Abstract
Agroindustrial waste can be valorized towards the obtaining of several products such as pigments, proteins, fibers, and polyphenolic compounds with antioxidant capacity. Orange peel waste is a promising source of polyphenolic compounds such as hesperidin. However, conventional extraction techniques present some environmental limitations such as high solvent consumption and high wastewater generation. Supercritical fluid extraction (SFE) has emerged as a green extraction technique due to the low use of solvent. The aim of this study was to maximize the hesperidin extraction based on theoretical predictions of the operating conditions from empirical thermodynamic models using SFE with carbon dioxide (CO2) as a solvent. The theoretical conditions were validated experimentally on a semi-pilot scale. The extracts were evaluated in terms of hesperidin content, total polyphenol content, and antioxidant capacity. Thermodynamic prediction of the operating conditions showed that the ethanol used as a co-solvent promotes hesperidin extraction. The optimum operating conditions were 25 °C, 80 bar, and a volumetric co-solvent concentration of 10%. The validation of the operating conditions resulted in a final hesperidin concentration of 11.5 ± 0.03 g/kg of orange peel waste. The experimental results were 30.26-times higher using 10% vol of ethanol than the extraction of hesperidin with pure ethanol as a co-solvent. The total polyphenol content and antioxidant capacity resulted in 831.92 ± 40.01 mg Galic acid/100 g orange peel waste, 15.41± 0.91 EC50/mL, and 5.31 ± 0.67 µMolTrox/100 g orange peel. Finally, the prediction of operating conditions from empirical thermodynamic models such as the Peng–Robinson equation of state with some modifications (Stryjek Vera) for solid–gas equilibrium solubility calculations, allows for maximizing the content of the polyphenolic compounds using SFE.
1. Introduction
Biotechnological processes have been one of the main bases of study in the food industry for formulating dietary, natural, and functional products [1]. Moreover, there is a growing scientific and industrial interest in the use of agroindustrial waste and agricultural by-products [2]. These trends promote the bioeconomy development in the framework of a circular economy based on biomass resources [3]. Through the development of the bioeconomy, achieving the sustainable development goals defined by the United Nations could be more than a dream [4]. The environmental and social impact becomes as crucial as the economic impact in the analysis and proposals of new agroindustrial waste valorization processes [5]. Then, the development of green processes has become a focus of research on the use of agroindustrial wastes [6]. Green processes involve the design of bioprocesses to promote environmental impact reduction and the generation of noxious products. Green processes can be applied in the transformation of biomass to a valuable product and for the recovery of valuable products such as hesperidin by means of novel extraction techniques [7].
One of the most important fruit crops in the world is the orange [8]. During the industrial processing of the fruit to produce orange juice, a large amount of waste is generated (peel, pulp, and seed). This waste is known as orange peel waste (OPW) [9]. At an industrial level, OPW is used for the production of essential oil and pectin [10]. Nevertheless, large amounts of OPW are destined for landfills causing environmental problems [11]. OPW contains a high content of polyphenolic compounds such as flavonoids, phenolic acids, and coumarins [12]. Therefore, a potential valorization route for OPW is the extraction of bioactive compounds [13].
Polyphenolic compounds are a highly diverse group of secondary metabolites and are present in several plants, fruits, and vegetables [14]. Polyphenolic compounds are characterized by high nutritional benefits and antioxidant properties [15]. For this reason, these compounds are widely generated at the industrial level [16]. They have applications across different sectors, such as the cosmetics, food, and the pharmaceutical industry [17]. The polyphenolic compounds market is expected to grow, increasing the interest of several industries in their production [16].
Polyphenolic compounds are obtained through different extraction processes [18]. Recent studies promote the catalytic or enzymatic conversion to synthesize polyphenols with higher antioxidant capacity and better sensory properties [19]. Hesperidin, gallic acid, ferulic acid, and p-coumaric acid are the most significant compounds found in OPW. The most abundant polyphenolic compound identified is hesperidin [20]. Hesperidin is a flavone glycoside (3,5,7-trihydroxyflavanone-7-rhamnoglucoside) with pharmacological properties [21]. Indeed, the pharmacological properties of citrus fruits are due to the presence of several compounds such as flavonoids, where hesperidin stands out [22]. Figure 1 presents the chemical structure of hesperidin.
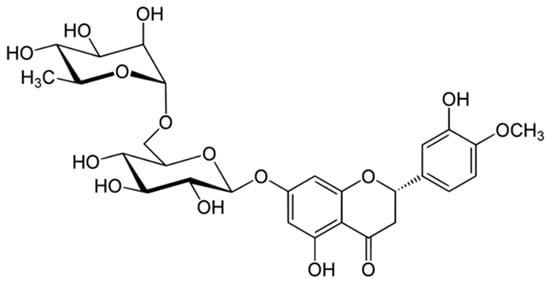
Figure 1.
Chemical structure of hesperidin.
Hesperidin contains an aglycone, a hesperidin (methyl eriodyctiol) attached to a rutinose. The rest of the glucosides of hesperidin are disaccharides that contain sugars such as rhamnose and glucose [22]. Orange pulp contains between 0.0256 and 0.393 g of hesperidin per kg of fresh pulp [22]. It has been reported that up to 4.1% hesperidin can be found in OPW [23]. As a result, investigating the extraction of hesperidin from OPW is of great interest. The pharmacological properties of hesperidin include antihyperlipidemic, cardioprotective, antihypertensive, and antidiabetic activities. These are mainly related to the antioxidant, anti-inflammatory, and insulin sensitivity-enhancing properties [24].
In conventional extraction techniques, a green process has to be defined. For instance, in solvent extraction, environmentally friendly and non-toxic compounds must be used to satisfy the technological requirements (production yields) [25]. Emerging extraction technologies are intended to further promote the technical and environmental development of these processes [26]. Supercritical fluid extraction (SFE) is an emerging technology and has been categorized as a green technology [27]. Nevertheless, the properties constantly change from gas to liquid as pressure and temperature are varied [28]. The use of supercritical fluids has been widely studied to extract different solutes [29]. Carbon dioxide (CO2) has been the solvent that has received the most attention [30].
Supercritical CO2 is a nonpolar solvent [31]. Generally, the solute solubility in supercritical CO2 is directly related to density. Thus, at high pressures, the solubility of the solute increases [32]. Thus, temperature generates two effects on the supercritical CO2 solute solubility. At high temperature and constant pressure, the solvent density decreases and, consequently, solubility decreases. When the solvent density is high, and the temperature increases, the vapor pressure of the solute increases, and solubility increases as well [33].
Bubalo et al. [32] defined as a guideline that the supercritical CO2 solubility of substances decreases with an increasing number of polar functional group. For polar solutes, the use of polar co-solvents is recommended to increase the solvation power [7]. The addition of water as a co-solvent does not improve the CO2 polarity. In fact, only 0.3% of the water is miscible with CO2. Co-solvents enhance solubility but also increase viscosity and density. This result implies that the diffusivity in the mobile phase decreased with a consequential mass transfer reduction. Besides, for a non-volatile solute, a higher temperature causes less extract recovery. One of the advantages of using SFE is the selectivity of the solute to the extract. Due to the wide range of polarity of the analytes, there is no universal solvent that can be used in a supercritical fluid extraction process. Trevisani Juchen P et al. [34] analyzed the extraction behavior using supercritical CO2 as the solvent and ethanol as a co-solvent to obtain rice bran oil. The highest extraction yield (25.48 wt.%) was obtained with a solid–liquid ratio of 1:1, 40 °C, and 200 bar. Likewise, several studies have been reported to evaluate the potential of aromatic co-solvents such as toluene in hydro-carbide recovery processes. Olukcu et al. [35] subjected Beypazari oil shale to supercritical fluid extraction with water and toluene. The results evidenced that using water as a co-solvent presented a higher degree of oil shale kerogen conversion than toluene. All these studies should encompass the research on using suitable co-solvents and achieving high extraction yields while maintaining low operating costs and environmental impacts.
The analysis of the phase behavior (PVT) of different solvents in the supercritical state has been previously investigated from different equations of state (EOSs) [36]. Indeed, Ajchariyapagorn [37] determined that the EOS of the Peng–Robinson, Redlich–Kwong–Soave, Lee–Kesler–Plocker, and Peng–Robinson with the Boston–Mathias alpha function are the most suitable for predicting PVT for CO2 in the supercritical state. The phase equilibrium between the solute, the solvent, and the co-solvent has been investigated from experimental works and mathematical modeling works [38]. The phase equilibrium study allows determining the solubility of the solute in the solvent in the supercritical state. In the literature, research to determine experimentally the solute solubility in a supercritical fluid exceeds the mathematical modeling research [39]. However, the correct mathematical prediction of the solute solubility in a supercritical fluid saves time and reagents. Most solubility modeling studies are based on empirical models or first principles models [37]. Empirical models incorporate experimentally obtained fitted parameters. First-principles models use properties of the pure compounds to calculate the EOS solubility. Hartono et al. [40] estimated the solubility of two compounds (b-carotene and cholesterol) in supercritical fluid (CO2) from the Vand der Waals and Redlich–Kwong EOS considering experimental data. Gordillo et al. [41] determined the phase equilibrium of penicillin in supercritical CO2 from experimental data. The data adjustment was carried out from the Van der Waals equation for the binary interaction parameters and the Lorentz–Berthelot mixing rule applied to the EOS Sánchez–Lacombe. Ceron et al. [42] estimated the solubility of cyanidin-3-glucoside in CO2 using the Soave–Redlich–Kwong EOS with the Van Wong–Sandler mixing rule.
This work aims to determine the optimal operating conditions for pressure and temperature based on theoretical predictions from an empirical model. Moreover, the volumetric concentration of the co-solvent was analyzed to increase the solubility of hesperidin as one of the most interesting polyphenolic compounds in OPW. For this purpose, mathematical modeling of the hesperidin solubility in supercritical CO2 using different types of co-solvents was carried out. The purpose was to maximize the hesperidin yields expected in SFE. Finally, these thermodynamic modeling results were validated at the experimental level to adjust these conditions.
2. Materials and Methods
The maximization of the hesperidin extraction with supercritical CO2 was analyzed in two stages. The first stage involved the theoretical prediction of operating conditions such as temperature, pressure, and volumetric concentration of the co-solvent. For this purpose, four co-solvents were analyzed. Thermodynamic models such as the Peng–Robinson equation of state with the Stryjek Vera modification have been used to calculate the hesperidin solubility. This approach allows selecting the most suitable co-solvent and operating conditions to carry out the hesperidin extraction. The second stage considered the experimental validation of the results obtained in the theoretical prediction for the co-solvent with better solubility values for hesperidin. The experiments were conducted in a pilot plant for supercritical CO2 extraction.
2.1. Raw Material and Chemicals
The raw material used was OPW from orange processing (Citrus sinensis) in a juice factory (FLP Procesados Company) located in the center of Colombia, Chinchina, Caldas (Colombia). The OPW was obtained from the mechanical extraction step of orange juice. Then, the OPW was frozen to conserve the properties. For the experimental tests, the OPW was dried at 40 °C to 10% moisture in a convection oven. Then, samples were ground in a rotary mill (SR200 Gusseisen, Redsch GmbH, Munich, Germany) to a diameter of 0.4 mm.
2.2. Thermodynamic Prediction of Operating Conditions for Supercritical Fluid Extraction
The theoretical prediction of the operating conditions for SFE was carried out based on the solubility of a solute in the supercritical fluid phase. The flavones, especially hesperidin, are the most abundant polyphenolic compounds in OPW [43,44]. For this reason, the solubility analysis was performed, having hesperidin as a component of the solid phase to be extracted.
The solute solubility in a phase is defined with thermodynamic models such as Raoult’s law. However, Raoult’s law is only applicable to molecularly similar compounds of low pressures and ideal behavior. Polyphenolic compounds are structurally complex molecules. As a result, Raoult’s law cannot be applied. However, the phase equilibrium can be calculated by using the fugacity of the mixture based on the chemical potential equation for each component in each phase at fixed temperature and pressure. The phase equilibrium between solute and supercritical CO2 is defined as a solid–gas equilibrium. As for the supercritical phase, the fugacity can be obtained by considering the fluid as an expanded liquid or a dense gas [45]. Then, a solid–gas equilibrium approach was proposed maintaining constant pressure and temperature, where the fugacity of the solid phase and the gas phase at equilibrium are equal. For this analysis, a binary system was considered. The binary system comprises a solute in the solid phase, the metabolite in the OPW (hesperidin), and CO2 in the gas phase. Equation (1) expresses the solute solubility in the gas phase from the iso-fugacity criterion.
where is the sublimation pressure of the solute, is the solid molar volume of the solute, is the sublimation fugacity coefficient of the solute (close to unity), and is the fugacity coefficient in the supercritical phase of the solute.
The thermodynamic solid–gas equilibria parameters and equation of state approach were used. For solubility calculations, the Peng–Robinson equation of state was used with some modifications (Stryjek Vera) and considering the Van der Waals (VDW) and Wong–Sandler (WS) mixing rules [46]. Table 1 shows the equations of state and mixing rules used for the calculation of solubility.

Table 1.
Equations for the calculation of solubility.
Phase equilibrium was determined by equating the chemical potentials of each component in each phase at constant pressure and temperature. Then, for pure substances, a vapor–solid equilibrium was considered, where the vapor phase represents the solid phase transition. In the WS mixing rules, the excess Helmholtz free energy at infinite pressure was calculated in the same manner as the excess Gibbs free energy obtained from activity models (UNIFAC-Functional Group Activity Coefficients) with some modifications (Dortmund-UNIFAC DORTMUND) [46].
The extraction was analyzed using ethanol, methanol, acetone, and hexane as co-solvents to increase the hesperidin concentration. Additionally, the use of each co-solvent at different volumetric concentrations was evaluated. These compounds were selected as co-solvents for the hesperidin extraction from orange peels due to the wide range of polarities they handle. Likewise, they are economical, and their recovery process is easier. The properties of the mixture (CO2, co-solvent, and hesperidin) were obtained from the Properties Analyzer tool in the software Aspen Plus V.9.0 (i.e., critical temperature, critical pressure, critical volume, normal boiling point, and acentric factor) as shown in Table 2. The thermodynamic method used was Peng–Robinson [37]. The equations system solution for the calculation of solubility was carried out using the Matlab R2020a software.

Table 2.
Compound properties obtained in Aspen Plus for solubility calculation.
2.3. Experimental Validation
The experimental validation of the theoretically predicted operating conditions was developed in a supercritical CO2 pilot plant. The co-solvent with the highest solubility of hesperidin was selected to corroborate the results. Additionally, the volumetric concentration of the selected co-solvent was varied to corroborate the trend shown in the prediction of operating conditions. Figure 2 shows the schematic representation of SFE equipment.
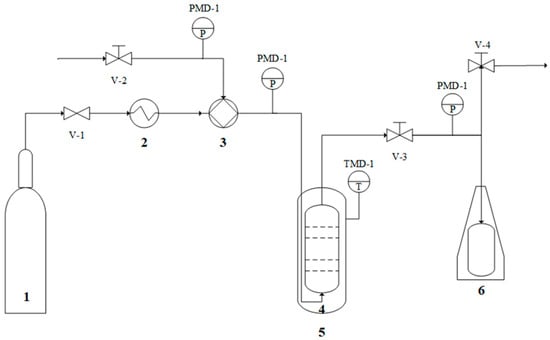
Figure 2.
Schematic representation of SFE equipment: (1) CO2 tank, (2) cooler, (3) high-pressure pump, (4) extractor tank, (5) extractor jacket, and (6) collecting tank. V: valve; PMD: pressure measurement device; TMD: temperature measurement device.
The SFE equipment consists of a CO2 conditioning line (cooler and high-pressure pump), the extractor (jacket and extractor tank), the extract collection, and the depressurization line (collecting tank). The capacity of the equipment and operation conditions were 254 mL, 350 bar, and 70 °C, respectively. The sample was introduced into a thimble and placed in an extraction vessel for 60 min. A solid–co-solvent ratio of 1:5 and a solid–solvent ratio of 1:18 were considered. The extracts were collected and stored at 4 °C. The extracts were characterized in terms of antioxidant activity via two methods (DPPH radical inhibition and ABTS+ radical decolorization) and total phenolic content.
2.3.1. Total Polyphenol Content (TPC) Identification
Total polyphenol content (TPC) was determined considering the Folin–Ciocalteu (1999) method with some modifications [47,48]. The procedure consisted of adding 150 µL of the collected extract into a vial and adding 150 µL of Folin–Ciocalteu reagent, distilled water (2.4 µL), and sodium carbonate solution (300 µL—20% w/v). Then, the solution was maintained in the dark for 2 h. The absorbance of the sample was determined at a wavelength of 765 nm from a spectrophotometer (UV/Visible Model 6405, Jenway, Essex, UK). The calibration curve was performed using different dilutions of gallic acid (10, 25, 50, 50, 75, and 100%) and the methodology described above. The TPC was expressed as mg of gallic acid equivalents (GA) per 100 g of dry sample.
2.3.2. Antioxidant Activity
Antioxidant activity was evaluated through DPPH (α,α-diphenyl-β-picrylhydrazyl) radical inhibition and ABTS+ (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) cationic radical decolorization assay).
The first method (DPPH radical inhibition) was performed based on the methodology described by Morinova et al. [49], Molyneux et al. [50] and Brand-Williams et al. [51]. Three dilutions of the sample (1:150, 1:300, and 1:500) in ethanol were performed. Then, 150 µL of each dilution was extracted and mixed with 3 mL of DPPH solution. The samples were placed in darkness for 1 h. Finally, the absorbance at 517 nm was determined in the spectrophotometer described above. The calibration curve was performed using different dilutions of a standard Trolox solution (10, 25, 50, 50, 75, and 100%) and the methodology described above. The control solution was prepared using ethanol instead of the extracted sample. The radical inhibition was calculated using Equation (17).
where Ao is the absorbance of the control, and Af is the absorbance of the sample after 60 min of reaction.
The percentage inhibition of the DPPH radical was plotted versus antioxidant concentration to determine the 50% inhibition concentration (IC50). The IC50 values were expressed in µmol Trolox per 100 g dry sample (TAA—µmol Trolox/100 g sample).
The second method (ABTS+ cation-radical decolorization) was performed considering the methodology proposed by Re et al. [52] and Ozgen et al. [53]. The same dilutions of the extract described above were considered for this method. Then, 150 µL of each dilution was extracted and mixed with 3 mL of a 60 µM ABTS solution. A calibration curve was prepared using Trolox® standard solutions diluted in ethanol. The inhibition of the ABTS+ radical cation was determined using Equation (17) but considering an absorbance of 734 nm. Finally, the IC50 values were expressed as µmol Trolox per 100 g dry sample (TAA—µmol Trolox/100 g sample).
3. Results
3.1. Thermodynamic Prediction of Operating Conditions for Supercritical Fluid Extraction
3.1.1. Thermodynamic Prediction of Operating Conditions Using CO2 as the Sole Solvent
Figure 3 shows the solubility behavior of hesperidin with supercritical CO2 extraction when varying the pressure and temperature without using a co-solvent. At high temperatures (35–40 °C), hesperidin was not solubilized at any pressure range tested. Reducing the temperature to values below 30 °C and handling pressures above 200 bar showed increased solubility. The highest solubility was achieved at high pressures (300 bar) and low temperatures (25 °C).
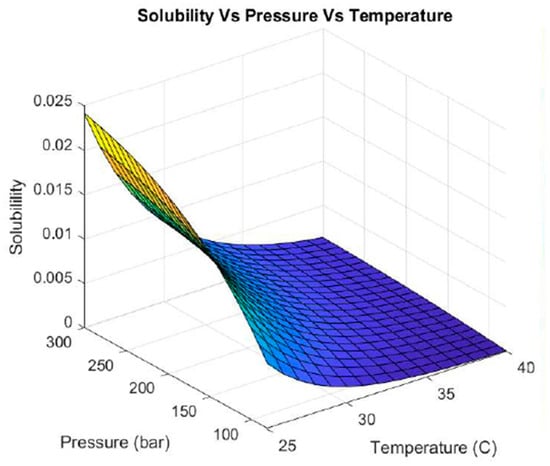
Figure 3.
Hesperidin solubility with supercritical CO2 as solvent and without co-solvent.
The relationship between the supercritical fluid polarity and the compound polarity to be extracted is crucial to determining the extraction efficiency. This principle follows the basic solubility principle, implying that supercritical fluids must have polar or apolar properties similar to those of the compound to be extracted to achieve efficient extraction [54]. CO2 is a nonpolar molecule as seen in Table 3 where the carbon atom has reached the noble gas atomic structure. Therefore, this compound does not participate in chemical reactions. Moreover, CO2 cannot form hydrogen bonds; consequently, there is a low physical solubility [55]. CO2 in a supercritical state combines the properties of a gas and liquid and acts as a gas in terms of diffusivity (can easily penetrate solids) and as a liquid in terms of dissolution capacity. Using supercritical CO2 is effective for dissolving apolar or slightly polar compounds. These properties can be adjusted by varying the pressure and temperature. By modifying these parameters, the density of CO2 is affected and generates a variation in the solute and solvent interactions [56].

Table 3.
Characteristics of the solvents used for hesperidin extraction.
At high temperatures (35–40 °C), the supercritical CO2 density decreases. Then, CO2 becomes less efficient in dissolving large and polar molecules such as hesperidin. This is because the solute–solvent interaction is weakened and as a consequence so is the solubility. In this case, even an increase in pressure is insufficient to compensate for the low density of the fluid. Therefore, hesperidin is not solubilized in any pressure range analyzed. On the contrary, by decreasing the temperature (25–30 °C) and increasing the pressure by more than 200 bar, the density of CO2 increases considerably. This improves the solubility of hesperidin, because the denser CO2 behaves as a liquid, favoring the solubilization of larger and polar molecules.
The variation of extreme operating conditions, in particular pressure, can be changed by using polar modifiers (co-solvents) in the process. The use of co-solvents, such as acetone, ethanol, or methanol, can increase the polarity of the supercritical fluid, allowing the solubilization of polar compounds [62]. For example, Pazuki G et al. [63] demonstrated that CO2 is more soluble in solvents with the carbonyl group (CO). Furthermore, the authors determined that CO2 is less soluble in less polar solvents with a symmetric structure.
3.1.2. Thermodynamic Prediction of Operating Conditions with the Addition of Different Co-Solvents
The results of the thermodynamic analysis to determine the best co-solvent and best volumetric concentration are shown in Figure 4. The co-solvents acetone, ethanol, hexane, and methanol were analyzed. Figure 4(a1–a4) shows the behavior of hesperidin solubility with different volumetric concentrations of acetone (30%, 50%, 70%, and 100%). Supercritical CO2 is an apolar or slightly polar solvent, ideal for dissolving apolar compounds. Moreover, CO2 has limitations for dissolving polar compounds. Adding a co-solvent, such as those evaluated in this work, increases the effective polarity of the system. In addition, the use of co-solvents allows operating conditions such as pressure and temperature to be reduced. This was evidenced in the obtained results. In contrast to using CO2 as the exclusive solvent, when 30% vol of acetone was added, the solubility was not affected in the pressure range evaluated; on the contrary, this parameter remained practically constant. Likewise, no hesperidin solubility was evidenced at temperatures above 35 °C. Maximum solubility was reached at 80 bar and 25 °C. The same trend was evident when using 50% acetone with an increase in solubility. By increasing the acetone concentration to 70%, the solubility of hesperidin increased in a temperature range of 25–30% by almost twice compared to the previous case. On the other hand, by using absolute acetone, the maximum solubilization of hesperidin was obtained.
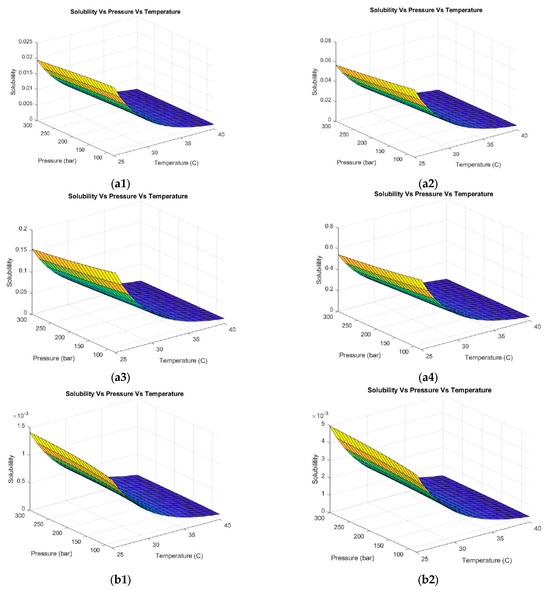
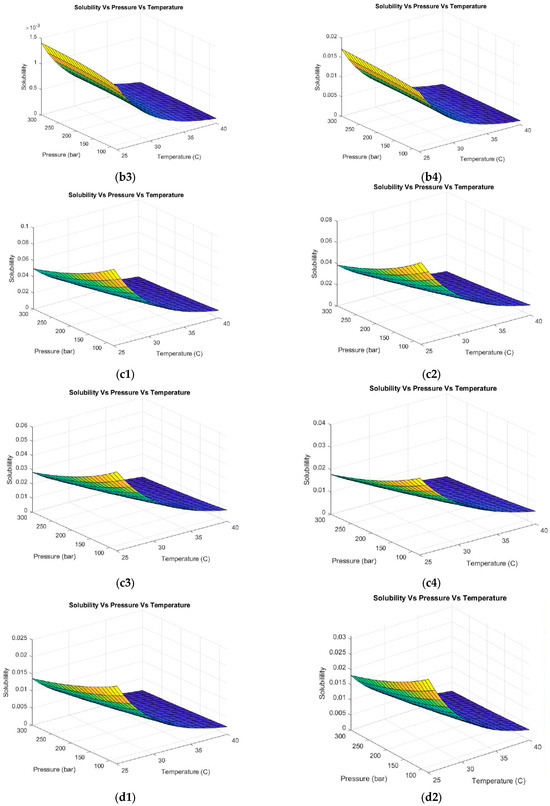
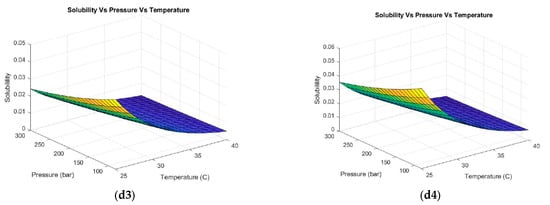
Figure 4.
Hesperidin solubility with different co-solvents at different volumetric concentrations. (a1–a4) Acetone (1) 30%, (2) 50%, (3) 70%, and (4) 100%. (b1–b4) Hexane (1) 30%, (2) 50%, (3) 70%, and (4) 100%. (c1–c4) Methanol (1) 30%, (2) 50%, (3) 70%, and (4) 100%. (d1–d4) Ethanol (1) 30%, (2) 50%, (3) 70%, and (4) 100%.
Using hexane as a co-solvent (Figure 4(b1–b4)) evidences the same trend of pressure and temperature behavior described above. Nevertheless, the representative numerical values of solubility decreased drastically compared to the case of using CO2 as the exclusive solvent. This was expected because of the characteristics of hexane (see Table 3). Hexane is a nonpolar solvent. When used as a co-solvent in a supercritical CO2 system, the effective polarity of the supercritical fluid is further decreased, resulting in negative implications when extracting polar compounds such as hesperidin [64]. As a result, the use of hexane as a co-solvent is not recommended because this compound does not optimize the operating conditions compared to more polar co-solvents.
When using methanol as a solvent (see Figure 4(c1–c4)), a positive trend in the hesperidin solubility was observed. An increasing behavior of solubility was evidenced with decreasing pressure and temperature for all methanol concentrations evaluated (10, 30, 50, and 100% vol). The highest solubility was obtained at low methanol concentrations (10–30% vol) as shown in Figure 4(c1,c2). This co-solvent allows the CO2 system to dissolve compounds with polar functional groups (such as hydroxyls or glycosides in hesperidin), leading to a higher extraction yield of polar compounds and a higher speed and efficiency of the process. However, one of the limitations of this solvent is toxicity. Regulatory authorities such as the Food and Drug Administration (FDA) restrict the use of methanol in the food and cosmetics industry. The use of safer solvents, such as ethanol or isopropanol, is preferred to minimize the risks to the consumer [65]. As a result, ethanol was evaluated as a co-solvent for the extraction of hesperidin from supercritical fluids as observed in Figure 4(d1–d4). The same pressure and temperature behavior was evidenced in the previous case. The highest solubility was obtained by working with low pressures and temperatures (80 bar and 25 °C) and ethanol concentrations of 10 and 30% vol. Ethanol is a solvent approved by international regulations [66] to be applied in processes involving as a final product a compound directed to the food or cosmetic industry due to low toxicity. Moreover, this solvent can be easily removed or evaporated at the end of the process. Kandi et al. [67] selected four types of organic solvents (methanol, ethanol, acetone, and water) based on the solubility of the bioactive compound ((−)-epicatechin). The solubility of the solvents that were measured in a temperature range from 298.15 to 338.15 K while maintaining a pressure of 0.1 MPa showed that 75% vol ethanol and acetone proved to be the most promising solvents for solubilizing (−)-epicatechin. Therefore, these solvents could be used to formulate products in several industries (chemical, pharmaceutical, and food) due to their non-toxicity and price. Xu R et al. [68] established that the hesperidin solubility depends mostly on the temperature and solvent composition. The authors evaluated ethanol, isopropanol, propylene glycol, and n-propanol as solvents. Ethanol was the solvent with the highest solubility of hesperidin.
Methanol, ethanol, and acetone were the co-solvents that enhanced the solubility of hesperidin. Ethanol presented the highest affinity for hesperidin. Solubility was determined to be a function of pressure. For each co-solvent tested, solubility decreased with increasing pressure. Thus, low pressures are recommended in supercritical fluid extraction, ensuring supercritical conditions of the solvent and co-solvent (120 bar). These results elucidate the potential of using co-solvents in the hesperidin extraction process due to the opportunities to handle lower operating conditions (pressure and temperature). This can be reflected in lower operating costs of the process.
3.2. Experimental Validation
The results obtained from the thermodynamic analysis were validated by performing the four extractions with the same relation: solid–solvent (CO2) ratio of 1:18 and a solid–co-solvent (ethanol) ratio of 1:5. The results of yield, antioxidant capacity, and total polyphenols are shown in Table 4.

Table 4.
Global yield, TPC, ABTS, and DPPH isotherms obtained via SFE.
Table 4 corroborates the trend obtained through thermodynamic analysis. The global yield results based on the amount of OPW lost are higher when volumetric concentrations of ethanol are lower (10% vol). This trend shows that there is a higher extraction of compounds under the conditions studied. There was a 60.36% increase in global yield when the extraction was performed with a volumetric concentration of 10% ethanol compared to using 100% ethanol. Espinosa-Prado et al. [69] analyzed the extraction of OPW polyphenolic compounds using supercritical CO2 without co-solvent and with co-solvent by varying the pressure (150–350 bar) and temperature (40–60 °C). These authors obtained a global yield between 2.01 and 2.62%. This difference is attributed to the variations between the pressure and temperature used. The authors observed that, when the pressure increases from 150 to 350 bar, the global yield decreased. Rozzi et al. [70] mentioned that flavonoids with high molecular mass are hardly soluble in pure CO2. However, the solubility of these molecules can increase by increasing pressure. This information helps to validate the results obtained in the present work in terms of global yield. Additionally, the resulting yields are higher than those reported in the open literature.
The TPC also followed this trend. At lower volumetric concentrations of ethanol, there was a higher concentration of TPC. This increase was more significant than the global yield. There was an increase of 70.47% of TPC when the extraction was performed with 10% vol of ethanol compared with pure ethanol. Espinosa-Prado et al. [69] reported TPC concentrations of 103 mg GAC/100 g RW at 250 bar, 60 °C, and a volumetric concentration of ethanol (co-solvent) of 90%. A similar result can be seen in Table 4 when the extraction with pure ethanol was evaluated as a co-solvent at 80 bar and 30 °C. However, this TPC concentration was the lowest compared with the result obtained in this work.
The antioxidant activity analyzed via the DPPH and ABTS tests did not follow the same trend. In general, the TPC has been linked to DPPH. Nevertheless, as shown in this work, the trend does not follow this tendency. Different authors, such as Barros et al. [71], Rapisarda et al. [72], and Franke et al. [73], suggest that the antioxidant activity of citrus extracts is directly related to the content of ascorbic acid, which is moderately soluble in ethanol. The highest DPPH radical removal activity was obtained for a 50% ethanol volumetric concentration. Moreover, the highest ABTS radical removal activity was obtained for 20% ethanol volumetric concentration.
4. Conclusions
This work successfully validated the agreement between the theoretical prediction of the operating conditions (pressure and temperature and volumetric concentration of co-solvents) of hesperidin extraction when using supercritical fluids (SFEs) and experimental results. Then, this methodology can be applied as a first stage to laboratory-scale SFE processes. Thermodynamic solid–gas equilibrium parameters and equations of state can be used. For example, the Peng–Robinson equation of state with some modifications (Stryjek Vera) is suitable for solubility calculations and Van der Waals (VDW) and Wong–Sandler (WS) mixing rules. Likewise, for pure substances, a vapor–liquid equilibrium can be considered. At a theoretical level, SFE using CO2 did not solubilize hesperidin at high temperatures (35–40 °C). In contrast, decreasing the temperature and handling pressures above 200 bar showed increased in solubility. The use of solvents such as acetone, methanol, and especially ethanol at low volumetric concentrations (10–30%) demonstrated an increase in the solubility of hesperidin as pressure and temperature decreased. In addition, for the first time, the efficient combination of theory and experimental results allowed obtaining higher hesperidin extraction yields when using SFE with CO2 compared to the results reported by other authors in the open literature.
Author Contributions
M.O.-S.: Investigation, Methodology, Analysis, Writing—original draft, Simulation; T.A.-P.: Investigation, Methodology, Analysis, Edition; C.A.C.A.: Funding acquisition, Conceptualization, Supervision, Writing, Edition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Mejoramiento de cadenas de valor agrícolas a partir del uso sostenible de sus residuos en biorrefinerías. Biosos-cv” code 59879.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vidovic, S.; Vladić, J.; Nastic, N.; Jokić, S. Subcritical and Supercritical Extraction in Food By-product and Food Waste Valorization. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 705–721. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Leipold, S.; Petit-Boix, A. The circular economy and the bio-based sector—Perspectives of European and German stakeholders. J. Clean. Prod. 2018, 201, 1125–1137. [Google Scholar] [CrossRef]
- Linser, S.; Lier, M. The contribution of sustainable development goals and forest-related indicators to national bioeconomy progress monitoring. Sustainability 2020, 12, 2898. [Google Scholar] [CrossRef]
- Donner, M.; Gohier, R.; de Vries, H. A new circular business model typology for creating value from agro-waste. Sci. Total Environ. 2020, 716, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Chen, Y.; Barzee, T.J.; Zhang, R.; Pan, Z. Citrus. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–242. [Google Scholar] [CrossRef]
- Berk, Z. Production of single-strength citrus juices. In Citrus Fruit Processing; Academic Press: Boca Raton, FL, USA, 2016; pp. 127–185. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass Convers. Biorefin. 2020, 11, 645–659. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Goula, A. Polyphenols in Agricultural Byproducts and Food Waste. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–44. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Arroyo, B.J.; de Almeida de Melo, E.; Campos, A.; Lins, L.; Santos, A.P.; Boyano-Orozco, L.C. Bioactive Compounds and Their Potential Use as Ingredients for Food and Its Application in Food Packaging. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–156. [Google Scholar] [CrossRef]
- Coelho, M.S.; Fernandes, S.S.; de las Mercedes Salas-Mellado, M. Association Between Diet, Health, and the Presence of Bioactive Compounds in Foods. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–183. [Google Scholar] [CrossRef]
- Gharaati Jahromi, S. Extraction Techniques of Phenolic Compounds from Plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- de la Rosa, J.D.P.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Enna, S.J.; Bylund, D.B. Hesperidin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–2. [Google Scholar] [CrossRef]
- Yumol, J.L.; Ward, W.E. The polyphenolic compound hesperidin and bone protection. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 431–440. [Google Scholar] [CrossRef]
- Chaudhri, V.K.; Hussain, Z.; Kumar, P.; Yadav, V.; Pandey, A.; Khan, R.; Srivastava, A.K. Isolation and Characterization of: Hesperidin from Orange Peel. Ethnopharmacol. Med. Foods 2016, 6, 15–18. [Google Scholar]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Cardiovascular Effects of Hesperidin: A Flavanone Glycoside. In Polyphenols in Human Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 989–992. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.L.; Smart, N.G.; Wai, C.M. Past, present, and possible future applications of supercritical fluid extraction technology. J. Chem. Educ. 1996, 73, 1163–1168. [Google Scholar] [CrossRef]
- Kiran, E.; Brennecke, J.F. Current State of Supercritical Fluid Science and Technology. In Supercritial Fluid Engineering Science; ACS Symposium Series: Washington DC, USA, 1992; pp. 1–8. [Google Scholar] [CrossRef]
- Lizcano, S.C.; Dávila, J.A.; Hernández, V. Fruit Agroindustrial Wastes for Preparing Beverages for Medicinal Purposes by Supercritical Fluid Extraction Technology: Andes Berry (Rubus glaucus benth) Case. In Production and Management of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–177. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Natural Extracts Using Supercritical Carbon Dioxide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Hrncic, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of supercritical and subcritical fluids in food processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Trevisani Juchen, P.; Nolasco Araujo, M.; Hamerski, F.; Corazza, M.L.; Pedersen Voll, F.A. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crop. Prod. 2019, 139. [Google Scholar] [CrossRef]
- Olukcu, N.; Yanik, J.; Saglam, M.; Yuksel, M.; Karaduman, M. Solvent effect on the extraction of Beypazari oil shale. Energy Fuels 1999, 13, 895–902. [Google Scholar] [CrossRef]
- de Loos, T.W. On the phase behaviour of asymmetric systems: The three-phase curve solid-liquid-gas. J. Supercrit. Fluids 2006, 39, 154–159. [Google Scholar] [CrossRef]
- Ajchariyapagorn, A.; Douglas, P.L.; Douglas, S.; Pongamphai, S.; Teppaitoon, W. Prediction of solubility of solid biomolecules in supercritical solvents using group contribution methods and equations of state. Am. J. Food Technol. 2008, 3, 275–293. [Google Scholar] [CrossRef]
- Rad, H.B.; Sabet, J.K.; Varaminian, F. Study of solubility in supercritical fluids: Thermodynamic concepts and measurement methods—A review. Brazilian J. Chem. Eng. 2019, 36, 1367–1392. [Google Scholar] [CrossRef]
- Tomberli, B.; Goldman, S.; Gray, C.G.; Saldaña, M.D.A.; Temelli, F. Using solute structure to predict solubility of organic molecules in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 37, 333–341. [Google Scholar] [CrossRef]
- Hartono, R.; Mansoori, G.A.; Suwono, A. Prediction of solubility of biomolecules in supercritical solvents. Chem. Eng. Sci. 2001, 56, 6949–6958. [Google Scholar] [CrossRef]
- Gordillo, M.D.; Blanco, M.A.; Pereyra, C.; Martínez De La Ossa, E.J. Thermodynamic modelling of supercritical fluid-solid phase equilibrium data. Comput. Chem. Eng. 2005, 29, 1885–1890. [Google Scholar] [CrossRef]
- Cerón, I.X.; Higuita, J.C.; Cardona, C.A. Design and analysis of antioxidant compounds from Andes Berry fruits (Rubus glaucus Benth) using an enhanced-fluidity liquid extraction process with CO2 and ethanol. J. Supercrit. Fluids 2012, 62, 96–101. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Lanza, C.M. Citrus fruit|Processed and Derived Products of Oranges. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1346–1354. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Semi-batch extraction of anthocyanins from red grape pomace in packed beds: Experimental results and process modelling. Chem. Eng. Sci. 2002, 57, 3831–3838. [Google Scholar] [CrossRef]
- Moncada, J.; Cardona, C.A.; Pisarenko, Y.A. Solubility of some phenolic acids contained in citrus seeds in supercritical carbon dioxide: Comparison of mixing rules, influence of multicomponent mixture and model validation. Theor. Found. Chem. Eng. 2013, 47, 381–387. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method. J. Anal. Appl. Pyrolysis 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Idarraga, A. Production of Biomolecules with Cosmetic Applications from Fruit Residues. Master’s Thesis, Universidad Nacional de Colombia-Sede Manizales, Manizales, Colombia, 2015. [Google Scholar]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Molyneux, P. The use of the stable radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2003, 26, 211–219. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Zvezdanovic, J.; Zizovic, I. Utilization of supercritical CO2 in bioactive principles isolation from Helichrysum italicum and their adsorption on selected fabrics. J. Supercrit. Fluids 2021, 171, 105197. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Quitain, A.T.; Tanaka, M.; Sasaki, M.; Goto, M. Reaction kinetics of hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide conditions. J. Supercrit. Fluids 2012, 66, 215–220. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Airgas. Carbon Dioxide Safety Data Sheet. 2023, Volume 1173, pp. 1–8. Available online: https://www.airgas.com/msds/001013.pdf (accessed on 2 October 2024).
- C. Roth GmbH. Acetone Safety Data Sheet. 2017. Available online: https://www.carlroth.com/medias/SDB-5025-IE-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyODI4NjJ8YXBwbGljYXRpb24vcGRmfGFEWmlMMmc1T1M4NU1UUXlNVEU0TURJek1UazRMMU5FUWw4MU1ESTFYMGxGWDBWT0xuQmtaZ3w4YmQ3MjEwOGI1Yjg3ZGZhNTk3ZTg0MGY1OTU2YTVhNjM0NjNmODM5ZGRiMTdmODg1MDk1MTJiMTgwYTY3Nzc3 (accessed on 2 October 2024).
- Carl Roth GmbH. Hexane Safety Data Sheet. 2017. Available online: https://www.carlroth.com/medias/SDB-1772-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyODgwNDF8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oMGUvaDIzLzkwNjkxNzMwMTQ1NTgucGRmfDg2YWQ1Yzc3ZjMwNmU4YzNmZjcwYzQwNmY2NmQ0OTI2NTU4OWI4YjZlM2NjYTE5NTA5MGJlNjI4MDc5YTBlZGU (accessed on 2 October 2024).
- Carl Roth GmbH. Methanol Safety Data Sheet. 2022. Available online: https://www.carlroth.com/medias/SDB-0082-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wzMzU5NTh8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oYzgvaDE5LzkxMTcxNDQ0MTYyODYucGRmfGE1MzI2ZWU4Yjg4YzZlNDVhZTZmYzUyMzg4NjljZmVjN2VlZDZiMWFhMzZlYzlkMGQ4YTE4ZWFjYWFhM2RkMWE (accessed on 2 October 2024).
- Carl Roth GmbH. Ethanol Safety Data Sheet. 2014. Available online: https://www.carlroth.com/medias/SDB-6746-IE-EN.pdf (accessed on 2 October 2024).
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Pazuki, G.R.; Pahlavanzadeh, H. Correlation and prediction of the solubility of CO2 in a mixture of organic solution solvents. Theor. Found. Chem. Eng. 2005, 39, 240–245. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef] [PubMed]
- Minsalud, “Maximum Residue Limits for Contaminants in Foods”, ERIA. 2022. Available online: https://www.ins.gov.co/BibliotecaDigital/limites-maximos-de-residuos-de-contaminantes-en-alimentos.pdf (accessed on 2 October 2024).
- Food and Drug Administration. Food Chemical Safety. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-chemical-safety (accessed on 2 October 2024).
- Kandi, S.; Charles, A.L. Measurement, correlation, and thermodynamic properties for solubilities of bioactive compound (−)-epicatechin in different pure solvents at 298.15 K to 338.15 K. J. Mol. Liq. 2018, 264, 269–274. [Google Scholar] [CrossRef]
- Xu, R.; Cong, Y.; Zheng, M.; Chen, G.; Chen, J.; Zhao, H. Solubility and Modeling of Hesperidin in Cosolvent Mixtures of Ethanol, Isopropanol, Propylene Glycol, and n-Propanol + Water. J. Chem. Eng. Data 2018, 63, 764–770. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K. Supercritical Fluids and the Food Industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Franke, S.I.R.; Ckless, K.; Silveira, J.D.; Rubensam, G.; Brendel, M.; Erdtmann, B.; Henriques, J.A.P. Study of antioxidant and mutagenic activity of different orange juices. Food Chem. 2004, 88, 45–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).