Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review
Abstract
1. Introduction
2. Pure MOF Catalysis
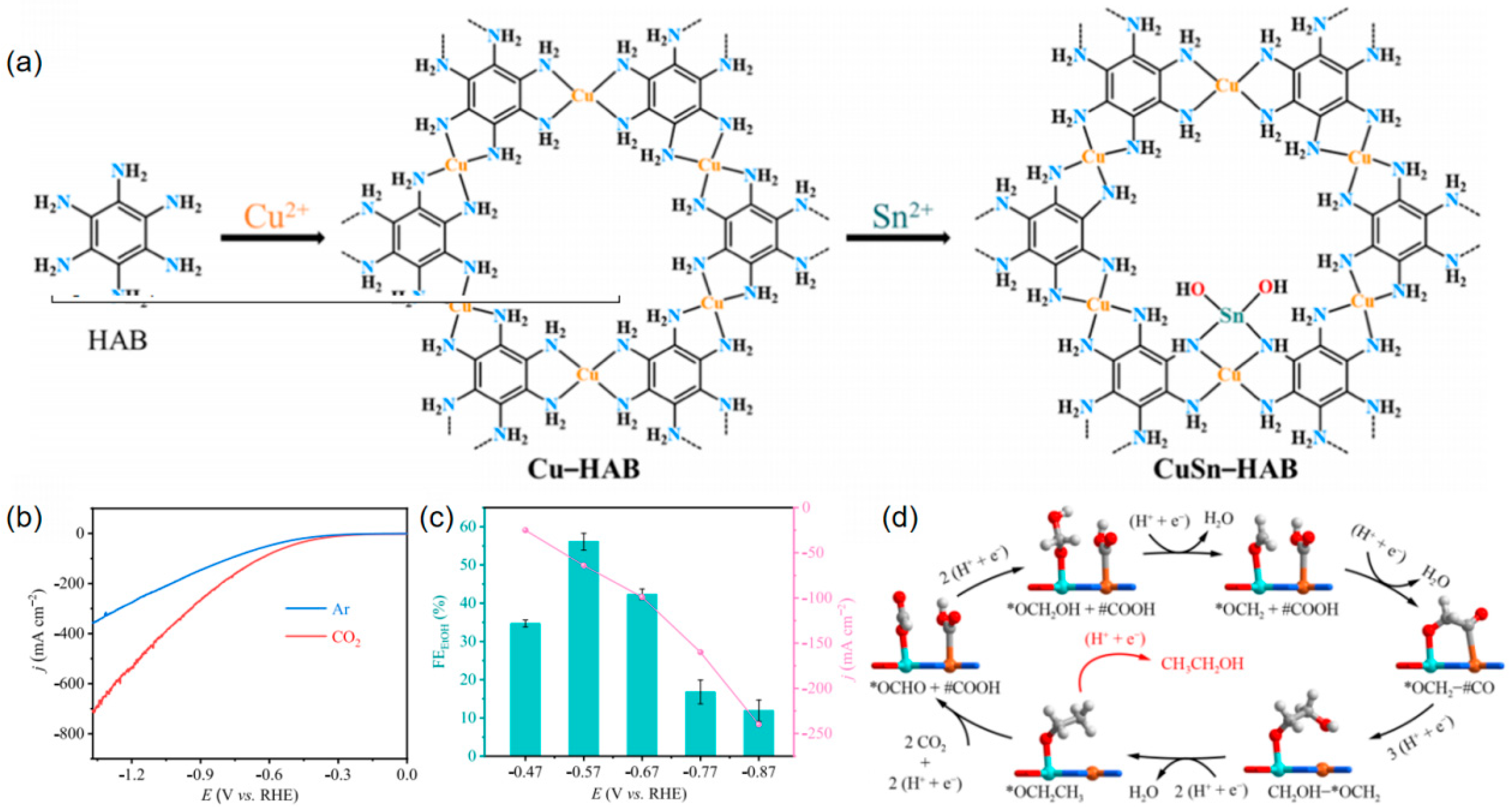
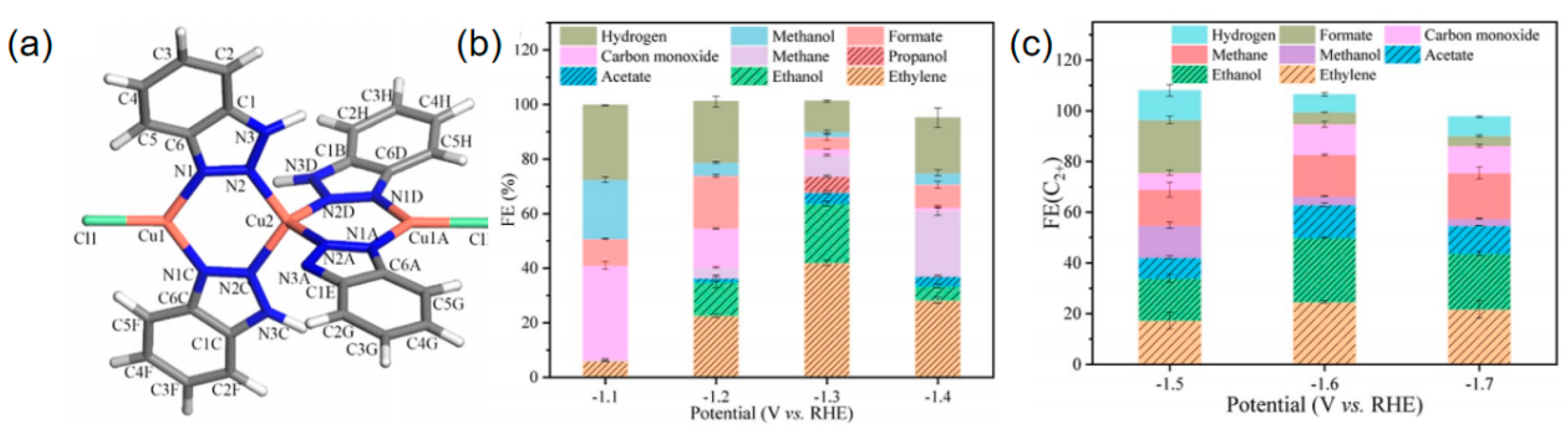
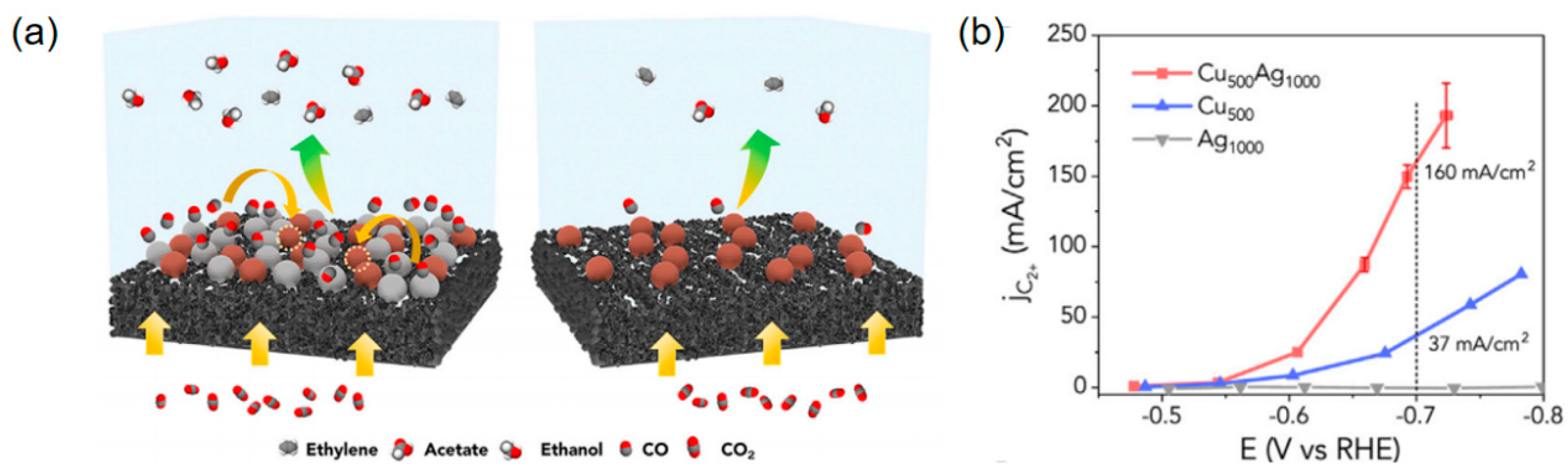
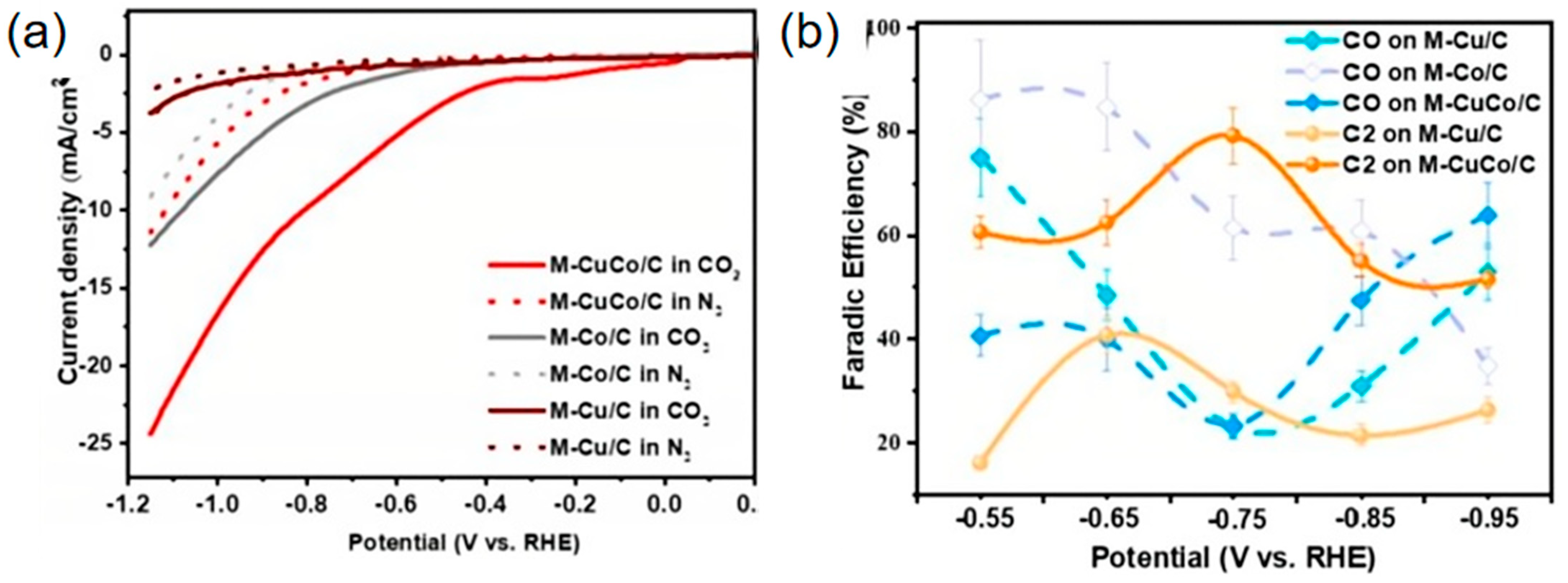
2.1. Collaborative Bimetallic Catalysis
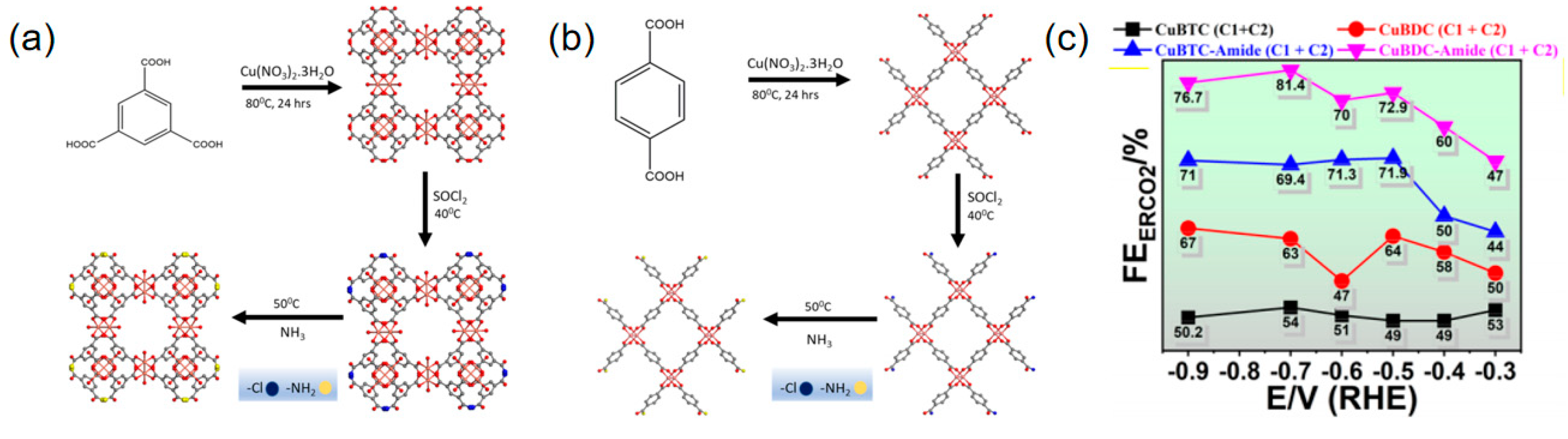
2.2. Doping Strategy
2.3. Building Heterogeneous Structures
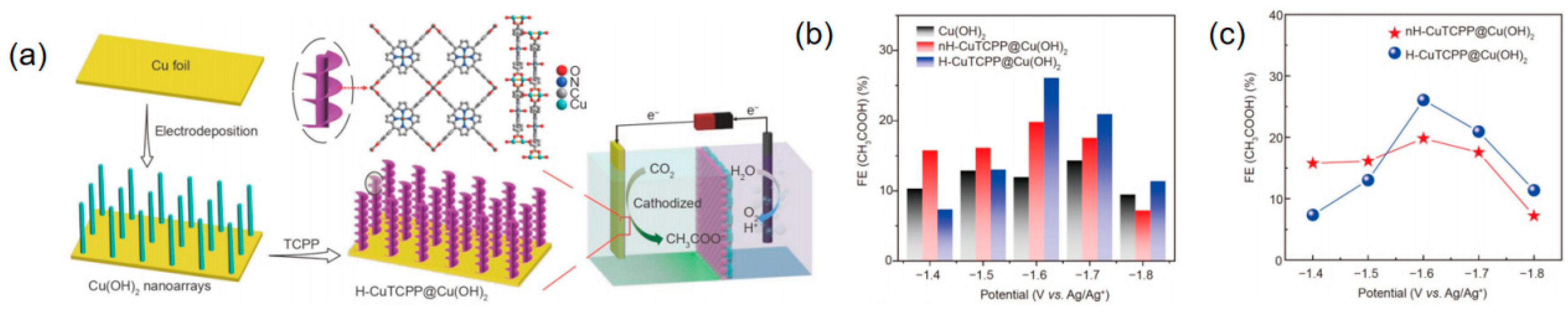
2.4. Change Local Environment
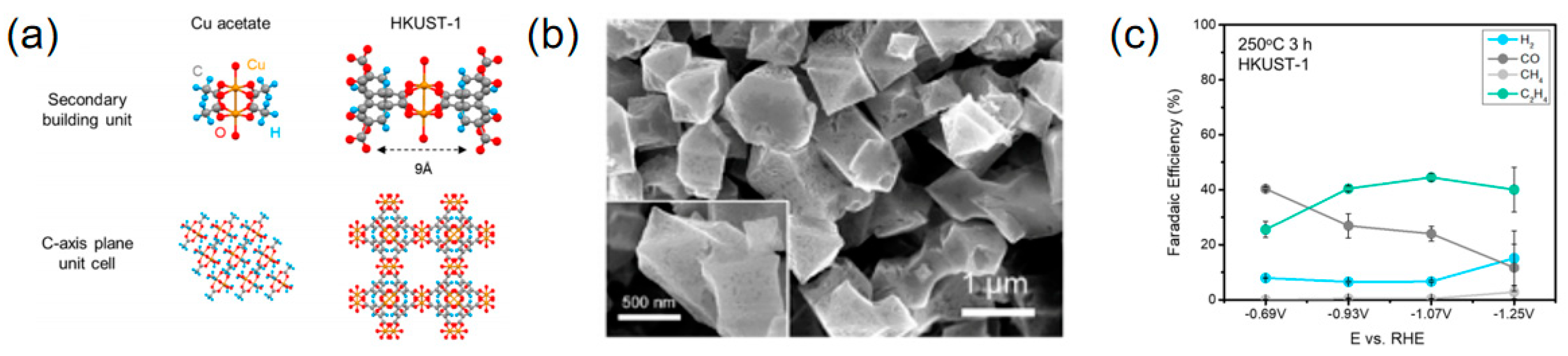
2.5. Adjusting and Controlling the Morphology
3. MOF-Derived Materials
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lian, Z.; Li, F.; He, X.; Chen, J.; Yu, R.C. Rising CO2 will increase toxicity of marine dinoflagellate Alexandrium minutum. J. Hazard. Mater. 2022, 431, 128627. [Google Scholar] [CrossRef] [PubMed]
- Kilner, C.L.; Carrell, A.A.; Wieczynski, D.J.; Votzke, S.; DeWitt, K.; Yammine, A.; Gibert, J.P. Temperature and CO2 interactively drive shifts in the compositional and functional structure of peatland protist communities. Glob. Chang. Biol. 2024, 30, e17203. [Google Scholar] [CrossRef] [PubMed]
- Albitar, K.; Borgi, H.; Khan, M.; Zahra, A. Business environmental innovation and CO2 emissions: The moderating role of environmental governance. Bus. Strategy Environ. 2023, 32, 1996–2007. [Google Scholar] [CrossRef]
- Özmen, İ.; Mutascu, M. Don’t look earth: Environmental taxes effect on CO2 emissions, evidence from moments quantile regression for EU countries. Environ. Dev. Sustain. 2023, 1–40. [Google Scholar] [CrossRef]
- Ajmal, S.; Kumar, A.; Tabish, M.; Selvaraj, M.; Alam, M.M.; Mushtaq, M.A.; Yasin, G. Modulating the microenvironment of single atom catalysts with tailored activity to benchmark the CO2 reduction. Mater. Today 2023, 67, 203–228. [Google Scholar] [CrossRef]
- Takagi, K.; Suzuki, N.; Hunge, Y.M.; Kuriyama, H.; Hayakawa, T.; Serizawa, I.; Terashima, C. Synergistic effect of Ag decorated in-liquid plasma treated titanium dioxide catalyst for efficient electrocatalytic CO2 reduction application. Sci. Total Environ. 2023, 902, 166018. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Lu, H.; Wang, L. How synthetic methods of single-atom electrocatalysts affect the catalytic performance of carbon dioxide reduction. Curr. Opin. Chem. Eng. 2023, 41, 100922. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Li, Y.; Li, C. The origins of catalytic selectivity for the electrochemical conversion of carbon dioxide to methanol. Nano Res. 2024, 17, 5–17. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, J.; Zhang, G.; Zhang, B.; He, Y. Electrochemical CO2-to-CO conversion: A comprehensive review of recent developments and emerging trends. Sep. Purif. Technol. 2024, 330, 125177. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, S.; Guo, Y.; Wang, H.; Wei, J.; Su, X.; Wang, J. Designing covalent organic frameworks with Co-O4 atomic sites for efficient CO2 photoreduction. Nat. Commun. 2023, 14, 1147. [Google Scholar] [CrossRef]
- Velty, A.; Corma, A. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 2023, 52, 1773–1946. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Mao, L.; Kang, X.; Dong, C.L.; Huang, Y.C.; Shen, S.; Guo, L. A high-cyano groups-content amorphous-crystalline carbon nitride isotype heterojunction photocatalyst for high-quantum-yield H2 production and enhanced CO2 reduction. Appl. Catal. B Environ. 2023, 331, 122733. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, R.; Lv, X.; Hu, Z.; Xia, D.; Li, C.; Liu, S. Modulating g-C3N4-based van der Waals heterostructures with spatially separated reductive centers for tandem photocatalytic CO2 methanation. Appl. Catal. B Environ. 2023, 330, 122666. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z. Electron regulation of single Indium atoms at the active oxygen vacancy of In2O3 (110) for production of acetic acid and acetone through direct coupling of CH4 with CO2. Chem. Asian J. 2022, 17, e202101383. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Yu, X.Y.; Kang, H.Z.; Zhang, H.X.; Gao, X.; Huang, Z.Q.; Chang, C.R. Design of Single-Atom and Frustrated-Lewis-Pair dual active sites for direct conversion of CH4 and CO2 to acetic acid. J. Catal. 2022, 408, 206–215. [Google Scholar] [CrossRef]
- Hou, S.L.; Dong, J.; Zhao, B. Formation of C-X Bonds in CO2 Chemical Fixation Catalyzed by Metal-Organic Frameworks. Adv. Mater. 2020, 32, 1806163. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling carbon dioxide through catalytic hydrogenation: Recent key developments and perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Tu, C.; Nie, X.; Chen, J.G. Insight into acetic acid synthesis from the reaction of CH4 and CO2. ACS Catal. 2021, 11, 3384–3401. [Google Scholar] [CrossRef]
- Bierbaumer, S.; Nattermann, M.; Schulz, L.; Zschoche, R.; Erb, T.J.; Winkler, C.K.; Glueck, S.M. Enzymatic conversion of CO2: From natural to artificial utilization. Chem. Rev. 2023, 123, 5702–5754. [Google Scholar] [CrossRef]
- Luan, L.; Ji, X.; Guo, B.; Cai, J.; Dong, W.; Huang, Y.; Zhang, S. Bioelectrocatalysis for CO2 reduction: Recent advances and challenges to develop a sustainable system for CO2 utilization. Biotechnol. Adv. 2023, 63, 108098. [Google Scholar] [CrossRef]
- Xiao, J.D.; Li, R.; Jiang, H.L. Metal–Organic Framework-Based Photocatalysis for Solar Fuel Production. Small Methods 2023, 7, 2201258. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Rabiee, H.; Ogunsakin, O.R.; Zhang, K. Metal-organic framework-derived transition metal chalcogenides (S, Se, and Te): Challenges, recent progress, and future directions in electrochemical energy storage and conversion systems. Coord. Chem. Rev. 2023, 480, 215030. [Google Scholar] [CrossRef]
- Zhang, L.; Nyahuma, F.M.; Zhang, H.; Cheng, C.; Zheng, J.; Wu, F.; Chen, L. Metal organic framework supported niobium pentoxide nanoparticles with exceptional catalytic effect on hydrogen storage behavior of MgH2. Green Energy Environ. 2023, 8, 589–600. [Google Scholar] [CrossRef]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Y.; Luan, B.; Wang, L.; Krishna, R.; Ni, H.; Zhang, Y. Benchmark single-step ethylene purification from ternary mixtures by a customized fluorinated anion-embedded MOF. Nat. Commun. 2023, 14, 401. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Yang, T.; Sridhar, D.; Algadi, H.; Xu, B.B.; Guo, Z. An overview of metal-organic frameworks and their magnetic composites for the removal of pollutants. Sep. Purif. Technol. 2023, 320, 124144. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Y.; Sun, Y.; Xian, J.; Long, Y.; Li, G. Ir nanoparticles anchored on metal-organic frameworks for efficient overall water splitting under pH-universal conditions. Angew. Chem. Int. Ed. 2023, 135, e202302220. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Eltaweil, A.S.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Omer, A.M. Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125 (Ti) composite: Process optimization, isotherms, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 49301–49313. [Google Scholar] [CrossRef]
- Jo, Y.M.; Jo, Y.K.; Lee, J.H.; Jang, H.W.; Hwang, I.S.; Yoo, D.J. MOF-based chemiresistive gas sensors: Toward new functionalities. Adv. Mater. 2023, 35, 2206842. [Google Scholar] [CrossRef]
- Liu, W.; Liu, C.; Wang, J.; Lv, J.; Lv, Y.; Yang, L.; Chu, P.K. Surface plasmon resonance sensor composed of microstructured optical fibers for monitoring of external and internal environments in biological and environmental sensing. Results Phys. 2023, 47, 106365. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Jiang, F.; Li, M.; Xu, Z. A dual-function [Ru(bpy)3]2+ encapsulated metal organic framework for ratiometric Al3+ detection and anticounterfeiting application. Dalton Trans. 2023, 52, 3846–3854. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Xu, K.; Zhong, Y.; He, C.; Jiang, L.; Xia, B.Y. Natural oxidase-mimicking copper-organic frameworks for targeted identification of ascorbate in sensitive sweat sensing. Nat. Commun. 2023, 14, 69. [Google Scholar] [CrossRef]
- Mohan, B.; Singh, G.; Chauhan, A.; Pombeiro, A.J.; Ren, P. Metal-organic frameworks (MOFs) based luminescent and electrochemical sensors for food contaminant detection. J. Hazard. Mater. 2023, 453, 131324. [Google Scholar] [CrossRef]
- Hussain, S.; Amu-Darko, J.N.O.; Wang, M.; Alothman, A.A.; Ouladsmane, M.; Aldossari, S.A.; Liu, G. CuO-decorated MOF derived ZnO polyhedral nanostructures for exceptional H2S gas detection. Chemosphere 2023, 317, 137827. [Google Scholar] [CrossRef]
- Fonseca, J.; Meng, L.; Imaz, I.; Maspoch, D. Self-assembly of colloidal metal–organic framework (MOF) particles. Chem. Soc. Rev. 2023, 52, 2528–2543. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Zhou, H.C.; Cheng, P.; Shi, W. Preparation and quantitative analysis of multicenter luminescence materials for sensing function. Nat. Protoc. 2023, 18, 1621–1640. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Yang, W.; Feng, X.; Wang, B. A rational design of functional porous frameworks for electrocatalytic CO2 reduction reaction. Chem. Soc. Rev. 2023, 52, 1382–1427. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xiong, H.; Zhang, Y.; Wu, K.; Liu, J.; Wang, J. Engineering pore environments of sulfate-pillared metal-organic framework for efficient C2H2/CO2 separation with record selectivity. Adv. Mater. 2023, 35, 2210415. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Patil, S.A.; Salunke, A.S.; Inamdar, A.I.; Im, H. Unprecedented urea oxidation on Zn@Ni-MOF with an ultra-high current density: Understanding the competition between UOR and OER, catalytic activity limitation and reaction selectivity. J. Mater. Chem. A 2023, 11, 14870–14877. [Google Scholar] [CrossRef]
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated bilayer MOF enables high-selectivity photocatalytic reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, L.; Liu, C.; Li, Z.; Zou, Y.; Zhang, X.; Yu, C. Crystal engineering enables cobalt-based metal–organic frameworks as high-performance electrocatalysts for H2O2 production. J. Am. Chem. Soc. 2023, 145, 7791–7799. [Google Scholar] [CrossRef]
- Zhao, D.; Li, W.; Wen, R.; Lei, N.; Li, W.; Liu, X.; Fan, L. Eu (III)-functionalized MOF-based dual-emission ratiometric sensor integrated with logic gate operation for efficient detection of hippuric acid in urine and serum. Inorg. Chem. 2023, 62, 2715–2725. [Google Scholar] [CrossRef]
- Wang, K.Y.; Zhang, J.; Hsu, Y.C.; Lin, H.; Han, Z.; Pang, J.; Zhou, H.C. Bioinspired framework catalysts: From enzyme immobilization to biomimetic catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liao, P.; Kang, J.; Liu, Q.; Wang, S.; Li, S.; Li, G. Locking effect in metal@MOF with superior stability for highly chemoselective catalysis. J. Am. Chem. Soc. 2023, 145, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, X.; Gu, Z.; Wu, G. MOF-derived Ni-Co bimetal/porous carbon composites as electromagnetic wave absorber. Adv. Compos. Hybrid Mater. 2023, 6, 28. [Google Scholar]
- Peng, Y.; Sanati, S.; Morsali, A.; García, H. Metal–organic frameworks as electrocatalysts. Angew. Chem. Int. Ed. 2023, 62, e202214707. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Wong, W.Y. Constructing 2D sandwich-like MOF/MXene heterostructures for durable and fast aqueous zinc-ion batteries. Angew. Chem. 2023, 135, e202218343. [Google Scholar] [CrossRef]
- Muthurasu, A.; Sampath, P.; Ko, T.H.; Lohani, P.C.; Pathak, I.; Acharya, D.; Kim, H.Y. Partial selenium surface modulation of metal organic framework assisted cobalt sulfide hollow spheres for high performance bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Appl. Catal. B Environ. 2023, 330, 122523. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, Q.; Liu, Y.; Zhang, Y.; Jia, Z.; Wu, G. Construction of self-assembly based tunable absorber: Lightweight, hydrophobic and self-cleaning properties. Nano-Micro Lett. 2023, 15, 137. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Li, X.; Duan, H.; Xie, S.; Dong, L.; Kang, F. Functional ultrathin separators proactively stabilizing zinc anodes for zinc-based energy storage. Adv. Mater. 2023, 35, 2300019. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Xuan, X.; Xia, W.; Feng, G.; Zhang, S.; Yamauchi, Y. Unlocking enhanced capacitive deionization of NaTi2(PO4)3/carbon materials by the yolk–shell design. J. Am. Chem. Soc. 2023, 145, 9242–9253. [Google Scholar] [CrossRef]
- Wei, T.; Lu, J.; Zhang, P.; Yang, G.; Sun, C.; Zhou, Y.; Tang, Y. Metal–organic framework-derived Co3O4 modified nickel foam-based dendrite-free anode for robust lithium metal batteries. Chin. Chem. Lett. 2023, 34, 107947. [Google Scholar] [CrossRef]
- Xu, X.; Eguchi, M.; Asakura, Y.; Pan, L.; Yamauchi, Y. Metal–organic framework derivatives for promoted capacitive deionization of oxygenated saline water. Energy Environ. Sci. 2023, 16, 1815–1820. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Hao, L.; Hong, S.; Robertson, A.W.; Sun, Z. Integration of ultrafine CuO nanoparticles with two-dimensional MOFs for enhanced electrochemical CO2 reduction to ethylene. Chin. J. Catal. 2022, 43, 1049–1057. [Google Scholar] [CrossRef]
- Yan, T.; Guo, J.H.; Liu, Z.Q.; Sun, W.Y. Metalloporphyrin encapsulation for enhanced conversion of CO2 to C2H4. ACS Appl. Mater. Interfaces 2021, 13, 25937–25945. [Google Scholar] [CrossRef]
- Yan, T.; Wang, P.; Sun, W.Y. Single-Site Metal–Organic Framework and Copper Foil Tandem Catalyst for Highly Selective CO2 Electroreduction to C2H4. Small 2023, 19, 2206070. [Google Scholar] [CrossRef]
- Liu, J.; Peng, L.; Zhou, Y.; Lv, L.; Fu, J.; Lin, J.; Qiao, J. Metal–Organic-Frameworks-Derived Cu/Cu2O catalyst with ultrahigh current density for continuous-flow CO2 electroreduction. ACS Sustain. Chem. Eng. 2019, 7, 15739–15746. [Google Scholar] [CrossRef]
- Mohammadi, T.; Hosseini, M.G.; Ashassi-Sorkhabi, H.; Sefidi, P.Y. Investigating performance of flower-like CoCu-MOF supported on carbon felt as a binder-free anode electrode in direct ethanol fuel cell. Synth. Met. 2023, 298, 117443. [Google Scholar] [CrossRef]
- Zou, X.; Li, A.; Ma, C.; Gao, Z.; Zhou, B.; Zhu, L.; Huang, Z. Nitrogen-doped carbon confined Cu-Ag bimetals for efficient electroreduction of CO2 to high-order products. Chem. Eng. J. 2023, 468, 143606. [Google Scholar] [CrossRef]
- Xu, H. Fundamental Study of Supported Metal Catalysts Prepared by Metal in Lithium Solution for Energy Science. Ph.D. Dissertation, Northern Illinois University, DeKalb, IL, USA, 2020. [Google Scholar]
- Jin, P.; Wang, Q.; Gu, X.; Huang, L.; Qin, W.; Chong, Y.; Lin, X. MOFs derived N doped CuNix@C dual metallic core-shell electrocatalysts for CO2 electrocatalytic reduction. Int. J. Electrochem. Sci. 2024, 19, 100427. [Google Scholar] [CrossRef]
- Fernández-Caso, K.; Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Electroreduction of CO2: Advances in the continuous production of formic acid and formate. ACS Energy Lett. 2023, 8, 1992–2024. [Google Scholar] [CrossRef]
- Jiang, Y.; Shan, J.; Wang, P.; Huang, L.; Zheng, Y.; Qiao, S.Z. Stabilizing oxidation state of SnO2 for highly selective CO2 electroreduction to formate at large current densities. ACS Catal. 2023, 13, 3101–3108. [Google Scholar] [CrossRef]
- Fan, Z.; Luo, R.; Zhang, Y.; Zhang, B.; Zhai, P.; Zhang, Y.; Hou, J. Oxygen-bridged indium-nickel atomic pair as dual-metal active sites enabling synergistic electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2023, 62, e202216326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, P.; Yuan, T.; Cheng, D.; Zhen, S.; Gao, H.; Gong, J. Modulation of *CHxO adsorption to facilitate electrocatalytic reduction of CO2 to CH4 over Cu-based catalysts. J. Am. Chem. Soc. 2023, 145, 6622–6627. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, S.; Liu, Q.; Qiu, Y.; Luo, J.; Liu, X. Oxygen vacancy-rich amorphous copper oxide enables highly selective electroreduction of carbon dioxide to ethylene. Acta Phys.-Chim. Sin. 2023, 39, 202207026. [Google Scholar] [CrossRef]
- Xia, W.; Xie, Y.; Jia, S.; Han, S.; Qi, R.; Chen, T.; Han, B. Adjacent copper single atoms promote C–C coupling in electrochemical CO2 reduction for the efficient conversion of ethanol. J. Am. Chem. Soc. 2023, 145, 17253–17264. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, Y.; Sun, F.; Wang, S.; Kang, Y.; Xu, X.; Li, H. Nanoarchitectonics of metallene materials for electrocatalysis. ACS Nano 2023, 17, 13017–13043. [Google Scholar] [CrossRef]
- Zhang, H.; Diao, J.; Ouyang, M.; Yadegari, H.; Mao, M.; Wang, M.; Riley, D.J. Heterostructured core–shell Ni–Co@Fe–Co nanoboxes of prussian blue analogues for efficient electrocatalytic hydrogen evolution from alkaline seawater. ACS Catal. 2023, 13, 1349–1358. [Google Scholar] [CrossRef]
- Lv, L.; Lu, R.; Zhu, J.; Yu, R.; Zhang, W.; Cui, E.; Mai, L. Coordinating the edge defects of bismuth with sulfur for enhanced CO2 electroreduction to formate. Angew. Chem. 2023, 135, e202303117. [Google Scholar] [CrossRef]
- Nie, W.; Heim, G.P.; Watkins, N.B.; Agapie, T.; Peters, J.C. Organic Additive-derived Films on Cu Electrodes Promote Electrochemical CO2 Reduction to C2+ Products Under Strongly Acidic Conditions. Angew. Chem. Int. Ed. 2023, 62, e202216102. [Google Scholar] [CrossRef]
- Moss, A.B.; Garg, S.; Mirolo, M.; Rodriguez, C.A.G.; Ilvonen, R.; Chorkendorff, I.; Seger, B. In operando investigations of oscillatory water and carbonate effects in MEA-based CO2 electrolysis devices. Joule 2023, 7, 350–365. [Google Scholar] [CrossRef]
- Shang, H.; Liu, D. Atomic design of carbon-based dual-metal site catalysts for energy applications. Nano Res. 2023, 16, 6477–6506. [Google Scholar] [CrossRef]
- Garg, S.; Xu, Q.; Moss, A.B.; Mirolo, M.; Deng, W.; Chorkendorff, I.; Seger, B. How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers. Energy Environ. Sci. 2023, 16, 1631–1643. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Dou, S. Key roles of interfacial OH– ion distribution on proton coupled electron transfer kinetics toward urea oxidation reaction. Small 2023, 19, 2302151. [Google Scholar] [CrossRef]
- Wu, H.; Singh-Morgan, A.; Qi, K.; Zeng, Z.; Mougel, V.; Voiry, D. Electrocatalyst microenvironment engineering for enhanced product selectivity in carbon dioxide and nitrogen reduction reactions. ACS Catal. 2023, 13, 5375–5396. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Tian, H.; Bian, L.; Liu, S.Z.; Liu, Y.; Wang, Z.L. Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products. J. Energy Chem. 2023, 83, 90–97. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhao, X.; Chen, S.; Nian, Q.; Luo, X.; Ren, X. Localized alkaline environment via in situ electrostatic confinement for enhanced CO2-to-ethylene conversion in neutral medium. J. Am. Chem. Soc. 2023, 145, 6339–6348. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, Z.; Wang, W.; Zhang, C.; Fu, L.; Zhu, Q.; Zhang, D. Nickel phosphide clusters sensitized TiO2 nanotube arrays as highly efficient photoanode for photoelectrocatalytic urea oxidation. Adv. Funct. Mater. 2023, 33, 2211169. [Google Scholar] [CrossRef]
- Yang, P.P.; Zhang, X.L.; Liu, P.; Kelly, D.J.; Niu, Z.Z.; Kong, Y.; Gao, M.R. Highly enhanced chloride adsorption mediates efficient neutral CO2 electroreduction over a dual-phase copper catalyst. J. Am. Chem. Soc. 2023, 145, 8714–8725. [Google Scholar] [CrossRef]
- Lin, X.; Chen, L.; Zhong, X.; BaQais, A.; Dang, W.; Amin, M.A.; Yang, Z. N-doped bimetallic phosphides composite catalysts derived from metal–organic frameworks for electrocatalytic water splitting. Adv. Compos. Hybrid Mater. 2023, 6, 79. [Google Scholar] [CrossRef]
- Yan, X.; Wang, X.; Han, J.; Du, X.; Liu, Z.; Yang, Y. A collaborative diffusion mechanism of multiple atoms during Cu–Ag bimetal surface reconstruction. Phys. Chem. Chem. Phys. 2023, 25, 10405–10416. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, X.; Gao, S.; Chen, F.; Lang, X.; Zhang, T.; Ma, G. Collaborative coupling catalytic interface enabling efficient hydrogen evolution in universal-pH electrolytes and seawater. Int. J. Hydrog. Energy 2024, 49, 1625–1632. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Huang, J.R.; Liao, P.Q.; Chen, X.M. Highly efficient electroreduction of CO2 to ethanol via asymmetric C–C coupling by a metal–organic framework with heterodimetal dual sites. J. Am. Chem. Soc. 2023, 145, 26783–26790. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Z.; Gao, G.; Chen, C.; Xue, Y.; Zhao, J.; Lu, X. Enhanced CO2 Electroreduction Selectivity toward Ethylene on Pyrazolate-Stabilized Asymmetric Ni–Cu Hybrid Sites. J. Am. Chem. Soc. 2023, 145, 26444–26451. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Bimetallic sites for catalysis: From binuclear metal sites to bimetallic nanoclusters and nanoparticles. Chem. Rev. 2023, 123, 4855–4933. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Zhu, J.; Ye, C.; Mao, Y.; Wang, B.; Wang, D. Charge-Separated Pdδ––Cuδ+ Atom Pairs Promote CO2 Reduction to C2. Nano Lett. 2023, 23, 2312–2320. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, X.; Feng, Y.; Zhang, H.; Zhang, G. Self-Polarization Triggered Multiple Polar Units Toward Electrochemical Reduction of CO2 to Ethanol with High Selectivity. Angew. Chem. 2023, 135, e202302241. [Google Scholar] [CrossRef]
- Zhu, H.L.; Chen, H.Y.; Han, Y.X.; Zhao, Z.H.; Liao, P.Q.; Chen, X.M. A porous π–π stacking framework with dicopper (I) sites and adjacent proton relays for electroreduction of CO2 to C2+ products. J. Am. Chem. Soc. 2022, 144, 13319–13326. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Y.; Chen, S.; Li, M.; Zheng, M.; Ma, L.; Duan, J. Less-Coordinated Atomic Copper-Dimer Boosted Carbon–Carbon Coupling During Electrochemical CO2 Reduction. Small 2023, 19, 2301536. [Google Scholar] [CrossRef]
- Wen, C.F.; Zhou, M.; Liu, P.F.; Liu, Y.; Wu, X.; Mao, F.; Yang, H.G. Highly Ethylene-Selective Electrocatalytic CO2 Reduction Enabled by Isolated Cu–S Motifs in Metal–Organic Framework Based Precatalysts. Angew. Chem. Int. Ed. 2022, 61, e202111700. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Ye, J.; Liu, L.; Li, L.; Li, Y.; Huang, X. Highly Selective CO2 Electroreduction to C2+ Products over Cu2O-Decorated 2D Metal–Organic Frameworks with Rich Heterogeneous Interfaces. Nano Lett. 2023, 23, 1474–1480. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, W.H.; Wang, Y.R.; Shen, F.C.; Lan, Y.Q. Tailoring the Hydrophobic Interface of Core–Shell HKUST-1@Cu2O Nanocomposites for Efficiently Selective CO2 Electroreduction. Small 2024, 20, 2307467. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Shekhah, O.; Ozden, A.; McCallum, C.; Li, F.; Wang, X.; Sargent, E.H. High-Rate and Selective CO2 Electrolysis to Ethylene via Metal–Organic-Framework-Augmented CO2 Availability. Adv. Mater. 2022, 34, 2207088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Zhu, H.L.; Huang, J.R.; Liao, P.Q.; Chen, X.M. Polydopamine coating of a metal–organic framework with Bi-copper sites for highly selective electroreduction of CO2 to C2+ products. ACS Catal. 2022, 12, 7986–7993. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Yu, S.; Louisia, S.; Jin, J.; Li, M.; Yang, P. Cu-Ag tandem catalysts for high-rate CO2 electrolysis toward multicarbons. Joule 2020, 4, 1688–1699. [Google Scholar] [CrossRef]
- Rashid, N.; Dar, F.A.; Bhat, M.A.; Ingole, P.P. Tailoring Motif and Channel Terminating Groups of Conventional Copper MOFs for Their Enhanced Activity, Selectivity, and Stability toward the Electroreduction of CO2 to Hydrocarbons. ACS Appl. Energy Mater. 2023, 6, 1378–1388. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, W.; Wang, J.; Li, X.; Li, Y.; Hu, X.; He, Y. High piezo/photocatalytic efficiency of Ag/Bi5O7I nanocomposite using mechanical and solar energy for N2 fixation and methyl orange degradation. Green Energy Environ. 2023, 8, 283–295. [Google Scholar] [CrossRef]
- Zhao, C.; Cai, L.; Wang, K.; Li, B.; Yuan, S.; Zeng, Z.; He, Y. Novel Bi2WO6/ZnSnO3 heterojunction for the ultrasonic-vibration-driven piezocatalytic degradation of RhB. Environ. Pollut. 2023, 319, 120982. [Google Scholar] [CrossRef]
- Lin, X.; Wu, H.; Zeng, S.; Peng, T.; Zhang, P.; Wan, X.; Yin, B. A self-designed device integrated with a Fermat spiral microfluidic chip for ratiometric and automated point-of-care testing of anthrax biomarker in real samples. Biosens. Bioelectron. 2023, 230, 115283. [Google Scholar] [CrossRef]
- Duan, X.; Xiao, J.; Zhou, A.; Wang, S.; Wen, J. Numerical investigation on flow and heat transfer performance of non-Newtonian Bingham fluid in novel spiral wound tube heat exchangers. Case Stud. Therm. Eng. 2023, 49, 103352. [Google Scholar] [CrossRef]
- Zhao, S.; Nie, Y.; Zhang, W.; Hu, R.; Sheng, L.; He, W.; Guo, K. Microfluidic field strategy for enhancement and scale up of liquid–liquid homogeneous chemical processes by optimization of 3D spiral baffle structure. Chin. J. Chem. Eng. 2023, 56, 255–265. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Zhang, Y.X.; Zhai, R.; Gu, Z.G.; Zhang, J. Helical copper-porphyrinic framework nanoarrays for highly efficient CO2 electroreduction. Sci. China Mater. 2022, 65, 1269–1275. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, Y.; Geng, S.; Peng, Z.; Yu, J.; Fan, Z. Simultaneous defect and size control of metal–organic framework nanostructures for highly efficient carbon dioxide electroreduction to multicarbon products. ACS Mater. Lett. 2023, 5, 2121–2130. [Google Scholar] [CrossRef]
- Nam, D.H.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Dinh, C.T.; Sargent, E.H. Metal–organic frameworks mediate Cu coordination for selective CO2 electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Yang, Y.; Zhang, Q.; Yang, M.; Zou, Q.; Du, J.; Liu, Z. The enhanced local CO concentration for efficient CO2 electrolysis towards C2 products on tandem active sites. Chem. Eng. J. 2022, 450, 138009. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.D.; Huang, J.M.; Guan, M.X.; Xu, M.; Gu, Z.Y. In situ reconstruction of Cu–N coordinated MOFs to generate dispersive Cu/Cu2O nanoclusters for selective electroreduction of CO2 to C2H4. ACS Catal. 2022, 12, 15230–15240. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, L.; Liu, J.; Wang, Y.; Ge, W.; Xiao, C.; Li, C. Toward High-Performance CO2-to-C2 Electroreduction via Linker Tuning on MOF-Derived Catalysts. Small 2022, 18, 2200720. [Google Scholar] [CrossRef]
- Ma, H.; Wang, F.; Shen, M.; Tong, Y.; Wang, H.; Hu, H. Advances of LiCoO2 in Cathode of Aqueous Lithium-Ion Batteries. Small Methods 2024, 8, 2300820. [Google Scholar] [CrossRef]
- Ma, H.; Tong, Y.; Wang, X.; Wang, H. Advancements and assessment of compressed carbon dioxide energy storage technologies: A comprehensive review. RSC Sustain. 2024, 2, 2731–2750. [Google Scholar] [CrossRef]
- Gholampour, N.; Ezugwu, C.I.; Younus, H.A.; Debecker, D.P.; Al Abri, M.; Al hajri, R.; Kao, C.; Verpoort, F. Recent Trends in CO2 Electroreduction over Metal–Organic Framework–Derived Materials: A Comprehensive Review. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Y.; Han, L.; Zhang, J.; Liu, Y.; Baeyens, J.; Lv, Y. Structure–performance relationships in MOF–derived electrocatalysts for CO2 reduction. Prog. Energy Combust. Sci. 2024, 104, 101175. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, J.; Wang, P.; Jin, H.; Zheng, Y.; Qiao, S.-Z. Recent Advances in Electrocatalytic Hydrogenation Reactions on Copper-Based Catalysts. Adv. Mater. 2024, 36, 2307913. [Google Scholar] [CrossRef] [PubMed]


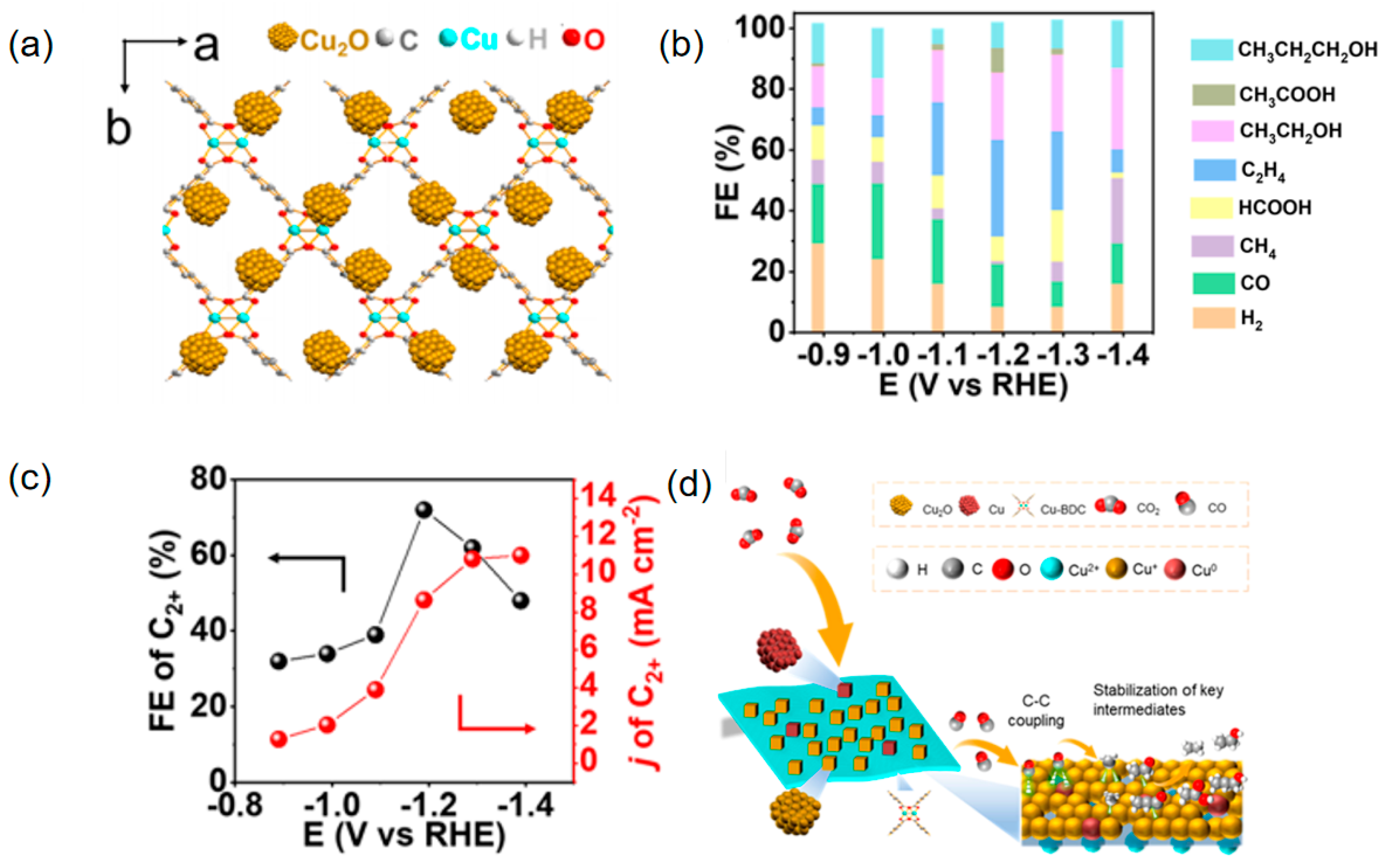
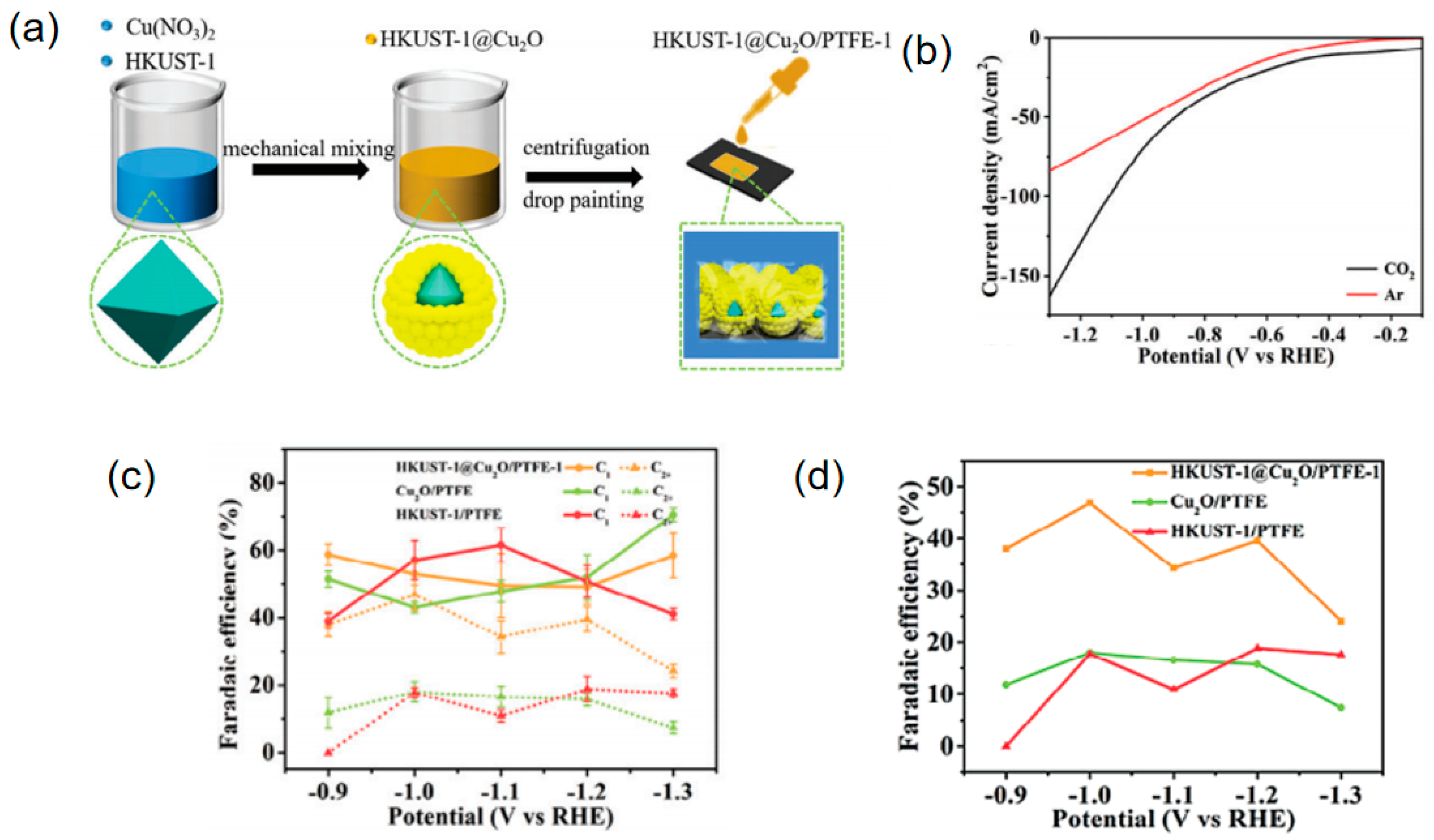
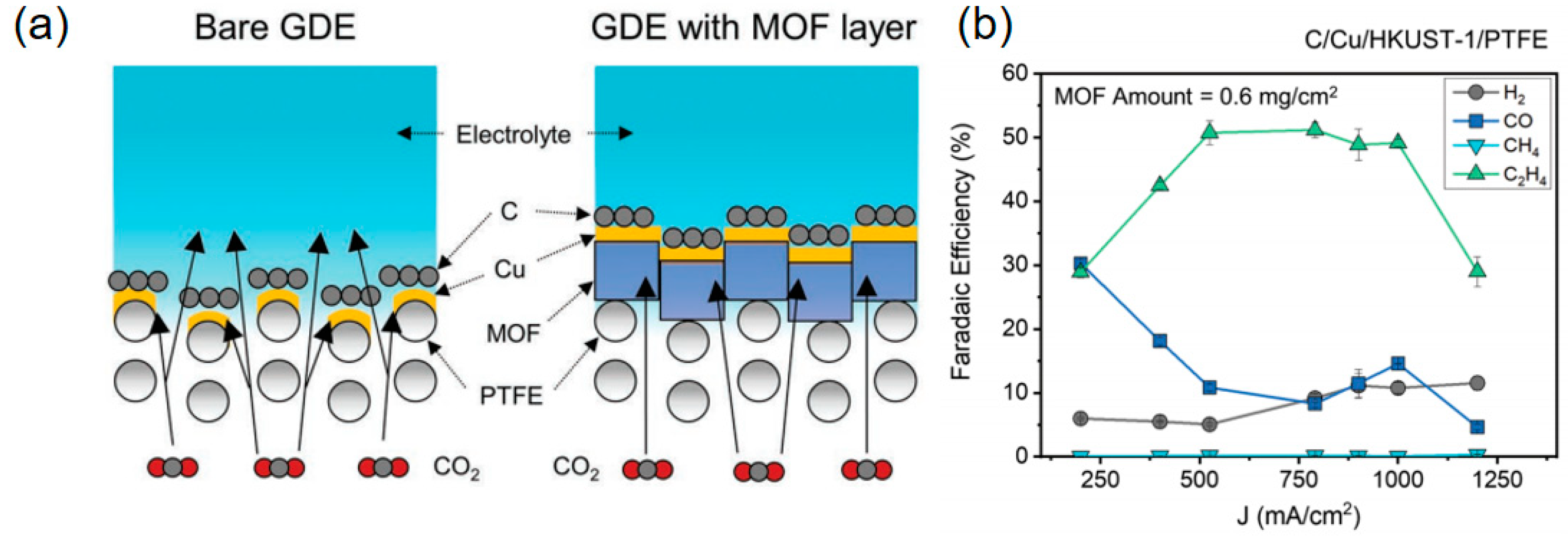
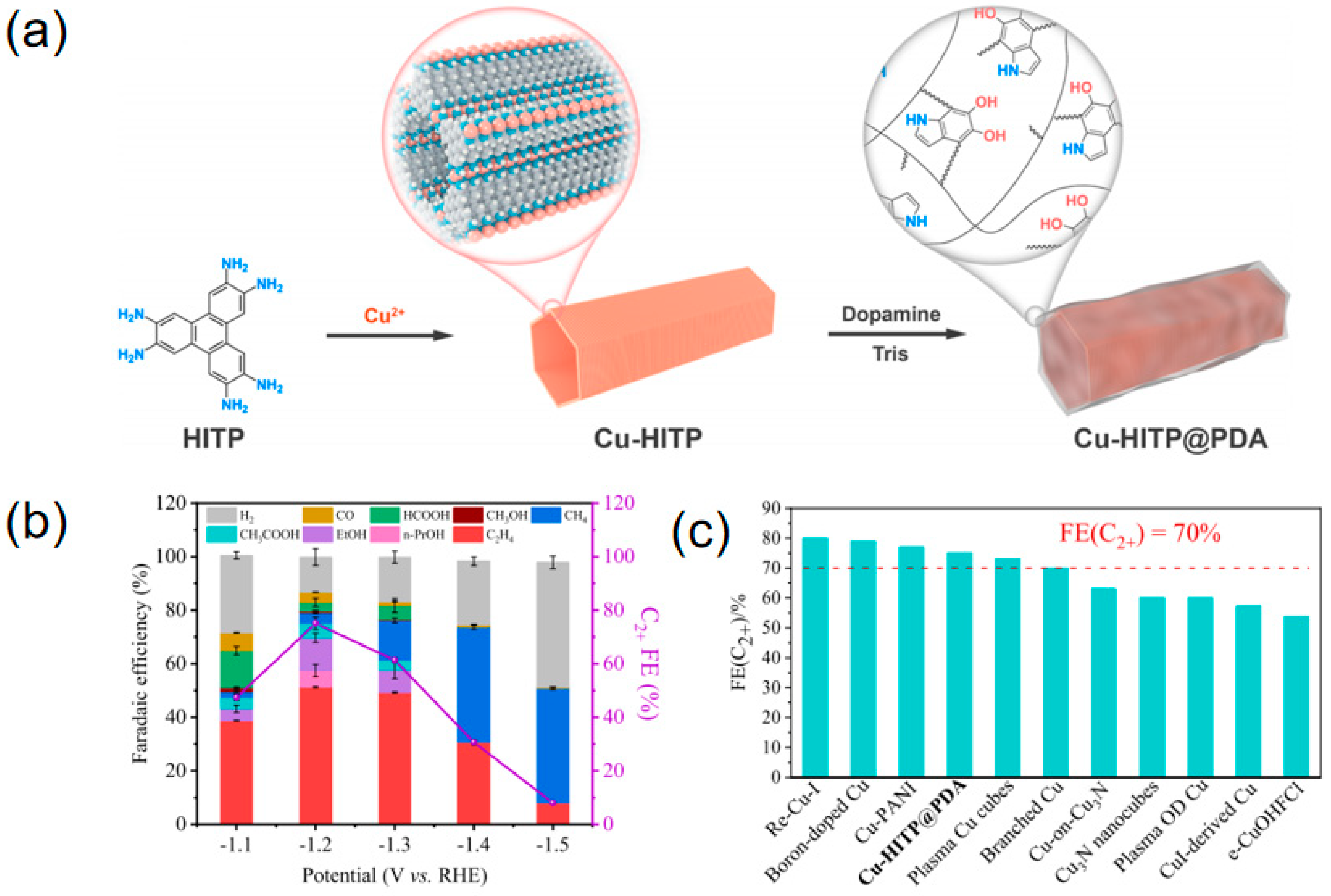



| Materials | Main Products | Electrolytes | FE (%) | Current Density (mA cm−2) | Refs |
|---|---|---|---|---|---|
| CuSn-HAB | CH3CH2OH | 1 M KOH | 56(2) | 68 | [84] |
| Cu1Ni-BDP | C2H4 | 1.0 M KOH | 52.7 | 530 | [85] |
| Cu2O@MOF/CF | CH3CH2OH | 0.5 M KHCO3 | 44.3 | >5 | [88] |
| CuBtz | C2+ | 0.1 M KHCO3 | 73.7 | 7.9 | [89] |
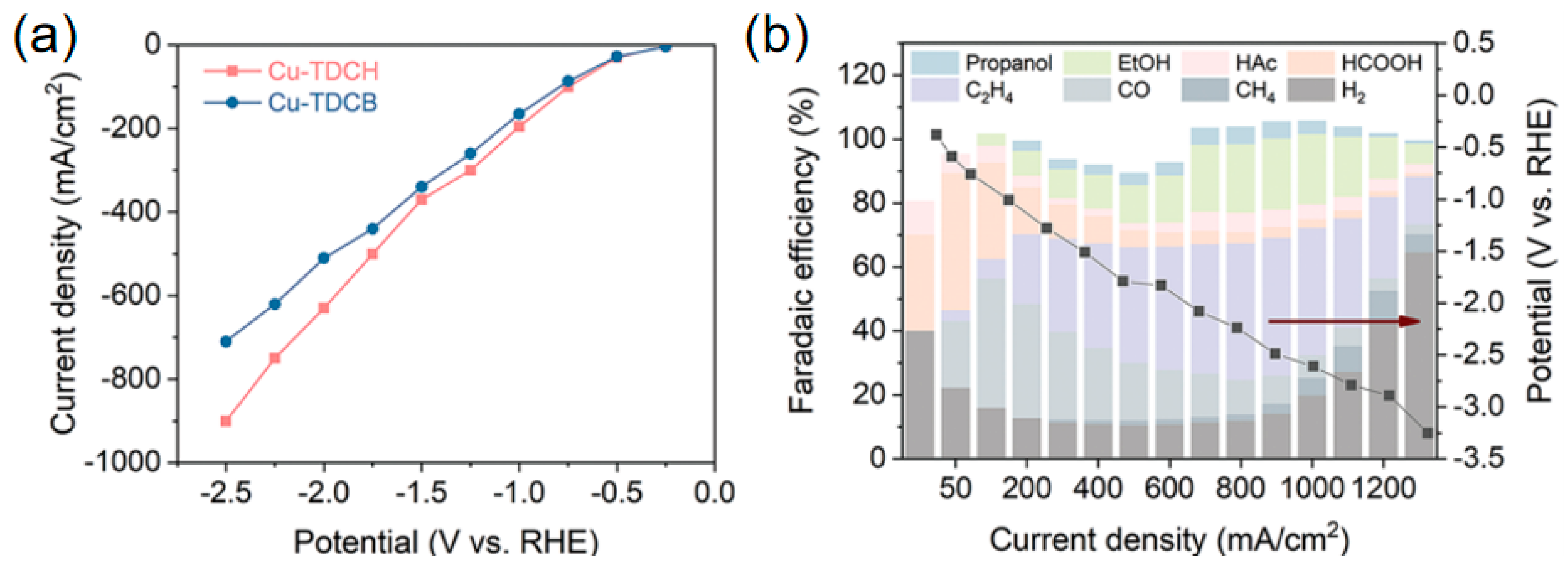
| Cu-TDCH | C2+ | 1 M KOH | 71 | 900 | [90] |
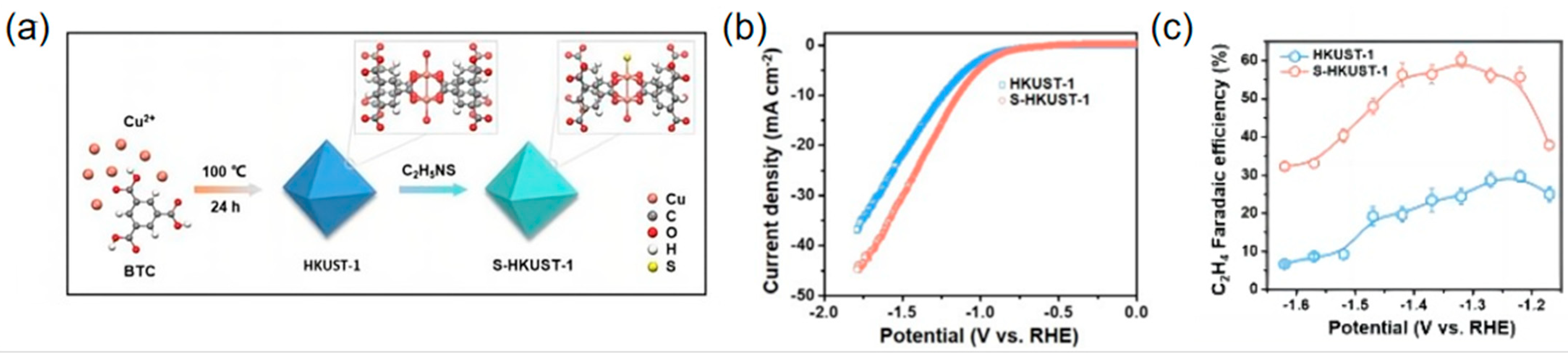
| S-HKUST-1 | C2H4 | 1.0 M KOH | 60 | 400 | [91] |
| Cu2O@Cu-BDC | C2+ | 0.1 M KBr | 72.1 | 11.1 | [92] |
| HKUST-1@Cu2O/PTFE-1 | C2H4 | 1 M KOH | 46.08 | 70.13 | [93] |
| C/Cu/HKUST-1/PTFE | C2H4 | 0.1 M KHCO3 | 56 | 400 | [94] |
| Cu-HITP@PDA | C2H4 | 0.1 M KHCO3 | 51 | 5.2 | [95] |
| Cu500Ag1000 | CH3CH2OH+ C2H4 | 1M KOH | \ | 160 | [96] |
| CuBDC-amide-MOF | C2H4 | 0.2 M KHCO3 | 18.90 | ~140 | [97] |
| Cu-MOF-CF | C2H4 | 0.1 M KHCO33 | 48.6 | ~8 | [56] |
| H-CuTCPP@Cu(OH)2 | CH3COOH | 0.5 mol L−1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4, 98%, Energy Chemical), acetonitrile (MeCN) solution, and 1 mol L−1 H2O | 26.1 | ~10 | [103] |
| CuTrz-109 nm | C2H4/C2+ | 0.1 M KHCO3 | 55.4%/81.8% | 11.7 | [104] |
| Materials | Main Products | Electrolytes | FE (%) | Current Density (mA cm−2) | Refs |
|---|---|---|---|---|---|
| 250 °C 3 h HKUST-1 | C2H4 | 1 M KOH | 45 | 262 | [105] |
| Co3O4-CuOx/C(M-CuCo/C) | C2+ | 0.1 M KHCO3 | 79.2 | 19.28 | [106] |
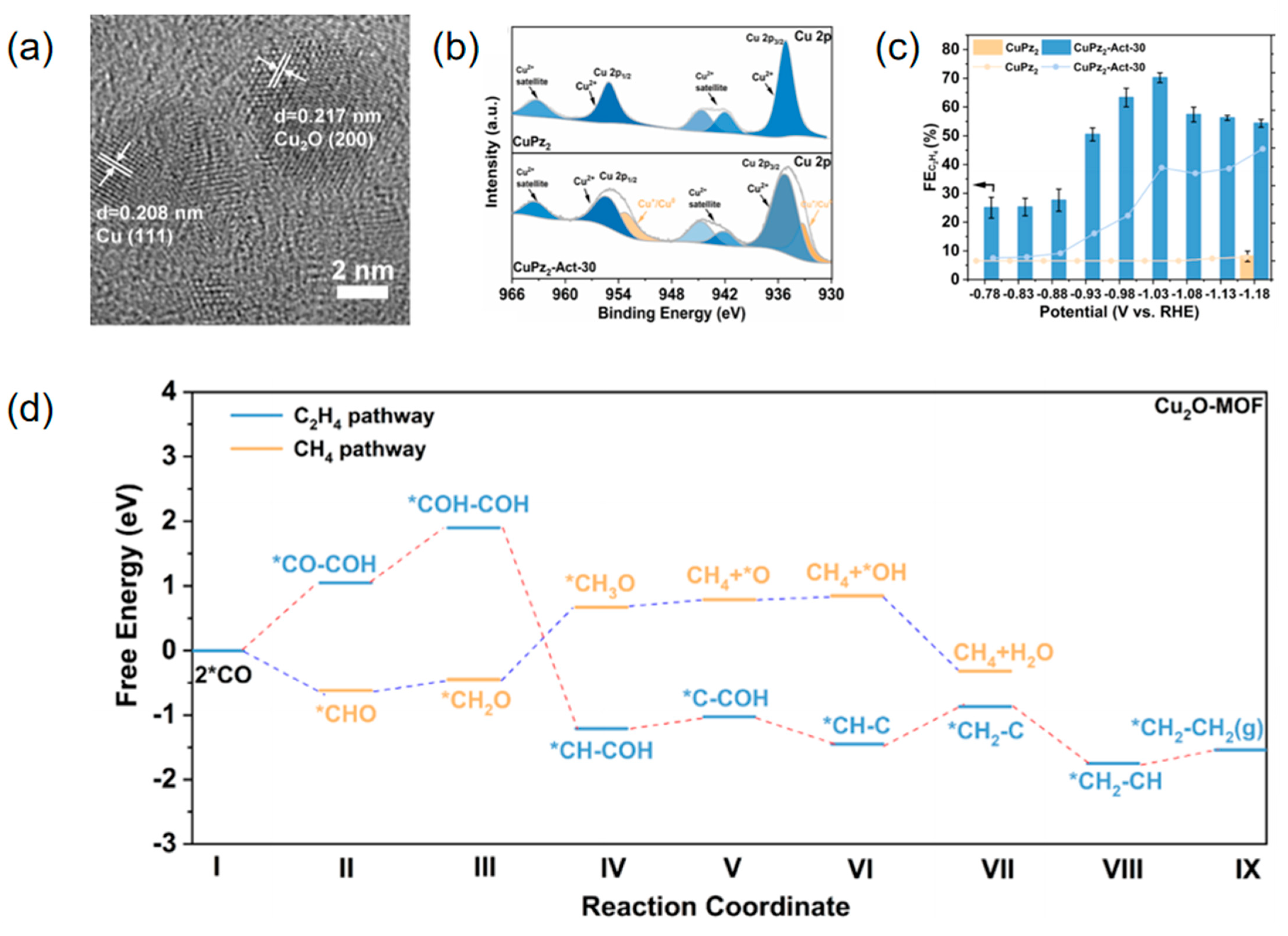
| Cupz2- act-30 | C2H4 | 0.1 M KCl | 70.2 ± 1.7 | 12.38 | [107] |
| 2F-Cu-BDC | C2+ | 1 M KOH | 63 | 150 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Ma, H.; Zhao, S. Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review. Processes 2024, 12, 2205. https://doi.org/10.3390/pr12102205
Fu H, Ma H, Zhao S. Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review. Processes. 2024; 12(10):2205. https://doi.org/10.3390/pr12102205
Chicago/Turabian StyleFu, Hongxin, Hailing Ma, and Shuaifei Zhao. 2024. "Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review" Processes 12, no. 10: 2205. https://doi.org/10.3390/pr12102205
APA StyleFu, H., Ma, H., & Zhao, S. (2024). Structural Control of Copper-Based MOF Catalysts for Electroreduction of CO2: A Review. Processes, 12(10), 2205. https://doi.org/10.3390/pr12102205







