Role of Sintering Aids in Electrical and Material Properties of Yttrium- and Cerium-Doped Barium Zirconate Electrolytes
Abstract
1. Introduction
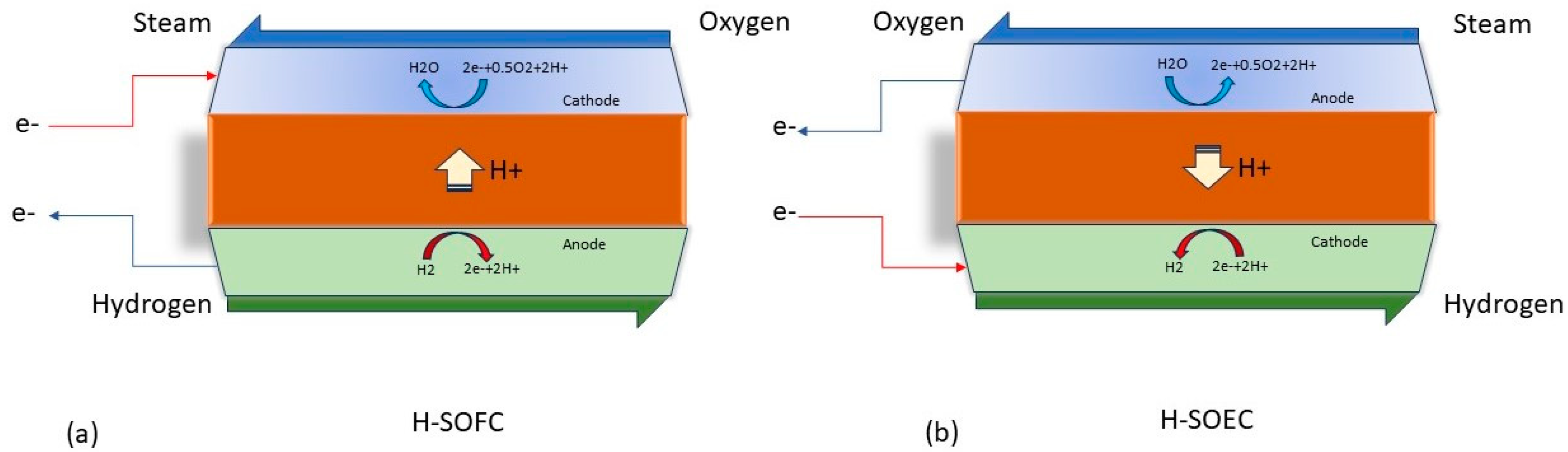
2. Current Challenges with BCZY Electrolyte
- Ions cannot migrate across the grains, thus increasing Rohm and lowering ionic conductivity.
- The gap between the individual grains manifests as pores or pinholes (depending on the size and number of such gaps) that can lead to gas cross-over, affecting the open-circuit voltage.
3. State-of-the-Art Sintering Aids
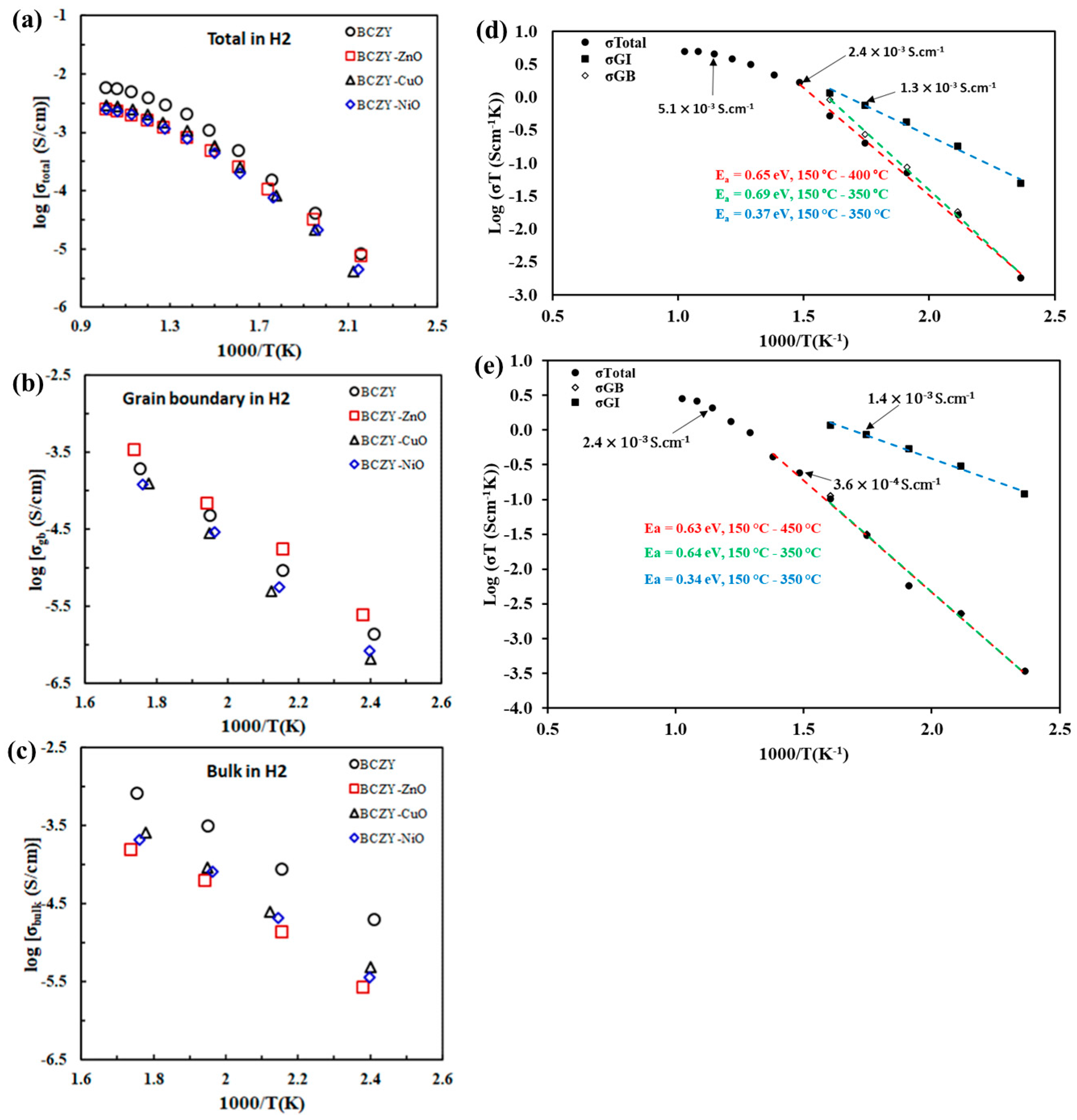
3.1. Nickel Oxide
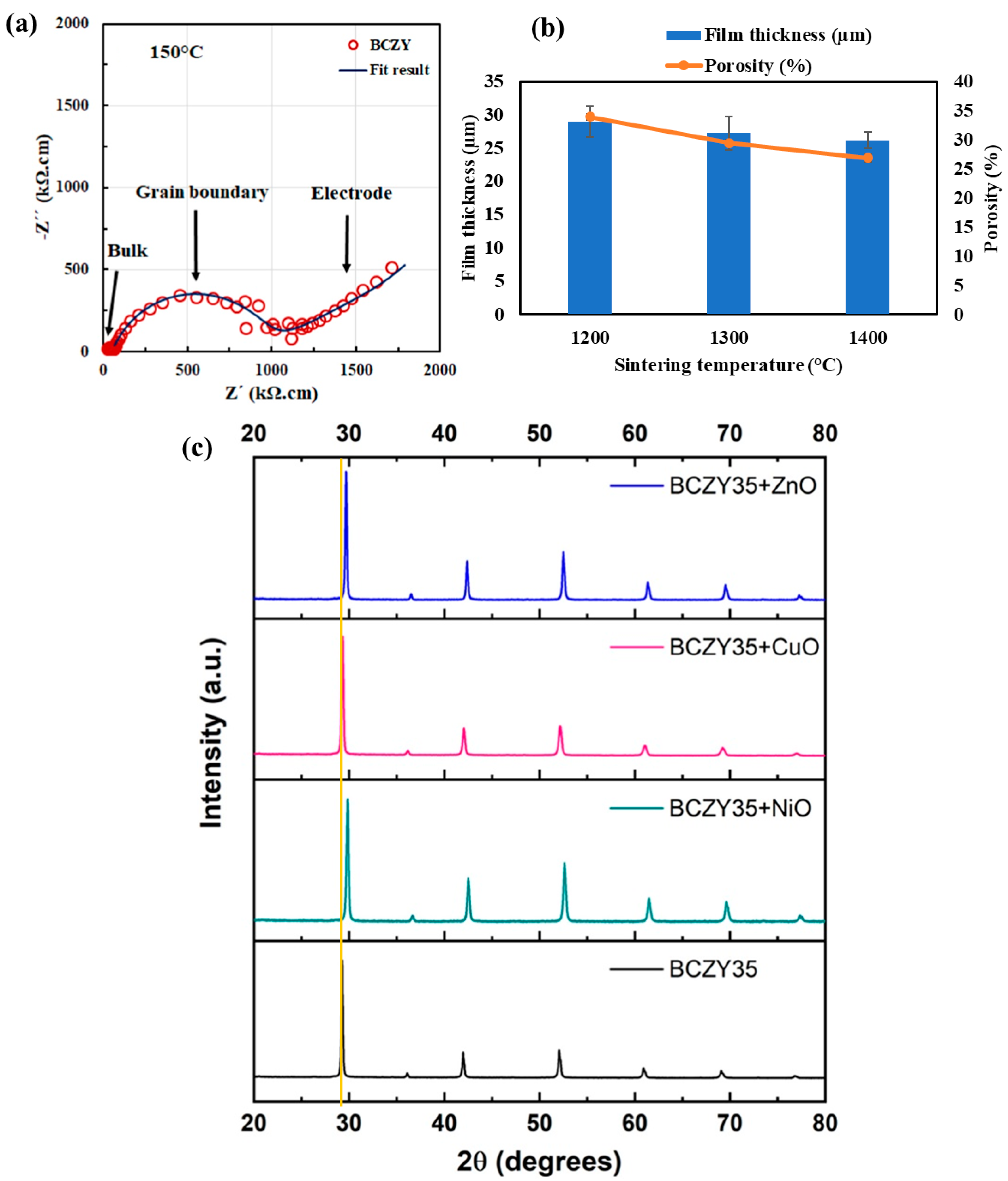
3.2. Oxides of Copper and Zinc
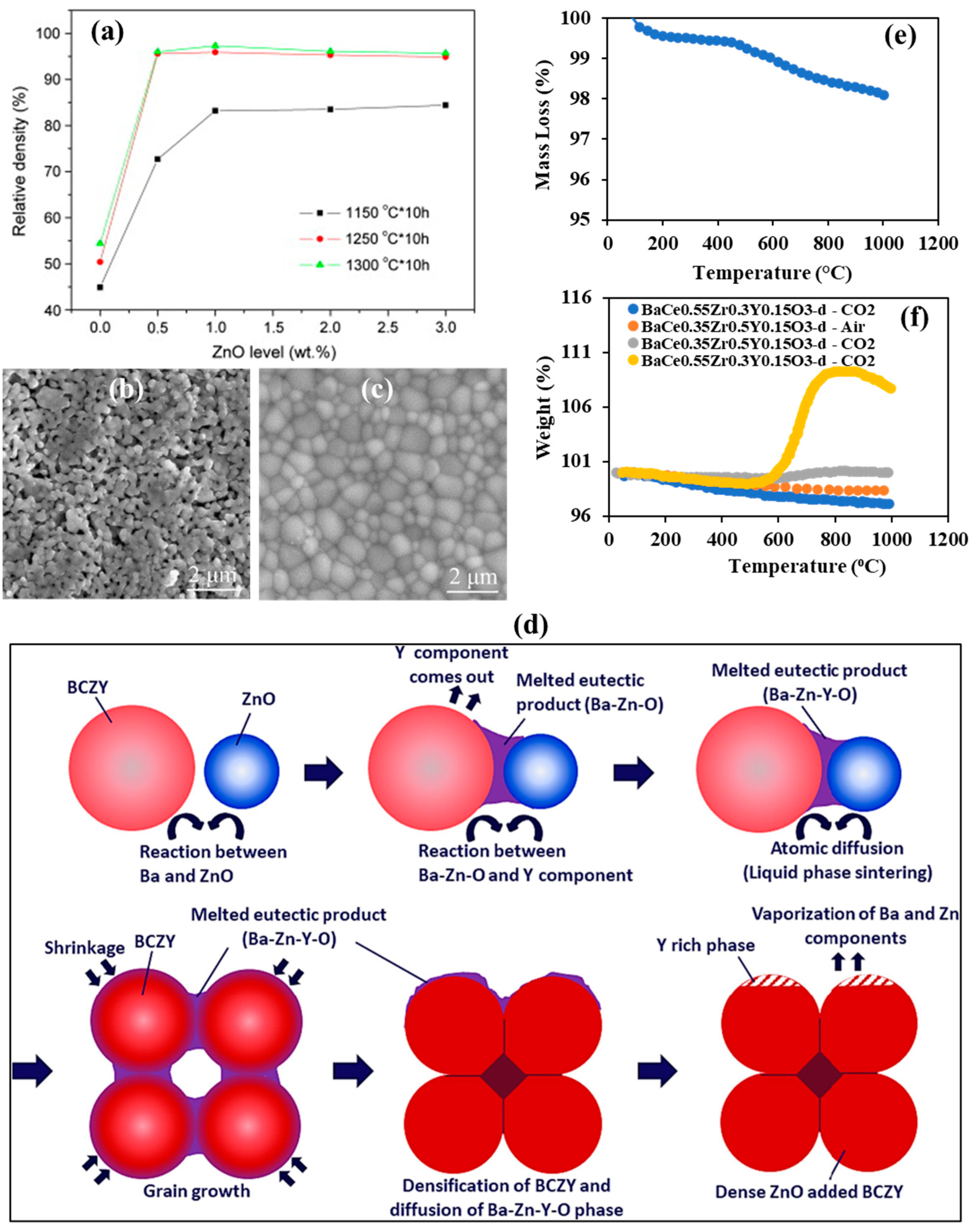
3.3. Oxides of Cobalt, Iron, and Other Metals

4. Future Recommendations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| σi | Ionic conductivity |
| σp | Protonic conductivity |
| σt | Total conductivity |
| Ar | Argon |
| Al2O3 | Aluminum (III) oxide |
| BaCeO3 | Barium cerate |
| BCZY63 | BaCe0.6Zr0.3Y0.1O3−δ |
| BCZY35 | BaCe0.3Zr0.55Y0.15O3−δ |
| BCZY2 | BaCe0.2Zr0.7Y0.1O3−δ—2 mol% ZnO |
| BCZY4 | BaCe0.2Zr0.7Y0.1O3−δ—4 mol% ZnO |
| BCFZY0.1 | BaCo0.4Fe0.4Zr0.1Y0.1O3−δ |
| B2O3 | Boron (III) oxide |
| BaO | Barium (II) oxide |
| BSCF | Ba0.5Sr0.5Co0.8Fe0.2O3−d |
| BZY10 | BaZr0.9Y0.1O3−d |
| BZY20 | BaZr0.8Y0.2O3−δ |
| BaZrO3 | Barium zirconate |
| BaZr1−x−yCexO3−δ | Ceria-doped barium zirconium |
| BaZr1−x−yYyO3−δ | Yttria-doped barium zirconate |
| BaZr1−x−yCexYyO3−δ | Barium zirconium cerium yttrium oxide |
| Cr2O3 | Chromium (III) oxide |
| CuO | Copper (II) oxide |
| DTGA | Differential thermogravimetric analysis |
| Dy | Dysprosium |
| EDTA | Ethylenediaminetetraacetic acid |
| Gd | Gadolinium |
| H-SOFC | Proton-conducting solid oxide fuel cell |
| H-SOEC | Proton-conducting solid oxide electrolytic cell |
| H2 | Hydrogen |
| LiF | Lithium fluoride |
| Li2O3 | Lithium (III) oxide |
| MnO2 | Manganese (IV) oxide |
| NiO | Nickel oxide |
| Ni1−xFex | Nickel (II) oxide–iron (II) oxide |
| Na2CO3 | Sodium carbonate |
| N2 | Nitrogen |
| OCV | Open-circuit voltage |
| PdO | Lead oxide |
| PLD | Pulsed laser deposition |
| SOFC | Solid oxide fuel cell |
| SOEC | Solid oxide electrolytic cell |
| Sm | Samarium |
| SnO2 | Tin (IV) oxide |
| SSR | Solid-state reaction |
| TGA | Thermogravimetric analysis |
| ZnO | Zinc oxide |
| ZnZr sites | Zinc zirconium sites |
| Y | Yttrium |
References
- Chelmehsara, M.E.; Mahmoudimehr, J. Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrogen Energy 2018, 43, 15521–15530. [Google Scholar] [CrossRef]
- Kalinci, Y.; Dincer, I. Analysis and performance assessment of NH3 and H2 fed SOFC with proton-conducting electrolyte. Int. J. Hydrogen Energy 2018, 43, 5795–5807. [Google Scholar] [CrossRef]
- Lei, L.; Tao, Z.; Hong, T.; Wang, X.; Chen, F. A highly active hybrid catalyst modified (La0.60Sr0.40)0.95Co0.20Fe0.80O3−δ cathode for proton conducting solid oxide fuel cells. J. Power Sources 2018, 389, 1–7. [Google Scholar] [CrossRef]
- Miao, L.; Hou, J.; Gong, Z.; Jin, Z.; Liu, W. A high-performance cobalt-free Ruddlesden-Popper phase cathode La1·2Sr0·8Ni0·6Fe0·4O4+δ for low temperature proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 7531–7537. [Google Scholar] [CrossRef]
- Wang, W.; Medvedev, D.; Shao, Z. Gas Humidification Impact on the Properties and Performance of Perovskite-Type Functional Materials in Proton-Conducting Solid Oxide Cells. Adv. Funct. Mater. 2018, 28, 1802592. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Gan, Y.; Xie, K.; Meng, G. Electrolysis of H2O and CO2 in an oxygen-ion conducting solid oxide electrolyzer with a La0.2Sr0.8TiO3+δ composite cathode. J. Power Sources 2012, 218, 244–249. [Google Scholar] [CrossRef]
- Preux, N.; Rolle, A.; Vannier, R.N. 12—Electrolytes and ion conductors for solid oxide fuel cells (SOFCs). In Functional Materials for Sustainable Energy Applications; Kilner, J.A., Skinner, S.J., Irvine, S.J.C., Edwards, P.P., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 370–401. [Google Scholar]
- Su, H.; Wu, D.; Li, C.; Li, C.; Zhang, C. Research advances on electrode materials for solid oxide electrolysis cells. Prog. Nat. Sci. Mater. Int. 2023, 33, 309–319. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Z.; Zhang, J. Solid Oxide Electrolysis of H2O and CO2 to Produce Hydrogen and Low-Carbon Fuels. Electrochem. Energy Rev. 2021, 4, 508–517. [Google Scholar] [CrossRef]
- Lee, K.-R.; Tseng, C.-J.; Chang, J.-K.; Wang, K.-W.; Huang, Y.-S.; Chou, T.-C.; Chiu, K.-C.; Tsai, L.-D.; Lee, S.-W. Ba1−xSrxCe0.8−yZryY0.2O3−δ protonic electrolytes synthesized by hetero-composition-exchange method for solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 22222–22227. [Google Scholar] [CrossRef]
- Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Licoccia, S.; Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−xZrx)Y0.2O3−δ protonic conductors for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFCs). Solid State Ion. 2008, 179, 558–564. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cinti, G.; Frattini, D.; Jannelli, E.; Desideri, U.; Bidini, G. Coupling Solid Oxide Electrolyser (SOE) and ammonia production plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, X.-y.; Shi, Y.; Ghoniem, A.F.; Cai, N. Exergy analysis of an integrated solid oxide electrolysis cell-methanation reactor for renewable energy storage. Appl. Energy 2018, 215, 371–383. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, O.S.; Shima, A.; Matsumoto, H.; Inoue, M.; Abe, T.; Matsuzaki, Y.; Sone, Y. Exergy valorization of a water electrolyzer and CO2 hydrogenation tandem system for hydrogen and methane production. Sci. Rep. 2019, 9, 6470. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.A.; Agnew, G.D.; Irvine, J.T.S. Green ammonia production via the integration of a solid oxide electrolyser and a Haber-Bosch loop with a series of solid electrolyte oxygen pumps. Energy Convers. Manag. 2023, 280, 116816. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Seitz, M.; von Storch, H.; Nechache, A.; Bauer, D. Techno economic design of a solid oxide electrolysis system with solar thermal steam supply and thermal energy storage for the generation of renewable hydrogen. Int. J. Hydrogen Energy 2017, 42, 26192–26202. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Wojcik, A.; Middleton, H.; Damopoulos, I.; Van Herle, J. Ammonia as a fuel in solid oxide fuel cells. J. Power Sources 2003, 118, 342–348. [Google Scholar] [CrossRef]
- Zhu, K.; Luo, B.; Liu, Z.; Wen, X. Recent advances and prospects of symmetrical solid oxide fuel cells. Ceram. Int. 2022, 48, 8972–8986. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Xia, C. A review on cathode processes and materials for electro-reduction of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2021, 493, 229713. [Google Scholar] [CrossRef]
- Königshofer, B.; Höber, M.; Nusev, G.; Boškoski, P.; Hochenauer, C.; Subotić, V. Accelerated degradation for solid oxide electrolysers: Analysis and prediction of performance for varying operating environments. J. Power Sources 2022, 523, 230982. [Google Scholar] [CrossRef]
- Motylinski, K.; Wierzbicki, M.; Kupecki, J.; Jagielski, S. Investigation of off-design characteristics of solid oxide electrolyser (SOE) operating in endothermic conditions. Renew. Energy 2021, 170, 277–285. [Google Scholar] [CrossRef]
- Cui, J.; Wang, J.; Zhang, X.; Li, G.; Wu, K.; Cheng, Y.; Zhou, J. Enhanced oxygen reduction reaction through Ca and Co Co-doped YFeO3 as cathode for protonic ceramic fuel cells. J. Power Sources 2019, 413, 148–157. [Google Scholar] [CrossRef]
- Katahira, K.; Kohchi, Y.; Shimura, T.; Iwahara, H. Protonic conduction in Zr-substituted BaCeO3. Solid State Ion. 2000, 138, 91–98. [Google Scholar] [CrossRef]
- Kindelmann, M.; Ebert, J.N.; Scheld, W.S.; Deibert, W.; Meulenberg, W.A.; Rheinheimer, W.; Bram, M.; Mayer, J.; Guillon, O. Cold sintering of BaZr0.7Ce0.2Y0.1O3−δ ceramics by controlling the phase composition of the starting powders. Scr. Mater. 2023, 224, 115147. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Ryu, K.H.; Haile, S.M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 1999, 125, 355–367. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Caboche, G. Water vapour solubility and conductivity study of the proton conductor BaCe(0.9−x)ZrxY0.1O(3−δ). Solid State Ion. 2009, 180, 990–997. [Google Scholar] [CrossRef]
- Ricote, S.; Caboche, G.; Estournès, C.; Bonanos, N. Synthesis, sintering, and electrical properties of BaCe0.9−xZrxY0.1O3−δ. J. Nanomater. 2008, 2008, 354258–354262. [Google Scholar] [CrossRef]
- Khan, K.; Babar, Z.u.D.; Qayyum, S.; Hanif, M.B.; Rauf, S.; Sultan, A.; Mosiałek, M.; Motola, M.; Lin, B. Design of efficient and durable symmetrical protonic ceramic fuel cells at intermediate temperatures via B-site doping of Ni in BaCe0.56Zr0.2Ni0.04Y0.2O3–δ. Ceram. Int. 2023, 49, 16826–16835. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Licoccia, S.; Traversa, E. Does the increase in Y-dopant concentration improve the proton conductivity of BaZr1−xYxO3−δ fuel cell electrolytes? Solid State Ion. 2010, 181, 1043–1051. [Google Scholar] [CrossRef]
- Saini, D.S.; Ghosh, A.; Tripathy, S.; Kumar, A.; Sharma, S.K.; Kumar, N.; Majumdar, S.; Bhattacharya, D. A Promising Proton Conducting Electrolyte BaZr1−xHoxO3−δ (0.05 ≤ x ≤ 0.20) Ceramics for Intermediate Temperature Solid Oxide Fuel Cells. Sci. Rep. 2020, 10, 3461. [Google Scholar] [CrossRef]
- Xu, X.; Bi, L.; Zhao, X.S. Highly-conductive proton-conducting electrolyte membranes with a low sintering temperature for solid oxide fuel cells. J. Membr. Sci. 2018, 558, 17–25. [Google Scholar] [CrossRef]
- Han, D.; Shinoda, K.; Tsukimoto, S.; Takeuchi, H.; Hiraiwa, C.; Majima, M.; Uda, T. Origins of structural and electrochemical influence on Y-doped BaZrO3 heat-treated with NiO additive. J. Mater. Chem. A 2014, 2, 12552–12560. [Google Scholar] [CrossRef]
- Babilo, P.; Haile, S.M. Enhanced Sintering of Yttrium-Doped Barium Zirconate by Addition of ZnO. J. Am. Ceram. Soc. 2005, 88, 2362–2368. [Google Scholar] [CrossRef]
- Hadi, N.H.; Somalu, M.R.; Samat, A.A.; Yusoff, W.N.A.W.; Muchtar, A.; Baharuddin, N.A.; Abdul, M.A.S.; Raharjo, J.; Khaerudini, D.S.; Abdalla, A.M.; et al. Understanding the Impact of Sintering Temperature on the Properties of Ni-BCZY Composite Anode for Protonic Ceramic Fuel Cell Application. Processes 2023, 11, 1902. [Google Scholar] [CrossRef]
- Almar, L.; Escolástico, S.; Navarrete, L.; Catalán-Martínez, D.; Ara, J.; Remiro-Buenamañana, S.; Quina, I.; Serra, J.M. Protonic Ceramic Electrolysis Cells (PCECs). In High Temperature Electrolysis; Laguna-Bercero, M.A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 245–276. [Google Scholar]
- Schober, T.; Bohn, H.G. Water vapor solubility and electrochemical characterization of the high temperature proton conductor BaZr0.9Y0.1O2.95. Solid State Ion. 2000, 127, 351–360. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, X.; Bi, L. Sintering aids for proton-conducting oxides—A double-edged sword? A mini review. Electrochem. Commun. 2020, 112, 106672. [Google Scholar] [CrossRef]
- Norby, T. (Invited) Advances in Proton Ceramic Fuel Cells, Steam Electrolyzers, and Dehydrogenation Reactors Based on Materials and Process Optimizations. ECS Trans. 2017, 80, 23. [Google Scholar] [CrossRef]
- Wang, M.; Hua, Y.; Gu, Y.; Yin, Y.; Bi, L. High-entropy design in sintering aids for proton-conducting electrolytes of solid oxide fuel cells. Ceram. Int. 2024, 50, 4204–4212. [Google Scholar] [CrossRef]
- Nasani, N.; Pukazhselvan, D.; Kovalevsky, A.V.; Shaula, A.L.; Fagg, D.P. Conductivity recovery by redox cycling of yttrium doped barium zirconate proton conductors and exsolution of Ni-based sintering additives. J. Power Sources 2017, 339, 93–102. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333–6341. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Hoban, M.; O’Hayre, R. Cost-effective solid-state reactive sintering method for high conductivity proton conducting yttrium-doped barium zirconium ceramics. Solid State Ion. 2010, 181, 496–503. [Google Scholar] [CrossRef]
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Wan Isahak, W.N.R. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 6605–6615. [Google Scholar] [CrossRef]
- Nasani, N.; Shakel, Z.; Loureiro, F.J.; Panigrahi, B.B.; Kale, B.B.; Fagg, D.P. Exploring the impact of sintering additives on the densification and conductivity of BaCe0.3Zr0.55Y0.15O3−δ electrolyte for protonic ceramic fuel cells. J. Alloys Compd. 2021, 862, 158640. [Google Scholar] [CrossRef]
- Jing, J.; Pang, J.; Chen, L.; Zhang, H.; Lei, Z.; Yang, Z. Structure, synthesis, properties and solid oxide electrolysis cells application of Ba(Ce, Zr)O3 based proton conducting materials. Chem. Eng. J. 2022, 429, 132314. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Jing, Y.; Matsumoto, H.; Aluru, N.R. Mechanistic insights into hydration of solid oxides. Chem. Mater. 2018, 30, 138–144. [Google Scholar] [CrossRef]
- Manning, P.; Sirman, J.; De Souza, R.; Kilner, J. The kinetics of oxygen transport in 9.5 mol% single crystal yttria stabilised zirconia. Solid State Ion. 1997, 100, 1–10. [Google Scholar] [CrossRef]
- Rasool, S.; Akbar, N.; Shah, M.Y.; Afzal, M.; Zhu, B. Insight of proton transport phenomena in semiconductor ionic materials. J. Power Sources 2024, 598, 234148. [Google Scholar] [CrossRef]
- Bonanos, N. Transport properties and conduction mechanism in high-temperature protonic conductors. Solid State Ion. 1992, 53, 967–974. [Google Scholar] [CrossRef]
- Hinojo, A.; Lujan, E.; Verdaguer, A.; Colominas, S.; Abella, J. ZnO sintering aid effect on proton conductivity of BaCe0.6Zr0.3Y0.1O3−δ electrolyte for hydrogen sensors. Ceram. Int. 2024, 50, 40205–40215. [Google Scholar] [CrossRef]
- Lybye, D.; Bonanos, N. Proton and oxide ion conductivity of doped LaScO3. Solid State Ion. 1999, 125, 339–344. [Google Scholar] [CrossRef]
- Shahid, M. Recent advances in protonconducting electrolytes for solid oxide fuel cells. Ionics 2022, 28, 3583–3601. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, Y.; Wang, B.; Zhai, G.; Yang, G.; Shao, Z.; Xiao, R.; Li, T. A review of progress in proton ceramic electrochemical cells: Material and structural design, coupled with value-added chemicals production. Energy Environ. Sci. 2023, 16, 5721–5770. [Google Scholar] [CrossRef]
- Lim, D.-K.; Park, C.-J.; Choi, M.-B.; Park, C.-N.; Song, S.-J. Partial conductivities of mixed conducting BaCe0.65Zr0.2Y0.15O3–δ. Int. J. Hydrogen Energy 2010, 35, 10624–10629. [Google Scholar] [CrossRef]
- Ji, H.-I.; Kim, B.-K.; Son, J.-W.; Yoon, K.J.; Lee, J.-H. Influence of sintering activators on electrical property of BaZr0.85Y0.15O3−δ proton-conducting electrolyte. J. Power Sources 2021, 507, 230296. [Google Scholar] [CrossRef]
- Odella, E.; Secor, M.; Reyes Cruz, E.A.; Guerra, W.D.; Urrutia, M.N.; Liddell, P.A.; Moore, T.A.; Moore, G.F.; Hammes-Schiffer, S.; Moore, A.L. Managing the redox potential of PCET in grotthuss-type proton wires. J. Am. Chem. Soc. 2022, 144, 15672–15679. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, J.-H.; An, H.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.-W.; Kim, B.-K.; Lee, H.-W.; Lee, J.-H. Fabrication of anode-supported protonic ceramic fuel cell with Ba(Zr0.85Y0.15)O3−δ–Ba(Ce0.9Y0.1)O3−δ dual-layer electrolyte. Int. J. Hydrogen Energy 2014, 39, 12812–12818. [Google Scholar] [CrossRef]
- He, F.; Song, D.; Peng, R.; Meng, G.; Yang, S. Electrode performance and analysis of reversible solid oxide fuel cells with proton conducting electrolyte of BaCe0.5Zr0.3Y0.2O3−δ. J. Power Sources 2010, 195, 3359–3364. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Mikhalev, S.; Kovalevsky, A.V.; Fagg, D.P. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3−δ electrolyte Protonic Ceramic Fuel Cell. J. Power Sources 2015, 278, 582–589. [Google Scholar] [CrossRef]
- Rafique, M.; Safdar, N.; Irshad, M.; Usman, M.; Akhtar, M.; Saleem, M.W.; Abbas, M.M.; Ashour, A.; Soudagar, M.E. Influence of Low Sintering Temperature on BaCe0.2Zr0.6Y0.2O3−δ; IT-SOFC Perovskite Electrolyte Synthesized by Co-Precipitation Method. Materials 2022, 15, 3585. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X. Exploring the role of NiO as a sintering aid in BaZr0.1Ce0.7Y0.2O3−δ electrolyte for proton-conducting solid oxide fuel cells. J. Power Sources 2018, 399, 207–214. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, I.; Meisel, C.; Herradón, C.; Rand, P.; Yang, J.; Kim, H.S.; Sullivan, N.; O’Hayre, R. Improving tubular protonic ceramic fuel cell performance by compensating Ba evaporation via a Ba-excess optimized proton conducting electrolyte synthesis strategy. J. Phys. Energy 2024, 6, 035004. [Google Scholar] [CrossRef]
- Mohamed Shibly, K. The Effect of Barium Non-Stoichiometry on the Phase Structure, Sintering and Electrical Conductivity of BaZr0.7Pr0.1Y0.2O3. Master’s Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2015. [Google Scholar]
- Yamaguchi, T.; Shimada, H.; Honda, U.; Kishimoto, H.; Ishiyama, T.; Hamamoto, K.; Sumi, H.; Suzuki, T.; Fujishiro, Y. Development of anode-supported electrochemical cell based on proton-conductive Ba(Ce,Zr)O3 electrolyte. Solid State Ion. 2016, 288, 347–350. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, T.; Sumi, H.; Yamaguchi, Y.; Nomura, K.; Fujishiro, Y. Effect of Ni diffusion into BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte during high temperature co-sintering in anode-supported solid oxide fuel cells. Ceram. Int. 2018, 44, 3134–3140. [Google Scholar] [CrossRef]
- Zhu, L.; Cadigan, C.; Duan, C.; Huang, J.; Bian, L.; Le, L.; Hernandez, C.H.; Avance, V.; O’Hayre, R.; Sullivan, N.P. Ammonia-fed reversible protonic ceramic fuel cells with Ru-based catalyst. Commun. Chem. 2021, 4, 121. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, K.M.; Carins, G.; Bayne, J.; Tupberg, C.; Irvine, G.J.; Irvine, J.T. Characterisation of direct ammonia proton conducting tubular ceramic fuel cells for maritime applications. J. Mater. Chem. A 2023, 11, 352–363. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Robinson, S.; Manerbino, A.; Coors, W.G. Galvanic hydrogen pumping in the protonic ceramic perovskite BaCe0.2Zr0.7Y0.1O3−δ. J. Membr. Sci. 2013, 446, 99–105. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Beyribey, B.; Bayne, J.; Persky, J. The effect of dip-coating parameters on the thickness and uniformity of BCZYZ electrolyte layer on porous NiO-BCZYZ tubular supports. Ceram. Int. 2022, 48, 6046–6051. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Zhou, M.; Cao, D.; Liu, P.; Wang, W.; Liu, M.; Huang, J.; Shao, J.; Liu, J. Multiple effects of iron and nickel additives on the properties of proton conducting yttrium-doped barium cerate-zirconate electrolytes for high-performance solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 50433–50445. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3−δ and comparison to protonic electrolyte materials. Solid State Ion. 2017, 299, 64–69. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Raimondi, G.; Maier, J. Mixed-Conducting Perovskites as Cathode Materials for Protonic Ceramic Fuel Cells: Understanding the Trends in Proton Uptake. Adv. Funct. Mater. 2018, 28, 1801241. [Google Scholar] [CrossRef]
- Hagy, L.S.; Ramos, K.; Gelfuso, M.V.; Chinelatto, A.L.; Chinelatto, A.S.A. Effects of ZnO addition and microwave sintering on the properties of BaCe0.2Zr0.7Y0.1O3−δ proton conductor electrolyte. Ceram. Int. 2023, 49, 17261–17270. [Google Scholar] [CrossRef]
- Hagy, L.; Ramos, K.; Gelfuso, M.; Chinelatto, A.; Chinelatto, A. Impact of microwave sintering and NiO additive on the densification and conductivity of BaCe0.2Zr0.7Y0.1O3−δ electrolyte for protonic ceramic fuel cell. Ceram. Int. 2024, 50, 40226–40236. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, B.; Meng, X.; Ye, X.; Luo, T.; Xin, X.; Wen, Z. Effect of NiO Addition on the Sintering and Electrochemical Properties of BaCe0.55Zr0.35Y0.1O3−δ Proton-Conducting Ceramic Electrolyte. Membranes 2024, 14, 61. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ion. 2013, 253, 201–210. [Google Scholar] [CrossRef]
- Lee, K.-R.; Tseng, C.-J.; Jang, S.-C.; Lin, J.-C.; Wang, K.-W.; Chang, J.-K.; Chen, T.-C.; Lee, S.-W. Fabrication of anode-supported thin BCZY electrolyte protonic fuel cells using NiO sintering aid. Int. J. Hydrogen Energy 2019, 44, 23784–23792. [Google Scholar] [CrossRef]
- Guo, Y.; Ran, R.; Shao, Z. A novel way to improve performance of proton-conducting solid-oxide fuel cells through enhanced chemical interaction of anode components. Int. J. Hydrogen Energy 2011, 36, 1683–1691. [Google Scholar] [CrossRef]
- Baral, A.K. Reduction in sintering temperature of stable proton conductor BaCe0.35Zr0.5Y0.15O3−δ prepared by sol–gel method and its transport properties. Solid State Ion. 2015, 272, 107–111. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T. A stable, easily sintered proton-conducting oxide electrolyte for moderate-temperature fuel cells and electrolyzers. Adv. Mater. 2006, 18, 1581–1584. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Conductivity studies of dense yttrium-doped BaZrO3 sintered at 1325 °C. J. Solid State Chem. 2007, 180, 3493–3503. [Google Scholar] [CrossRef]
- Chen, M.; Chen, D.; Wang, K.; Xu, Q. Densification and electrical conducting behavior of BaZr0.9Y0.1O3−δ proton conducting ceramics with NiO additive. J. Alloys Compd. 2019, 781, 857–865. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Blanc, F.; Okuyama, Y.; Buannic, L.; Lucio-Vega, J.C.; Grey, C.P.; Haile, S.M. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 2013, 12, 647–651. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Lee, K.-R.; Bhavanari, M.; Su, P.-C.; Osman, N.; Lee, S.-W.; Tseng, C.-J. Enhancing protonic ceramic fuel cell performance through nanomilling of BCZY electrolyte powder. Ceram. Int. 2023, 49, 32172–32180. [Google Scholar] [CrossRef]
- Guo, J.-H.; Li, X.-D.; Cheng, X.-L.; Liu, H.-Y.; Li, S.-J.; Chen, G. The theoretical study of the bimetallic Ni/Pd, Ni/Pt and Pt/Pd catalysts for hydrogen spillover on penta-graphene. Int. J. Hydrogen Energy 2018, 43, 19121–19129. [Google Scholar] [CrossRef]
- Ren, K.; Jia, F.; Zhang, C.; Xing, E.; Li, Y. Ni as the promoter on hydrogen spillover for better hydrogenative regeneration on Pt/Y based catalysts. Fuel 2023, 335, 127047. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Z.; Tian, H.; Li, J. Effect of bismuth oxide on the microstructure and electrical conductivity of yttria stabilized zirconia. Sensors 2016, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Influence of Bi2O3 additive on the electrical conductivity of calcia stabilized zirconia solid electrolyte. J. Eur. Ceram. Soc. 2015, 35, 1485–1493. [Google Scholar]
- Yeh, T.; Kusuma, G.; Suresh, M.; Chou, C. Effect of sintering process on the microstructures of Bi2O3-doped yttria stabilized zirconia. Mater. Res. Bull. 2010, 45, 318–323. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Yun, D.S.; Joo, J.H.; Yu, J.H. The effects of NiO addition on the structure and transport properties of proton conducting BaZr0.8Y0.2O3−δ. J. Alloys Compd. 2015, 621, 263–267. [Google Scholar] [CrossRef]
- Shimada, H.; Li, X.; Hagiwara, A.; Ihara, M. Proton-Conducting Solid Oxide Fuel Cells with Yttrium-Doped Barium Zirconate for Direct Methane Operation. J. Electrochem. Soc. 2013, 160, F597. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Manerbino, A.; Sullivan, N.P.; Coors, W.G. Effects of the fabrication process on the grain-boundary resistance in BaZr0.9Y0.1O3−δ. J. Mater. Chem. A 2014, 2, 16107–16115. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, M.; Liu, Z.; Liu, J. A comparative investigation on protonic ceramic fuel cell electrolytes BaZr0.8Y0.2O3−δ and BaZr0.1Ce0.7Y0.2O3−δ with NiO as sintering aid. Ceram. Int. 2022, 48, 17208–17216. [Google Scholar] [CrossRef]
- Mu, S.; Huang, H.; Ishii, A.; Zhao, Z.; Zou, M.; Kuzbary, P.; Peng, F.; Brinkman, K.S.; Xiao, H.; Tong, J. Rapid laser reactive sintering of BaCe0.7Zr0.1Y0.1Yb0.1O3−δ electrolyte for protonic ceramic fuel cells. J. Power Sources Adv. 2020, 4, 100017. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Liu, M.; Liu, J. Enhancing sinterability and electrochemical properties of Ba(Zr0.1Ce0.7Y0.2)O3−δ proton conducting electrolyte for solid oxide fuel cells by addition of NiO. Int. J. Hydrogen Energy 2018, 43, 13501–13511. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; Duan, C.; O’Hayre, R. Ionic transport modification in proton conducting BaCe0.6Zr0.3Y0.1O3−δ with transition metal oxide dopants. Solid State Ion. 2016, 294, 37–42. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Hanif, M.B.; Gao, J.-T.; Li, C.-J.; Li, C.-X. Sintering behavior of BaCe0.7Zr0.1Y0.2O3−δ electrolyte at 1150 °C with the utilization of CuO and Bi2O3 as sintering aids and its electrical performance. Int. J. Hydrogen Energy 2022, 47, 7403–7414. [Google Scholar] [CrossRef]
- Loureiro, F.J.A.; Nasani, N.; Reddy, G.S.; Munirathnam, N.R.; Fagg, D.P. A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for Protonic Ceramic Fuel Cells. J. Power Sources 2019, 438, 226991. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, J.-H.; Lee, H.-W.; Kim, B.-K. Estimation of the protonic concentration and mobility in Ba(Zr0.81Yb0.15Zn0.04)O3−δ ceramic. Solid State Ion. 2011, 192, 88–92. [Google Scholar] [CrossRef]
- Coors, W.G. Protonic ceramic fuel cells for high-efficiency operation with methane. J. Power Sources 2003, 118, 150–156. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wu, X.; Hu, J.; Xia, C. Sintering behavior and conductivity study of yttrium-doped BaCeO3–BaZrO3 solid solutions using ZnO additives. J. Am. Ceram. Soc. 2009, 92, 2623–2629. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Xu, N.; Li, X.; Chen, N. Influence of ZnO addition on the properties of high temperature proton conductor Ba1.03Ce0.5Zr0.4Y0.1O3−δ synthesized via citrate–nitrate method. Int. J. Hydrogen Energy 2009, 34, 2739–2746. [Google Scholar] [CrossRef]
- Matsuda, R.M.; Nakamura, K.; Mori, M.; Dailly, J. Sintering mechanism and electrical conductivity of ZnO added BaCe0.8Zr0.1Y0.1O3−δ proton conducting perovskites. Solid State Ion. 2023, 403, 116407. [Google Scholar] [CrossRef]
- Taillades, G.; Battochi, P.; Taillades, M.; Jones, D.; Roziére, J. Chemically Stable Electrolytes and Advanced Electrode Architectures for Efficient Proton Ceramic Fuel Cells. ECS Trans. 2011, 35, 805. [Google Scholar] [CrossRef]
- Thabet, K.; Le Gal La Salle, A.; Quarez, E.; Joubert, O. High Performance Dense Proton Ceramic Electrolyte Material Obtained by Cold Sintering Process. ECS Trans. 2019, 91, 983. [Google Scholar] [CrossRef]
- Baral, A.K.; Tsur, Y. Sintering aid (ZnO) effect on proton transport in BaCe0.35Zr0.5Y0.15O3−δ and electrode phenomena studied by distribution function of relaxation times. J. Am. Ceram. Soc. 2019, 102, 239–250. [Google Scholar] [CrossRef]
- Baral, A.K.; Choi, S.; Kim, B.K.; Lee, J.-H. Processing and characterizations of a novel proton-conducting BaCe0.35Zr0.50Y0.15O3−δ electrolyte and its nickel-based anode composite for anode-supported IT-SOFC. Mater. Renew. Sustain. Energy 2014, 3, 35. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Wang, C.; Liu, Y.; Shao, Z.; An, J.; Liu, C. Stable and easily sintered BaCe0.5Zr0.3Y0.2O3−δ electrolytes using ZnO and Na2CO3 additives for protonic oxide fuel cells. Electrochim. Acta 2013, 95, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Wang, L.; Zhu, J.; Meng, W.; He, Z.; Dai, L. Improvement of sinterability of BaZr0.8Y0.2O3−δ for H2 separation using Li2O/ZnO dual-sintering aid. Ceram. Int. 2018, 44, 15935–15943. [Google Scholar] [CrossRef]
- Castellani, P.; Quarez, E.; Nicollet, C.; Joubert, O.; Gautier, N.; Pers, P.; Taillades, G.; Le Gal La Salle, A. Cold-sintering and Li doped ZnO sintering aid for the densification of BaZr0.7Ce0.2Y0.1O3−δ proton conducting ceramics. Int. J. Hydrogen Energy 2023, 54, 1343–1356. [Google Scholar] [CrossRef]
- Reddy, G.S.; Bauri, R. A novel route to enhance the sinterability and its effect on microstructure, conductivity and chemical stability of BaCe0.4Zr0.4Y0.2O3−δ proton conductors. Mater. Chem. Phys. 2018, 216, 250–259. [Google Scholar] [CrossRef]
- Zhou, G.; Li, Y.; Luo, Y.; Huang, W.; Li, B. Effect of A-site modification on electrical properties and chemical stability of Ba1−xCaxCe0.5Zr0.3Y0.2O3−δ proton-conducting electrolyte. Ceram. Int. 2023, 49, 11184–11196. [Google Scholar] [CrossRef]
- Jing, Y.; Aluru, N. The role of A-site ion on proton diffusion in perovskite oxides (ABO3). J. Power Sources 2020, 445, 227327. [Google Scholar] [CrossRef]
- Kim, H.-W.; Seo, J.; Yu, J.H.; Yun, K.S.; Joo, J.H.; Moon, J.; Park, H.J. Effect of cerium on yttrium-doped barium zirconate with a ZnO sintering aid: Grain and grain boundary protonic conduction. Ceram. Int. 2021, 47, 32720–32726. [Google Scholar] [CrossRef]
- Jeong, S.; Kobayashi, T.; Kuroda, K.; Kwon, H.; Zhu, C.; Habazaki, H.; Aoki, Y. Evaluation of thin film fuel cells with Zr-rich BaZrxCe0.8−xY0.2O3−δ electrolytes (x ≥ 0.4) fabricated by a single-step reactive sintering method. RSC Adv. 2018, 8, 26309–26317. [Google Scholar] [CrossRef]
- Nasani, N.; Dias, P.A.N.; Saraiva, J.A.; Fagg, D.P. Synthesis and conductivity of Ba(Ce,Zr,Y)O3−δ electrolytes for PCFCs by new nitrate-free combustion method. Int. J. Hydrogen Energy 2013, 38, 8461–8470. [Google Scholar] [CrossRef]
- Bu, J.; Jönsson, P.G.; Zhao, Z. Electrical conductivities of translucent BaZrxCe0.8−xY0.2O3−δ (x = 0.5, 0.6, 0.7) ceramics. Scr. Mater. 2016, 115, 87–90. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Wani, B.; Bharadwaj, S. Synthesis, stability and conductivity of BaCe0.8−xZrxY0.2O3−δ as electrolyte for proton conducting SOFC. Int. J. Hydrogen Energy 2012, 37, 3848–3856. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Manerbino, A.; Coors, W.G. Conductivity study of dense BaCexZr(0.9−x)Y0.1O(3−δ) prepared by solid state reactive sintering at 1500 °C. Int. J. Hydrogen Energy 2012, 37, 7954–7961. [Google Scholar] [CrossRef]
- Zhao, L.; Tan, W.; Zhong, Q. The chemical stability and conductivity improvement of protonic conductor BaCe0.8−xZrxY0.2O3−δ. Ionics 2013, 19, 1745–1750. [Google Scholar] [CrossRef]
- Bentzer, H.K.; Bonanos, N.; Phair, J.W. EMF measurements on mixed protonic/electronic conductors for hydrogen membrane applications. Solid State Ion. 2010, 181, 249–255. [Google Scholar] [CrossRef]
- Sutija, D.P.; Norby, T.; Björnbom, P. Transport number determination by the concentration-cell/open-circuit voltage method for oxides with mixed electronic, ionic and protonic conductivity. Solid State Ion. 1995, 77, 167–174. [Google Scholar] [CrossRef]
- Badwal, S. Grain boundary resistivity in zirconia-based materials: Effect of sintering temperatures and impurities. Solid State Ion. 1995, 76, 67–80. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Radenahmad, N.; Zakaria, A.K.M.; Zaini, J.H.; Rahman, S.M.H.; Eriksson, S.G.; Irvine, J.T.S.; Azad, A.K. Highly dense and chemically stable proton conducting electrolyte sintered at 1200 °C. Int. J. Hydrogen Energy 2018, 43, 894–907. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Zaini, J.H.; Savaniu, C.D.; Irvine, J.T.S.; Azad, A.K. Highly dense and novel proton conducting materials for SOFC electrolyte. Int. J. Hydrogen Energy 2017, 42, 27308–27322. [Google Scholar] [CrossRef]
- Lin, B.; Hu, M.; Ma, J.; Jiang, Y.; Tao, S.; Meng, G. Stable, easily sintered BaCe0.5Zr0.3Y0.16Zn0.04O3−δ electrolyte-based protonic ceramic membrane fuel cells with Ba0.5Sr0.5Zn0.2Fe0.8O3−δ perovskite cathode. J. Power Sources 2008, 183, 479–484. [Google Scholar] [CrossRef]
- Luisetto, I.; Licoccia, S.; D’Epifanio, A.; Sanson, A.; Mercadelli, E.; Di Bartolomeo, E. Electrochemical performance of spin coated dense BaZr0.80Y0.16Zn0.04O3−δ membranes. J. Power Sources 2012, 220, 280–285. [Google Scholar] [CrossRef]
- Peng, C.; Melnik, J.; Li, J.; Luo, J.; Sanger, A.R.; Chuang, K.T. ZnO-doped BaZr0.85Y0.15O3−δ proton-conducting electrolytes: Characterization and fabrication of thin films. J. Power Sources 2009, 190, 447–452. [Google Scholar] [CrossRef]
- Toriumi, H.; Jeong, S.; Kitano, S.; Habazaki, H.; Aoki, Y. Enhanced performance of protonic solid oxide steam electrolysis cell of Zr-rich side BaZr0.6Ce0.2Y0.2O3−δ electrolyte with an anode functional layer. ACS Omega 2022, 7, 9944–9950. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xie, H.; Su, P.-C. Spray coating of dense proton-conducting BaCe0.7Zr0.1Y0.2O3 electrolyte for low temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 6516–6525. [Google Scholar] [CrossRef]
- Zuo, C.; Zha, S.; Liu, M.; Hatano, M.; Uchiyama, M. Ba(Zr0.1Ce0.7Y0.2)O3–δ as an Electrolyte for Low-Temperature Solid-Oxide Fuel Cells. Adv. Mater. 2006, 18, 3318–3320. [Google Scholar] [CrossRef]
- Leng, Z.; Huang, Z.; Zhou, X.; Zhang, B.; Bai, H.; Zhou, J.; Wang, S. The effect of sintering aids on BaCe0·7Zr0·1Y0.1Yb0.1O3−δ as the electrolyte of proton-conducting solid oxide electrolysis cells. Int. J. Hydrogen Energy 2022, 47, 33861–33871. [Google Scholar] [CrossRef]
- Soares, H.S.; Antunes, I.; Loureiro, F.J.A.; Pérez-Coll, D.; Willinger, M.-G.; Brandão, A.D.; Mather, G.C.; Fagg, D.P. Effect of the addition mechanism of ZnO sintering aid on densification, microstructure and electrical properties of Ba(Zr,Y)O3−δ proton-conducting perovskite. Int. J. Hydrogen Energy 2021, 46, 26466–26477. [Google Scholar] [CrossRef]
- Antunes, I.; Brandão, A.; Figueiredo, F.M.; Frade, J.R.; Gracio, J.; Fagg, D.P. Mechanosynthesis of nanopowders of the proton-conducting electrolyte material Ba(Zr, Y)O3−δ. J. Solid State Chem. 2009, 182, 2149–2156. [Google Scholar] [CrossRef]
- Fu, X.-Z.; Luo, J.-L.; Sanger, A.R.; Luo, N.; Chuang, K.T. Y-doped BaCeO3−δ nanopowders as proton-conducting electrolyte materials for ethane fuel cells to co-generate ethylene and electricity. J. Power Sources 2010, 195, 2659–2663. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N. Enhanced sintering and conductivity study of cobalt or nickel doped solid solution of barium cerate and zirconate. Solid State Ion. 2010, 181, 694–700. [Google Scholar] [CrossRef]
- Norby, T.; Widerøe, M.; Glöckner, R.; Larring, Y. Hydrogen in oxides. Dalton Trans. 2004, 19, 3012–3018. [Google Scholar] [CrossRef]
- Münch, W.; Kreuer, K.D.; Seifert, G.; Maier, J. Proton diffusion in perovskites: Comparison between BaCeO3, BaZrO3, SrTiO3, and CaTiO3 using quantum molecular dynamics. Solid State Ion. 2000, 136–137, 183–189. [Google Scholar] [CrossRef]
- Gui, L.; Ling, Y.; Li, G.; Wang, Z.; Wan, Y.; Wang, R.; He, B.; Zhao, L. Enhanced sinterability and conductivity of BaZr0.3Ce0.5Y0.2O3−δ by addition of bismuth oxide for proton conducting solid oxide fuel cells. J. Power Sources 2016, 301, 369–375. [Google Scholar] [CrossRef]
- Wan, Y.; He, B.; Wang, R.; Ling, Y.; Zhao, L. Effect of Co doping on sinterability and protonic conductivity of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ for protonic ceramic fuel cells. J. Power Sources 2017, 347, 14–20. [Google Scholar] [CrossRef]
- Liu, S.; Tan, X.; Li, K.; Hughes, R. Preparation and characterisation of SrCe0.95Yb0.05O2.975 hollow fibre membranes. J. Membr. Sci. 2001, 193, 249–260. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Lin, B.; Ding, H.; Wang, S.; Ling, Y.; Peng, R.; Meng, G.; Liu, X. High performance of proton-conducting solid oxide fuel cell with a layered PrBaCo2O5+δ cathode. J. Power Sources 2009, 194, 835–837. [Google Scholar] [CrossRef]
- Viechineski, F.N.; Ramos, K.; Chinelatto, A.L.; Chinelatto, A.S.A. Optimizing the densification of BaCe0.2Zr0.7Y0.1O3−δ proton conducting electrolyte using Fe2O3, Mn2O3 and ZnO sintering aids. J. Mater. Sci. Mater. Electron. 2023, 34, 2165. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Kong, L.B.; Chan, S.H.; Hing, P.; Kilner, J.A. Iron oxide as an effective sintering aid and a grain boundary scavenger for ceria-based electrolytes. Solid State Ion. 2004, 167, 203–207. [Google Scholar] [CrossRef]
- Lin, X.-L.; Babar, Z.U.D.; Gao, Y.; Gao, J.-T.; Li, C.-X. Influence of Triple Sintering Additives (BaO-CuO-B2O3) on the Sintering Behavior and Conductivity of the Proton-Conducting BaZr0.1Ce0.7Y0.2O3−δ Electrolyte Sintered at 1150 °C. ACS Appl. Energy Mater. 2023, 6, 4833–4843. [Google Scholar] [CrossRef]
- Wei, M.; Fan, Z.; Li, J.; Xu, D. Influences of B2O3/CuO additions on the sintering behavior, microstructure and microwave dielectric properties of 6Nd[(Zn0.7Co0.3)0.5Ti0.5]O3–4(Na0.5Nd0.5)TiO3 ceramics. Ceram. Int. 2021, 47, 27545–27552. [Google Scholar] [CrossRef]
- Tsai, C.L.; Kopczyk, M.; Smith, R.J.; Schmidt, V.H. Low temperature sintering of Ba(Zr0.8−xCexY0.2)O3−δ using lithium fluoride additive. Solid State Ion. 2010, 181, 1083–1090. [Google Scholar] [CrossRef]
- Cao, L.; Chai, Y.; Li, P.; Shen, Z.; Wu, J. Efficient self-assembly of transition metal oxide nanoclusters on silicon substrates. Thin Solid Film. 2005, 492, 13–18. [Google Scholar] [CrossRef]
- Haussonne, J.M.; Desgardin, G.; Herve, A.; Boufrou, B. Dielectric ceramics with relaxors and a tetragonal tungsten bronze. J. Eur. Ceram. Soc. 1992, 10, 437–452. [Google Scholar] [CrossRef]
- Pollet, M.; Marinel, S. Low temperature sintering of CaZrO3 using lithium fluoride addition. J. Eur. Ceram. Soc. 2003, 23, 1925–1933. [Google Scholar] [CrossRef]
- Sneha, B.R.; Thangadurai, V. Synthesis of nano-sized crystalline oxide ion conducting fluorite-type Y2O3-doped CeO2 using perovskite-like BaCe0.9Y0.1O2.95 (BCY) and study of CO2 capture properties of BCY. J. Solid State Chem. 2007, 180, 2661–2669. [Google Scholar] [CrossRef]
- Tao, Z.; Zhu, Z.; Wang, H.; Liu, W. A stable BaCeO3-based proton conductor for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 3481–3484. [Google Scholar] [CrossRef]


| Electrolyte Composition | Sintering Aids | mol% or weight% Added | Sintering Temperature (°C), Duration | Relative Density (%) | Atmosphere | T (°C) | Conductivity (×10−3 Scm−1) | T (°C) | Peak Power Density (mWcm−2) | Open-Circuit Voltage (V) | Synthesis Method of Powder |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BaZr0.1Ce0.66Ni0.04Y0.2O3−δ [72] | NiO | 4% (mol) | 1400 °C, 5 h | N/A | Wet H2 | 600 | 6.30 | 600 | 477 | 1.053 | Wet chemistry |
| BaZr0.1Ce0.66Ni0.04Y0.2O3− δ [72] | NiO | 4% (mol) | 1400 °C, 5 h | N/A | Wet H2 | 600 | 6.30 | 650 | 674 | 1.038 | Wet chemistry |
| BaZr0.85Y0.15O3−δ [72] | NiO | 4% (mol) | 1450 °C, 8 h | >95% | Humid N2 atmosphere (pH2O = 0.026 atm) | 800 | 1.03 | N/A | N/A | N/A | Wet chemistry |
| BaZr0.91Ni0.01Y0.08O3− δ [94] | NiO | 1% (mol) | 1600 °C, 5 h | >99% | Wet 1% H2 + Ar | 900 | >1.00 | N/A | N/A | 1.000 | Solid-state reaction |
| BaZr0.8Y0.2O3− δ [103] | NiO | 2% (wt) | 1450 °C, 5 h | >95% | 3% H2O/Ar | 600 | 3.10 | 700 | 5.5 | N/A | Conventional solid-state reaction |
| BaZr0.9Y0.1O3− δ [104] | NiO | 0.6% (mol) | 1600 °C, 4 h | 97.3 | 3% humidified H2 | 600 | 1.20 | 900 | 2.35 | 1.124 | Conventional solvent mixing |
| BaZr0.9Y0.1O3− δ [104] | NiO | 0.9% (mol) | 1500 °C, 4 h | 96.3 | 3% humidified H2 | 600 | 5.90 | 900 | 2.35 | 0.794 | Conventional solvent mixing |
| BaZr0.9Y0.1O3− δ [104] | NiO | 1% (mol) | 1450 °C, 8 h | N/A | Dry air | 600 | ~7.50 | N/A | N/A | N/A | Conventional solvent mixing |
| BaZr0.9Y0.1O3− δ [104] | NiO | 1% (mol) | 1450 °C, 8 h | N/A | Humid air | 600 | 3.70 | N/A | N/A | N/A | Conventional solvent mixing |
| BaZr0.9Y0.1O3− δ [104] | NiO | 1% (mol) | 1450 °C, 8 h | N/A | 5% H2/Ar | 600 | 2.00 | N/A | N/A | N/A | Conventional solvent mixing |
| BaZr0.9Y0.1O3− δ [104] | NiO | 1.5% (mol) | 1500 °C, 4 h | 96.5 | 3% humidified H2 | 600 | 0.44 | 900 | 2.35 | 0.793 | Conventional solvent mixing |
| BaZr0.9Y0.1O3−d [105] | NiO | 1% (wt) | 1600 °C, 12 h | N/A | 5% H2 in N2 | 600 | 1.03 | N/A | N/A | N/A | Solid-state reactive sintering |
| BaZr0.9Y0.1O3−d [105] | NiO | 1% (wt) | 1600 °C, 12 h | N/A | 5% H2 in N3 | 500 | 1.10 | N/A | N/A | N/A | Solid-state reactive sintering |
| BaZr0.8Y0.2O3− δ [79] | NiO | 1% (wt) | 1450 °C | N/A | H2/air | N/A | N/A | 500 | 335 | 1.050 | Synthesis from raw precursor |
| BaCe0.7Zr0.1Y0.1Yb0.1O3− δ [79] | NiO | 1% (wt) | 1400 °C | N/A | H2/air | N/A | N/A | 500 | 455 | >1.050 | Solution infiltration |
| BaZr0.1Ce0.7Y0.2O3− δ [106] | NiO | 2% (mol) | 1400 °C, 6 h | N/A | Wet air Wet H2 | 700 | 25 19 | 700 | 855 | 1.000 | Solid-state reaction |
| BaZr0.8Y0.2O3−δ [106] | NiO | 2% (mol) | 1400 °C, 6 h | N/A | Wet air Wet H2 | 700 | 14 4 | 700 | 360 | 0.950 | Solid-state reaction |
| BaZr0.8Y0.2O3−δ [14] | NiO | 1% (wt) | 1450 °C, 18 h | N/A | Humidified H2 and air | N/A | N/A | 600 | 660 | N/A | Solid-state reactive sintering |
| BaCe0.7Zr0.1Y0.1Yb0.1O3−δ [107] | NiO | 1% (wt) | rapid laser reactive sintering defocus distance: 20 mm laser energy: 95 W scan speed: 0.1 mm/s | N/A | Humidified H2 and air | 600 | 3.70 | 600 | 121 | 0.970 | Solid-state reactive sintering + rapid laser reactive sintering |
| BaCe0.7Zr0.1Y0.2O3−δ [108] | NiO | 0.5% (wt) | 1400 °C, 6 h | ~98 | Humidified H2 and air | N/A | N/A | 600 | 60 | 1.110 | Solid-state reaction |
| BaZr0.8Y0.2O3−δ [14] | NiO | 1% (wt) | 1450 °C, 18 h | N/A | Humidified H2 and air | N/A | N/A | 600 | 660 | N/A | Solid-state reactive sintering |
| Electrolyte Composition | Sintering Temperature (°C), Duration | Atmosphere | T (°C) | Total Conductivity, σTotal (S/cm) | Synthesis Procedure |
|---|---|---|---|---|---|
| BZCY20 [128] | 1400 °C, 12 h | humidified 10%-H2/Ar | 600 °C | ~0.00520 | solid-state reactive sintering |
| BZCY20 [129] | 1500 °C, 8 h | humidified N2 | 600 °C | ~0.00170 | new nitrate-free acetate–H2O2 combustion method |
| BZCY40 [129] | 1500 °C, 8 h | humidified N2 | 600 °C | ~0.00550 | new nitrate-free acetate–H2O2 combustion method |
| BZCY20 [130] | 1350 °C + sintering pressure of 50 MPa | humidified N2 + H2 | 600 °C | ~0.00310 | solid-state reaction with spark plasma sintering |
| Sample | Time (h) | Temperature (°C) | Grain Size (µm) | Densification (%) | Bulk Conductivity in Wet Air (Scm−1), 500 °C |
|---|---|---|---|---|---|
| BCZY | 5 | 1450 | 0.48 | 85 | 0.50 10−3 |
| 10 | 0.54 | 89 | 0.65 10−3 | ||
| 15 | 1.05 | 92 | 0.78 10−3 | ||
| BCZY-ZnO | 10 | 1300 | 0.91 | 97 | 0.65 10−3 |
| Electrolyte Composition | Sintering Aids | mol% or weight% of Sintering Aid | Sintering Temperature (°C), Duration | Relative Density (%) | Atmosphere | T (°C) | Conductivity (×10−3 Scm−1) | T (°C) | Peak Power Density (mWcm−2) | Open-Circuit Voltage (V) | Synthesis Method of Powder |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BaCe0.7Zr0.1Y0.1Zn0.1O3− δ [137] | ZnO | 10% (wt) | 1200 °C, 2 h | 97.47 | wet 5% H2 | 600 | 8.59 | N/A | N/A | N/A | conventional solid-state reaction |
| BaCe0.7Zr0.1Y0.1Zn0.1O3− δ [137] | ZnO | 10% (wt) | 1200 °C, 2 h | 97.47 | dry H2 | 600 | 6.67 | N/A | N/A | N/A | conventional solid-state reaction |
| BaCe0.6Zr0.2Y0.15Sm0.05O3− δ [138] | ZnO | 4% (wt) | 1400 °C, 10 h | 98.96 | wet 5% H2 | 700 | 3.89 | 700 | 420 | 1.01 | conventional solid-state reaction |
| BaCe0.5Zr0.3Y0.16Zn0.04O3− δ [139] | ZnO | 4% (mol) | 1200 °C, 5 h | 97.4 | humidified hydrogen (∼3% H2O) | 700 | 2.73 | 700 | 240 | 1.00 | modified Pechini method—using citrate and ethylenediamine tetraacetic acid (EDTA), which are used as parallel complexing agents |
| BaZr0.80Y0.2O3− δ [94] | N/A | N/A | 1400 °C, 10 h | 68 | dry 5% H2 | 900 | 9.00 | N/A | N/A | N/A | solid-state reaction |
| BaZr0.80Y0.2O3− δ [94] | ZnO | 4% (mol) | 1325 °C, 10 h | 96 | dry 5% H2 | 600 | 1.00 | N/A | N/A | N/A | solid-state reaction |
| BaCe0.5Zr0.3Y0.2O2.9 [114] | ZnO | 4% (mol) | 1300 °C, 10 h | 98.5 | humidified hydrogen (3%H2O + 97%H2) | 600 | 13.5 | N/A | N/A | N/A | solid-state reaction |
| BaCe0.5Zr0.3Y0.2O2.9 [114] | ZnO | 7% (mol) | 1300 °C, 10 h | 96 | humidified hydrogen (3%H2O + 97%H2) | 600 | 10.0 | N/A | N/A | N/A | solid-state reaction |
| BaCe0.5Zr0.3Y0.2O2.9 [114] | ZnO | 20% (mol) | 1300 °C, 10 h | 94 | humidified hydrogen (3%H2O + 97%H2) | 600 | 71.1 | N/A | N/A | N/A | solid-state reaction |
| BaZr0.80Y0.16Zn0.04O3− δ [140] | ZnO | 4% (mol) | 1450 °C, 5 h | N/A | H2 (~3% H2O) | 600 | 600 | ~75 | 0.99 | citric acid–nitrate auto-combustion method | |
| BaZr0.85Y0.15O3−δ [141] | ZnO | 1% (wt) | 1500 °C, N/A | >95 | (3% H2O) 5% H2 atmosphere | 650 | 10.0 | 800 | 24.5 | 1.00 | solid-state reaction |
| BaZr0.80Y0.16Zn0.04O3−δ [141] | ZnO | 1% (wt) | 1400 °C, 5 h | N/A | humidified H2 and air | N/A | N/A | 800 | 27 | 1.00 | solid-state reaction |
| BaZr0.80Y0.16Zn0.04O3−δ [141] | ZnO | 1% (wt) | 1450 °C, 5 h | N/A | humidified H2 and air | N/A | N/A | 600 | 75 | N/A | solid-state reaction |
| BaCe0.6Zr0.3Y0.1O3−δ [34] | ZnO | 5% (mol) | 1400 °C, 12 h | 98 | N/A | N/A | N/A | 500 | N/A | 1.094 | solid-state reaction |
| BaZr0.4Ce0.4Y0.2O3−δ [128] | Zn(NO3)2 | 3.56% (wt) | 1400 °C, 8 h | >97 | humidified H2/Ar and air | 600 | 4.20 | 600 | 279 | 1.07 | solid-state reactive sintering |
| BaZr0.6Ce0.2Y0.2O3−δ [128] | Zn(NO3)2 | 3.56% (wt) | 1400 °C, 8 h | >97 | humidified H2/Ar and air | 600 | 5.2 | 600 | 336 | 1.08 | solid-state reactive sintering |
| BaZr0.7Ce0.1Y0.2O3−δ [128] | Zn(NO3)2 | 3.56% (wt) | 1400 °C, 8 h | >97 | humidified H2/Ar and air | 600 | 2.7 | 600 | 111 | 1.05 | solid-state reactive sintering |
| BaZr0.6Ce0.2Y0.2O3−δ [142] | Zn(NO3)2 | 3.56% (wt) | 1400 °C, 12 h | >97 | humidified H2/Ar and air | 600 | 3.00 | 600 | 336 | 0.92 | solid-state reaction + single-step cofiring (electrolyte) |
| Parameter (mS·cm−1) | BZY10 | BZY10 + 1% Co | BZY10 + 2% Co | BZY10 + 1% Ni | BZY10 + 2% Ni | BCZY27 | BCZY27 + 1% Co | BCZY27 + 2% Co | BCZY27 + 1% Ni | BCZY27 + 2% Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| σi measured | 1.66 | 0.17 | 0.49 | 0.36 | 1.02 | 1.89 | 0.98 | 1.23 | 1.77 | 1.4 |
| σi corrected | 1.88 | 0.18 | 0.53 | 0.39 | 1.16 | 2.04 | 1.04 | 1.28 | 1.93 | 1.53 |
| σp measured | 2.48 | 0.39 | 1.71 | 1.85 | 9.41 | 2.32 | 1.65 | 1.73 | 4.09 | 3.78 |
| σp corrected | 2.81 | 0.42 | 1.84 | 1.99 | 10.68 | 2.5 | 1.74 | 1.81 | 4.46 | 4.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loganathan, S.; Biswas, S.; Kaur, G.; Giddey, S. Role of Sintering Aids in Electrical and Material Properties of Yttrium- and Cerium-Doped Barium Zirconate Electrolytes. Processes 2024, 12, 2278. https://doi.org/10.3390/pr12102278
Loganathan S, Biswas S, Kaur G, Giddey S. Role of Sintering Aids in Electrical and Material Properties of Yttrium- and Cerium-Doped Barium Zirconate Electrolytes. Processes. 2024; 12(10):2278. https://doi.org/10.3390/pr12102278
Chicago/Turabian StyleLoganathan, Shivesh, Saheli Biswas, Gurpreet Kaur, and Sarbjit Giddey. 2024. "Role of Sintering Aids in Electrical and Material Properties of Yttrium- and Cerium-Doped Barium Zirconate Electrolytes" Processes 12, no. 10: 2278. https://doi.org/10.3390/pr12102278
APA StyleLoganathan, S., Biswas, S., Kaur, G., & Giddey, S. (2024). Role of Sintering Aids in Electrical and Material Properties of Yttrium- and Cerium-Doped Barium Zirconate Electrolytes. Processes, 12(10), 2278. https://doi.org/10.3390/pr12102278








