Effect of Rhizobacteria Application on Nutrient Content, Bioactive Compounds, Antioxidant Activity, Color Properties and Fruit Characteristics of Strawberry Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
2.3. Pomological Analysis
2.4. Biochemical Analysis
2.5. Nutrient Analysis
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liston, A.; Cronn, R.; Ashman, T.L. Fragaria: A genus with deep historical roots and ripe for evolutionary and ecological insights. Am. J. Bot. 2014, 101, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yan, Q.; Chen, L.; Song, Y.; Li, J.; Fu, C.; Dong, M. Effects of ploidy level and haplotype on variation of photosynthetic traits: Novel evidence from two Fragaria species. PLoS ONE 2017, 12, e0179899. [Google Scholar] [CrossRef]
- Isobe, S.N.; Shirasawa, K.; Nagano, S.; Hirakawa, H. Current status of octoploid strawberry (Fragaria × ananassa) genome study. In The Genomes of Rosaceous Berries and Their Wild Relatives; Compendium of Plant Genomes; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 129–137. [Google Scholar]
- Oğuz, F.G.; Pırlak, L. Determination of strawberry planting times and cultivars in Eskişehir conditions. J. Bahri Dagdas Crop Res. 2019, 8, 148–157. [Google Scholar]
- López-Aranda, J.M.; Soria, C.; Santos, B.M.; Miranda, L.; Dominguez, P.; Medina-Mínguez, J.J. Strawberry Production in Mild Climates of the World: A Review of Current Cultivar Use. Int. J. Fruit Sci. 2011, 11, 232–244. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Database. Available online: https://www.fao.org/home/en (accessed on 19 March 2024).
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sarıdaş, M.A. Farklı Dozlarda Kalsiyum Uygulamalarının Bazı Çilek Çeşitlerinde Meyve Verim ve Kalite Kriterleri ile Yapraklardaki Besin Element Konsantrasyonları Üzerine Etkileri. Master’s Thesis, University of Çukurova, Adana, Türkiye, 2013. [Google Scholar]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-Lόpez, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Roussos, P.A.; Triantafillidis, A.; Kepolas, E.; Peppas, P.; Piou, A.; Zoti, M.; Gasparatos, D. Effects of integrated and organic management on strawberry (cv. Camarosa) plant growth, nutrition, fruit yield, quality, nutraceutical characteristics, and soil fertility status. Horticulturae 2022, 8, 184. [Google Scholar] [CrossRef]
- Verma, P.P.; Shelake, R.M.; Das, S.; Sharma, P.; Kim, J.Y. Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF): Potential biological control agents of diseases and pests. In Microbial Interventions in Agriculture and Environment; Springer: Singapore, 2019; pp. 281–311. [Google Scholar]
- Maharana, R.; Singh, B.S.M.; Mandal, K.; Dhal, N.K. Microbial-Mediated Mechanism to Improve Rock Phosphate Solubilization and Its Agronomic Implications. In Enzymes for Pollutant Degradation; Microorganisms for Sustainability; Mulla, S.I., Bharagava, R.N., Eds.; Springer Nature: Singapore, 2022; pp. 327–339. ISBN 9789811645747. [Google Scholar]
- Nadarajah, K.; Abdul Rahman, N.S.N. The Microbial Connection to Sustainable Agriculture. Plants 2023, 12, 2307. [Google Scholar] [CrossRef]
- Arıkan, Ş.; İpek, M.; Pırlak, L. Effects ofplant growth promoting rhizobacteria (PGPR) on yield and fruit quality of quince. In Proceedings of the International Conference on Agriculture and Biotechnology, Kuala Lumpur, Malaysia, 29–30 December 2013. [Google Scholar]
- İpek, M.; Arıkan, Ş.; Eşitken, A.; Pırlak, L. The effect of plant growth promoting rhizobacteria on plant development, yield and fruit quality of raspberry (Rubus idaeus L.) cultivar “Heritage”. Yüzüncü Yıl Univ. J. Agric. Sci. 2018, 28, 42–48. [Google Scholar]
- Atılgan, H.; Mısırlı, A.; Özaktan, H.; Şen, F.; Bilgin, N.A. Effects of bacteria and compost tea applications on fruit characteristics, yield and nutrient content on Salihli sweet cherry variety. J. Agric. Fac. Ege Univ. 2019, 56, 409–415. [Google Scholar]
- Arıkan, Ş.; Pırlak, L. Effects of plant growth promoting rhizobacteria (PGPR) on growth, yield and fruit quality of sour cherry (Prunus cerasus L.). Erwerbs-Obstbau 2016, 58, 221–226. [Google Scholar] [CrossRef]
- Yaman, M.; Yildiz, E.; Sumbul, A.; Ercisli, S.; Sonmez, O.; Gunes, A.; Say, A.; Kece, Y.M.; Unsal, H.T. The Effect of PGPR applications on bioactive content and fruit characteristics of different apple scion–rootstock combinations. Erwerbs-Obstbau 2023, 65, 1267–1273. [Google Scholar] [CrossRef]
- Yildiz, E.; Yaman, M.; Ercisli, S.; Sumbul, A.; Sonmez, O.; Gunes, A.; Bozhuyuk, M.R.; Kviklys, D. Effects of rhizobacteria application on leaf and fruit nutrient content of different apple scion–rootstock combinations. Horticulturae 2022, 8, 550. [Google Scholar] [CrossRef]
- Yildiz, E.; Yaman, M.; Sümbül, A.; Sönmez, O. The Effect of Rhizobacterial application on fruit quality parameters in different rootstock-cultivar combinations in apple. Çukurova J. Agric. Food Sci. 2022, 37, 21–29. [Google Scholar]
- Pii, Y.; Graf, H.; Valentinuzzi, F.; Cesco, S.; Mimmo, T. The Effects of Plant Growth-Promoting Rhizobacteria (PGPR) on the Growth and Quality of Strawberries. Acta Hortic. 2018, 1217, 231–238. [Google Scholar] [CrossRef]
- Seema, K.; Mehta, K.; Singh, N. Studies on the effect of plant growth promoting rhizobacteria (PGPR) on growth, physiological parameters, yield and fruit quality of strawberry cv. chandler. J. Pharmacogn. Phytochem. 2018, 7, 383–387. [Google Scholar]
- Kumar, P.; Sharma, N.; Sharma, S.; Gupta, R. Rhizosphere stochiometry, fruit yield, quality attributes and growth response to PGPR transplant amendments in strawberry (Fragaria × ananassa Duch.) growing on solarized soils. Sci. Hortic. 2020, 265, 109215. [Google Scholar] [CrossRef]
- Hosseini, A.; Hosseini, M.; Schausberger, P. Plant Growth-Promoting Rhizobacteria Enhance Defense of Strawberry Plants against Spider Mites. Front. Plant Sci. 2022, 12, 783578. [Google Scholar] [CrossRef]
- Kim, M.-J.; Shim, C.-K.; Ko, B.-G.; Kim, J. Effect of the Microalga Chlorella fusca CHK0059 on Strawberry PGPR and Biological Control of Fusarium Wilt Disease in Non-Pesticide Hydroponic Strawberry Cultivation. J. Microbiol. Biotechnol. 2020, 30, 708–716. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Chizhevskaya, E.P.; Vorobyov, N.I.; Bobkova, V.V.; Pomyaksheva, L.V.; Khomyakov, Y.V.; Konovalov, S.N. The Quality and Productivity of Strawberry (Fragaria × ananassa Duch.) Improved by the Inoculation of PGPR Bacillus velezensis BS89 in Field Experiments. Agronomy 2022, 12, 2600. [Google Scholar] [CrossRef]
- García-López, J.V.; Redondo-Gómez, S.; Flores-Duarte, N.J.; Zunzunegui, M.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Mateos-Naranjo, E. Exploring through the use of physiological and isotopic techniques the potential of a PGPR-based biofertilizer to improve nitrogen fertilization practices efficiency in strawberry cultivation. Front. Plant Sci. 2023, 14, 1243509. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Gulluce, M.; von Wirén, N.; Sahin, F. Yield promotion and phosphorus solubilization by plant growth–promoting rhizobacteria in extensive wheat production in Turkey. J. Plant Nut. Soil Sci. 2012, 175, 818–826. [Google Scholar] [CrossRef]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Kochem, A.-J.; Disqué, C.; Mühl, H.; Gebert, S.; Winter, J.; Matten, J.; Sakka, S.G. Diagnosis of Bacteremia in Whole-Blood Samples by Use of a Commercial Universal 16S rRNA Gene-Based PCR and Sequence Analysis. J. Clin. Microbiol. 2009, 47, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Giusti, M.M.; Rodriguez-Saona, L.E.; Wrolstad, R.E. Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. J. Agric. Food Chem. 1999, 47, 4631–4637. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wissen. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lees, R. Laboratory Handbook of Methods of Food Analysis, 2nd ed.; Leonard Hill Books: London, UK, 1971. [Google Scholar]
- Kacar, B. Bitki ve Toprağın Kimyasal Analizleri. III. Toprak Analizleri; No: 3; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları: Ankara, Turkey, 1995. [Google Scholar]
- Kacar, B.; İnal, A. Bitki Analizleri; Nobel Yayınları: Ankara, Turkey, 2008. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Çiylez, S. Bazi Mikoriza ve Bakteri Irklarinin Birlikte ve Tek Olarak Bazi Çilek Çeşitlerinde Büyüme ve Verim Üzerine Etkileri. Master’s Thesis, University of Selçuk, Konya, Türkiye, 2019. [Google Scholar]
- Erturk, Y.; Ercisli, S.; Cakmakci, R. Yield and growth response of strawberry to plant growth-promoting Rhizobacteria inoculation. J. Plant Nutr. 2012, 35, 817–826. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant growth-promoting rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high-calcareous soil conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E.; et al. Impact of Beneficial Microorganisms on Strawberry Growth, Fruit Production, Nutritional Quality, and Volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef] [PubMed]
- Balcı, G.; Koç, A.; Ertürk, Y.; Keles, H.; Kılıç, T.; Bakoğlu, N. Effects of the combined inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria on yield and quality in organic strawberry cultivation in alkaline soils. Harran Tarım Gıda Bilimleri Dergisi 2021, 25, 448–456. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP Pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef]
- Morais, M.C.; Mucha, Â.; Ferreira, H.; Gonçalves, B.; Bacelar, E.; Marques, G. Comparative study of plant growth-promoting bacteria on the physiology, growth and fruit quality of strawberry. J. Sci. Food Agric. 2019, 99, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- PeŠaković, M.; Karaklajić-Stajić, Ž.; Milenković, S.; Mitrović, O. Biofertilizer affecting yield related characteristics of strawberry (Fragaria × ananassa Duch.) and soil micro-organisms. Sci. Hortic. 2013, 150, 238–243. [Google Scholar] [CrossRef]
- Pırlak, L.; Köse, M. Effects of Plant Growth Promoting Rhizobacteria on Yield and Some Fruit Properties of Strawberry. J. Plant Nutr. 2009, 32, 1173–1184. [Google Scholar] [CrossRef]
- Castellanos-Morales, V.; Villegas, J.; Wendelin, S.; Vierheilig, H.; Eder, R.; Cárdenas-Navarro, R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria × ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010, 90, 1774–1782. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Dönmez, M.F.; Ertürk, Y.; Erat, M.; Haznedar, A.; Sekban, R. Diversity and metabolic potential of culturable bacteria from the rhizosphere of Turkish tea grown in acidic soils. Plant Soil 2010, 332, 299–318. [Google Scholar] [CrossRef]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Figen Donmez, M.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Ağgün, Z.; Geçer, M.K.; Aslantaş, R. The effects on fruit yield and fruit properties of plant growth promoting bacteria applications on some strawberry cultivars. Int. J. Agric. Wildl. Sci. 2018, 4, 20–25. [Google Scholar]
- Ünal, N. Topraksız Çilek Yetiştiriciliğinde Yetiştirme Ortamları ve Faydalı Bakteri Kullanımının Verim ve Kaliteye Etkileri. Master’s Thesis, University of Ege, İzmir, Türkiye, 2019. [Google Scholar]
- Öztürk, A.; Öztürk, B. Samsun ekolojisinde yetiştirilen standart bazı elma çeşitlerinin fenolojik ve pomolojik özelliklerinin belirlenmesi. Anadolu Tarım Bilimleri Derg. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Ansari, M.H.; Hashemabadi, D.; Mahdavi, M.; Kaviani, B. The role of Pseudomonas strains and arbuscular mycorrhiza fungi as organic phosphate–solubilizing in the yield and quality improvement of strawberry (Fragaria × ananassa Duch cv. Selva) fruit. Acta Sci. Pol. Hortorum Cultus 2018, 17, 93–107. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria × ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef] [PubMed]
- Pešaković, M.; Milenković, S.; Đukić, D.; Mandić, L.; Karaklajić-Stajić, Ž.; Tomić, J.; Miletić, N. Phenolic composition and antioxidant capacity of integrated and conventionally grown strawberry (Fragaria × ananassa Duch.). Hortic. Sci. 2016, 43, 17–24. [Google Scholar] [CrossRef]
- Tomic, J.M.; Milivojevic, J.M.; Pesakovic, M.I. The response to bacterial inoculation is cultivar-related in strawberries. Turk. J. Agric. For. 2015, 39, 332–341. [Google Scholar] [CrossRef]
- Arıkan, S. Tuzlu Toprak Koşullarında Faydalı Rhizobacteria Tedavilerinin Elma ve Kiraz Üzerine Etkileri. Ph.D. Thesis, University of Selçuk, konya, Türkiye, 2017. [Google Scholar]
- Esitken, A.; Karlidag, H.; Ercisli, S.; Turan, M.; Sahin, F. The effect of spraying a growth-promoting bacterium on the yield, growth, and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Arıkan, S. Bitki Büyümesini Destekleyen Rhizobakterilerin Vişnenin Büyümesi, Verimi ve Meyve Kalitesi Üzerine Etkileri. Master’s Thesis, University of Selçuk, Konya, Türkiye, 2012. [Google Scholar]
- Ertürk, A.S. Bitki Gelişimini Destekleyen Rizobakterilerin (PGPR) Ayvanın Meyve ve Bitki Özellikleri Üzerine Etkileri (Cv. Esme). Master’s Thesis, University of Gaziosmanpaşa, Tokat, Türkiye, 2015. [Google Scholar]
- Erdoğan, U. Armut Sürgünlerinin Vejetatif Gelişme Özelliklerinde Bitki Büyümesini Destekleyen Azot Sabitleyici ve Fosfat Çözücü Bakteri Kombinasyonlarının Etkilerinin Belirlenmesi. Master’s Thesis, University of Bozok, Yozgat, Türkiye, 2017. [Google Scholar]
- Tuzlaci, H.İ. The Using Facilities of Application of Plant Growth Promoting Rhizobacteria in Strawberry Culture on Greenhouse and Field Conditions. Master’s Thesis, University of Ataturk, Erzurum, Türkiye, 2014. [Google Scholar]
- Choi, H.G. Correlation among phenotypic parameters related to the growth and photosynthesis of strawberry (Fragaria × ananassa Duch.) grown under various light intensity conditions. Front. Plant Sci. 2021, 12, 647585. [Google Scholar] [CrossRef]
- Fagherazzi, A.F.; Suek Zanin, D.; Soares dos Santos, M.F.; Martins de Lima, J.; Welter, P.D.; Francis Richter, A.; Regianini Nerbass, F.; Anneliese Kretzschmar, A.; Rufato, L.; Baruzzi, G. Initial crown diameter influences on the fruit yield and quality of strawberry Pircinque. Agronomy 2021, 11, 184. [Google Scholar] [CrossRef]
- Jo, J.S.; Kim, D.S.; Jo, W.J.; Sim, H.S.; Lee, H.J.; Moon, Y.H.; Woo, U.J.; Jung, S.B.; Kim, S.; Mo, X.; et al. Prediction of strawberry fruit yield based on cultivar-specific growth models in the tunnel-type greenhouse. Hortic. Environ. Biotechnol. 2022, 63, 467–476. [Google Scholar] [CrossRef]
- Saridas, M.A.; Simsek, O.; Donmez, D.; Kacar, Y.A.; Kargi, S.P. Genetic diversity and fruit characteristics of new superior hybrid strawberry (Fragaria × ananassa Duchesne ex Rozier) genotypes. Genet. Resour. Crop Evol. 2021, 68, 741–758. [Google Scholar] [CrossRef]
- Milosavljević, D.M.; Mutavdžić, D.R.; Radotić, K.; Milivojević, J.M.; Maksimović, V.M.; Maksimović, J.J.D. Phenolic Profiling of 12 Strawberry Cultivars Using Different Spectroscopic Methods. J. Agric. Food Chem. 2020, 68, 4346–4354. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; dos Reis Correia, P.M.; Ferrão, A.C.; Gonçalves, F.; Lerat, C.; El-Idrissi, T.; Rodrigo, E. Evaluation of phenolic and antioxidant properties of strawberry as a function of extraction conditions. Braz. J. Food Technol. 2020, 23, e2019142. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, R.K.; Patil, R.T.; Sharma, R.R.; Asrey, R.; Kumar, A.; Jangra, K.K. Sequential foliar application of vermicompost leachates improves marketablefruit yield and quality of strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2010, 124, 34–39. [Google Scholar] [CrossRef]
- Khalil, N.H.; Hammoodi, J.K. Effect of nitrogen, potassium and calcium in strawberry fruit quality. Int. J. Agric. Stat. 2021, 16, 1967–1972. [Google Scholar]
- Negi, Y.K.; Sajwan, P.; Uniyal, S.; Mishra, A.C. Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 2021, 283, 110038. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Liu, B.; Wang, Z.; Yu, Y.; Bo, L.; Li, B. Accumulation, migration and health risk of trace metals in a soil-strawberry-human system of the Yangtze River Delta region, China. Environ. Res. 2023, 231, 116310. [Google Scholar] [CrossRef] [PubMed]
- Benlioğlu, B.; Demirel, F.; Türkoğlu, A.; Haliloğlu, K.; Özaktan, H.; Kujawa, S.; Niedbała, G. Insights into drought tolerance of tetraploid wheat genotypes in the germination stage using machine learning algorithms. Agriculture 2024, 14, 206. [Google Scholar] [CrossRef]
- Lambrecht, D.M.; Lúcio, A.D.C.; Diel, M.I.; Schmidt, D.; de Lima Tartaglia, F.; Tischler, A.L. Differences between strawberry cultivars based on principal component analysis. Int. J. Innov. Educ. Res. 2020, 8, 136–145. [Google Scholar] [CrossRef]
- Barth, E.; Resende, J.T.V.d.; Moreira, A.F.P.; Mariguele, K.H.; Zeist, A.R.; Silva, M.B.; Stulzer, G.C.G.; Mafra, J.G.M.; Simões Azeredo Gonçalves, L.; Roberto, S.R.; et al. Selection of experimental hybrids of strawberry using multivariate analysis. Agronomy 2020, 10, 598. [Google Scholar] [CrossRef]
- Amiri, A.; Mortazavi, S.M.H.; Mahmoodi Sourestani, M.; Mottaghipisheh, J. Assessment of physico-chemical characteristics of strawberry (Fragaria x ananasa Duch cv Camarosa) during fruit growth and development stages using principal component analysis. J. Horti. Postharvest Res. 2022, 5, 285–296. [Google Scholar]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Changes in Quality Characteristics of Strawberry Juice After Equivalent High Pressure, Ultrasound, and Pulsed Electric Fields Processes. Food Eng. Rev. 2021, 13, 601–612. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; Júnior, E.P.L.; D’Agostini, M.; Nardi, F.S.; Trentin, T.d.S.; Dornelles, A.G.; Huzar-Novakowiski, J.; Calvete, E.O. Horticultural Potential of Nine Strawberry Cultivars by Greenhouse Production in Brazil: A View through Multivariate Analysis. Sci. Hortic. 2021, 279, 109738. [Google Scholar] [CrossRef]
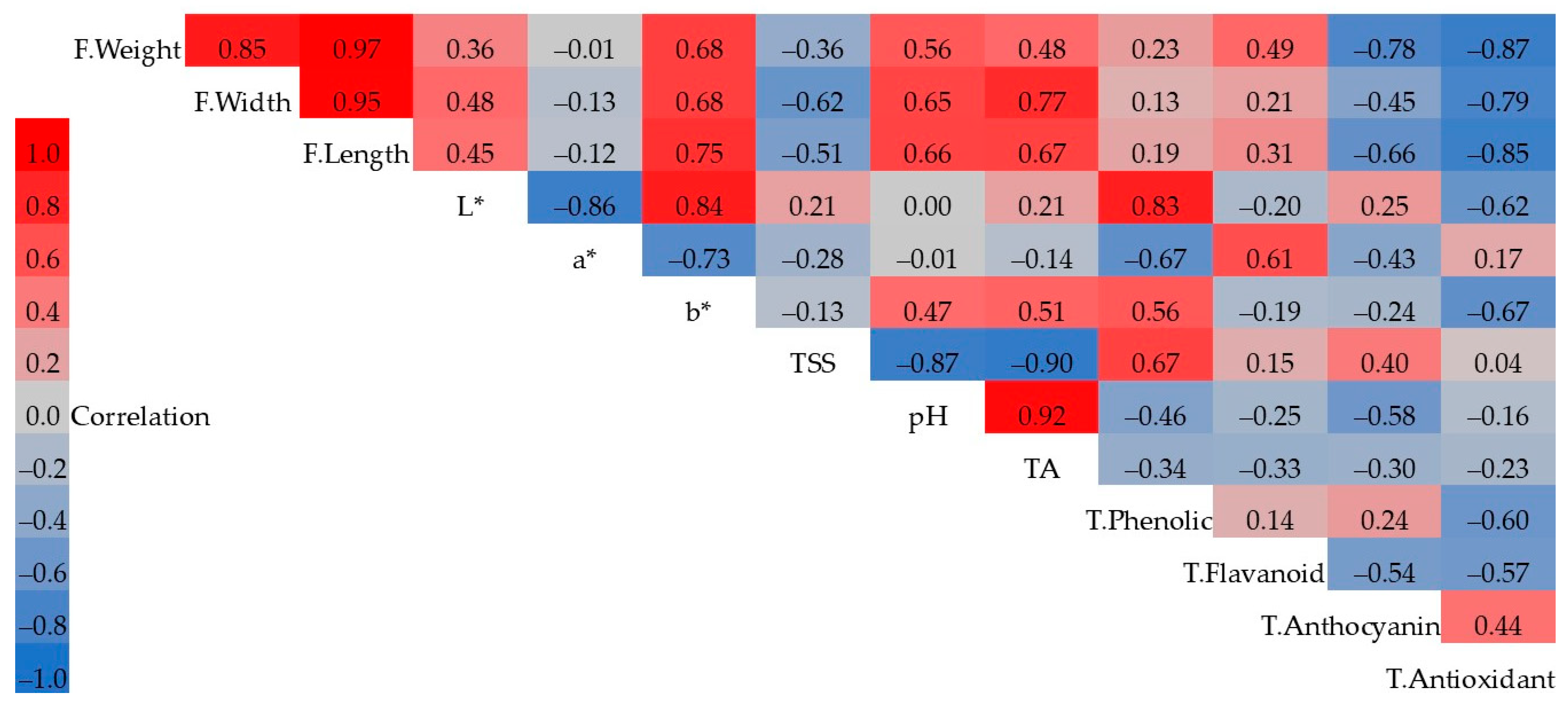
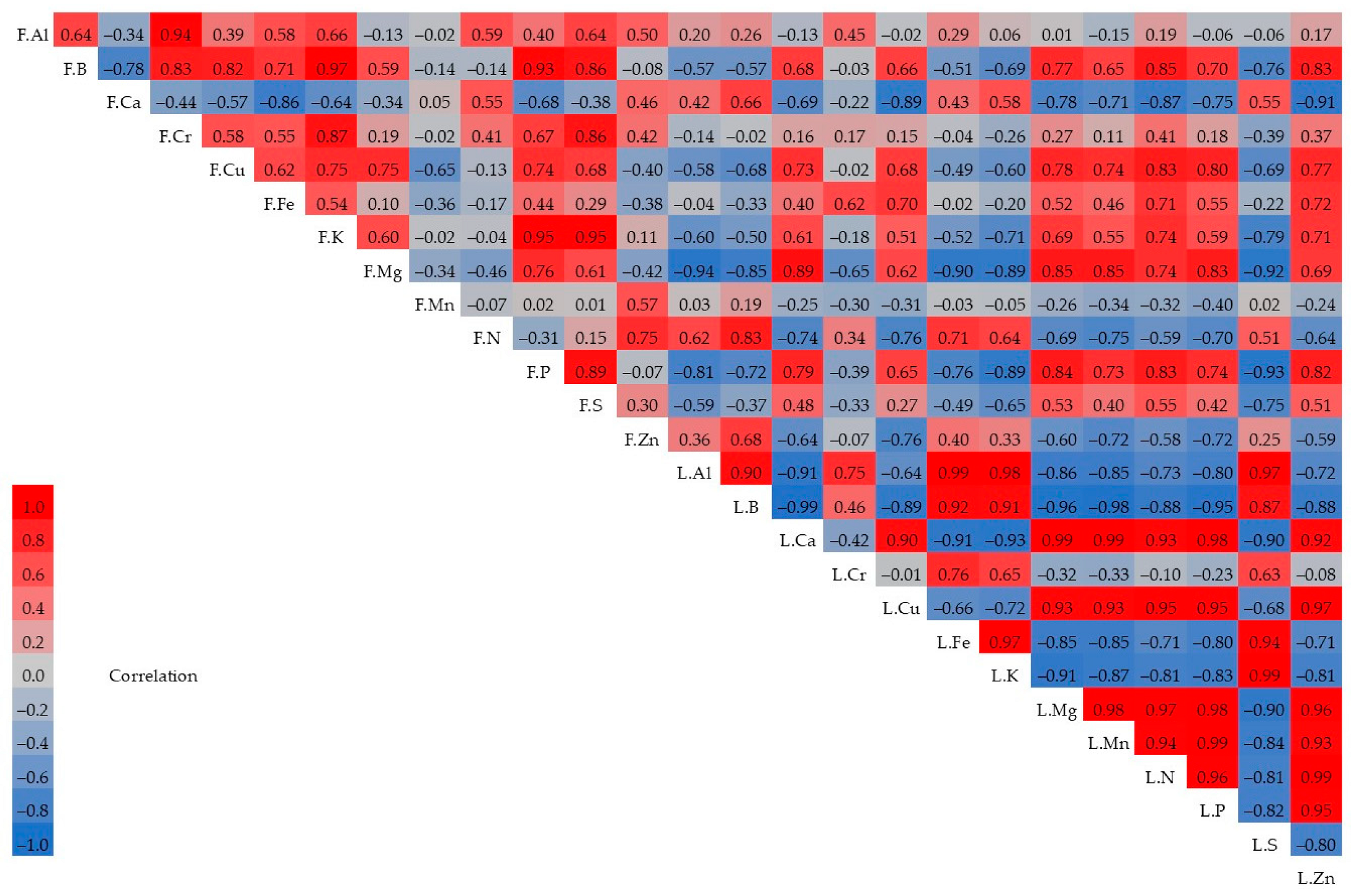
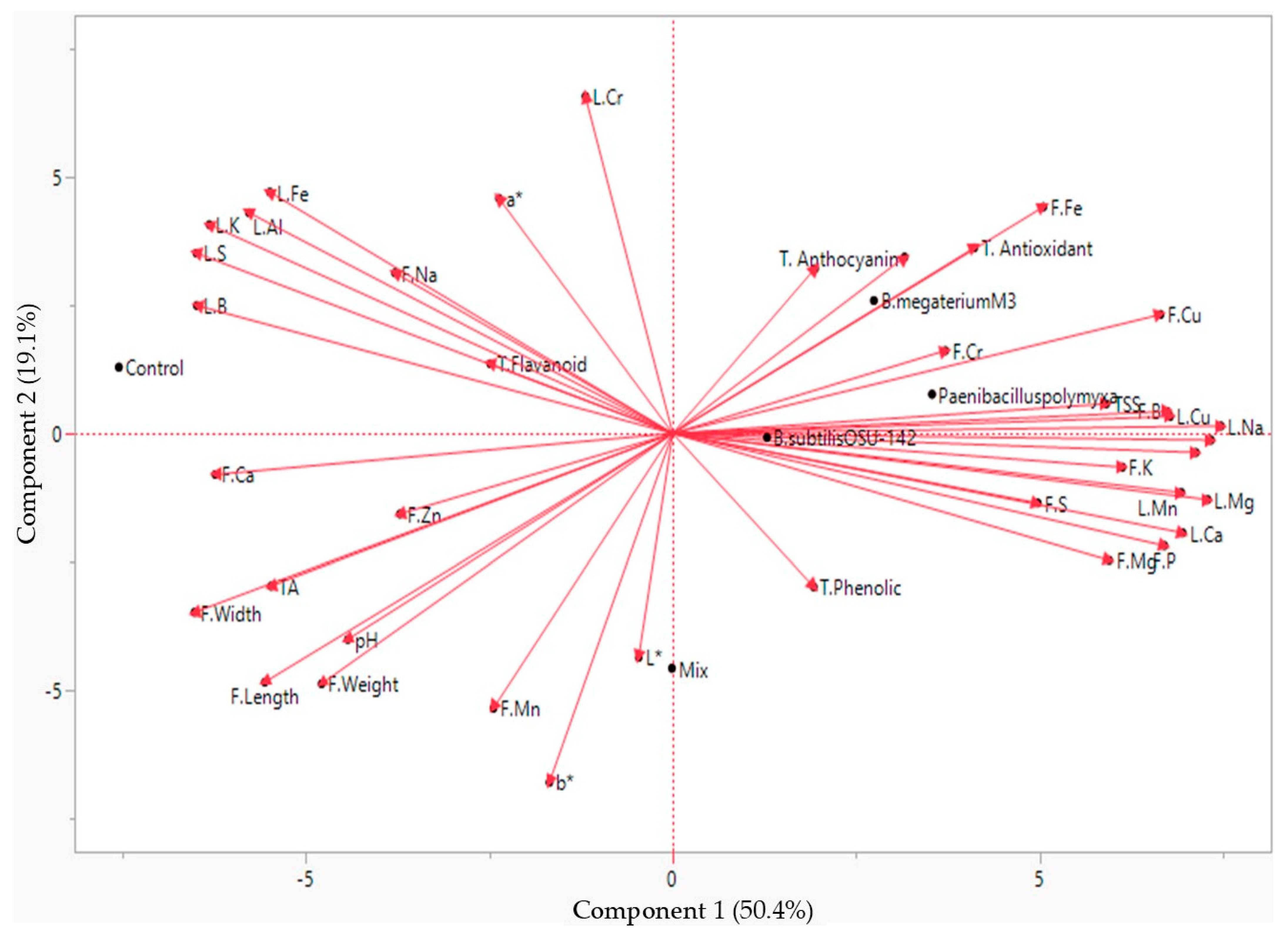
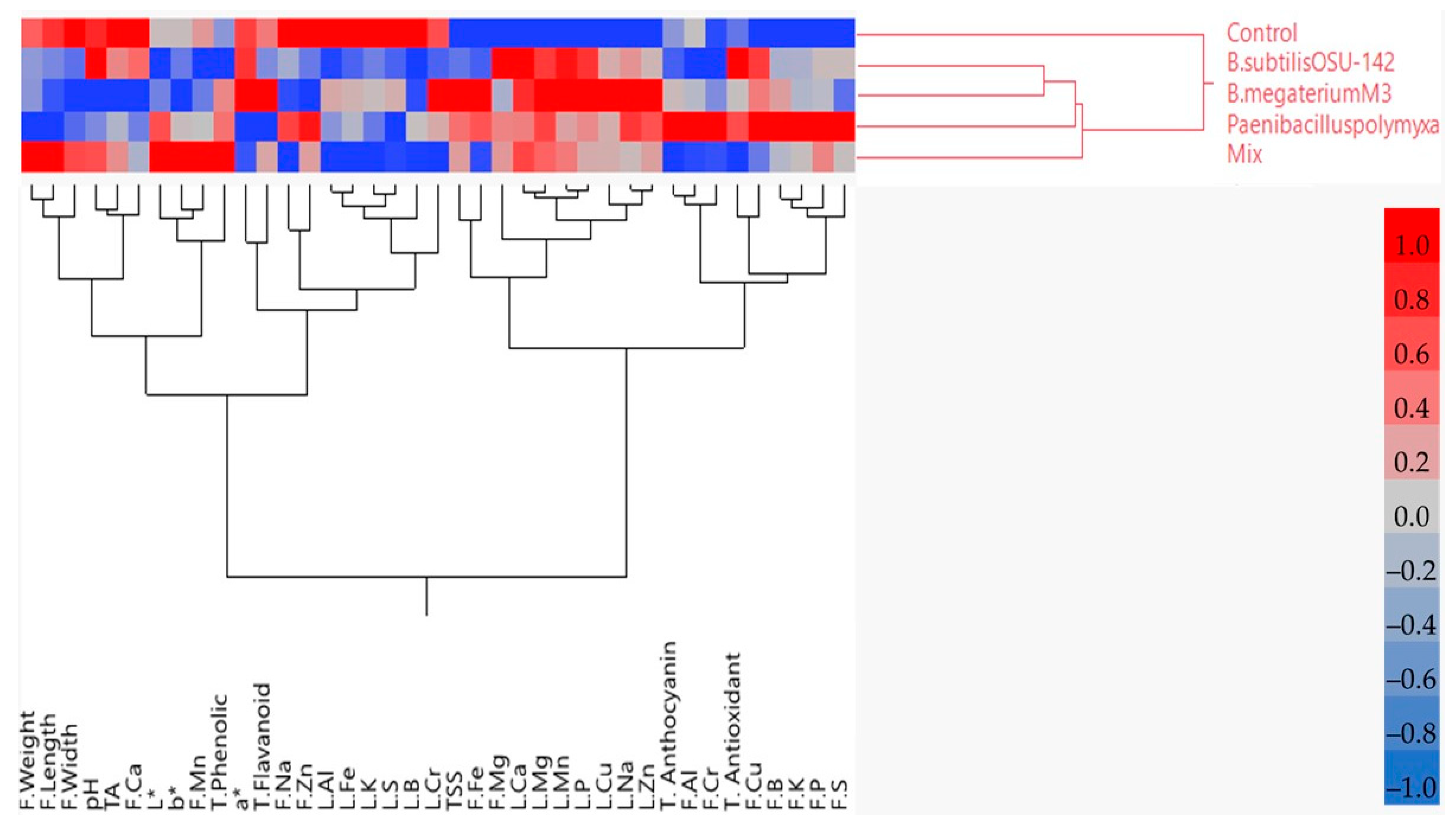
| Cultivar | Treatment | Fruit Weight (g) | Fruit Width (mm) | Fruit Length (mm) | TSS (%) | pH | TA | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|---|
| Albion | Control | 8.87 ab* | 22.44 b | 29.45 ab | 9.10 d | 3.72 | 0.93 a | 33.04 a | 80.13 abc | 14.44 b |
| B. subtilis OSU-142 | 7.85 b | 23.18 b | 28.84 c | 10.16 ab | 3.66 | 0.88 ab | 29.81 b | 86.78 a | 14.65 b | |
| P. polymyxa | 10.88 ab | 22.63 b | 28.81 c | 9.76 bc | 3.75 | 0.86 abc | 31.97 a | 83.53 ab | 15.19 ab | |
| B. megaterium M3 | 11.79 a | 26.65 a | 31.43 ab | 10.76 a | 3.65 | 0.79 c | 33.26 a | 79.07 bc | 16.58 a | |
| Mix | 10.78 ab | 25.02 ab | 33.49 a | 9.53 cd | 3.76 | 0.84 bc | 32.89 a | 75.98 c | 15.32 ab | |
| Monterey | Control | 9.56 ab | 20.97 c | 31.33 ab | 9.73 b | 3.91 ab | 0.58 | 31.94 ab | 89.17 ab | 14.07 bc |
| B. subtilis OSU-142 | 8.80 b | 21.67 bc | 27.82 b | 9.26 b | 4.02 a | 0.57 | 30.52 b | 83.01 ab | 13.74 bc | |
| P. polymyxa | 6.99 b | 22.00 bc | 25.72 b | 11.13 a | 3.73 b | 0.55 | 35.62 a | 70.06 c | 16.48 b | |
| B. megaterium M3 | 6.57 b | 24.96 ab | 26.28 b | 10.90 a | 3.72 b | 0.51 | 29.45 b | 92.65 a | 11.42 c | |
| Mix | 12.67 a | 25.31 a | 34.73 a | 11.03 a | 3.88 ab | 0.61 | 35.86 a | 79.61 bc | 20.89 a |
| Cultivar | Treatment | Total Phenolic (mg GAE/100 g) | Total Flavonoid (mg CAE/100 g) | Total Anthocyanin (mg cyn-3-gluc/100 g) | Antioxidant Activity (% Inhibition) |
|---|---|---|---|---|---|
| Albion | Control | 377.01 c* | 61.91 b | 220.47 bc | 30.35 b |
| B. subtilis OSU-142 | 333.79 c | 64.31 ab | 173.87 d | 31.53 ab | |
| P. polymyxa | 473.64 ab | 63.18 b | 283.84 a | 32.368 a | |
| B. megaterium M3 | 430.29 b | 72.54 a | 230.190 b | 30.52 ab | |
| Mix | 500.30 a | 67.21 ab | 190.51 cd | 30.30 b | |
| Monterey | Control | 350.37 | 71.47 | 193.87 | 33.06 ab |
| B. subtilis OSU-142 | 320.45 | 64.61 | 223.60 | 37.04 a | |
| P. polymyxa | 327.23 | 62.36 | 223.78 | 35.04 ab | |
| B. megaterium M3 | 360.53 | 64.02 | 200.58 | 34.88 ab | |
| Mix | 347.45 | 64.91 | 197.21 | 31.72 b |
| Macronutrients | Micronutrients | Heavy Metals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Treatment | N | P | K | Ca | Mg | S | B | Fe | Mn | Zn | Cu | Al | Cr |
| Albion | Control | 230.86 | 2199.96 | 7749.59 ab* | 1857.66 a | 1193.38 | 408.75 b | 11.01 | 36.25 a | 15.35 c | 10.47 a | 1.17 | 12.64 c | 0.41 b |
| B. subtilis OSU-142 | 224.26 | 2106.91 | 7659.51 b | 1414.62 ab | 1109.41 | 392.75 b | 10.80 | 29.78 b | 18.84 c | 8.11 ab | 1.44 | 16.85 abc | 0.63 ab | |
| P. polymyxa | 224.33 | 2476.26 | 8896.53 a | 1540.04 ab | 1232.76 | 461.83 a | 13.10 | 31.77 ab | 25.18 b | 10.80 a | 1.54 | 22.31 a | 0.98 b | |
| B. megaterium M3 | 213.49 | 2203.56 | 8206.24 a | 1294.06 b | 1145.44 | 405.08 b | 12.10 | 34.42 a | 17.41 c | 9.34 ab | 1.45 | 20.17 ab | 1.48 a | |
| Mix | 203.25 | 2172.85 | 7851.68 ab | 1457.68 ab | 1120.76 | 380.55 b | 11.26 | 27.21 b | 35.59 a | 9.25 ab | 1.23 | 14.34 bc | 0.57 ab | |
| Monterey | Control | 276.45 | 1547.35 | 5577.27 b | 940.12 b | 737.37 b | 313.24 | 7.60 b | 30.21 b | 19.12 a | 8.56 | 0.66 b | 21.28 a | 0.72 a |
| B. subtilis OSU-142 | 224.74 | 1899.01 | 6703.46 a | 1295.27 a | 1014.39 a | 387.01 | 9.94 a | 39.41 b | 5.95 c | 9.50 | 1.07 a | 10.49 b | 0.33 b | |
| P. polymyxa | 267.00 | 1715.17 | 7081.86 a | 1011.70 ab | 836.74 ab | 398.95 | 10.56 a | 56.61 a | 7.15 b | 8.15 | 1.06 a | 24.41 a | 0.68 a | |
| B. megaterium M3 | 206.55 | 1781.01 | 6197.52 ab | 1183.22 a | 880.68 ab | 337.29 | 9.51 a | 61.30 a | 10.93 ab | 7.79 | 0.95 a | 13.27 b | 0.44 b | |
| Mix | 211.70 | 1891.03 | 6709.26 a | 1150.93 a | 939.91 ab | 398.03 | 9.61 a | 40.44 b | 6.10 bc | 9.12 | 0.80 ab | 13.62 b | 0.63 a | |
| Macronutrients | Micronutrients | Heavy Metals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Treatment | N | P | K | Ca | Mg | S | B | Fe | Mn | Zn | Cu | Al | Cr |
| Albion | Control | 146.22 b* | 1257.88 b | 6209.45 | 2496.27 b | 869.33 b | 642.29 | 35.35 | 96.84 | 18.93 b | 5.75 b | 1.74 b | 95.96 | 3.27 a |
| B. subtilis OSU-142 | 227.54 a | 1954.54 a | 5013.82 | 4806.97 a | 1505.61 a | 513.47 | 43.20 | 87.44 | 38.63 a | 8.45 a | 3.33 a | 101.56 | 2.82 ab | |
| P. polymyxa | 269.67 a | 1792.87 ab | 5175.90 | 4119.10 a | 1444.64 a | 498.21 | 37.42 | 83.39 | 29.72 ab | 10.38 a | 3.21 a | 91.16 | 2.81 ab | |
| B. megaterium M3 | 264.67 a | 1883.53 a | 5208.31 | 4319.38 a | 1411.43 a | 496.11 | 39.46 | 91.20 | 39.21 a | 10.10 a | 3.32 a | 95.96 | 2.53 b | |
| Mix | 203.23 ab | 1694.55 ab | 5191.68 | 4300.66 a | 1345.79 a | 527.82 | 44.87 | 80.92 | 35.36 a | 8.77 a | 3.33 a | 97.72 | 2.31 b | |
| Monterey | Control | 150.25 c | 810.32 c | 7162.59 a | 2051.69 b | 690.87 c | 688.42 a | 62.98 a | 79.94 | 21.08 b | 4.94 | 1.40 b | 155.75 a | 3.81 |
| B. subtilis OSU-142 | 165.72 b | 938.67 b | 5128.02 bc | 2629.25 a | 733.01 c | 488.05 b | 40.15 b | 74.22 | 30.03 a | 5.96 | 3.06 ab | 70.83 b | 1.66 | |
| P. polymyxa | 173.88 b | 860.89 bc | 5226.35 bc | 2532.83 a | 778.39 b | 473.53 b | 49.93 ab | 98.06 | 30.60 a | 6.17 | 3.16 ab | 97.23 ab | 3.33 | |
| B. megaterium M3 | 204.94 a | 1134.29 a | 5800.18 b | 2861.38 a | 922.49 a | 609.68 a | 44.32 ab | 97.87 | 30.33 a | 8.03 | 5.02 a | 109.59 ab | 5.25 | |
| Mix | 169.23 b | 868.85 bc | 4503.55 c | 2734.17 a | 815.84 b | 452.60 b | 38.56 b | 72.07 | 27.41 a | 6.11 | 3.05 ab | 73.22 b | 1.62 | |
| PCA1 | % Cont. | PCA2 | % Cont. | PCA3 | % Cont. | PCA4 | % Cont. | |
|---|---|---|---|---|---|---|---|---|
| Fruit Weight | −0.14 | 2.05 | −0.24 | 5.59 | −0.11 | 1.20 | 0.14 | 1.99 |
| Fruit Width | −0.19 | 3.80 | −0.17 | 2.85 | 0.05 | 0.30 | 0.07 | 0.42 |
| Fruit Length | −0.17 | 2.76 | −0.23 | 5.52 | −0.03 | 0.09 | 0.09 | 0.81 |
| L* | −0.01 | 0.02 | −0.21 | 4.47 | 0.25 | 6.42 | 0.21 | 4.32 |
| a* | −0.07 | 0.50 | 0.22 | 4.95 | −0.27 | 7.42 | −0.04 | 0.17 |
| b* | −0.05 | 0.25 | −0.33 | 10.86 | 0.13 | 1.57 | 0.08 | 0.59 |
| Total Soluble Solids | 0.18 | 3.12 | 0.03 | 0.08 | 0.01 | 0.01 | 0.28 | 7.89 |
| pH | −0.13 | 1.75 | −0.19 | 3.78 | −0.04 | 0.14 | −0.27 | 7.53 |
| Titratable acidity | −0.16 | 2.68 | −0.14 | 2.07 | 0.09 | 0.86 | −0.23 | 5.35 |
| Total Phenolic | 0.06 | 0.33 | −0.14 | 2.09 | 0.14 | 2.01 | 0.36 | 13.18 |
| Total Flavonoid | −0.07 | 0.55 | 0.07 | 0.43 | −0.24 | 5.73 | 0.31 | 9.41 |
| Total Anthocyanin | 0.09 | 0.89 | 0.17 | 2.78 | 0.30 | 8.73 | 0.01 | 0.02 |
| Total Antioxidant | 0.12 | 1.51 | 0.18 | 3.08 | 0.00 | 0.00 | −0.31 | 9.80 |
| Fruit Al | 0.06 | 0.34 | 0.16 | 2.43 | 0.32 | 10.55 | 0.04 | 0.16 |
| Fruit B | 0.20 | 4.06 | 0.02 | 0.04 | 0.17 | 2.80 | 0.02 | 0.04 |
| Fruit Ca | −0.19 | 3.47 | −0.04 | 0.15 | −0.01 | 0.02 | −0.25 | 6.33 |
| Fruit Cr | 0.11 | 1.23 | 0.08 | 0.61 | 0.32 | 10.07 | −0.02 | 0.03 |
| Fruit Cu | 0.20 | 3.95 | 0.11 | 1.27 | 0.03 | 0.08 | −0.16 | 2.57 |
| Fruit Fe | 0.15 | 2.28 | 0.21 | 4.59 | 0.05 | 0.26 | 0.20 | 3.97 |
| Fruit K | 0.18 | 3.35 | −0.03 | 0.10 | 0.22 | 4.66 | −0.03 | 0.09 |
| Fruit Mg | 0.18 | 3.16 | −0.12 | 1.43 | −0.07 | 0.43 | −0.22 | 5.01 |
| Fruit Mn | −0.07 | 0.53 | −0.26 | 6.73 | 0.14 | 1.95 | 0.23 | 5.28 |
| Fruit N | −0.11 | 1.28 | 0.15 | 2.30 | 0.25 | 6.22 | −0.17 | 2.85 |
| Fruit P | 0.20 | 4.01 | −0.11 | 1.12 | 0.13 | 1.76 | −0.03 | 0.08 |
| Fruit S | 0.15 | 2.21 | −0.07 | 0.43 | 0.25 | 6.29 | −0.14 | 1.84 |
| Fruit Zn | −0.11 | 1.23 | −0.08 | 0.58 | 0.32 | 9.97 | −0.05 | 0.21 |
| Leaf Al | −0.17 | 2.98 | 0.21 | 4.36 | 0.06 | 0.31 | 0.12 | 1.39 |
| Leaf B | −0.19 | 3.76 | 0.12 | 1.46 | 0.15 | 2.16 | 0.00 | 0.00 |
| Leaf Ca | 0.21 | 4.31 | −0.09 | 0.88 | −0.11 | 1.17 | −0.03 | 0.08 |
| Leaf Cr | −0.04 | 0.13 | 0.32 | 10.16 | 0.04 | 0.13 | 0.21 | 4.28 |
| Leaf Cu | 0.20 | 4.10 | 0.02 | 0.03 | −0.13 | 1.64 | 0.13 | 1.58 |
| Leaf Fe | −0.16 | 2.69 | 0.23 | 5.21 | 0.09 | 0.74 | 0.08 | 0.62 |
| Leaf K | −0.19 | 3.56 | 0.20 | 3.90 | 0.02 | 0.02 | 0.04 | 0.16 |
| Leaf Mg | 0.22 | 4.75 | −0.06 | 0.39 | −0.07 | 0.53 | 0.00 | 0.00 |
| Leaf Mn | 0.21 | 4.29 | −0.06 | 0.31 | −0.14 | 1.89 | −0.01 | 0.02 |
| Leaf N | 0.22 | 4.99 | 0.01 | 0.01 | −0.04 | 0.13 | 0.05 | 0.23 |
| Leaf P | 0.21 | 4.54 | −0.02 | 0.03 | −0.12 | 1.49 | −0.01 | 0.01 |
| Leaf S | −0.19 | 3.77 | 0.17 | 2.91 | −0.02 | 0.06 | 0.09 | 0.81 |
| Leaf Zn | 0.22 | 4.81 | −0.01 | 0.00 | −0.04 | 0.17 | 0.09 | 0.90 |
| Eigenvalues | 19.64 | 7.46 | 7.05 | 4.83 | ||||
| Variance (%) | 50.36 | 19.14 | 18.10 | 12.39 | ||||
| Cumulative Variance (%) | 50.36 | 69.50 | 87.61 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elikara, A.U.; Popescu, G.C.; Demirel, S.; Sümbül, A.; Yaman, M.; Demirel, F.; Say, A.; Güneş, A. Effect of Rhizobacteria Application on Nutrient Content, Bioactive Compounds, Antioxidant Activity, Color Properties and Fruit Characteristics of Strawberry Cultivars. Processes 2024, 12, 2242. https://doi.org/10.3390/pr12102242
Elikara AU, Popescu GC, Demirel S, Sümbül A, Yaman M, Demirel F, Say A, Güneş A. Effect of Rhizobacteria Application on Nutrient Content, Bioactive Compounds, Antioxidant Activity, Color Properties and Fruit Characteristics of Strawberry Cultivars. Processes. 2024; 12(10):2242. https://doi.org/10.3390/pr12102242
Chicago/Turabian StyleElikara, Alper Umut, Gheorghe Cristian Popescu, Serap Demirel, Ahmet Sümbül, Mehmet Yaman, Fatih Demirel, Ahmet Say, and Adem Güneş. 2024. "Effect of Rhizobacteria Application on Nutrient Content, Bioactive Compounds, Antioxidant Activity, Color Properties and Fruit Characteristics of Strawberry Cultivars" Processes 12, no. 10: 2242. https://doi.org/10.3390/pr12102242
APA StyleElikara, A. U., Popescu, G. C., Demirel, S., Sümbül, A., Yaman, M., Demirel, F., Say, A., & Güneş, A. (2024). Effect of Rhizobacteria Application on Nutrient Content, Bioactive Compounds, Antioxidant Activity, Color Properties and Fruit Characteristics of Strawberry Cultivars. Processes, 12(10), 2242. https://doi.org/10.3390/pr12102242








