Abstract
This study is devoted to the synthesis of aerated concrete by a non-autoclave method using ash from thermal power plants and a nanopreparation. Fullerenol-m was used as a nanopreparation. The fullerenol-m content in the sealing water of aerated concrete changed in the range of 0.00 ÷ 0.03 mas.%. The main performance characteristics of the nanostructured aerated concrete were studied, namely the compressive strength, impact toughness, thermal conductivity, density and moisture content. A significant improvement in the performance characteristics of the nanomodified aerated concrete compared to unmodified samples was demonstrated, which was most clearly manifested as an increase in impact toughness by several (three to five) times. The best performance characteristics of the modified aerated concrete were observed at a fullerenol-m concentration relative to the added cement within 0.022–0.028 wt.%. The authors attribute such a strong change and improvement in the physical, chemical and operational properties of aerated concrete when modified with fullerenol-m to the fact that fullerenol-m (a few thousandths of wt.%) has a very strong structuring effect on the sealing water and, as a consequence, on the resulting aerated concrete.
1. Introduction
The most popular building material in low-rise construction is aerated concrete, which is also called cellular concrete. The increase in the demand for aerated concrete is facilitated by the growth of electricity tariffs, and this in turn stimulates the use of building materials with low thermal conductivity [1]. Aerated concrete is used to construct partitions inside buildings, as well as for the insulation of external walls. It has good thermal insulation, which allows a reduction in the cost of heating a building. Over the past few decades, many authors have studied the possibility of improving the characteristics of various concretes using nanomaterials such as nano-TiO2, nano-SiO2 and carbon nanotubes [2,3,4,5,6]. Nanotechnology makes it possible to influence the properties of materials if the sizes of the nanomaterials are in the range of 1–100 nm (such a size can be fractional or fractal) [3], and this research topic is becoming increasingly popular among researchers due to the possibility of new scientific and practical applications [7,8]. The review in [9] presents new achievements in research related to increasing the durability of concrete using nanomaterials. It is shown that the inclusion of nanomaterials in concrete has a positive effect in terms of increasing its mechanical strength and durability, and it also leads to energy savings due to reduced cement consumption in the production of concrete. It is known that in order to increase the strength and improve the pore structure in cellular concrete, it is possible to modify the composition with carbon-containing nanomaterials, the introduction of which results in the effect of reinforcing the viscous mineral matrix [10]. The work in [11] shows the effect of additives at ultra-low doses (0.006–0.042% by weight of the binder), such as dispersed multi-layer carbon nanotubes, in changing the physical and mechanical properties of cement concrete. Recently, much attention has been paid to the use of waste generated during coal combustion in thermal power plants as a silica component for the production of aerated concrete blocks. Attention has also been paid to the use of thermal power plant (TPP) ash as a filler for aerated concrete [12,13]. It should be noted that earlier, in [14], the possibility of modifying standard concrete and gypsum using water-soluble carbon nanomaterials was considered. The authors demonstrated a significant improvement in the strength characteristics of building materials (primarily the impact toughness).
This article is the first to investigate the possibility of using water-soluble carbon-containing nanopreparations for the production of aerated concrete by a non-autoclave method, using ash and slag waste from thermal power plants as a filler. The main objective of this study is to improve the main strength characteristics (and a number of others, such as the density, thermal conductivity and humidity).
This study has great potential, since the operation of TPPs results in the formation of a huge amount of ash and slag waste (ASW), which occupies huge areas and negatively affects the environment. The production of aerated concrete blocks based on ash and slag waste is associated with obvious environmental and economic advantages—the utilization of ash and slag waste from thermal power plants and the reduction of the costs of their maintenance and storage.
2. Materials and Methods of Material Synthesis
2.1. Concrete M400
For the preparation of aerated concrete samples, we used cement of the M400 brand, produced by the Bukhtarma Cement Company LLP. This is one of the most affordable cement grades used in the northeast of Kazakhstan and is produced on its own resource base. The chemical and phase compositions of M400 cement, as presented by the manufacturer, are given in Table 1.

Table 1.
Average chemical and phase compositions of M400 cement (Bukhtarma Cement Company, Kazakhstan).
Additionally, a fairly fast and reliable method for the analysis of various materials was used—the scanning electron microscopy (SEM) method. We conducted an electron microscopic study of M400 cement on a JSM-6390LV scanning electron microscope with an INCA microanalysis system. Figure 1 shows an image of the microstructure of the M400 cement and the quantitative composition of the chemical elements detected.
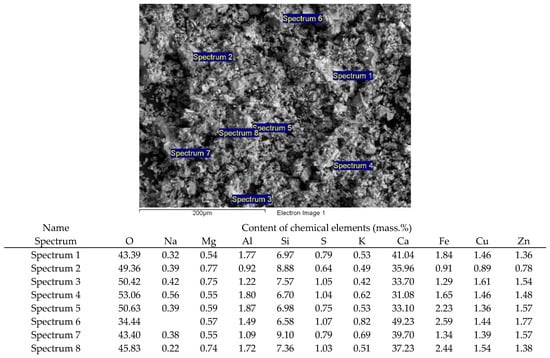
Figure 1.
M400 cement analysis with JSM-6390LV scanning electron microscope and INCA microanalysis system (magnification: 300).
The main chemical elements are calcium, oxygen and silicon, which is confirmed by the data given in Table 1. Visually, the presence of these chemical elements in the studied sample of M400 cement is clearly visible in the energy spectrum obtained with the EDS microanalyzer (Figure 2).
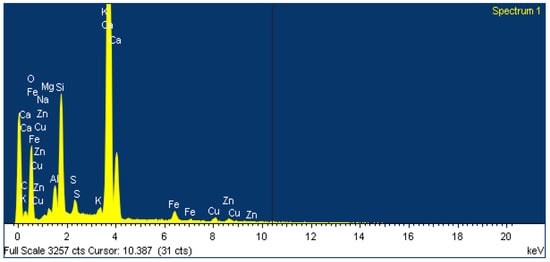
Figure 2.
Results of element analysis via electron microscopy of M400 cement.
The presence of such elements as copper and zinc in the spectrum can be explained by the fact that the company uses waste slag from metallurgical plants in the Republic of Kazakhstan in the production of M400 cement.
2.2. Ash and Slag Waste—ASW
The authors used ash and slag waste (ASW; ash dump of boiler house No. 2 of Ust-Kamenogorsk Thermal Power Plant (Ust-Kamenogorsk, Kazakhstan)). This waste is not only free but also has a negative cost, since it requires disposal. The results of the elemental analysis of the ASW are presented below in Table 2.

Table 2.
Average chemical and phase analysis of ash and slag waste—ASW (ash dump of boiler room No. 2 of Ust-Kamenogorsk Thermal Power Plant (Ust-Kamenogorsk, Kazakhstan)).
Ash and slag waste is characterized by a complex composition. In terms of the chemical composition, the basis of ash and slag waste is silicon, aluminum and iron oxides. The content of alkali and alkaline earth metal oxides is approximately 6.4%. We additionally conducted an electron microscopic study of the ash and slag waste using the JSM-6390LV scanning electron microscope with the INCA microanalysis system. Figure 3 shows an image of the ash and slag waste microstructure and the quantitative composition of the chemical elements detected.
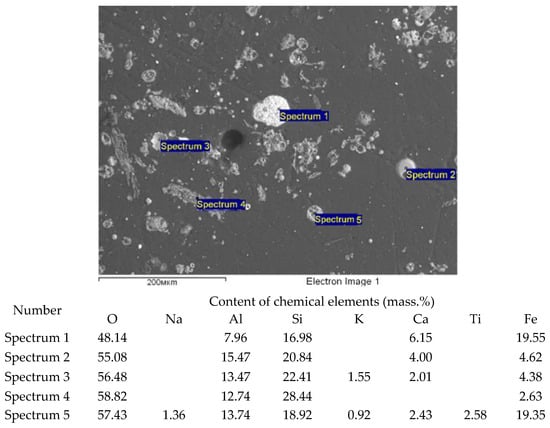
Figure 3.
Ash and slag waste (ASW) analysis using JSM-6390LV scanning electron microscope with INCA microanalysis system (magnification: 300).
In addition to aluminosilicate aggregates, the composition of fly ash includes spheroidal particles of magnetite (Fe3O4) mixed with hematite (Fe2O3), which constitute the magnetic fraction of ASW.
Figure 4 shows the X-ray diffraction pattern of the ASW obtained using an Aeris Research diffractometer.
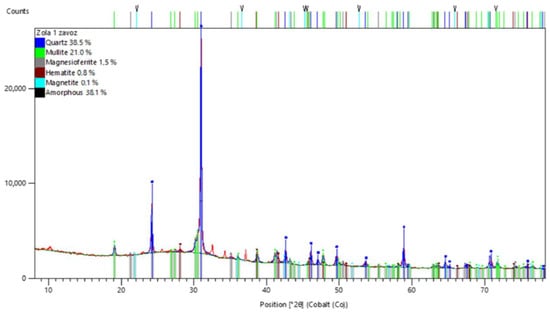
Figure 4.
X-ray diffraction spectrum of ASW sample obtained on an Aeris Research diffractometer.
The comparison of the study results with the diffraction database showed that the studied sample of ASW was represented by the following: amorphous phase—38.1%; mullite—21.0%; quartz—38.5%; magnesioferrite—1.5%; magnetite—0.1%; hematite—0.8%.
2.3. Fullerenol-m
By themselves, individual, well-purified fullerenols (for example, probably the most popular fullerenol, C60 (OH)24; C70 (OH)12) [15,16] are quite expensive and cannot be effectively used in construction, even in the form of microadditives. Therefore, in our work, we used the much more accessible fullerenol-m. This nanopreparation was obtained using a previously developed method for the synthesis of mixed fullerenols (fullerenol-mix-ss) directly from fullerene soot [17]. Hence, the name of the nanopreparation used is fullerenol-mix or fullerenol-m. The stages in the synthesis of fullerenol-m and the characteristics of the intermediates at the different stages of synthesis are presented in Table 3. The research on this product was carried out via a number of methods associated with physicochemical analysis (Table 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11).

Table 3.
Stages and characteristics of fullerenols-m synthesis.

Table 4.
Physical–chemical investigation of fullerenol-m product.
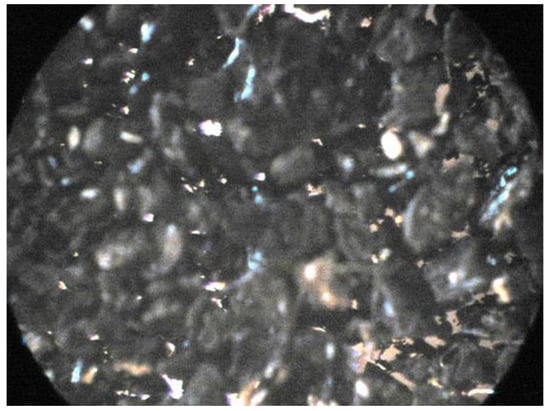
Figure 5.
Optical microscopy photo (magnification: 50) of crystals of fullerenol-m.
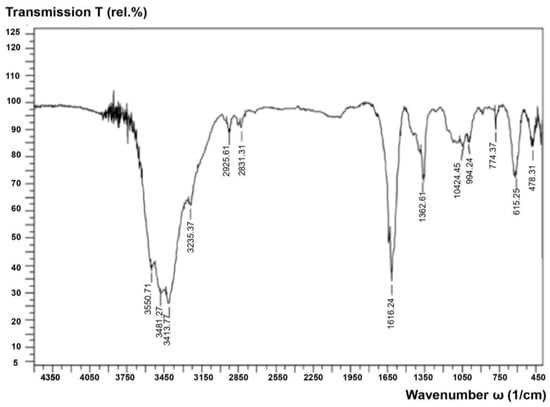
Figure 6.
IR spectrum of fullerenol-m.
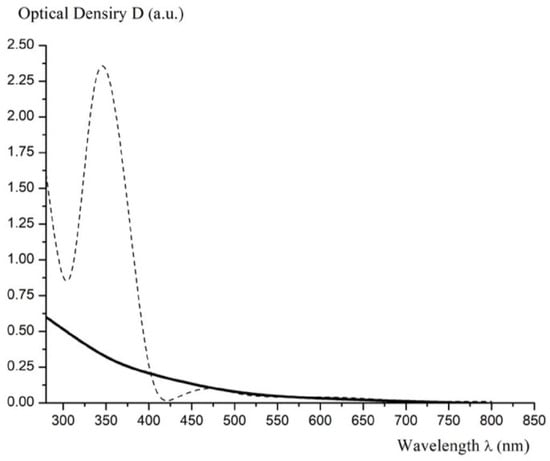
Figure 7.
Electronic spectrum of fullerenol-m (solid line); spectrum of C60—dot.
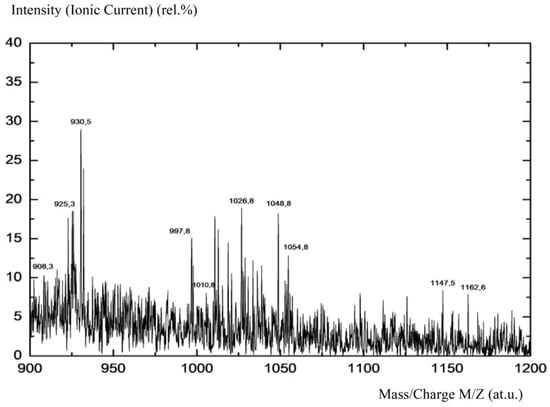
Figure 8.
Mass spectrum of fullerenol-m (fragment).
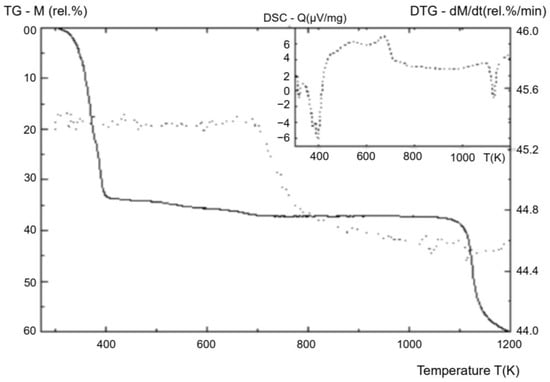
Figure 9.
Thermogram of fullerenol-m (TG curve—solid, DTG curve—points).
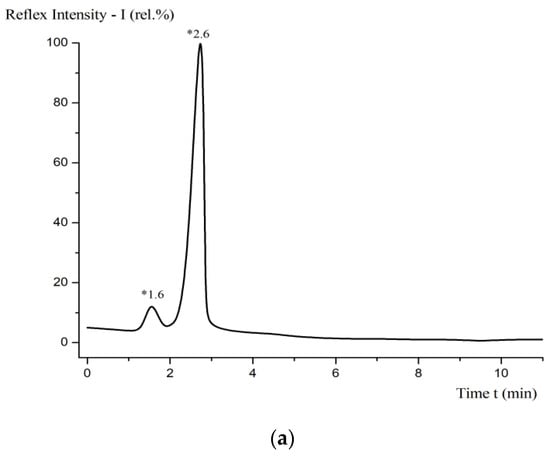
Figure 10.
HPLC of the solution of fullerenol-m (a); basic line without fullerenol-m (b).
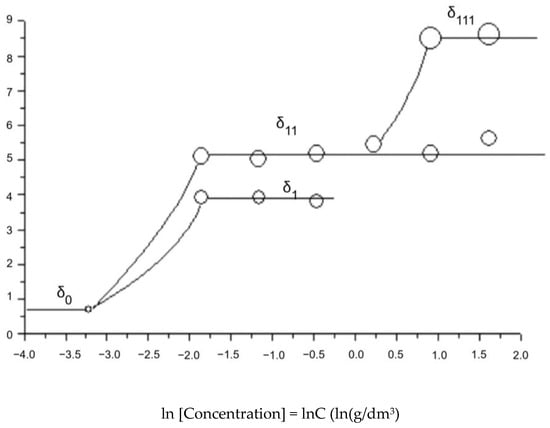
Figure 11.
Dynamic light scattering of the solution of fullerenol-m (associate size distribution).
According to Figure 5 (optical microscope Min-5), the morphology of fullerenol-m corresponds to a three-dimensionally soldered polycrystalline formation with an average linear crystallite size of several hundred microns. The linear size of the soldered crystallite in the photo of 1 cm corresponds to a real size of about 200 microns. The fullerenol-m synthesized by us was studied for identification by infrared spectrophotometry using a Shimadzu FTIR8400S device (Figure 6 and Table 4). The study of the obtained spectrum allowed us to identify the main absorption peaks at the given frequencies, corresponding to the O-H, C-O and C-O-H groups (Table 4).
The data on the identification of fullerenol-m using the SPECORD M-32 electron spectrometer are presented in Table 4 and Figure 7.
As can be clearly seen from Figure 7, the electron spectrum of fullerenol-m has no visible absorption peaks, and only monotonously increasing absorption is observed when shifted to the short-wavelength region of the spectrum. In particular, there is no intense absorption peak common to light fullerenes at λ = 335.7 nm (see the electronic absorption spectrum for the solution of C60, represented in Figure 7, for comparison).
Using mass spectrometry with the Mibcrotof device (Bruker), ionization–electronic impact studies were conducted to identify fullerenol-m, the results of which are presented in Table 4 and Figure 8.
Figure 8 clearly shows the presence of mass-spectrometric peaks at M/Z = 925–1163 a.u., corresponding to sodium forms of polyalcohols Cn(OH)mOp(ONa)q. The study of the thermal stability of fullerenol-m was carried out using a device for the thermal testing of materials from Shanghai Jiahang Instruments Co., Ltd., in an air atmosphere at normal pressure in the temperature range T = 25 ÷ 1150 °C (the results are presented in Table 4 and Figure 9).
The thermogram shows that, at T = 100 ÷ 130 °C, the decomposition of the fullerenol-m crystal hydrate occurs. At T = 170 ÷ 830 °C, the oxidative destruction of fullerenol-m occurs, consisting of dehydroxylation and decarboxylation processes, with the formation of semi-ketones and the rearrangement of the pinacol-type hydroxyl groups and their degradation. At T = 850 ÷ 880 °C, the loss of all hydroxyl groups is observed; at T ≥ 900 °C, the oxidation of the fullerene nuclei begins.
Figure 10 shows the HPLC of the solution of fullerenol-m (a); in the basic line without fullerenol-m (b), it was removed using an Agilent liquid chromatograph. Table 4 shows the experimental conditions.
In Figure 10, it can be found that, at t = 2.6 min, the strongest peak of fullerenol-m is observed. The width of the main peak at half-height is d_(1/2) ≈ 0.25 min. The chromatographic purity of fullerenol-m is ≈ 98 ÷ 99 wt%.
Figure 11 and Table 4 show the data on the size distribution of the associates, obtained using the Malvern Zetasizer Nano ZS90 device.
The classical stage-by-stage hierarchical association of nanoclusters formed by molecules creating a mixed nanopreparation of fullerenol-m was found. Here, zeroth-order associates (monomers with linear dimensions δ ≈ 2 nm) at the lowest gross concentrations of fullerenol-m are found; at C fullerenol-m < 0.01 g/dm3, first-order associates with δ ≈ 25 ± 10 nm are formed; at C fullerenol-m = 0.05 ÷ 0.15 g/dm3, second-order associates with δ ≈ 230 ± 50 nm are formed from first-order associates; at C fullerenol-m = 0.15 ÷ 3.0 g/dm3, third-order associates with δ ≈ 3 ± 1 nm are formed from second-order associates; and at C fullerenol-m = 3.0 ÷ 10 g/dm3, a microcolloid solution is formed, based on third-order associates.
2.4. Aerated Concrete
For the synthesis of aerated concrete, the following reagents were additionally used: technical grade A soda ash with sodium carbonate containing at least 99.4% Na2CO3; grade A5 aluminum powder; and fullerenol-m.
The research methodology included the production and testing of experimental aerated concrete samples (cubes with an edge size of 15 cm) with different amounts of fullerenol-m, including control samples (with a three-fold repetition of the experiment). The scheme of the synthesis process is presented in Figure 12.
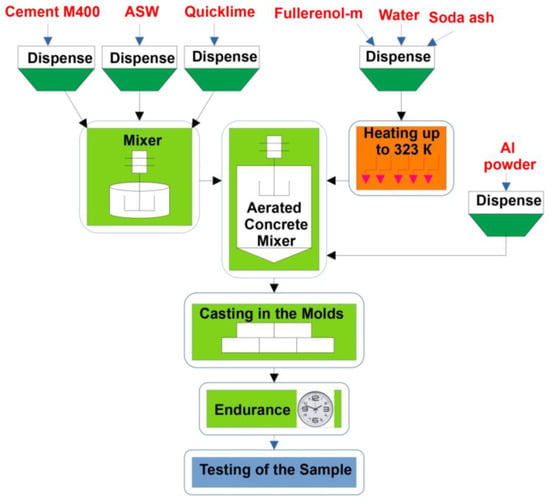
Figure 12.
The scheme of the synthesis of aerated concrete, based on the ash of thermal power plants, nanostructured with water-soluble fullerenols.
Concrete, ASW and other dry materials were dosed by weight (Figure 12). First, cement, ASW and quicklime were mixed. Separately, a mixing solution was prepared containing fullerenol-m at the required concentration [16], soda ash (Na2CO3) and water. Then, the mixing solution was heated to 50 °C. The heated mixing solution was added to the dry mixture consisting of cement, ASW and quicklime and mixed for 3 min. After this, aluminum powder was added to the resulting mixture and mixed for 2 min. When the aluminum powder was introduced into the liquid suspension, active gas formation began, due to which pores were formed. The finished mixture was poured into molds to two thirds of the height. The lifting of the aerated concrete and the minimum gain in strength occurred under normal conditions; then, the samples were placed in a normal curing chamber for storage for 28 days.
In our experiments, we used different ratios of ASW, cement and lime:
- -
- cement/slag waste/lime 1.0/1.0/0.0 (in grams 2200/2200/0);
- -
- cement/slag waste/lime 1.0/0.9/0.1 (in grams 2200/1980/220);
- -
- cement/slag waste/lime 1.0/0.8/0.2 (in grams 2200/1760/440).
The amounts of soda ash and aluminum powder in all experiments were the same at 10 g and 3.6 g, respectively. The volume of the mixing solution in the experiments was 2450 mL.
Figure 13 shows the appearance of the aerated concrete samples obtained by the non-autoclave method with the same concentration of fullerenol-m (0.028 wt.% in relation to cement).

Figure 13.
The appearance of aerated concrete obtained by a non-autoclave method with the same concentration of fullerenol-m (0.028 mass.% relative to cement).
Figure 14 shows an image of the microstructure of the synthesized aerated concrete sample, obtained using an electron microscope. Table 5 shows the minimum and maximum concentrations of the chemical elements found in the aerated concrete when examined using the scanning electron microscopy method.
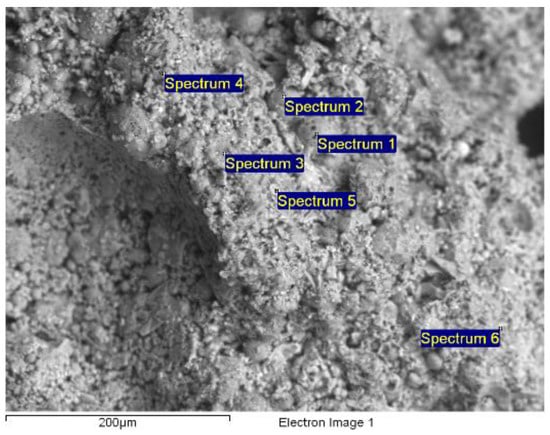
Figure 14.
Aerated concrete analysis using JSM-6390LV scanning electron microscope with INCA microanalysis system (magnification: 300).

Table 5.
Average chemical analysis of aerated concrete, with maximum and minimum element content.
The main chemical elements are oxygen, calcium, aluminum and silicon. Visually, the presence of the chemical elements in the studied sample of aerated concrete was confirmed by the energy spectrum obtained with the EDS microanalyzer (Figure 15).
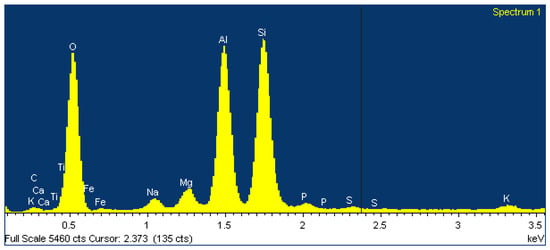
Figure 15.
The results of the element analysis of the aerated concrete using an electron microscope (example).
2.5. Testing and Research Methods
2.5.1. Specific Impact Strength
The classical methods of Charpy and Izod are rarely applied to construction materials, i.e., cement/beton, because of the relatively low impact strength and high mechanical heterogeneity of the latter. In the testing of cement/beton by these methods, it is practically impossible to obtain stable and internally correlated results. For the impact resistance testing of nanomodified construction material samples, we used a method that was methodically close to Gardner’s test (method of falling metal ball) [24]. The scheme of the apparatus is shown in Figure 16. As a result, we obtained data on the so-called specific impact resistance, which has the unit [kJ/m3], or the classical impact resistance in ·m−1. We determine the so-called first specific impact resistance (when the first crack appears) and the second one (when complete sample destruction is observed).
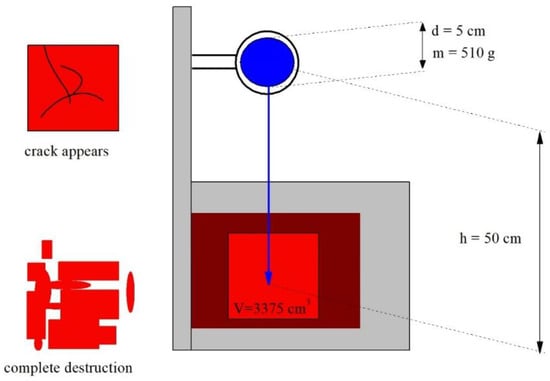
Figure 16.
Schematic of the device for the measurement of the specific impact resistance. Falling spherical steel weight (blue), r—weight radius, m—weight mass, h—drop height; gray parallelepiped—platform; brown parallelepiped—stop inserter of the sample; red cube—aerated concrete sample with the volume V = 15∙15∙15 = 375 cm3. Schematic images of samples: with crack (top left), with complete destruction (bottom left).
2.5.2. Compressive Strength
Compressive strength tests of the samples were carried out using a PGM-100MG4 hydraulic press (small-sized hydraulic press, Russian [25]). The measurements were carried out in accordance with [26]. Axis compression cubes with certain dimensions (≈150 mm × 50 mm × 150 mm) were studied.
2.5.3. Density
The density of the aerated concrete was determined by the direct weighing of the cubes, taking into account the imperfection of the shape (average of 3 measurements).
2.5.4. Humidity
The humidity of the aerated cement was measured by a humidity meter, the electronic moisture meter MG4-U [27]. The measurements were carried out in accordance with [28].
2.5.5. Thermal Conductivity
The thermal conductivity of the aerated cement was measured by a thermal conductivity meter ITP-MG4 [29]. The measurements were carried out in accordance with [29].
3. Results
The strength characteristics and some physical–chemical properties of the aerated concrete nanostructured with fullerenols are presented in Table 6 and Figure 17 and Figure 18.

Table 6.
Strength characteristics and some physical–chemical properties of aerated concrete nanostructured with fullerenols.
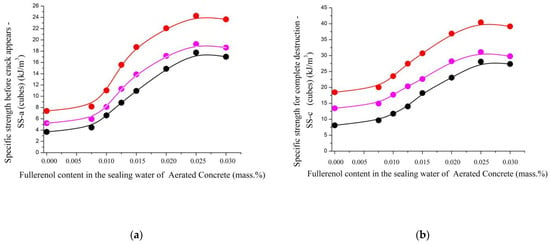
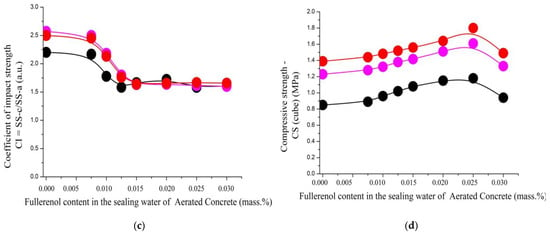
Figure 17.
The dependence of the strength characteristics of aerated concrete nanostructured with fullerenols: (a) specific strength before crack appears (top); (b) specific strength for complete destruction (middle); (c) coefficient of impact strength (bottom); (d) compressive strength of fullerenol content in sealing water at different mass ratios of cement ash/slag waste/lime: 1.0/1.0/0.0 (black); 1.0/0.9/0.1 (magenta); 1.0/0.8/0.2 (red).
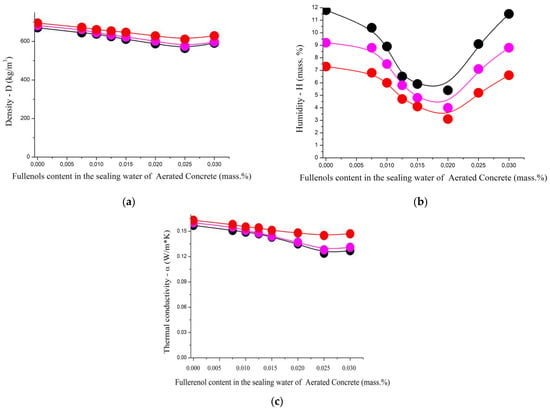
Figure 18.
The dependence of some properties of aerated concrete nanostructured with fullerenols, namely the (a) density (middle top), (b) humidity (middle bottom), and (c) thermal conductivity (bottom), on the fullerenol content in the sealing water at different mass ratios of cement ash/slag waste/lime: 1.0/1.0/0.0 (black); 1.0/0.9/0.1 (magenta); 1.0/0.8/0.2 (red).
4. Discussion
The increase in the influence of the nanopreparation added during the synthesis of the aerated concrete samples on their physical and mechanical characteristics is well demonstrated by Figure 17 and Figure 18.
The dependencies presented in Figure 17a,b,d have the same configuration, showing an increase in the strength characteristics of the aerated concrete with the amount of the added nanopreparation. The maximum values are achieved at a fullerenol-m concentration of 0.022–0.028% based on the mass of the cement.
The dependencies of the density and thermal conductivity of the aerated concrete (Figure 18a,c) also have similar configurations. With an increase in the amount of the added nanopreparation, the strength and thermal conductivity of the aerated concrete decrease. The maximum decrease is achieved at a fullerenol concentration of 0.022–0.028% based on the mass of the cement.
In Figure 19, we present the most significant changes in the strength and some physical characteristics of the fullerenol-m-modified aerated concrete in comparison with unmodified samples against the fullereneol-m concentration in the sealing water.
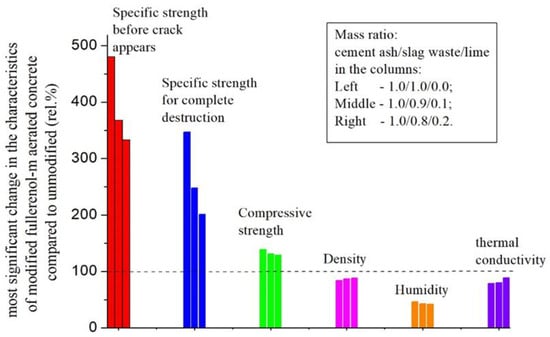
Figure 19.
Most significant changes in the strength and some physical characteristics of fullerenol-m-modified aerated concrete in comparison with unmodified samples against fullereneol-m concentration in sealing water (mass ratio of cement ash/slag waste/lime in the column: 1.0/1.0/0.01—left, 1.0/0.9/0.1—middle, 1.0/0.8/0.2—right).
We can see that the introduction of water-soluble fullerenol-m into the composition of aerated concrete (in quantities of 0.0075–0.03 mass.%) has a significant effect on the strength and physicochemical properties of the concrete:
- The specific impact strength increases ≈3 ÷ 5 times (crack appears, maximum effect);
- The specific impact strength increases ≈2 ÷ 3 times (complete destruction);
- The compressive strength increases by ≈20 ÷ 30 rel.%;
- The density decreases by ≈12 ÷ 16 rel.%;
- The humidity decreases by ≈55 ÷ 60 rel.%;
- The thermal conductivity decreases by ≈10 ÷ 20 rel.%.
The maximum effect of the modification was observed with the minimal introduction of quicklime or in the absence thereof. All effects are positive regarding the exploitation characteristics of aerated concrete.
The conducted studies have shown the possibility of obtaining aerated concrete samples with improved characteristics, i.e., with increased strength and with a reduced density and thermal conductivity.
5. Conclusions
Thus, the introduction of water-soluble fullerenol-m into the composition of aerated concrete in micro-quantities has a significant positive effect on the specific impact strength (maximum increase is 3–5 times) and humidity (maximum decrease is 2–2.5 times) and has also a positive effect on the compressive strength, density and thermal conductivity (decrease is tens rel. %).
The effect of the additive’s introduction is extreme. It is the maximum at a fullerenol-m concentration of 0.022–0.028 wt.% in relation to the mass of the cement, which corresponds to the minimum introduction of quicklime.
We believe that the unexpectedly strong effect of extremely low fullerenol-m concentrations on the strength and other physicochemical properties of aerated concrete is due to the strong structuring effect of even insignificant amounts of fullerenol-m on the sealing aqueous solution. Thus, it was previously experimentally shown (by densimetry) that, even in very dilute fullerenol solutions, the partial molar volumes of fullerenol have huge negative values, which are ten times higher in modulus than the average molar volume of fullerenol. This indirectly indicates the very strong ordering of aqueous solutions by fullerenols, partly due to the formation of a strong system of hydrogen bonds.
Author Contributions
Conceptualization, O.V.R., N.A.K. and M.A.S.; methodology, N.A.K., N.A.C. and D.K.A.; validation, O.V.R., N.A.K., N.A.C., M.A.S., D.K.A. and E.K.; formal analysis, O.V.R., M.A.S. and E.K.; investigation, O.V.R., N.A.K., M.A.S. and D.K.A.; resources, N.A.C. and N.A.K.; data curation, O.V.R.; writing—original draft preparation, O.V.R.; writing—review and editing, O.V.R., N.A.K., M.A.S. and N.A.C.; supervision, O.V.R. and D.K.A.; project administration, O.V.R., D.K.A. and E.K.; funding acquisition, O.V.R. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan and financed by grant No. BR21882292—“Integrated development of sustainable construction industries: innovative technologies, production optimization, efficient use of resources and creation of a technology park”.
Data Availability Statement
The data presented in this study are available in this article (tables and figures).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sabitov, Y.Y.; Dyussembinov, D.S.; Zhumagulova, A.A.; Bazarbayev, D.O.; Lukpanov, R.E. Composite non-autoclaved aerated concrete based on an emulsion. Mag. Civ. Eng. 2021, 106, 10605. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ou, J. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Alnuaimi, N.A. Recent Advancements in the Nanomaterial Application in Concrete and Its Ecological Impact. Materials 2021, 14, 6387. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Huseien, G.F. A Review on Concrete Composites Modified with Nanoparticles. J. Compos. Sci. 2023, 7, 67. [Google Scholar] [CrossRef]
- Alhassan, M.; Alkhawaldeh, A.; Betoush, N.; Alkhawaldeh, M.; Huseien, G.F.; Amaireh, L.; Elrefae, A. Life Cycle Assessment of the Sustainability of Alkali-Activated Binders. Biomimetics 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Gołaszewski, J.; Klemczak, B.; Smolana, A.; Gołaszewska, M.; Cygan, G.; Mankel, C.; Peralta, I.; Röser, F.; Koenders, E.A.B. Effect of Foaming Agent, Binder and Density on the Compressive Strength and Thermal Conductivity of Ultra-Light Foam Concrete. Buildings 2022, 12, 1176. [Google Scholar] [CrossRef]
- Al-Saffar, F.; Wong, L.; Paul, S. An Elucidative Review of the Nanomaterial Effect on the Durability and Calcium-Silicate-Hydrate (C-S-H) Gel Development of Concrete. Gels 2023, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, G.; Kerien, J.; Plechanova, T.; Krutikov, V. Nanobewehrung von Schaumbeton. Beton- und Stahl. Стрoительные Материалы 2007, 102, 120–124. [Google Scholar]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Urkhanova , L.A.; Rosina, V.E. High-Strength Concrete with the Use of Fly Ash and Microsilica. Proceedings of Irkutsk State Technical University. 2011. № 10. С. 97–100. Available online: https://journals.istu.edu/vestnik_irgtu/journals/2011/10 (accessed on 8 July 2024).
- Fediuk, R.S.; Yushin, A.M. The use of fly ash the thermal power plants in the construction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 93, 012070. [Google Scholar] [CrossRef]
- Zolotarev, А.; Lushin, A.; Charykov, A.; Semenov, K.; Namazbaev, V.; Keskinov, V.; Kritchenkov, A. Impact Resistance of Cement and Gypsum Plaster Nanomodified by Water-Soluble Fullerenols. Ind. Eng. Chem. Res. 2013, 52, 14583–14591. [Google Scholar] [CrossRef]
- Podolsky, N.E.; Lelet, M.I.; Ageev, S.V.; Petrov, A.V.; Mazur, A.S.; Iamalova, N.R.; Zakusilo, D.N.; Charykov, N.A.; Vasina, L.V.; Semenov, K.N.; et al. Thermodynamic properties of the C70(OH)12 fullerenol in the temperature range T = 9.2 K to 304.5 K. J. Chem. Thermodyn. 2020, 144, 106029. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical study of water-soluble C60(OH)24 fullerenol. J. Mol. Liq. 2020, 311, 113360–113411. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Letenko, D.G.; Nikitin, V.A.; Namazbaev, V.I. Synthesis and identification of mixed fullerenol, obtained by the method of the direct oxidation of fullerene soot. Russ. J. Phys.Chem. 2011, 65, 1108–1115. [Google Scholar]
- Kratschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon; Nature: London, UK, 1990; Volume 347, pp. 354–358. [Google Scholar]
- Smalley, R.E.; Haufler, R.E. Electric arc Process for Making Fullerenes. United. States Patent № 5227038, 13 July 1993. [Google Scholar]
- Gruzinskaya, E.A.; Keskinov, V.A.; Keskinova, M.V.; Semenov, K.N.; Charykov, N.A. Fullerenovaya Sazha Elektrodugovogo Sinteza. Nanosistemy Fiz. Khimiya Mat. 2012, 3, 83–90. (In Russia) [Google Scholar]
- Hasanshin, I.Y.; Popkova, O.S. Synthesis of fullerene soot by plasma enhanced chemical vapour deposition in impulsing discharge at atmospheric pressure from liquid hydrocarbon. Sci. J. Kuban State Agrar. Univ. 2012, 80, 1–13. [Google Scholar]
- Abduguev, R.M.; Alekhin, O.S.; Gerasimov, V.I.; Losev, G.M.; Nekrasov, K.V.; Nikonov, Y.A.; Charykov, N.A. Device for Producing a Fullerene-Containing Black // WO2005087662 (A1), МПК C01B31/02, № PCT/RU2005/000119; published 22.09.2005.
- Abduguevб, R.M.; Alekhin, O.S.; Gerasimov, V.I.; Losev, G.M.; Nekrasov, K.V.; Nikonov, Y.A.; Soroka, A.I.; Charykov, N.A. Method for Production a Fullerene-Containing Black // WO2005070826 (A1), МПК C01B31/02, C09C1/48, № PCT/RU2005/000025; published 04.08.2005.
- Federal Agency for Technical Regulation and Metrology of the Russian Federation Certificate of Approval of the Type of Measuring Instruments. Federal Agency for Technical Regulation of Metrology. Russia. Chelyabinsk. 2017.RU.C.28.059.A. N 45609. 49 P.
- GOST 25485-89; Cellular Concretes. Technical Conditions. State Construction Committee of the USSR: Moscow, Russia, 1990; 36p.
- Limited Liability Company "Special Design Bureau Stroypribor". Humidity Meter Electronic Moisture Meter-MG4-U; Federal Agency for Metrology: Chelyabinsk, Russia, 2015; 36p. (In Russia) [Google Scholar]
- GOST 30515; The Interstate Council for Standardization, Metrology and Certification. CEMENTS General Technical Conditions: Moscow, Russia, 2013; 37p. (In Russia)
- Limited Liability Company "Special Design Bureau Stroypribor". Thermal Conductivity Meter ITP-MG4; Federal Agency for Metrology: Chelyabinsk, Russia, 2020; 39p. (In Russia) [Google Scholar]
- Brooks, J. Elasticity, shrinkage, creep and thermal movement. In Advanced Concrete Technology; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).