Theoretical and Experimental Study of the Stability of Thiamethoxam Under Different Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Part
2.1.1. Preparation of Thiamethoxam Solution and Quantification
2.1.2. Assessment of Thiamethoxam Stability at Different Temperatures
2.1.3. Assessment of the Stability of Thiamethoxam Exposed to Solar Radiation
2.1.4. Ecotoxicological Evaluation Using the Vibrio fischeri Bioindicator
2.2. Theoretical Calculations
3. Results and Discussion
Experimental Part
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbosa, F.R.G.M.; Duarte, V.N.; Staduto, J.A.R.; Kreter, A.C. Land-Use Dynamics for Agricultural and Livestock in Central-West Brazil and Its Reflects on the Agricultural Frontier Expansion. Clean. Circ. Bioecon. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Relatório Conjuntura dos Recursos Hídricos no Brasil Atualiza Informações Sobre Águas do País. Available online: https://www.gov.br/ana/pt-br/assuntos/noticias-e-eventos/noticias/relatorio-conjuntura-dos-recursos-hidricos-no-brasil-atualiza-informacoes-sobre-aguas-do-pais (accessed on 20 September 2024).
- BRASIL. Lei nº 7802, de 11 de Julho de 1989. Dispõe Sobre a Pesquisa, a Experimentação, a Produção, a Embalagem e Rotulagem, o Transporte, o Armazenamento, a Comercialização, a Propaganda Comercial, a Utilização, a Importação, a Exportação, o Destino Final dos Resíduos e Embalagens, o Registro, a Classificação, o Controle, a Inspeção e a Fiscalização de Agrotóxicos, Seus Componentes e Afins, e dá Outras Providências. Presidência da República Casa Civil Subchefia para Assuntos Jurídicos. 1989. Available online: https://www.planalto.gov.br/ccivil_03/leis/l7802.htm (accessed on 15 April 2024).
- Sharma, A.; Kumar, V.; Hukral, A.K.; Bhardwaj, R. Responses of Plants to Pesticide Toxicity: An Overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef]
- Santana, C.M.; da Costa, A.R.; Nunes, R.M.P.; Nunes, N.M.F.; Peron, A.P.; Melo-Cavalcante, A.A.d.C.; Ferreira, P.M.P. Exposição ocupacional de trabalhadores rurais a agrotóxicos. Cad. Saúde Colet. 2016, 24, 301–307. [Google Scholar] [CrossRef]
- Baird, C.; Cann, M. Química Ambiental, 4th ed.; Bookman: Porto Alegre, Brazil, 2011. [Google Scholar]
- dos Santos, L.M.G.; Barata-Silva, C.; Neto, S.A.V.; Magalhães, C.D.; Pereira, R.A.; Malheiros, J.; da Silva, A.L.O.; do Couto Jacob, S. Assessment of Horticultural Products Whose Crops Allow the Use of Copper-Based Pesticides by Inductively Coupled Plasma Optical Emission Spectrometry. J. Food Compos. Anal. 2023, 119, 105272. [Google Scholar] [CrossRef]
- Cassiana, C.M.; Cristiane, V.; Raphael, D.A. Contaminantes emergentes em matrizes aquáticas do brasil: Cenário atual e aspectos analíticos, ecotoxicológicos e regulatórios. Quim. Nova 2017, 40, 1094–1110. [Google Scholar]
- Medeiros, J.F.; Acayabaa, R.D.; Montagner, C.C. A química na avaliação do impacto à saúde humana diante da exposição aos pesticidas. Quim. Nova 2021, 44, 584–598. [Google Scholar] [CrossRef]
- Hilton, M.J.; Jarvis, T.D.; Ricketts, D.C. The degradation rate of thiamethoxam in European field studies. Pest. Manag. Sci. 2016, 72, 388–397. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Chen, X.; Xi, S.; Cui, K.; Li, J.; Dong, D.; Wu, F.; Wu, Z. Efficient degradation of thiamethoxam pesticide in water by iron and manganese oxide composite biochar activated persulfate. Chem. Eng. J. 2023, 473, 145051. [Google Scholar] [CrossRef]
- Phenol. Available online: https://wwwn.cdc.gov/TSP/substances/ToxSubstance.aspx?toxid=27 (accessed on 20 September 2024).
- Agrotóxico. Available online: https://www.gov.br/inca/pt-br/assuntos/causas-e-prevencao-do-cancer/exposicao-no-trabalho-e-no-ambiente/agrotoxico (accessed on 20 September 2024).
- Cruz, Á.B.; Francisco De Carvalho, R.; Silva, T.S.; De Almeida Sarmento, R.; Cavallini, G.S.; Pereira, D.H. Adsorptive Capacity of a G-C3N4 Matrix for Thiamethoxam Removal: A DFT Study. Comput. Theor. Chem. 2022, 1215, 113816. [Google Scholar] [CrossRef]
- de Araújo, K.S.; Antonelli, R.; Gaydeczka, B.; Granato, A.C.; Malpass, G.R.P. Processos oxidativos avançados: Uma revisão de fundamentos e aplicações no tratamento de águas residuais urbanas e efluentes industriais. Rev. Ambiente Água 2016, 11, 387–401. [Google Scholar] [CrossRef]
- Pereira, A.K.S.; Silva, L.F.; Barbosa, G.A.F.; Miranda, T.G.; Sousa, R.R.; Sarmento, R.A.; Souza, N.L.G.D.; Pereira, D.H.; Cavallini, G.S. The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil. Water 2023, 15, 1608. [Google Scholar] [CrossRef]
- Barbosa, R.d.S. Avaliação Ecotoxicológica e Química da Degradação do Neonicotinóide Tiametoxam Cruiser® pelo Processo Foto-Fenton. Master’s Dissertation, Universidade Federal do Tocantins, Palmas, Brazil, 2021. [Google Scholar]
- Lebik-Elhadi, H.; Frontistis, Z.; Ait-Amar, H.; Madjene, F.; Mantzavinos, D. Degradation of Pesticide Thiamethoxam by Heat—Activated and Ultrasound—Activated Persulfate: Effect of Key Operating Parameters and the Water Matrix. Process Saf. Environ. Prot. 2020, 134, 197–207. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton., A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF Density Functionals for Conformationally Flexible Anionic Clusters: M06 Functionals Perform Better than B3LYP for a Model System with Dispersion and Ionic Hydrogen-Bonding Interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Accounts 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-shell Atoms and Hydrides of the First-row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A Complete Basis Set Model Chemistry. II. Open-shell Systems and the Total Energies of the First-row Atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D.01; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Ojha, J.K.; Ramesh, G.; Reddy, B.V. Structure, Chemical Reactivity, NBO, MEP Analysis and Thermodynamic Parameters of Pentamethyl Benzene Using DFT Study. Chem. Phys. Impact 2023, 7, 100280. [Google Scholar] [CrossRef]
- Zhang, Y.; Vecchio, R.D.; Blough, N.V. Investigating the Mechanism of Hydrogen Peroxide Photoproduction by Humic Substances. Environ. Sci. Technol. 2012, 46, 11836–11843. [Google Scholar] [CrossRef]
- Das Neves, A.P.N.; Carlos, T.D.; Bezerra, L.B.; Alceno, W.D.; Sarmento, R.A.; De Souza, N.L.G.D.; Pereira, D.H.; Cavallini, G.S. Carbonate Anion Photolyzed by Solar Radiation or Combined with Peracetic Acid to Form Reactive Species for Dye Degradation. J. Photochem. Photobiol. A Chem. 2021, 420, 113511. [Google Scholar] [CrossRef]
- Nelson, P.N. A DFT Mechanistic Study of Two Possible Hydrolytic Evolution Pathways of Thiamethoxam; Implications in Food and Environmental Safety. Comput. Theor. Chem. 2021, 1202, 113333. [Google Scholar] [CrossRef]
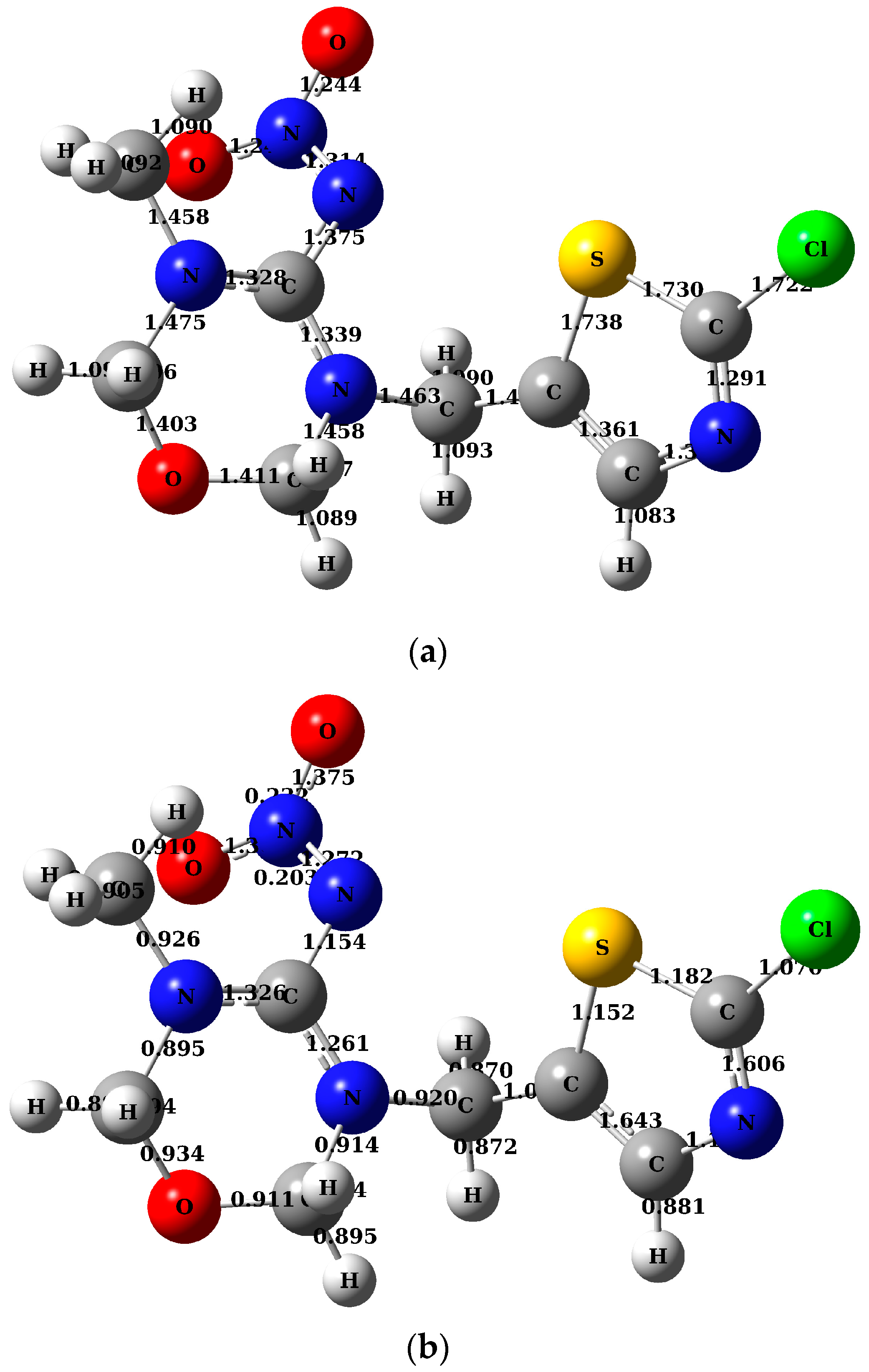


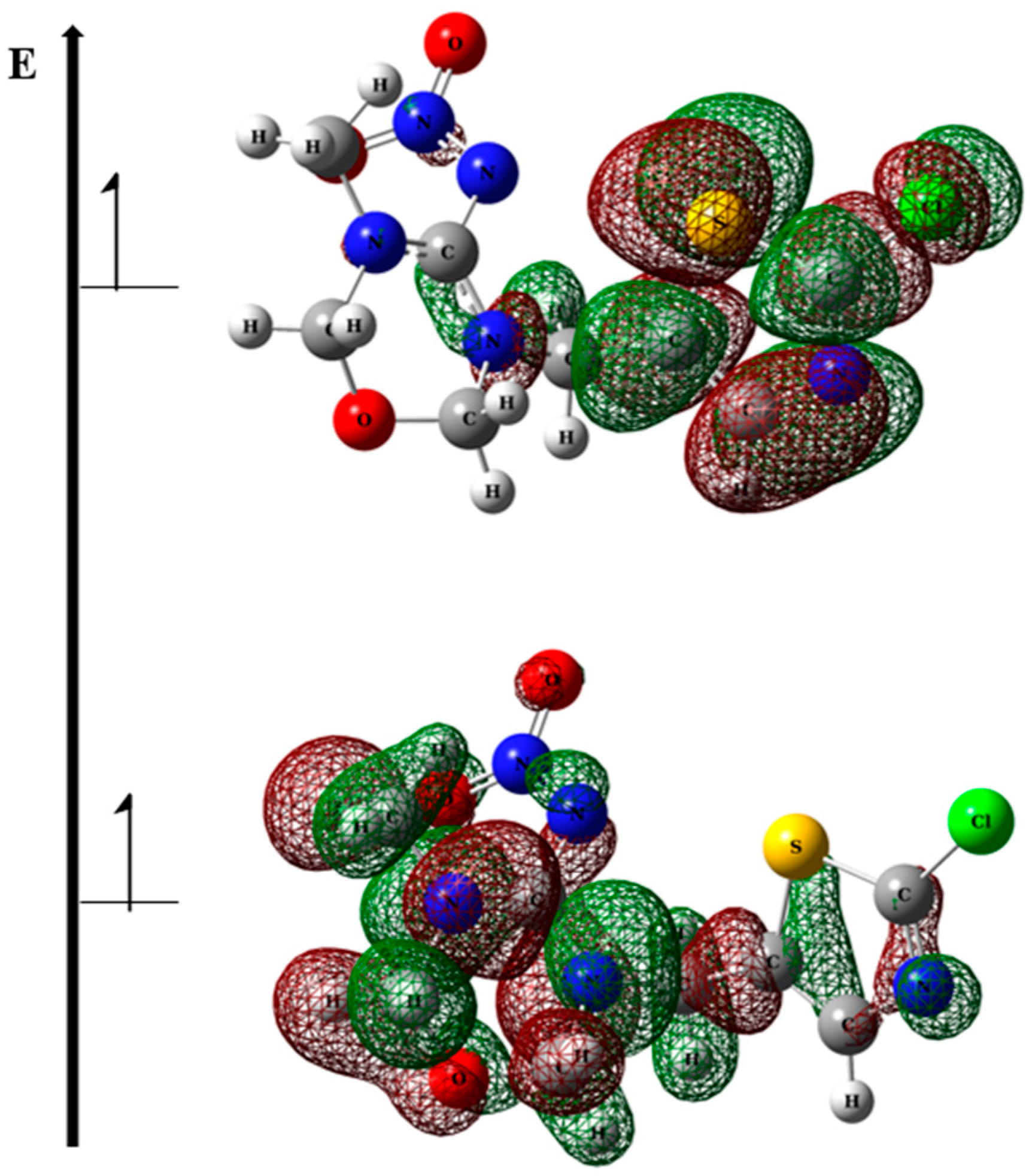
| TMX Concentration (mg·L−1) | |||
|---|---|---|---|
| Time (min) | 24 °C | 40 °C | 60 °C |
| 0 | 16.5 ± 0.05 | 16.4 ± 0.1 | 16.5 ± 0.1 |
| 30 | 16.5 ± 0.1 | 16.5 ± 0.1 | 16.4 ± 0.4 |
| 60 | 16.6 ± 0.02 | 16.6 ± 0.1 | 16.5 ± 0.2 |
| 90 | 16.6 ± 0.04 | 16.7 ± 0.1 | 16.6 ± 0.1 |
| 120 | 16.6 ± 0.1 | 16.6 ± 0.1 | 16.7 ± 0.6 |
| 150 | 16.6 ± 0.1 | 16.6 ± 0.1 | 16.7 ± 0.2 |
| 180 | 16.6 ± 0.04 | 16.7 ± 0.1 | 16.7 ± 0.2 |
| pH | Day 1 | Day 12 | Degradation |
|---|---|---|---|
| 3 | 0.9987 | 1.0135 | ±1% |
| 5 | 0.9990 | 0.9903 | ±1% |
| 6 | 0.9827 | 0.9706 | ±1% |
| 7 | 0.9798 | 0.9610 | ±2% |
| 9 | 0.9780 | 0.9580 | ±2% |
| Pseudo-First-Order | Pseudo-Second-Order | |||
|---|---|---|---|---|
| k (Day−1) | R2 | k (dm3 mol−1 Day−1) | R2 | |
| pH 3 + Solar radiation | 0.1367 | 0.9972 | 0.0197 | 0.9558 |
| pH 5 + Solar radiation | 0.1596 | 0.9907 | 0.0262 | 0.9427 |
| pH 6 + Solar radiation | 0.1337 | 0.9957 | 0.0184 | 0.9422 |
| pH 7 + Solar radiation | 0.1183 | 0.9955 | 0.0148 | 0.9412 |
| pH 9 + Solar radiation | 0.1031 | 0.9933 | 0.0115 | 0.9588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, R.F.; Silva, T.S.; Pereira, A.K.d.S.; Cavallini, G.S.; Pereira, D.H. Theoretical and Experimental Study of the Stability of Thiamethoxam Under Different Environmental Conditions. Processes 2024, 12, 2328. https://doi.org/10.3390/pr12112328
de Carvalho RF, Silva TS, Pereira AKdS, Cavallini GS, Pereira DH. Theoretical and Experimental Study of the Stability of Thiamethoxam Under Different Environmental Conditions. Processes. 2024; 12(11):2328. https://doi.org/10.3390/pr12112328
Chicago/Turabian Stylede Carvalho, Raimundo Francisco, Thiago Soares Silva, Anna Karla dos Santos Pereira, Grasiele Soares Cavallini, and Douglas Henrique Pereira. 2024. "Theoretical and Experimental Study of the Stability of Thiamethoxam Under Different Environmental Conditions" Processes 12, no. 11: 2328. https://doi.org/10.3390/pr12112328
APA Stylede Carvalho, R. F., Silva, T. S., Pereira, A. K. d. S., Cavallini, G. S., & Pereira, D. H. (2024). Theoretical and Experimental Study of the Stability of Thiamethoxam Under Different Environmental Conditions. Processes, 12(11), 2328. https://doi.org/10.3390/pr12112328






