Abstract
Welding current is an essential parameter for submerged arc welding process. In submerged arc welding, the enormous heat generated by the current promotes the decomposition of the oxides in the flux, releasing oxygen and increasing the oxygen level in the metal, which further affects the microstructure and mechanical properties of the weld joint. Although previous studies have developed various models to evaluate oxygen content, the thermodynamic mechanism by which current influences oxygen levels in metal remains inadequately understood. This study integrates CALPHAD technology with welding thermodynamics to predict and simulate the impact of the welding current on oxygen content in metals. By combining experimental data with thermodynamic modeling, the research investigates how different current settings affect oxygen content in the metal across various welding zones, specifically when using CaO-Al2O3 fluxes with low and high basicity indices for the welding of typical carbon steel. This study selected two current values, 300 A and 600 A, for modeling analyses of the welding process, along with two typical fluxes with basicity indices of 1.6 and 0.4. The results indicate that the proposed method outperforms the BI model and can predict the metallurgical effects of current on oxygen content in the droplet and molten pool zones. The thermodynamic mechanisms that govern the metal oxygen level are also evaluated. These findings aim to enhance the understanding of the thermodynamic mechanism that governs oxygen behavior under different current conditions, thereby contributing to the optimization of submerged arc welding process.
1. Introduction
In thick steel plate welding, submerged arc welding (SAW) is notable for its efficiency and high-quality metal joining [1,2]. Such a welding method operates under a flux layer that conceals the arc and weld pool, thereby preventing metal exposure to air and minimizing oxidation and/or contamination [3,4]. SAW is particularly advantageous for applications requiring high deposition rates, making it ideal for heavy industry sectors such as shipbuilding, bridge construction, and pressure vessel fabrication [5].
Current is an essential process parameter for SAW operations [6]. Aside from influencing the morphology of the weld metal (WM), the current affects the reaction temperature of the entire SAW metallurgical system, which in turn determines the composition, microstructure, and mechanical properties of the weldment [7].
For SAW, controlling metal composition is vital to ensuring the quality of the weldment, since the metal composition fundamentally determines the service performance of the WM [6,8]. SAW is an extremely complex metallurgical system, involving high temperatures and multiple phases, making composition control a persistent technical challenge [1,3]. Furthermore, to accommodate different welding conditions, SAW must be performed under various current settings, further complicating the challenge of composition control for the WM [9].
Oxygen (O) is the most essential factor influencing the microstructure and properties of the submerged arc welded metal [6]. Unlike other arc welding methods, the high-temperature arc plasma in SAW causes the oxides in the flux to decompose, releasing O2 and substantially increasing the O content in the metal [10,11]. Excessive O content may lead to reduced toughness and hardenability. Conversely, a WM with low O levels often has poorer mechanical properties, especially toughness, due to the absence of inclusions that promote the formation of acicular ferrite (AF)—a microstructure that effectively inhibits crack propagation through cleavage [12]. As such, a considerable amount of research has been undertaken to elucidate the transfer of O in the SAW process induced by changes of welding current [1,6].
Initially, Mitra et al. [13,14,15] proposed a metallurgical model for the SAW process, suggesting that welding current is an important factor influencing the temperature of chemical reactions and the behavior of element transfer in SAW. Subsequently, Lau et al. [10,16] measured the O content in the metal under different welding currents, identifying both the welding current and the O potential of the flux as significant factors affecting O transfer behavior. Further, several regression models have been developed to explore the impact of the welding current on the transfer behavior of O in the SAW process [17].
In fact, SAW is an enclosed black-box system involving flux (slag), metal, arc plasma, and various generated gases [1]. During this process, flux particles cover the molten pool, slag, and arc plasma, which restricts researchers to qualitative analyses of the physical and chemical reactions through simulations [6].
Achieving thermodynamic equilibrium in SAW processes is generally unlikely due to significant temperature gradients, the presence of multiple phases, and considerable radiative energy transfers [3,18,19]. Nevertheless, equilibrium considerations can provide critical constraints on the chemical reactions involved [6,8]. A prevalent method is to assume local thermodynamic equilibrium, predicated on the notion that high temperatures and high surface-to-volume ratios can compensate for the brief reaction times available [1,20]. Based on these hypotheses, models like the basicity index (BI) model, slag–metal equilibrium model, and gas–slag–metal equilibrium model have been developed to predict the O transfer behavior subject to submerged arc welding process [6]. Recently, the cross-zone model has been developed to simulate the variation roadmap of O content during the SAW process [20]. This model takes into account the significant O uptake in the droplet reaction zone, providing a more comprehensive understanding of O behavior in the total SAW process.
However, no metallurgical model has yet been proposed to analyze and simulate the mechanism by which welding current affects the transfer behavior of O, despite the fact that current is an essential factor in determining the temperature of chemical reactions in SAW [1,6]. Traditionally, models used to predict the O content in the SAW process include the BI model and the (gas–)slag–metal equilibrium model [6]. The BI model is a typical empirical model based on steelmaking theory and assumes that flux is the primary factor determining the O level in the metal [6]. However, the BI model is essentially a regression model and does not account for the thermodynamic factors influencing flux O potential [21]. Subsequently, the (gas–)slag–metal equilibrium model was developed based on the scientific hypothesis of local thermodynamic equilibrium in the weld pool reaction zone [6]. Nevertheless, the equilibrium model does not consider the significant O uptake in the droplet zone of SAW. To address this issue, the cross-zone thermodynamic model has been proposed based upon the rich O layer theory and CALPHAD technology [20]. The present study aims to simulate and evaluate the impact of the welding current on the transfer behavior of O in the SAW process via the cross-zone thermodynamic model and the “effective equilibrium temperature” (EET) database. This study will focus on typical carbon steel submerged arc welding, using CaO-Al2O3 fluxes with both high and low basicity, to gain a deeper understanding of the thermodynamic mechanisms by which current affects the O content in the metal subject to the SAW process.
2. Simulation and Modeling
2.1. Models and Validation Data
Figure 1 illustrates the mechanism diagrams of the cross-zone model and the BI model. The cross-zone model is based on the theory of local thermodynamic equilibrium (Figure 1c,d) while also considering O enrichment in the droplet zone (Figure 1b). On the other hand, the BI model primarily takes into account the basicity index of the flux and uses the BI to predict the O potential of the flux (Figure 1e).
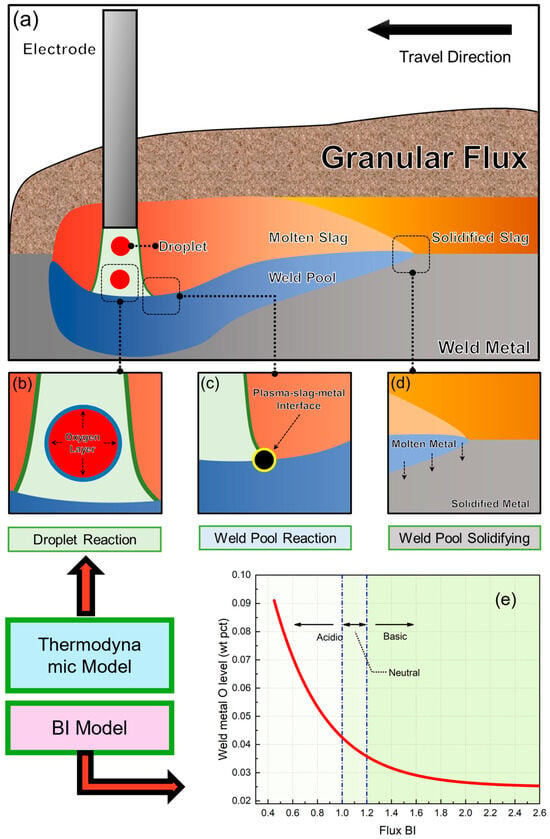
Figure 1.
Model mechanism diagram [20,22]. (a) Comprehensive schematic diagram of SAW process, (b) droplet interaction zone, (c) weld pool interaction zone, (d) weld pool solidification zone, and (e) illustration of BI model.
The experimental data and procedures used for simulation and validation have been detailed in previous works [10,20]. The compositions estimated based on previous works of the BM, EL, and flux can be found in Table 1 and Table 2 [10,20]. To ensure the comprehensiveness, simulation analyses will be conducted for fluxes with high and low BIs. The composition measurements have been reported in earlier works and will be referenced in this paper for model validation and analysis [10,20]. Then, the O level in the metal is to be simulated and predicted via the BI and the cross-zone models.

Table 1.
Compositions of employed fluxes (wt pct) [10,20].

Table 2.
Compositions of base metal and electrode (wt pct) [10,20].
2.2. Prediction and Simulation via BI Model
The BI is a model used to predict the O content from the basicity value of the flux. The BI is calculated from Equation (1) [21].
The O content in the WM is predicted via the BI model under the following process:
- The BI value is calculated from Equation (1).
- The O content in the WM is predicted by the BI value via Figure 1e.
2.3. Prediction and Simulation via Cross-Zone Model
For SAW, directly measurement for the chemical reaction temperature during the welding process is impractical since the molten pool is completely covered by flux particles and slag. Additionally, the temperature in some zones of welding can exceed 2000 °C, which further complicates direct measurement [6]. To facilitate the research, extensive simulation methods have been employed to explore the relationship between the welding current and the EET [6]. As previously noted, while the actual EET cannot be measured technically, recent studies suggest that EET levels generally rise with an increased current. Based on such scientific hypotheses, the EET data of the droplet zone and the weld pool zone are introduced into the cross-zone model to simulate the thermodynamic impact of current on O content [23].
In this study, FactSage 7.3 is utilized for modeling and calculations, leveraging its integrated databases, calculation modules, and interactive interface to explore chemical reactions, phase equilibria, and thermochemical modeling [24]. The Equilib module is employed to conduct equilibrium calculations. Specifically, the databases FactPS, Fstel, and FToxide are used. FactPS database offers thermodynamic data for pure substances, Fstel database provides data for steel and related alloys, and FToxide database covers various oxide systems [25]. The calculation process is as follows [23,24]:
2.3.1. Droplet Zone
- Step 1: Database Selection and Phase Simulation
- The primary databases chosen for this process include FToxid, Fstel, and FactPS.
- These databases were configured in the Equilib module for subsequent phase simulations.
- Step 2: Setting the Equilibrium Temperature for the SAW Process
- To accurately model the SAW process, equilibrium temperatures were set at 2300 °C and 2500 °C, corresponding to the EET in the weld pool zone.
- The input metal chemistries were based on the BM compositions.
To predict the oxygen concentration in the droplet, an equilibrium calculation was conducted using iron and oxygen as the primary metal constituents. The PO2 values from Table 3 were utilized in this calculation, which details the oxygen concentration in molten droplets and the corresponding equilibrium partial pressures of O2.

Table 3.
The predicted O content and O2 partial pressure in the droplet zone.
2.3.2. Weld Pool Zone
The nominal compositions were calculated and set as the input stream for the model applied to the weld pool zone. The FactSage Equilib module was used to perform gas–slag–metal equilibrium calculations to predict the compositions of the submerged arc welded metal.
- Database Selection: The FToxid, Fstel, and FactPS databases were selected. The solution phases included ASlag-liq (all oxides), S (FToxid-SLAGA), and LIQUID (FStel-Liqu) to model the molten slag and steel phases.
- Temperature Settings: Equilibrium temperatures were set at 1700 °C and 2000 °C to simulate conditions relevant to the weld pool zone.
- Input Compositions: The nominal compositions served as the input metal chemistries, while the flux compositions listed in Table 1 were used as the flux input.
- The predicted O data are summarized in Table 4.
 Table 4. Predicted O content via different models (ppm).
Table 4. Predicted O content via different models (ppm).
3. Results and Discussion
3.1. The Influence of Current on O Content
In SAW, the increase in O content begins in the droplet zone and undergoes further changes in the weld pool zone [6]. Figure 2 illustrates the variation in O content within the metal under low and high currents. It can be observed that significant O uptake occurs in the droplet zone, regardless of whether a high-basicity flux (flux F-1) or a low-basicity flux (flux F-2) is used. Subsequently, the metallurgical process from the droplet zone to the weld pool zone is a deoxidation process. This observation is consistent with the thermodynamic analysis by Lau et al. [10,16]. As shown by the arrows in Figure 2a,b, for both types of fluxes, the O content in the metal increases with the increasing welding current, whether in the droplet region or the weld pool zone.
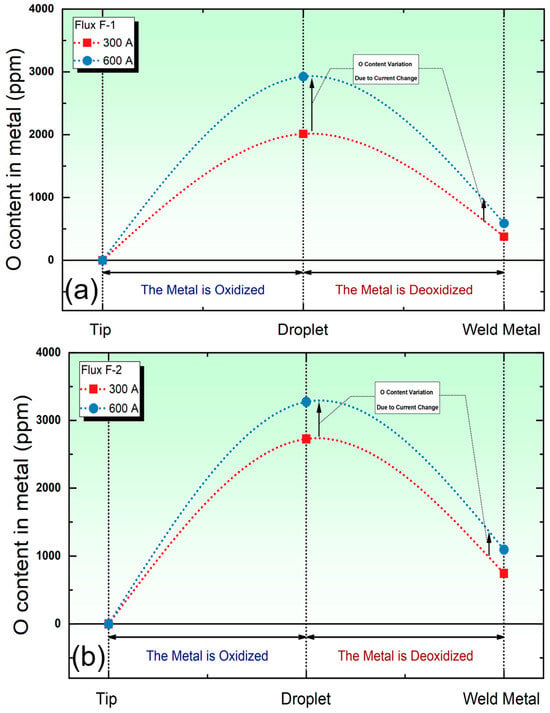
Figure 2.
Trends in the O Content in the metal under different currents and different fluxes. (a) O content subject to flux F-1; (b) O content subject to flux F-2.
Figure 3 illustrates the predicted O content using the cross-zone model. By observing Figure 3, it can be seen that this model accurately predicts the trend in the O content within the metal, with a sharp increase in the droplet region and a subsequent decrease in the weld pool. Notably, the model is able to predict the influence of current on O content. As shown in Figure 3a,b, with the increasing current, the O content increases in both the droplet zone and the weld pool zone for flux F-1 and F-2.
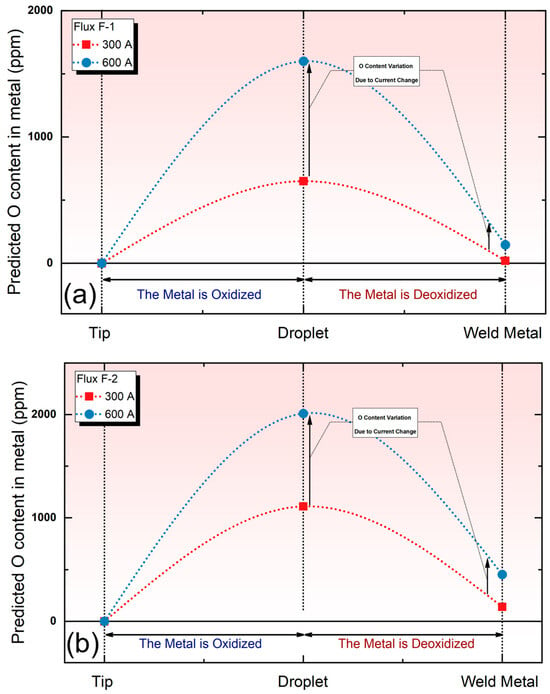
Figure 3.
Trends in the simulated O content in the metal under different currents and different fluxes. (a) Simulated O content subject to flux F-1; (b) simulated O content subject to flux F-2.
It is widely recognized that in the SAW process, the flux plays a crucial role in determining the O content in the metal. During SAW, the high-temperature metallurgical system causes oxides in the flux to decompose, releasing O2 and substantially raising the O level in the hot metal. For example, SiO2 and MnO in the flux may decompose through Reactions (2) and (3), releasing O2 gas [6]. Lau et al. [10,16] and Chai et al. [26] proposed a scientific hypothesis suggesting that the degree of oxide decomposition in the flux, specifically the O2 partial pressure, is a key factor in determining the O content in the metal. To validate this hypothesis, the simulated O2 partial pressure and the predicted O content in the metal are plotted in Figure 4.
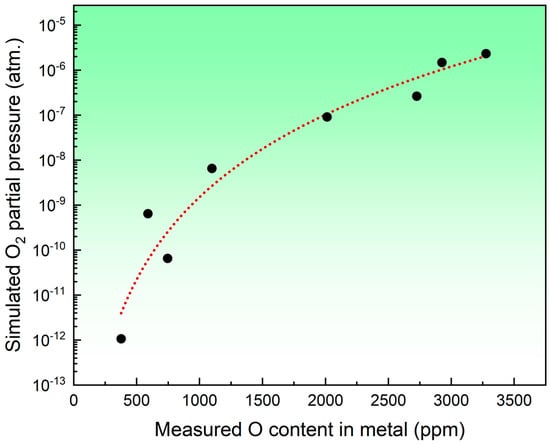
Figure 4.
A diagram of the relationship between the O2 partial pressure and the O content in the metal.
As noted earlier, direct measurement of the O2 partial pressure is not feasible. However, as illustrated in Figure 4, an increase in the O2 partial pressure corresponds with a rise in the O content in the metal, supporting the earlier hypothesis that the O2 partial pressure resulting from oxide decomposition is an essential factor in determining the O content in the metal [10,16]. Consequently, even without direct measurements, this model provides valuable insights for the gas analysis of the SAW metallurgical process. Furthermore, it introduces an approach for investigating gas partial pressures under different currents and their relationship with the metal’s O content.
3.2. Comparative Analysis of Models
This section discusses the thermodynamic model’s ability to distinguish the O potential between high- and low-basicity fluxes under different welding currents. Figure 5 presents the O content in the metal across various welding reaction zones for flux F-1 (high basicity) and F-2 (low basicity) at welding currents of 300 A and 600 A, respectively. Analyzing Figure 5 reveals that, regardless of the welding current, the low-basicity flux demonstrates a stronger O supply to the metal. This observation suggests that a lower basicity is associated with a higher O potential of the flux.
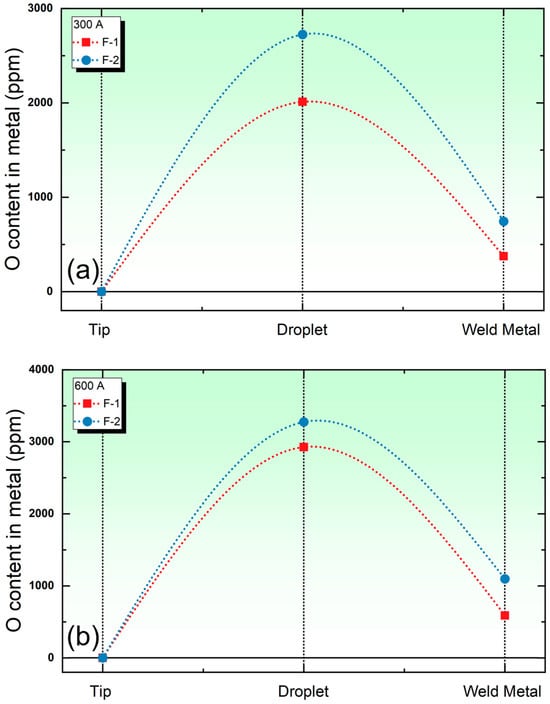
Figure 5.
The evolution of the O content in the metal under different welding currents. (a) O content subject to 300 A; (b) O content subject to 600 A.
Figure 6 shows the predicted O content values using the cross-zone and BI models under different currents. As shown, regardless of the welding conditions at 300 A or 600 A, and whether in the droplet reaction zone or the weld pool reaction zone, the O content in the metal increases with the decrease in flux basicity, as illustrated in Figure 6a,b. Figure 6c indicates the trend of the O content in the metal predicted by the BI model. It can be seen that the BI model can also predict the influence of flux basicity in the weld pool reaction zone on the O content in the metal subject to the SAW process.
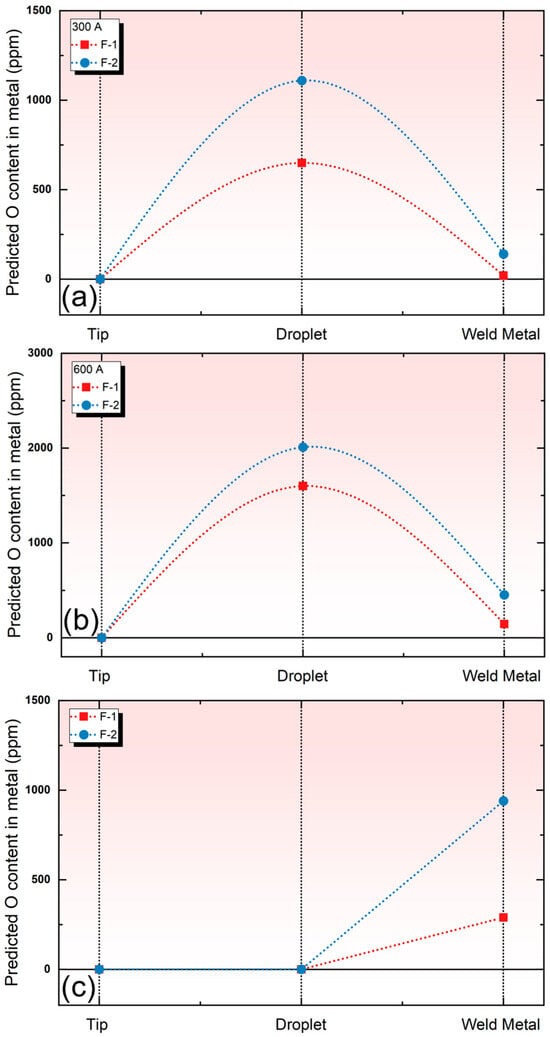
Figure 6.
The evolution of the simulated O content calculated via the models in the metal under different welding currents. (a) O content subject to 300 A; (b) O content subject to 600 A; (c) O predicted via BI model.
However, it is important to note that the BI model is essentially an empirical model, which only reflects the relationship between flux basicity and the oxygen content in the weld metal. As shown in Figure 6c, the BI model does not account for the O increase in the droplet reaction zone during the SAW process. Therefore, the oxidation–reduction pathway reflected by the BI model suggests that oxidation occurs only in the weld pool zone, which contradicts the actual oxidation–reduction pathway, where oxidation occurs in the droplet zone and then reduction occurs in the weld pool zone [10,16,20].
To ensure welding quality, SAW is typically conducted under varying conditions. However, the welding current tends to significantly influence the composition of the WM, which in turn affects the microstructure and mechanical properties of the welded joint [6]. O is a crucial factor in determining the microstructure and properties of the WM. However, the traditional BI model is unable to predict the impact of the welding current on O content, limiting effective guidance for material matching and selection in SAW under different welding currents. Therefore, this study employs a thermodynamic model to provide a predictive method for O content in the droplet and molten pool zones as a function of current, which may facilitate electrode matching, optimize welding processes, and enhance welding process modeling.
However, it should be noted that there are other welding parameters related to the SAW process, particularly voltage and welding speed. Mitra et al. [13] highlight that voltage and welding speed are kinetic factors influencing the metallurgy of SAW, determining how each welding zone deviates from thermodynamic equilibrium. Therefore, to further improve the predictive accuracy of the model, these factors should be included in future models to enhance precision.
It is noted that the proposed model may not be applicable to other welding processes since SAW typically lacks gas protection. Additionally, the use of flux and the high-temperature arc cause the oxides in the flux to decompose, significantly increasing the O content in the metal [1]. Other welding processes, such as TIG or MIG welding, do not exhibit these metallurgical characteristics; therefore, this model is currently not suitable for those processes.
This model should also be applicable to other steel grades, as the flux serves as the primary source of O in the SAW process [6]. In the droplet zone, O uptake can reach up to thousands of ppm [10]. Previous studies have shown that while alloying elements can affect the final O content, their impact is relatively minor compared to the O contribution from the flux [6]. Therefore, the method proposed in this study could also be used to predict the effect of current on O content in other steel grades.
4. Conclusions
Based on the cross-zone thermodynamic model, this study proposes a method for predicting and simulating the effect of current on the O content in the metal subject to the SAW process. The prediction results are validated against typical carbon steel under both high- and low-basicity fluxes and are compared with the traditional BI model. The present study concludes as follows:
- The proposed model is capable of predicting the impact of the welding current on the O content in the metal within both the droplet zone and the molten pool zone during the SAW process. Thermodynamic analysis indicates that at higher current levels, the decomposition of oxides in the flux is enhanced, leading to an increase in the partial pressure O2 and the O content in the metal. Under the same welding current, this model can also predict the effect of flux basicity on the O content in the metal in both the droplet and molten pool zones.
- Although the BI model can differentiate the O content levels in the metal within the weld pool zone, it cannot distinguish the O content within the droplet zone. Moreover, the BI model is unable to accurately predict the complete oxygen/reduction roadmap throughout the SAW process. Such limitations can be addressed by the model proposed in this study.
Author Contributions
Conceptualization, J.Z., J.F., and D.Z.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 50474085), the Initial Fund of Suqian University (No. 2022XRC040), and the Suqian Science & Technology Project (No. K202239).
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar]
- Kanjilal, P.; Pal, T.; Majumdar, S. Prediction of Element Transfer in Submerged Arc Welding. Weld. J 2007, 10, 40. [Google Scholar]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Sharma, L.; Chhibber, R. Study of Weld Bead Chemical, Microhardness & Microstructural Analysis Using Submerged Arc Welding Fluxes for Linepipe Steel Applications. Ceram. Int. 2020, 46, 24615–24623. [Google Scholar]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. ASM Int. ASM Handb. 1993, 6, 55–63. [Google Scholar]
- Zhang, J.; Shao, G.; Fan, J.; Wang, L.; Zhang, D. A Review on Parallel Development of Flux Design and Thermodynamics Subject to Submerged Arc Welding. Processes 2022, 10, 2305. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Liby, A.; Dixon, R.; Olson, D. Welding: Theory and Practice; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Mitra, U. Kinetics of Slag Metal Reactions during Submerged Arc Welding of Steel; Massachusetts Institute of Technology: Cambridge, MA, USA, 1984. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding Using CaO-Al2O3 Bbased Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J 1985, 64, 140–151. [Google Scholar]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Pandey, N.; Bharti, A.; Gupta, S. Effect of Submerged Arc Welding Parameters and Fluxes on Element Transfer Behaviour and Weld-metal Chemistry. J. Mater. Process. Technol. 1994, 40, 195–211. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions during Submerged Arc Welding with FeO-MnO-SiO2 fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D. Thermodynamic Simulation of O Content Variation Roadmap in Submerged Arc Welding Process: From Droplet to Weld Metal. Processes 2023, 11, 784. [Google Scholar] [CrossRef]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Zhang, D. Optimizing Elemental Transfer Predictions in Submerged Arc Welding via CALPHAD Technology under Varying Heat Inputs: A Case Study into SiO2-Bearing Flux. Processes 2024, 12, 1541. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).