content and predominance of silicate minerals further corroborate the limited acid-generating capacity of the tailings. Additionally, plagioclase, as a source of calcium, can release calcium ions under acidic conditions, contributing to acid neutralization. Limonite, a hydroxide mineral, may also participate in neutralization reactions [
28]. These findings enhance our understanding of the roles of various mineral phases in metal release and retention. Furthermore, factors such as particle size, pH, and weathering degree influence the mobility of heavy metals, particularly the leaching behavior of Pb, Zn, and Cd under simulated acid rain conditions.
3.1.3. Particle Morphology and Leaching Behavior
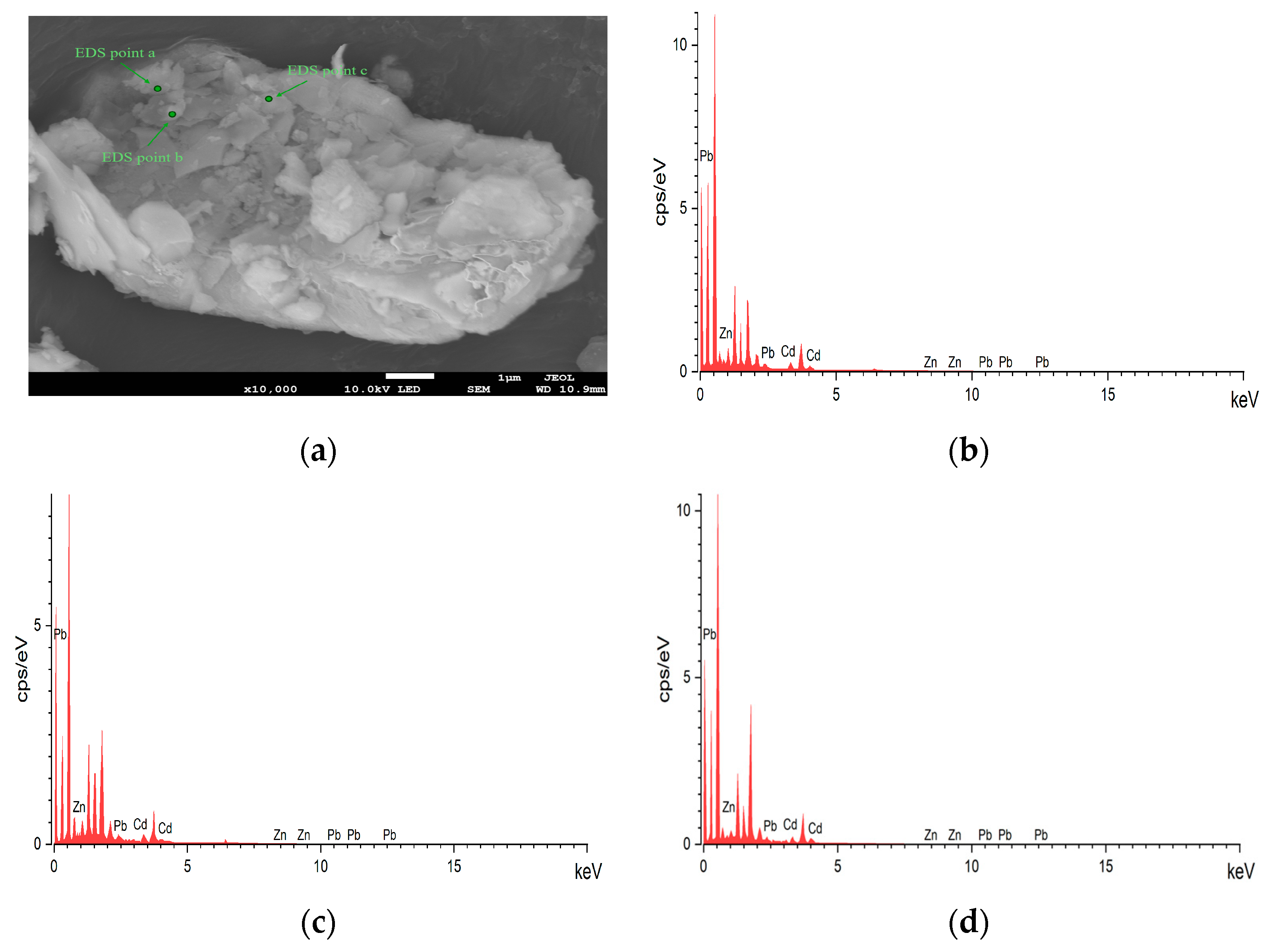
The scanning electron microscopy (SEM) images (
Figure 3a) revealed that the tailing particles exhibited irregular shapes and a wide particle size distribution. This variation in particle morphology and size suggested a complex internal structure and surface characteristics, which could influence the leaching behavior of heavy metals [
29]. These morphological characteristics could significantly influence the leaching behavior of heavy metals under acidic conditions, as irregular shapes and higher surface areas increase metal release potential. Further analysis through energy-dispersive X-ray spectroscopy (EDS) (as shown in the elemental mapping of Cd, Zn, and Pb in
Figure 3b–d) identified elemental concentrations of Zn (56.31%), Cd (5.48%), and Pb (38.21%), confirming their significant presence within the tailings. These results align with the inductively coupled plasma (ICP) measurements, thereby reinforcing the association between these metals and mineral phases.
This comprehensive characterization provides stronger evidence for metal mobilization mechanisms. Existing studies provide evidence of the association between heavy metals and sulfide minerals in similar tailings. For example, Saedi et al. and Liu et al. showed that in tailings from a lead–zinc mining area, Pb and Zn mainly exist in the forms of galena (PbS) and sphalerite (ZnS) [
30,
31]. In addition, He et al. confirmed through SEM-EDS analysis the coexistence of heavy metals with sulfide minerals [
32]. Our findings also demonstrate the coexistence of heavy metals with sulfide minerals, particularly the strong association of Zn, Cd, and Pb with sulfides, as shown by the EDS elemental mapping results. This further strengthens the conclusion about the association between heavy metals and sulfide minerals in our samples.
3.2. Heavy Metal Partitioning in Tailings
Building upon the basic physicochemical properties of tailings, this section delves into the chemical speciation of heavy metals, which directly influences their environmental risk and mobility. The environmental risk posed by heavy metal contamination in tailings is influenced not just by the overall metal content but also by their chemical speciation, since distinct forms of metal species demonstrate varying levels of mobility and bioavailability. Therefore, it is essential to understand the distribution of heavy metal speciation within tailings to accurately predict and assess their environmental impact.
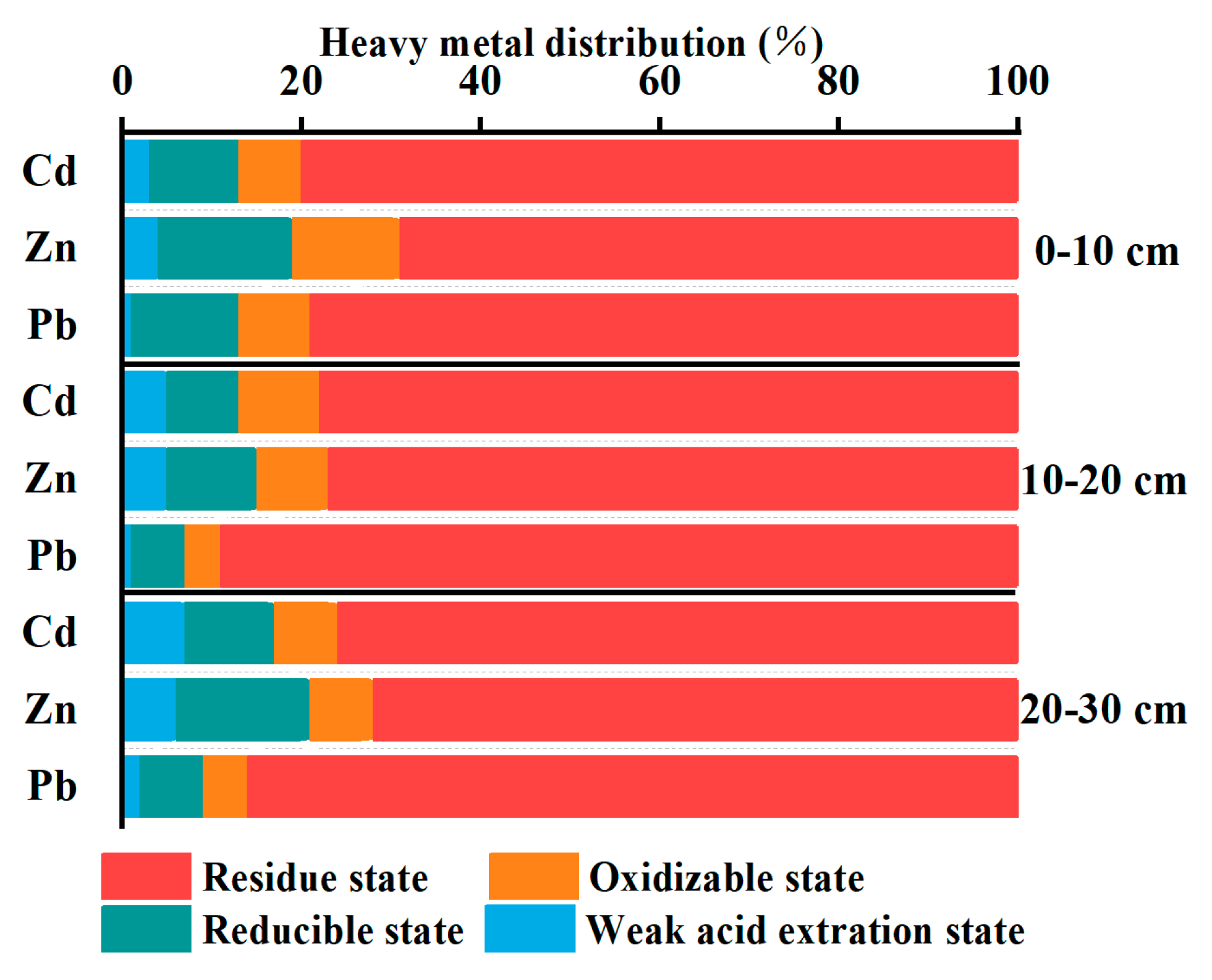
Figure 4 illustrates the proportion of various heavy metal species present in the surface, middle, and deep layers of the disturbed layer in the tailing pond.
In different depths of the disturbed layer, most of the heavy metals were found predominantly in the residual fraction, indicating that a large proportion of heavy metals in the tailings had already undergone oxidation. The maximum proportions of residual fractions for Pb, Zn, and Cd in the upper, middle, and lower layers were 85.25%, 69.3%, and 72.6%, respectively. The high proportion of Pb in the residual fraction (85.25%) suggests that Pb has a relatively low potential for mobility in these tailings. The proportion of reducible fractions for all three metals was significantly lower than that of oxidizable fractions, which was related to the low organic matter content in the tailings [
33].
To provide a clearer understanding of the oxidation states and chemical forms of these metals, we analyzed their distribution across different fractions. Lead (Pb) is predominantly present in the residual fraction, suggesting it is incorporated into stable mineral structures, possibly as sulfide minerals like PbS (lead sulfide). In the oxidizable fraction, Pb is associated with organic matter and sulfide minerals, which may oxidize under environmental conditions, potentially releasing Pb into more mobile forms. Zinc (Zn) is similarly found mainly in the residual fraction, possibly as ZnS (zinc sulfide), and in the oxidizable fraction associated with organic matter and sulfides. Cadmium (Cd) is primarily present in the oxidizable fraction, indicating its association with organic matter and sulfides, which, upon oxidation, may release Cd into more mobile and bioavailable forms, contributing to its higher environmental risk.
The non-residual fraction of heavy metals indicates their potential mobility under varying environmental conditions, as heavy metals in oxidizable and reducible forms can transform into exchangeable forms, thereby increasing their likelihood of release. Among the three metals, Cd had the highest proportion of non-residual fractions at 27.4%, suggesting that Cd may have a relatively high mobility and a greater potential for environmental contamination [
34]. Based on the content of non-residual fractions, the potential mobility of the heavy metals in these tailings followed the following order: Cd > Zn > Pb.
3.3. Impact of Acid Generation Potential of Tailings
The acid generation potential of the tailings was evaluated, with the results summarized in
Table 4. The acid neutralization capacity (ANC) values for the two samples were 195.51 kg H
2SO
4/t, respectively, indicating a robust capacity for neutralizing external acidity [
35]. This substantial neutralization capacity can be attributed to the high content of calcium and magnesium oxides, hydroxides, and carbonate compounds, as revealed by XRF analysis.
In contrast, the net acid generation (NAG) values were negative, measured at −1.59 kg H2SO4/t, indicating a stronger acid neutralization potential that surpasses the net acid production. This suggests that the tailings possess an overall net capacity to neutralize potential acid production, thereby significantly lowering the likelihood of long-term acidification. While NAG values are still related to potential long-term acidification through the slow oxidation of residual sulfides, the negative results indicate that the risk of acid production over prolonged exposure is minimal. Thus, while immediate acidification is unlikely, the overall potential for long-term acidification has been notably reduced due to the enhanced neutralization capacity.
The analysis was conducted on all nine tailings samples to provide a comprehensive view of the acid generation and neutralization characteristics. The study reflects the average values across these nine samples, offering a balanced and representative assessment of the tailings’ geochemical behavior in terms of sulfur content, metal concentrations, and their variability. This approach ensures a broader understanding of the acid generation and neutralization potential observed in the collected samples [
36,
37,
38]. Considering that these tailings have been exposed to weathering for many years and still demonstrate a capacity for acid production, and given the significant volume of material stored in the tailing pond, the risk of acid-related pollution cannot be entirely dismissed. The combined assessment of net acid producing potential (NAPP) and NAG-pH values classifies the tailings as non-acid-generating materials [
39]. This classification can be explained by two primary factors: (1) the long-term closure of the tailings pond, leading to the depletion of sulfur in various migratory forms; and (2) the inherently low initial sulfur content in the tailings. Consequently, while the high ANC values suggest a reduced risk of immediate acidification, the potential for long-term acid generation remains a concern that warrants continuous monitoring. The findings suggest that despite the strong acid neutralization capacity (ANC) of low-sulfur tailings, there is still a risk of heavy metal leaching under extreme acidic conditions, necessitating a closer examination of the influence of acid rain pH on metal leaching behavior.
3.4. Effect of Simulated Acid Rain pH on Leachate Characteristics
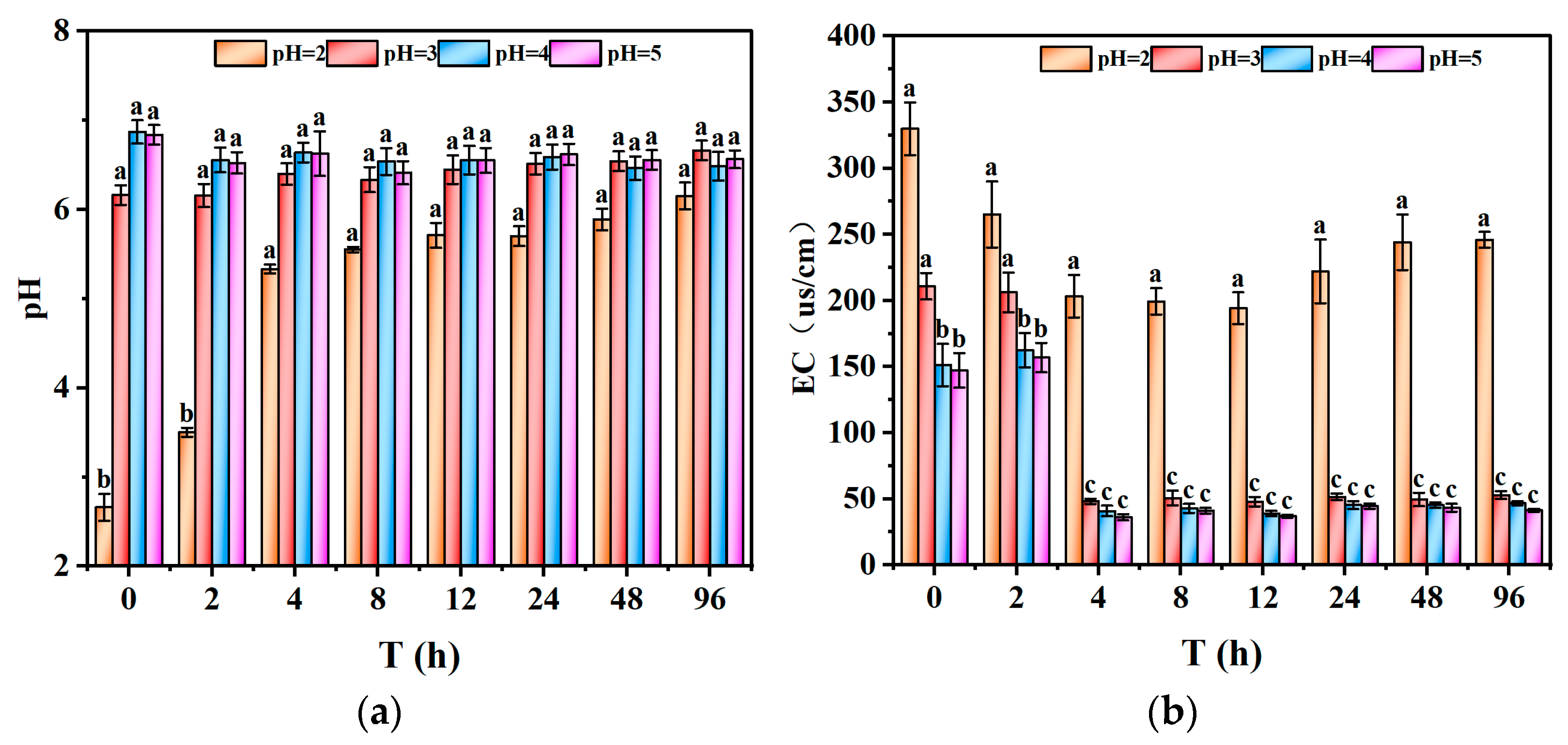
After evaluating the potential for acid generation and its implications on metal release, it is essential to investigate the direct impact of acid rain pH on the leaching characteristics of heavy metals. The static leaching experiments were conducted using the surface layer of tailing samples sieved through a 100-mesh screen to evaluate the impact of external acid rain solutions (pH = 2, 3, 4, and 5) on the pH and electrical conductivity (EC) of the leachates. The results are presented in
Figure 5a,b, and the relationship between the leaching concentrations of heavy metals (Pb, Zn, and Cd) and acid rain pH is depicted in
Figure 6a–c.
As illustrated in
Figure 5a, the pH of the leachates increased rapidly to approximately 6.0 within 12 h of exposure to acid rain. For initial acid rain pH values of 3, 4, and 5, the leachate pH rose to around 6.3 immediately upon contact, indicating that the initial pH of the acid rain had a limited impact on the final leachate pH. This observation suggested that the tailings possessed a strong neutralization capacity against external acidic inputs. The rapid pH rise at t = 0 for pH 3 demonstrates the effective buffering action of the tailings, driven by the presence of acid-neutralizing minerals. However, at pH 4 and 5, the buffering response was slower, likely due to the smaller pH gradient, resulting in a more gradual neutralization process. In the study region, acid rain is relatively frequent, with pH levels typically ranging from 3.5 to 4.5, but occasional extreme events with pH as low as 2.3 have been recorded, making the buffering capacity observed at pH 3 environmentally relevant.
In contrast, at pH 2, the stronger acidity leads to more aggressive disruption of acid-sensitive minerals such as carbonates and oxides, causing a slower initial pH increase and a lower equilibrium pH compared to pH 3. The higher proton concentration at pH 2 enhances mineral dissolution and increases the release of adsorbed heavy metals, which explains the discrepancy between pH 2 and pH 3 leachate curves. This behavior highlights the non-linear nature of acid-tailing interactions, where small pH decreases can result in disproportionately higher metal mobilization due to the increased dissolution and exchange reactions.
Over time, the pH of the leachate remained relatively stable at elevated levels and did not exhibit a decreasing trend, even under conditions of pH 2 acid rain. During the initial 4 h, the tailings rapidly neutralized the acid rain pH from 2 to around 6, after which the pH remained stable or slightly increased. Under pH 3 and 4 conditions, stability was achieved even more rapidly. This observation aligns with the high ANC and low NAG values of the tailings, further confirming their strong neutralization capacity against external acidic inputs. This robust buffering capacity could be attributed to two primary factors: (1) the high calcium and magnesium content in the tailings (10.967%), which suggested a substantial presence of acid-consuming minerals such as oxides, hydroxides, and carbonates [
40]; and (2) the inherently low sulfur content, which limited acid generation potential [
41].
As illustrated in
Figure 5b, the EC of the leachates initially exhibited a decrease before stabilizing. This trend can be explained by the initial release of surface-adsorbed salts and heavy metal ions, which resulted in elevated initial EC values. As the dissolution and adsorption processes approached equilibrium, the rate of ionic release diminished, resulting in the stabilization of the EC [
41]. However, due to ongoing chemical reactions, some insoluble components continued to dissolve over prolonged acid rain exposure, leading to minor fluctuations in EC. These fluctuations in electrical conductivity were consistent with variations observed in pH values.
The comparison between
Figure 5a,b clearly shows that leachates with lower pH values exhibited higher EC, indicating that a decrease in acid rain pH promoted the leaching of soluble salts and heavy metal ions from the tailings. The correlation between pH and EC suggests that increasing acidity in the leachate leads to the mobilization of more metal ions and soluble salts, which results in elevated EC values.
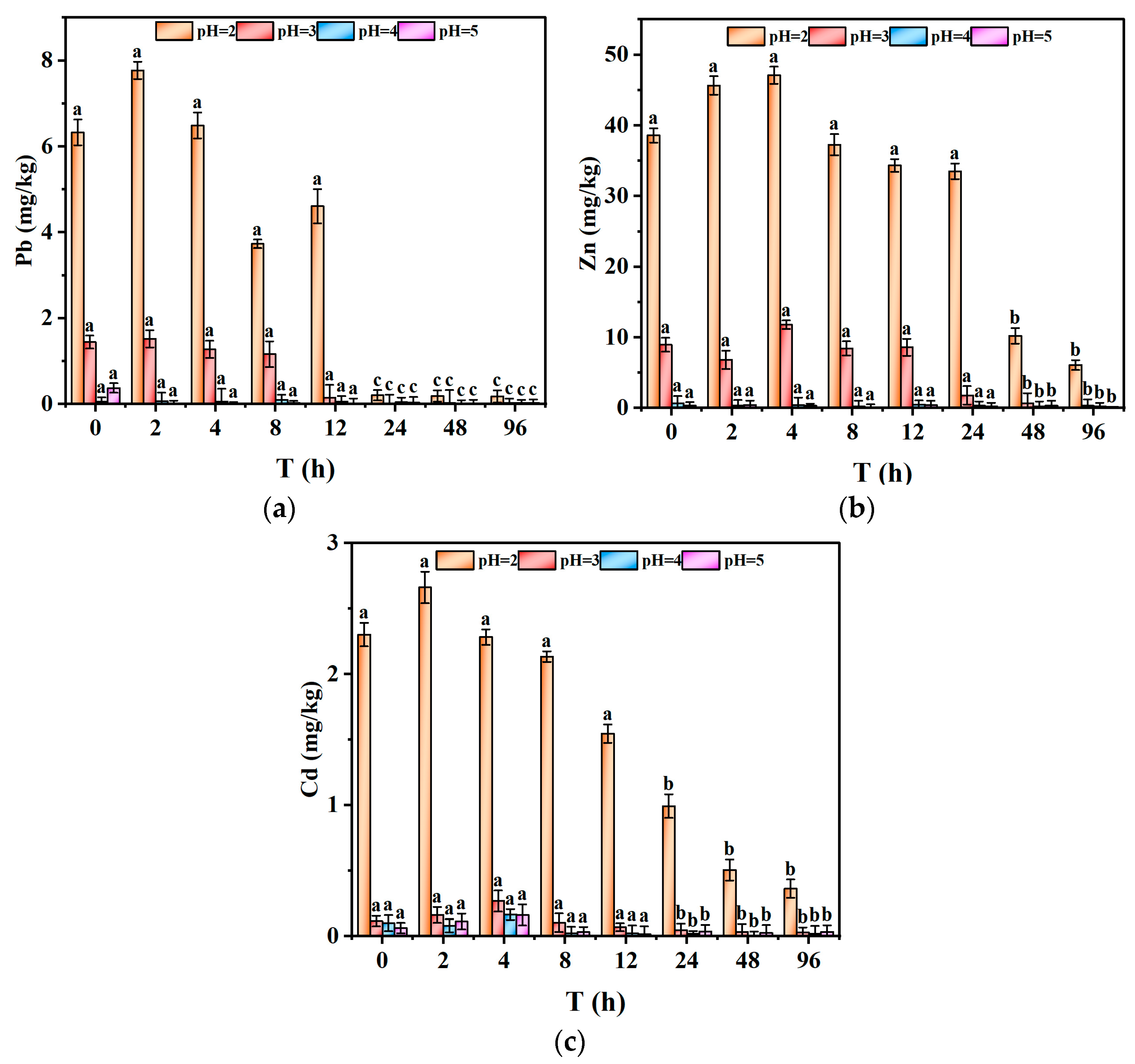
Figure 6a–c illustrated the leaching patterns of Pb, Zn, and Cd under different acid rain pH levels. It is clear that the pH of acid rain strongly affected the leaching concentrations of these heavy metals, as lower pH values lead to a marked increase in their release [
42]. For instance, at pH 5, the leaching concentration of Zn was only 0.076 mg/L, while at pH 2, it surged dramatically to 7.53 mg/L. This significant increase in metal release under pH 2 conditions emphasizes the higher leaching potential associated with extreme acidic inputs, which could pose greater environmental risks if similar conditions arise. Continuous leaching of the three heavy metals was observed under pH 2 and 3 conditions, whereas minimal to no leaching occurred under pH 4 and 5 conditions. This indicated that lower pH values can significantly enhance the leaching of heavy metals from tailings, which poses a greater threat to the surrounding environment. This is consistent with existing studies. These studies indicate that under acidic conditions, especially when the pH is below 3, the solubility of metal ions such as Pb, Zn, and Cd increases, thereby enhancing their mobility. Additionally, increasing pH induces the precipitation of metal hydroxides or carbonates, which reduces metal mobility [
43]. Under high pH conditions, these speciation changes significantly decrease the bioavailability of heavy metals [
44].
This phenomenon may be associated with the speciation distribution of heavy metals, as Zn and Cd exhibited higher proportions within the weak acid-extractable fraction [
45]. Under acidic leaching conditions, oxidizable fractions can transform into weak acid-extractable fractions, thereby increasing metal mobility. While this study focused on absolute leaching concentrations, future work should consider normalizing the results to the mass of metal contributed by the 4 g of sample or its non-refractory fractions. This approach would allow for a more accurate assessment of the relative leaching behavior of each metal and help clarify the mechanisms driving metal mobilization under acidic conditions. Establishing standardized curves and normalization protocols would also enhance the comparability of results across different studies, providing more robust conclusions about the potential environmental impacts of tailings leaching.
The leaching profiles of the three metals displayed a biphasic pattern, featuring a rapid initial release stage succeeded by a more gradual release phase [
46]. This pattern can be attributed to the initial rapid dissolution of soluble salts and surface-adsorbed heavy metal ions into the leachate, while the subsequent stage involved the gradual release of exchangeable heavy metal ions. It is important to note that the samples were dried at a low temperature (≤40 °C) to minimize alterations in metal speciation; however, results might differ slightly with undried samples. Additionally, potential metal losses onto the flask walls, particularly at higher pH levels, could contribute to the observed decrease in metal concentrations. Visible precipitates, especially of Zn and Pb, were observed at more neutral pH levels, suggesting the formation of metal hydroxides or carbonates under these conditions. The rapid release phase observed under pH 2 conditions reflects the stronger initial dissolution of minerals and desorption of heavy metals due to higher proton concentrations. As the pH of the leachate increased over time, the oxidative dissolution of the tailings diminished, and the release and desorption of heavy metals reached a state of equilibrium, resulting in a reduced leaching rate. This two-stage release behavior is consistent with the trend observed in the EC data, where EC initially decreased before stabilizing.
The comparison between heavy metal release concentrations and leachate pH (
Figure 6a) showed that within the initial 9 h under pH 2 acid rain conditions, the leachate pH increased rapidly, accompanied by a rise in the concentrations of the three heavy metals. This observation suggested that the strong acid neutralization capacity of the tailings was insufficient to completely prevent the release of heavy metals. Notably, a strong acid neutralization capacity does not imply that the tailings do not contribute to environmental contamination. During the initial stage, when the leachate pH was low, heavy metals were rapidly released into the leachate at high concentrations. In contrast, during the later stages, when the leachate pH was elevated, heavy metal concentrations in the leachate were significantly lower or even undetectable. This indicated that acidic conditions facilitate the leaching of heavy metals from the tailings [
47].
In summary, one of the key factors influencing the release of heavy metals from tailings is the acidification of the tailing pile. China, particularly the southern regions, is a region prone to acid rain, characterized by both high frequency and intensity. For example, in Guangxi, the lowest recorded pH of acid rain has reached as low as 2.32 [
48]. Although acid rain with pH values below 3.0 is relatively rare—occurring in less than 5% of total acid rain events and primarily during extreme pollution episodes—it still poses a potential risk for heavy metal leaching from tailings. Therefore, effective management of tailings acidification is crucial to prevent the release of heavy metals and to mitigate the associated risks to the surrounding environment and human health. Tailings management strategies should prioritize minimizing exposure to extreme acidic conditions, especially those approaching pH 2, to limit heavy metal mobilization and reduce environmental hazards.
3.5. Effect of Tailings Particle Size on Leaching Behavior
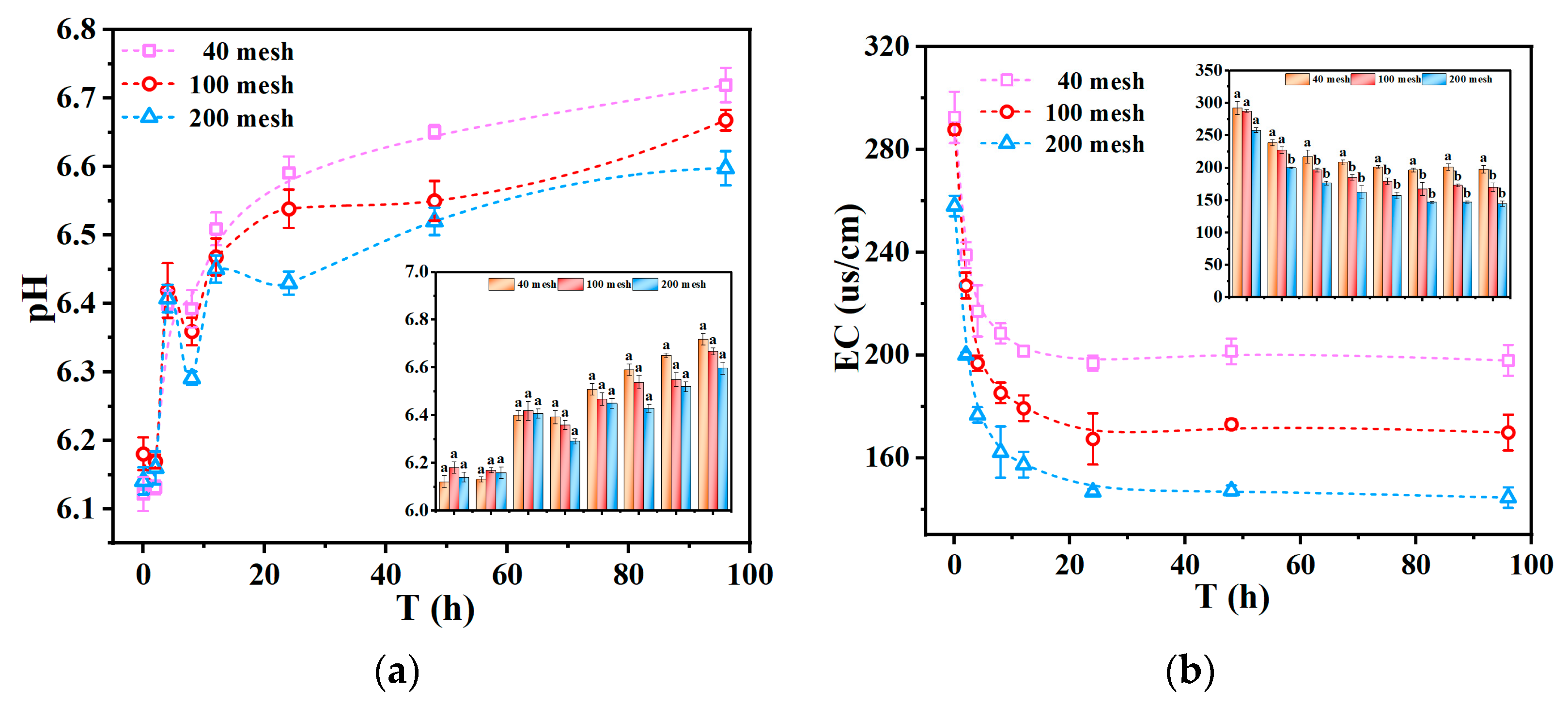
To further understand the influence of physical properties on metal leaching, this section evaluates the effect of tailing particle size, which determines the exposure and interaction area between tailings and acid rain. Under the condition of simulated acid rain, at pH 3, the variations in leachate pH and EC over time for upper layer tailings of varying particle sizes (40 mesh, 100 mesh, and 200 mesh) are shown in
Figure 7a,b. Additionally, the relationship between the leaching concentrations of heavy metals (Pb, Zn, and Cd) and particle size is illustrated in
Figure 8a–c.
As shown in
Figure 7a, the leachate pH rapidly increased initially and then gradually stabilized, indicating that the tailings have a strong acid neutralization capacity. The neutralization capacity of tailings with different particle sizes followed the following order: 40 mesh > 100 mesh > 200 mesh. Due to the larger contact area between the larger tailing particles and the acid rain, there is an increased ability to dissolve soluble components, resulting in higher conductivity values. This is consistent with the fact that larger tailing particles provide a higher effective contact area [
49]. This trend was attributed to the larger effective contact area of coarse tailing particles compared to finer ones [
50]. Although larger particles have a smaller specific surface area, they are less susceptible to agglomeration during acid rain leaching, which allows for a greater effective contact area with the leachate.
As depicted in
Figure 7b, the EC of leachate increased with particle size, following the following order: 40 mesh > 100 mesh > 200 mesh. This could be attributed to the larger tailing particles providing a greater effective contact area with acid rain, which enhanced the dissolution of soluble components, thereby resulting in elevated EC values. Over time, the EC of the leachate showed an initial decrease followed by stabilization. This trend may be explained by the initial release of ions adsorbed on the surface of the tailings when the leachate pH was lower, leading to higher EC values. As the soluble components on the tailings’ surface were gradually depleted and an adsorption–desorption equilibrium was reached, the EC of the leachate decreased and eventually stabilized [
51].
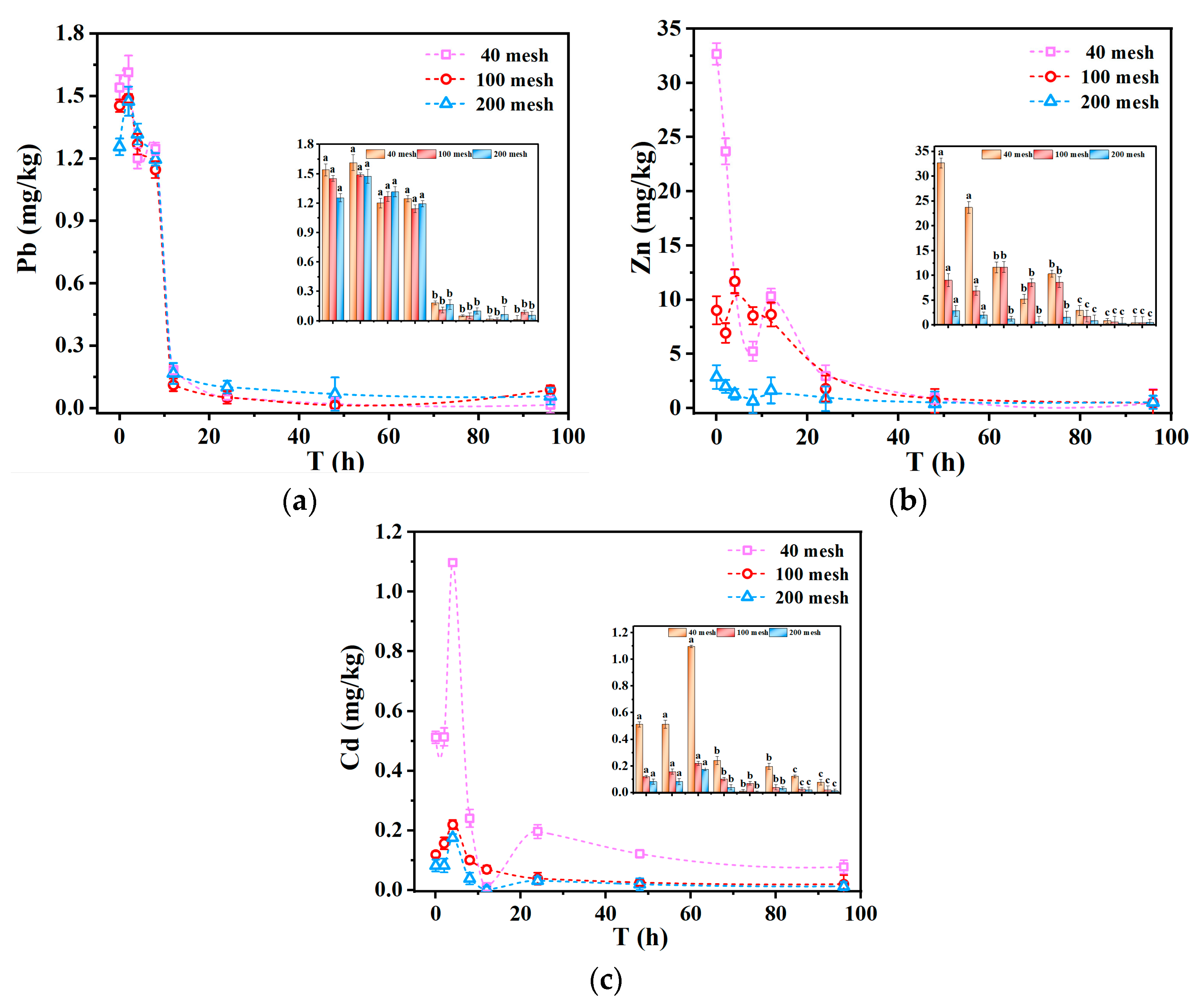
The trends in leaching concentration for Pb, Zn, and Cd with varying particle sizes, as shown in
Figure 8a–c, demonstrate a consistent pattern; the leaching concentration increased with larger particle size, following the following hierarchy of order: 40 mesh > 100 mesh > 200 mesh. This phenomenon could be attributed to the more effective and extensive interaction between larger tailing particles and simulated acid rain, enabling the heavy metals to be adsorbed on the surfaces of coarser particles. Conversely, finer tailing particles tend to agglomerate significantly upon exposure to acid rain, which can block interstitial spaces between particles and reduce the effective contact area with the leachate. This observation is based on indirect evidence from leaching behavior. Future research should include particle size distribution analysis of the leachate to confirm the extent of agglomeration and its influence on metal mobility. Additionally, analyzing the initial metal content of different tailing fractions would provide further insights into how agglomeration affects metal leaching dynamics. The reduction in effective contact area due to agglomeration likely limited ion-exchange reactions between H
+ in the acid rain and heavy metal ions adsorbed on the tailing surface [
52]. Similarly, studies have shown that smaller particles have a larger specific surface area, leading to faster metal release. This is because smaller particles provide more reactive surfaces, thereby accelerating the leaching process of heavy metals [
53]. In contrast, larger particles have greater pore space, which not only affects water infiltration but also alters the leaching dynamics [
54]. Moreover, due to the increased reactive area of larger particles under acidic conditions, their metal release rate can also be faster. As a result, the concentrations of leached heavy metals from finer tailings were lower than those observed in coarser particles, which had greater effective surface contact with the leachate.
Comparing the effects of acid rain pH and particle size on the leaching concentrations of heavy metals, it can be inferred that particle size had a less significant influence on leaching concentrations than acid rain pH. Specifically, the differences in leaching concentrations observed among tailings of varying particle sizes are relatively minor, while the variations in concentrations under different pH conditions can be substantially greater—sometimes by an order of magnitude [
55]. Therefore, when assessing the risk of heavy metal pollution from tailings, priority should be given to the influence of acid rain pH over that of particle size.
3.6. Effect of Disturbed Layer Depth on Heavy Metal Leaching Under Acidic Conditions
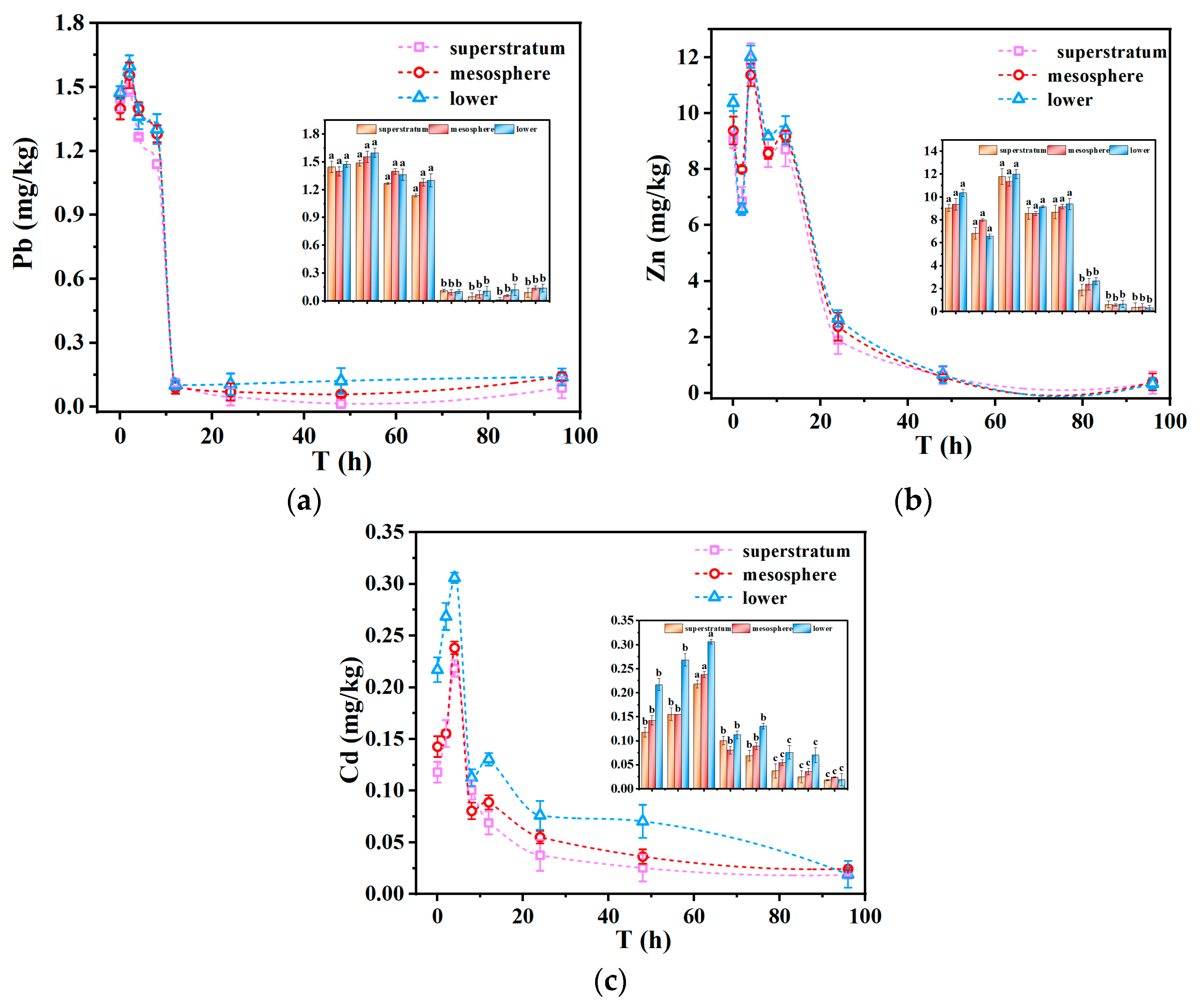
Considering that both chemical and physical properties influence heavy metal leaching, this section investigates the effect of depth on metal release to better understand the vertical migration patterns within the tailing pond. This experiment evaluated the impact of pH 3 simulated acid rain on the leaching concentrations of heavy metals (Pb, Zn, and Cd) in tailings from different depths within this disturbed layer (0 cm, 15 cm, and 30 cm). The results are presented in
Figure 9a–c. As observed, the leaching concentrations of the three heavy metals showed a consistent trend with depth; as the depth of the disturbed layer increased, the leaching concentrations of heavy metals also increased. This phenomenon may be attributed to the long-term leaching of rainwater, which facilitated the downward migration of heavy metals, especially those adsorbed on the surface of the upper tailings, resulting in higher concentrations in the lower layers. The weathering process plays a significant role here, as it transforms primary sulfide minerals into secondary oxides or hydroxides over time, changing metal speciation and increasing their mobility [
56]. For example, some studies have demonstrated that deeper, more weathered layers show higher solubility of metal ions, particularly under acidic conditions [
57]. These findings align with the results of this study, where greater weathering was associated with increased mobility of Cd and Zn. Another contributing factor could be the depletion of surface layers through runoff and leaching, which removes heavy metals from the uppermost parts of the tailings over time. Additionally, variations in mineral phases at different depths could affect the association and mobility of metals, leading to distinct distribution patterns along the vertical profile. This trend was most pronounced for Cd, aligning with findings from previous studies [
58]. However, the trend was not entirely consistent across all samples, possibly due to the complex physical and chemical changes that have occurred in the disturbed layer over the years, influenced by factors such as wind erosion and hydraulic flushing, especially considering that the tailing pond had remained inactive for an extended period.
The three heavy metals demonstrated comparable trends concerning both depth and time. Notably, the leaching concentrations of Pb and Zn almost completely overlapped after 12 h of immersion, suggesting that the chemical composition of tailings at different depths within the disturbed layer may be relatively uniform. Furthermore, the variations in the leaching concentrations of the three heavy metals across different depths were not particularly pronounced, indicating a potential lack of strong correlation between the vertical depth of the tailings and heavy metal leaching concentrations. Alternatively, it could be due to the relatively minor differences in sampling depth within the disturbed layer [
59].
The leaching behavior of the three heavy metals exhibited a two-stage pattern: an initial rapid release phase occurring within the first 12 h, followed by a slower release phase. This suggested that the heavy metals migrating downward are likely those that were adsorbed on the tailing surfaces. As shown in
Figure 9c, even after 12 h of exposure to acid rain, Cd still exhibited noticeable differences in leaching concentrations at various depths, suggesting a notable potential for downward migration. This suggested that Cd possesses the highest downward mobility among the three metals, while Pb and Zn displayed comparatively weaker downward mobility.
This behavior may be associated with the chemical speciation of these metals within the tailings. The non-residual fraction is generally regarded as an indicator of the potential mobility of heavy metals. The non-residual fraction of Cd was 37.8%, significantly higher than that of Pb, which was only 14.7%. This suggests that Cd has a greater potential for migration compared to Pb, thereby making it more susceptible to leaching and contamination under acidic conditions.
4. Conclusions
The findings suggest that the pH of simulated acid rain (SAR) significantly influences the release behavior of heavy metals, particularly for Pb, Zn, and Cd. However, it is important to note that the pronounced mobilization of heavy metals was primarily observed at extremely low pH (pH 2), which is less common in natural settings. Under highly acidic conditions (pH 2–3), the concentrations of these metals in the leachate increased by 4–6 times compared to neutral conditions (pH 7), with Cd showing the highest release potential. This indicates that the ANC of low-sulfur tailings is not sufficient to prevent heavy metal leaching under extreme acidic conditions. It should also be noted that the leaching data have not been normalized to the quantities of metals originally present in the tailings, which could affect the interpretation of the results. Moreover, particle size also plays a role in heavy metal leaching, as coarser particles exhibited higher leaching concentrations due to increased surface area contact; however, the effect of particle size is less pronounced than that of SAR pH. Analysis of the disturbed layer (0–30 cm) further indicated that heavy metal concentrations increased with depth, particularly for Cd, whose non-residual fraction was significantly higher, indicating a strong potential for downward migration. These results highlight the need for effective tailing management strategies that consider not only ANC but also particle size distribution and vertical leaching patterns to mitigate environmental risks. Future studies should aim to refine the experimental protocols, especially in terms of simulating rainwater contact and normalizing metal mobilization data, to provide more representative results for field conditions.