Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results
Abstract
1. Introduction
2. Materials and Methods
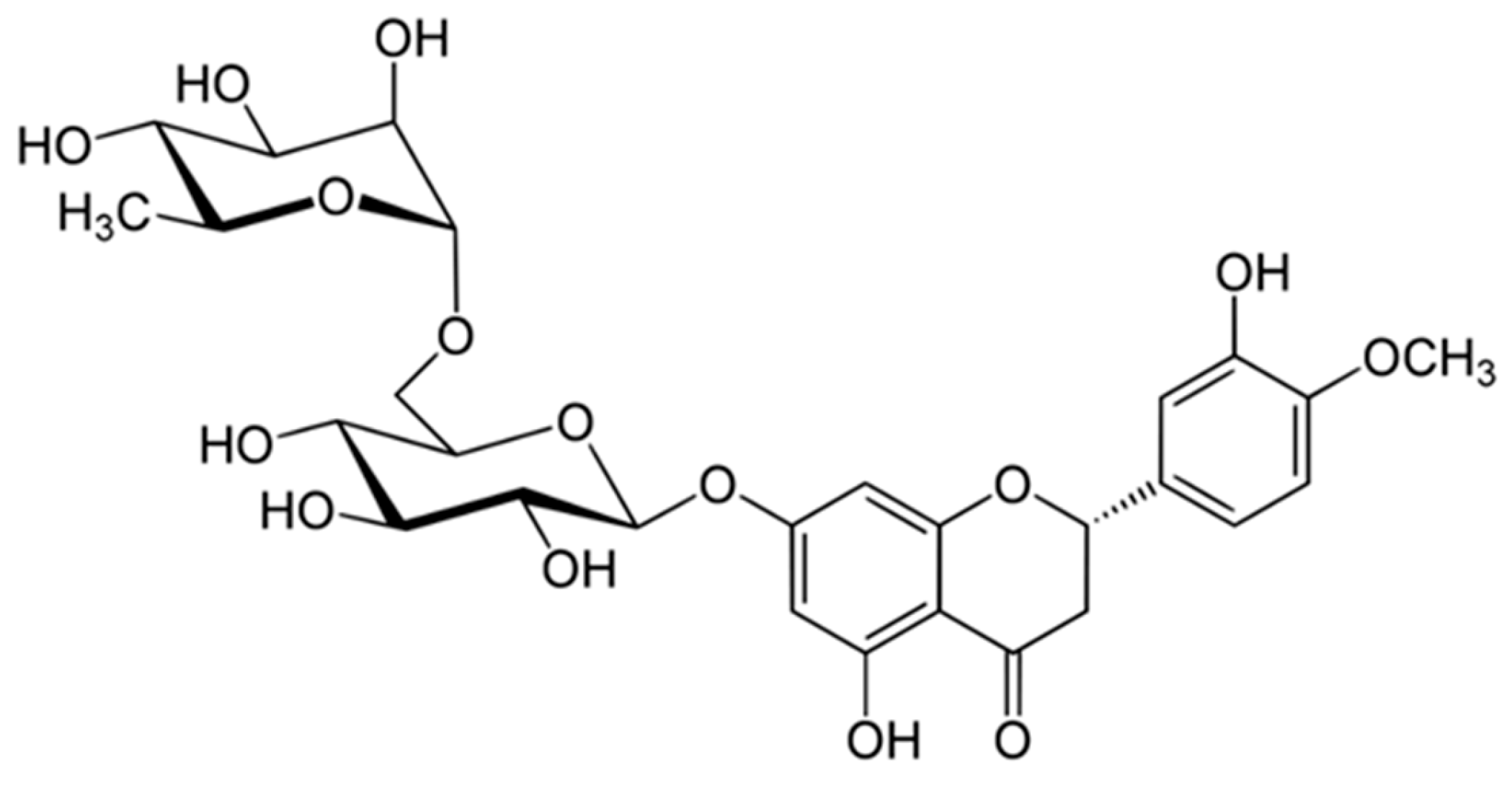
2.1. Raw Material and Chemicals
2.2. Thermodynamic Prediction of Operating Conditions for Supercritical Fluid Extraction
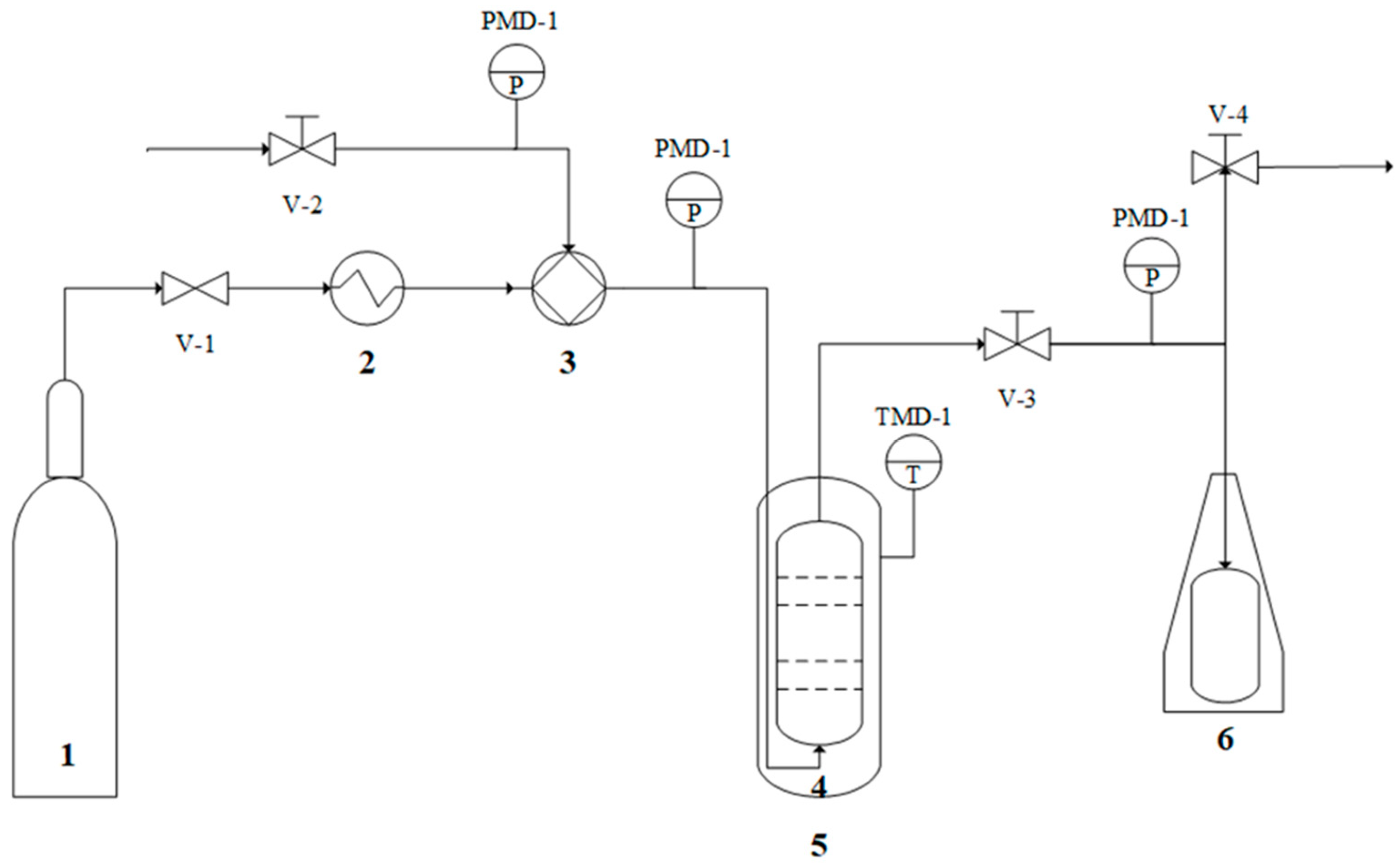
2.3. Experimental Validation
2.3.1. Total Polyphenol Content (TPC) Identification
2.3.2. Antioxidant Activity
3. Results
3.1. Thermodynamic Prediction of Operating Conditions for Supercritical Fluid Extraction
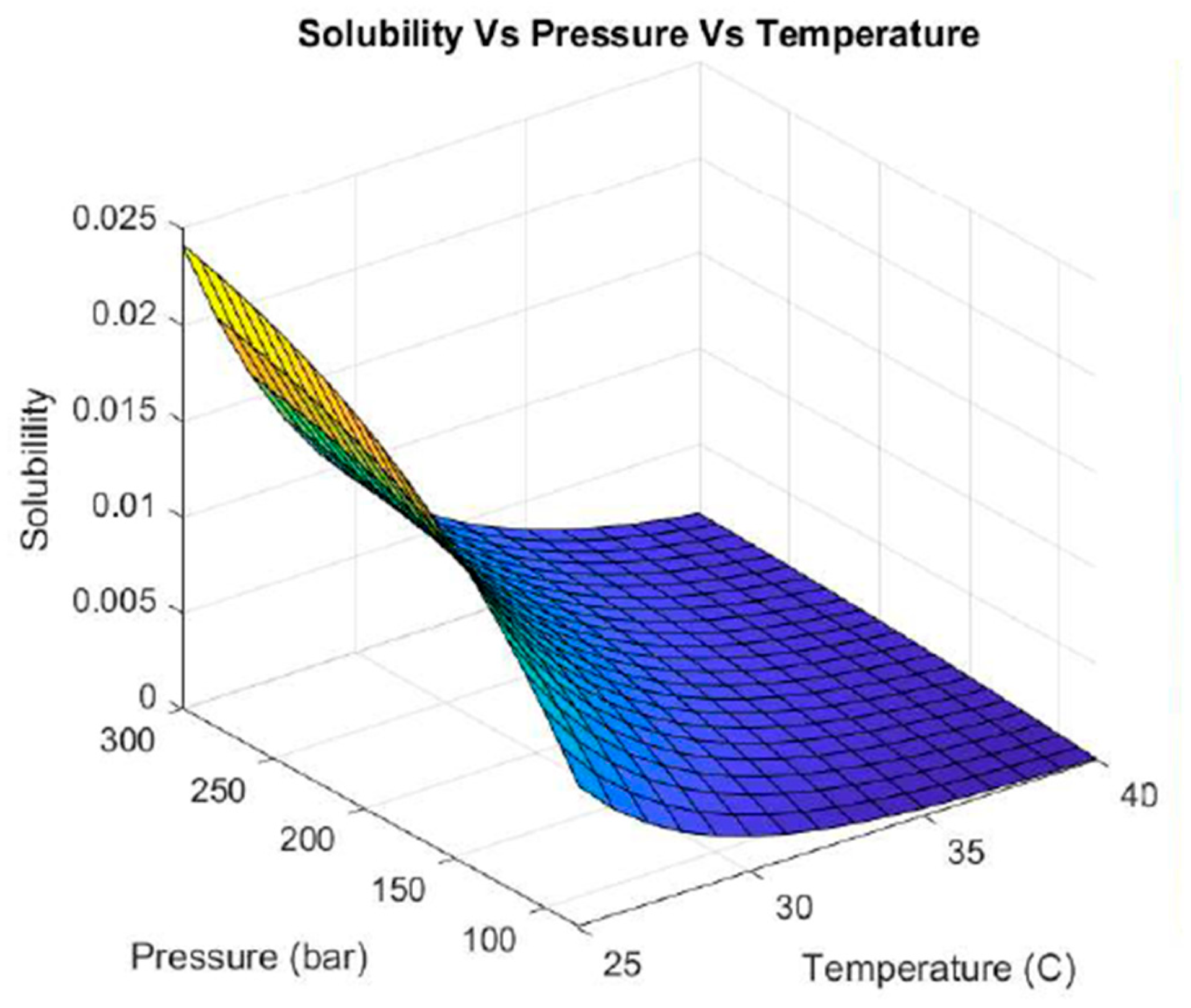
3.1.1. Thermodynamic Prediction of Operating Conditions Using CO2 as the Sole Solvent
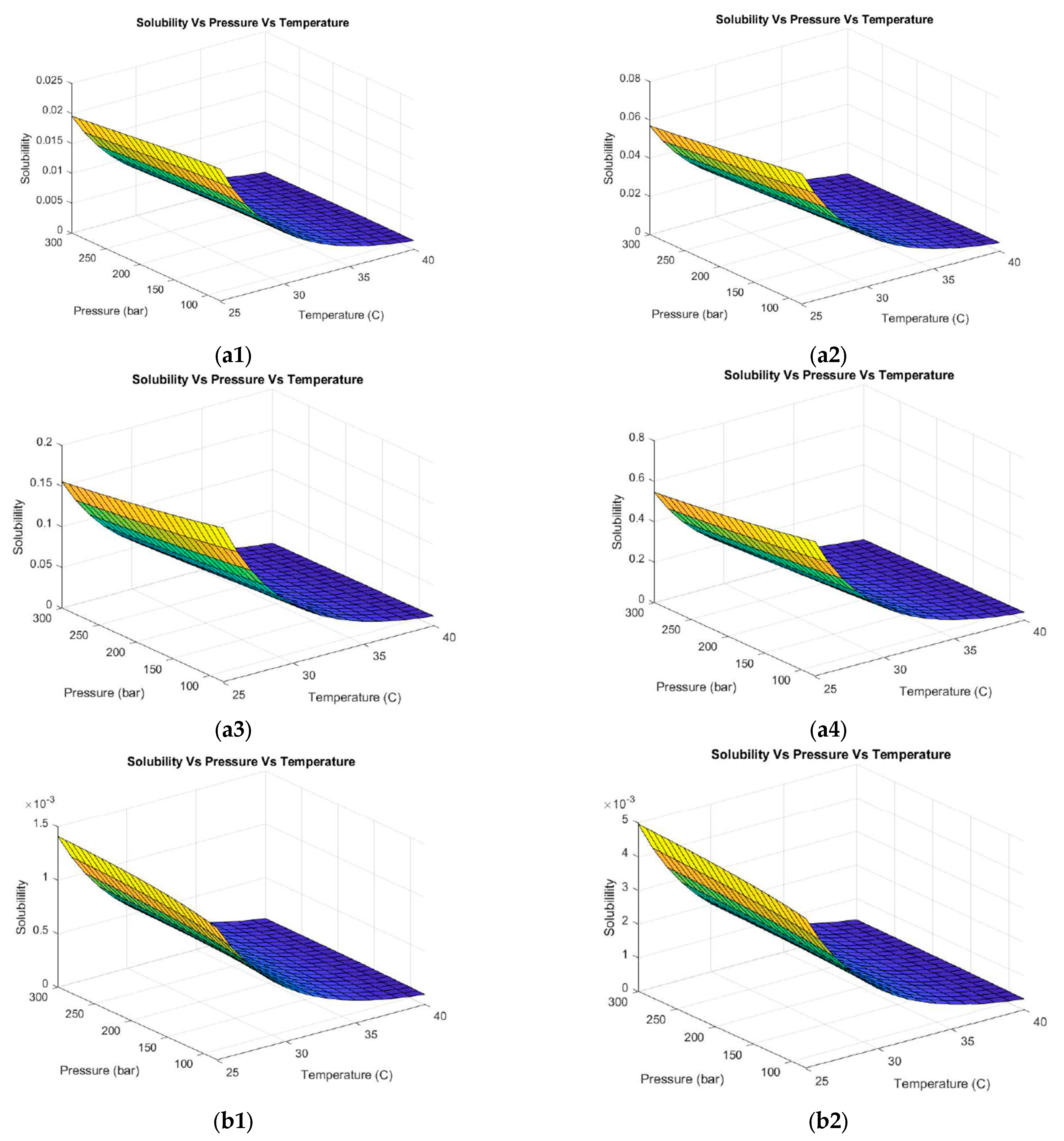
3.1.2. Thermodynamic Prediction of Operating Conditions with the Addition of Different Co-Solvents
3.2. Experimental Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vidovic, S.; Vladić, J.; Nastic, N.; Jokić, S. Subcritical and Supercritical Extraction in Food By-product and Food Waste Valorization. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 705–721. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Leipold, S.; Petit-Boix, A. The circular economy and the bio-based sector—Perspectives of European and German stakeholders. J. Clean. Prod. 2018, 201, 1125–1137. [Google Scholar] [CrossRef]
- Linser, S.; Lier, M. The contribution of sustainable development goals and forest-related indicators to national bioeconomy progress monitoring. Sustainability 2020, 12, 2898. [Google Scholar] [CrossRef]
- Donner, M.; Gohier, R.; de Vries, H. A new circular business model typology for creating value from agro-waste. Sci. Total Environ. 2020, 716, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Chen, Y.; Barzee, T.J.; Zhang, R.; Pan, Z. Citrus. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–242. [Google Scholar] [CrossRef]
- Berk, Z. Production of single-strength citrus juices. In Citrus Fruit Processing; Academic Press: Boca Raton, FL, USA, 2016; pp. 127–185. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass Convers. Biorefin. 2020, 11, 645–659. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Goula, A. Polyphenols in Agricultural Byproducts and Food Waste. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–44. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Arroyo, B.J.; de Almeida de Melo, E.; Campos, A.; Lins, L.; Santos, A.P.; Boyano-Orozco, L.C. Bioactive Compounds and Their Potential Use as Ingredients for Food and Its Application in Food Packaging. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–156. [Google Scholar] [CrossRef]
- Coelho, M.S.; Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Association Between Diet, Health, and the Presence of Bioactive Compounds in Foods. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–183. [Google Scholar] [CrossRef]
- Gharaati Jahromi, S. Extraction Techniques of Phenolic Compounds from Plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- de la Rosa, J.D.P.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Enna, S.J.; Bylund, D.B. Hesperidin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–2. [Google Scholar] [CrossRef]
- Yumol, J.L.; Ward, W.E. The polyphenolic compound hesperidin and bone protection. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 431–440. [Google Scholar] [CrossRef]
- Chaudhri, V.K.; Hussain, Z.; Kumar, P.; Yadav, V.; Pandey, A.; Khan, R.; Srivastava, A.K. Isolation and Characterization of: Hesperidin from Orange Peel. Ethnopharmacol. Med. Foods 2016, 6, 15–18. [Google Scholar]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Cardiovascular Effects of Hesperidin: A Flavanone Glycoside. In Polyphenols in Human Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 989–992. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.L.; Smart, N.G.; Wai, C.M. Past, present, and possible future applications of supercritical fluid extraction technology. J. Chem. Educ. 1996, 73, 1163–1168. [Google Scholar] [CrossRef]
- Kiran, E.; Brennecke, J.F. Current State of Supercritical Fluid Science and Technology. In Supercritial Fluid Engineering Science; ACS Symposium Series: Washington DC, USA, 1992; pp. 1–8. [Google Scholar] [CrossRef]
- Lizcano, S.C.; Dávila, J.A.; Hernández, V. Fruit Agroindustrial Wastes for Preparing Beverages for Medicinal Purposes by Supercritical Fluid Extraction Technology: Andes Berry (Rubus glaucus benth) Case. In Production and Management of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–177. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Natural Extracts Using Supercritical Carbon Dioxide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of supercritical and subcritical fluids in food processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Trevisani Juchen, P.; Nolasco Araujo, M.; Hamerski, F.; Corazza, M.L.; Pedersen Voll, F.A. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crop. Prod. 2019, 139. [Google Scholar] [CrossRef]
- Olukcu, N.; Yanik, J.; Saglam, M.; Yuksel, M.; Karaduman, M. Solvent effect on the extraction of Beypazari oil shale. Energy Fuels 1999, 13, 895–902. [Google Scholar] [CrossRef]
- de Loos, T.W. On the phase behaviour of asymmetric systems: The three-phase curve solid-liquid-gas. J. Supercrit. Fluids 2006, 39, 154–159. [Google Scholar] [CrossRef]
- Ajchariyapagorn, A.; Douglas, P.L.; Douglas, S.; Pongamphai, S.; Teppaitoon, W. Prediction of solubility of solid biomolecules in supercritical solvents using group contribution methods and equations of state. Am. J. Food Technol. 2008, 3, 275–293. [Google Scholar] [CrossRef]
- Rad, H.B.; Sabet, J.K.; Varaminian, F. Study of solubility in supercritical fluids: Thermodynamic concepts and measurement methods—A review. Brazilian J. Chem. Eng. 2019, 36, 1367–1392. [Google Scholar] [CrossRef]
- Tomberli, B.; Goldman, S.; Gray, C.G.; Saldaña, M.D.A.; Temelli, F. Using solute structure to predict solubility of organic molecules in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 37, 333–341. [Google Scholar] [CrossRef]
- Hartono, R.; Mansoori, G.A.; Suwono, A. Prediction of solubility of biomolecules in supercritical solvents. Chem. Eng. Sci. 2001, 56, 6949–6958. [Google Scholar] [CrossRef]
- Gordillo, M.D.; Blanco, M.A.; Pereyra, C.; Martínez De La Ossa, E.J. Thermodynamic modelling of supercritical fluid-solid phase equilibrium data. Comput. Chem. Eng. 2005, 29, 1885–1890. [Google Scholar] [CrossRef]
- Cerón, I.X.; Higuita, J.C.; Cardona, C.A. Design and analysis of antioxidant compounds from Andes Berry fruits (Rubus glaucus Benth) using an enhanced-fluidity liquid extraction process with CO2 and ethanol. J. Supercrit. Fluids 2012, 62, 96–101. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Lanza, C.M. Citrus fruit|Processed and Derived Products of Oranges. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1346–1354. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Semi-batch extraction of anthocyanins from red grape pomace in packed beds: Experimental results and process modelling. Chem. Eng. Sci. 2002, 57, 3831–3838. [Google Scholar] [CrossRef]
- Moncada, J.; Cardona, C.A.; Pisarenko, Y.A. Solubility of some phenolic acids contained in citrus seeds in supercritical carbon dioxide: Comparison of mixing rules, influence of multicomponent mixture and model validation. Theor. Found. Chem. Eng. 2013, 47, 381–387. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method. J. Anal. Appl. Pyrolysis 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Idarraga, A. Production of Biomolecules with Cosmetic Applications from Fruit Residues. Master’s Thesis, Universidad Nacional de Colombia-Sede Manizales, Manizales, Colombia, 2015. [Google Scholar]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Molyneux, P. The use of the stable radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2003, 26, 211–219. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Zvezdanovic, J.; Zizovic, I. Utilization of supercritical CO2 in bioactive principles isolation from Helichrysum italicum and their adsorption on selected fabrics. J. Supercrit. Fluids 2021, 171, 105197. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Quitain, A.T.; Tanaka, M.; Sasaki, M.; Goto, M. Reaction kinetics of hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide conditions. J. Supercrit. Fluids 2012, 66, 215–220. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Airgas. Carbon Dioxide Safety Data Sheet. 2023, Volume 1173, pp. 1–8. Available online: https://www.airgas.com/msds/001013.pdf (accessed on 2 October 2024).
- C. Roth GmbH. Acetone Safety Data Sheet. 2017. Available online: https://www.carlroth.com/medias/SDB-5025-IE-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyODI4NjJ8YXBwbGljYXRpb24vcGRmfGFEWmlMMmc1T1M4NU1UUXlNVEU0TURJek1UazRMMU5FUWw4MU1ESTFYMGxGWDBWT0xuQmtaZ3w4YmQ3MjEwOGI1Yjg3ZGZhNTk3ZTg0MGY1OTU2YTVhNjM0NjNmODM5ZGRiMTdmODg1MDk1MTJiMTgwYTY3Nzc3 (accessed on 2 October 2024).
- Carl Roth GmbH. Hexane Safety Data Sheet. 2017. Available online: https://www.carlroth.com/medias/SDB-1772-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyODgwNDF8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oMGUvaDIzLzkwNjkxNzMwMTQ1NTgucGRmfDg2YWQ1Yzc3ZjMwNmU4YzNmZjcwYzQwNmY2NmQ0OTI2NTU4OWI4YjZlM2NjYTE5NTA5MGJlNjI4MDc5YTBlZGU (accessed on 2 October 2024).
- Carl Roth GmbH. Methanol Safety Data Sheet. 2022. Available online: https://www.carlroth.com/medias/SDB-0082-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wzMzU5NTh8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oYzgvaDE5LzkxMTcxNDQ0MTYyODYucGRmfGE1MzI2ZWU4Yjg4YzZlNDVhZTZmYzUyMzg4NjljZmVjN2VlZDZiMWFhMzZlYzlkMGQ4YTE4ZWFjYWFhM2RkMWE (accessed on 2 October 2024).
- Carl Roth GmbH. Ethanol Safety Data Sheet. 2014. Available online: https://www.carlroth.com/medias/SDB-6746-IE-EN.pdf (accessed on 2 October 2024).
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Pazuki, G.R.; Pahlavanzadeh, H. Correlation and prediction of the solubility of CO2 in a mixture of organic solution solvents. Theor. Found. Chem. Eng. 2005, 39, 240–245. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef] [PubMed]
- Minsalud, “Maximum Residue Limits for Contaminants in Foods”, ERIA. 2022. Available online: https://www.ins.gov.co/BibliotecaDigital/limites-maximos-de-residuos-de-contaminantes-en-alimentos.pdf (accessed on 2 October 2024).
- Food and Drug Administration. Food Chemical Safety. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-chemical-safety (accessed on 2 October 2024).
- Kandi, S.; Charles, A.L. Measurement, correlation, and thermodynamic properties for solubilities of bioactive compound (−)-epicatechin in different pure solvents at 298.15 K to 338.15 K. J. Mol. Liq. 2018, 264, 269–274. [Google Scholar] [CrossRef]
- Xu, R.; Cong, Y.; Zheng, M.; Chen, G.; Chen, J.; Zhao, H. Solubility and Modeling of Hesperidin in Cosolvent Mixtures of Ethanol, Isopropanol, Propylene Glycol, and n-Propanol + Water. J. Chem. Eng. Data 2018, 63, 764–770. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K. Supercritical Fluids and the Food Industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Franke, S.I.R.; Ckless, K.; Silveira, J.D.; Rubensam, G.; Brendel, M.; Erdtmann, B.; Henriques, J.A.P. Study of antioxidant and mutagenic activity of different orange juices. Food Chem. 2004, 88, 45–55. [Google Scholar] [CrossRef]


| Equation | Abbreviations | ||
|---|---|---|---|
| PRSV | (2) | P: Absolute pressure T: Absolute temperature R: Ideal gas constant a, and b = the energy and size parameters w: Acentric factor : Characteristic parameter of each substance | |
| (3) | |||
| (4) | |||
| (5) | |||
| (6) | |||
| (7) | |||
| VDW | (8) | : Binary interaction parameter y: Molar fraction | |
| (9) | |||
| (10) | |||
| WS | (11) | : Binary interaction parameter y: Molar fraction (solubility) : Excess Helm-holtz free energy at infinite pressure | |
| (12) | |||
| (13) | |||
| (14) | |||
| (15) | |||
| (16) | |||
| Parameter | Hesperidin | Ethanol | Methanol | Hexane | Acetone |
|---|---|---|---|---|---|
| Critical Temperature (K) | 1066.45 | 514 | 512.5 | 507.69 | 508.2 |
| Critical Pressure (bar) | 14.65 | 61.37 | 80.84 | 30.25 | 47.01 |
| Acentric factor | 0.79 | 0.64 | 0.56 | 0.30 | 0.31 |
| Compound | Polarity | Molecular Weight (g/mol) | Critical Pressure (atm) | Critical Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| CO2 | Nonpolar | 44.01 | 73.8 | 31.0 | [57] |
| Acetone | Polar | 58.08 | 47.0 | 235.0 | [58] |
| Hexane | Nonpolar | 86.18 | 30.2 | 234.5 | [59] |
| Methanol | Polar | 32.04 | 79.9 | 240.0 | [60] |
| Ethanol | Polar | 46.07 | 63.0 | 241.0 | [61] |
| Ethanol Concentration (% vol) | Global Yield | TPC (mgGA/100gRM *) | DPPH (EC50/mL) | ABTS (µMolTrolox/100gRM *) | Hesperidin (g/kgRM *) |
|---|---|---|---|---|---|
| 100 | 13.27 ± 1.92 | 245.61 ± 14.78 | 3.97 ± 0.71 | 14.64 ± 1.62 | 0.38 ± 0.05 |
| 50 | 16.09 ± 0.76 | 689.52 ± 22.91 | 21.88 ± 0.94 | 1.51 ± 0.35 | 0.85 ± 0.07 |
| 20 | 17.75 ± 1.02 | 749.83 ± 30.13 | 16.19 ± 0.32 | 19.34 ± 0.84 | 8.18 ± 0.01 |
| 10 | 21.28 ± 1.45 | 831.92 ± 40.01 | 15.41± 0.91 | 5.31 ± 0.67 | 11.5 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Sanchez, M.; Agudelo-Patiño, T.; Cardona Alzate, C.A. Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results. Processes 2024, 12, 2457. https://doi.org/10.3390/pr12112457
Ortiz-Sanchez M, Agudelo-Patiño T, Cardona Alzate CA. Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results. Processes. 2024; 12(11):2457. https://doi.org/10.3390/pr12112457
Chicago/Turabian StyleOrtiz-Sanchez, Mariana, Tatiana Agudelo-Patiño, and Carlos Ariel Cardona Alzate. 2024. "Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results" Processes 12, no. 11: 2457. https://doi.org/10.3390/pr12112457
APA StyleOrtiz-Sanchez, M., Agudelo-Patiño, T., & Cardona Alzate, C. A. (2024). Maximizing the Hesperidin Extraction Using Supercritical Carbon Dioxide and Ethanol: Theoretical Prediction and Experimental Results. Processes, 12(11), 2457. https://doi.org/10.3390/pr12112457









