Effects of the PMMA Molecular Weight on the Thermal and Thermo-Oxidative Decomposition as the First Chemical Stage of Flaming Ignition
Abstract
1. Introduction
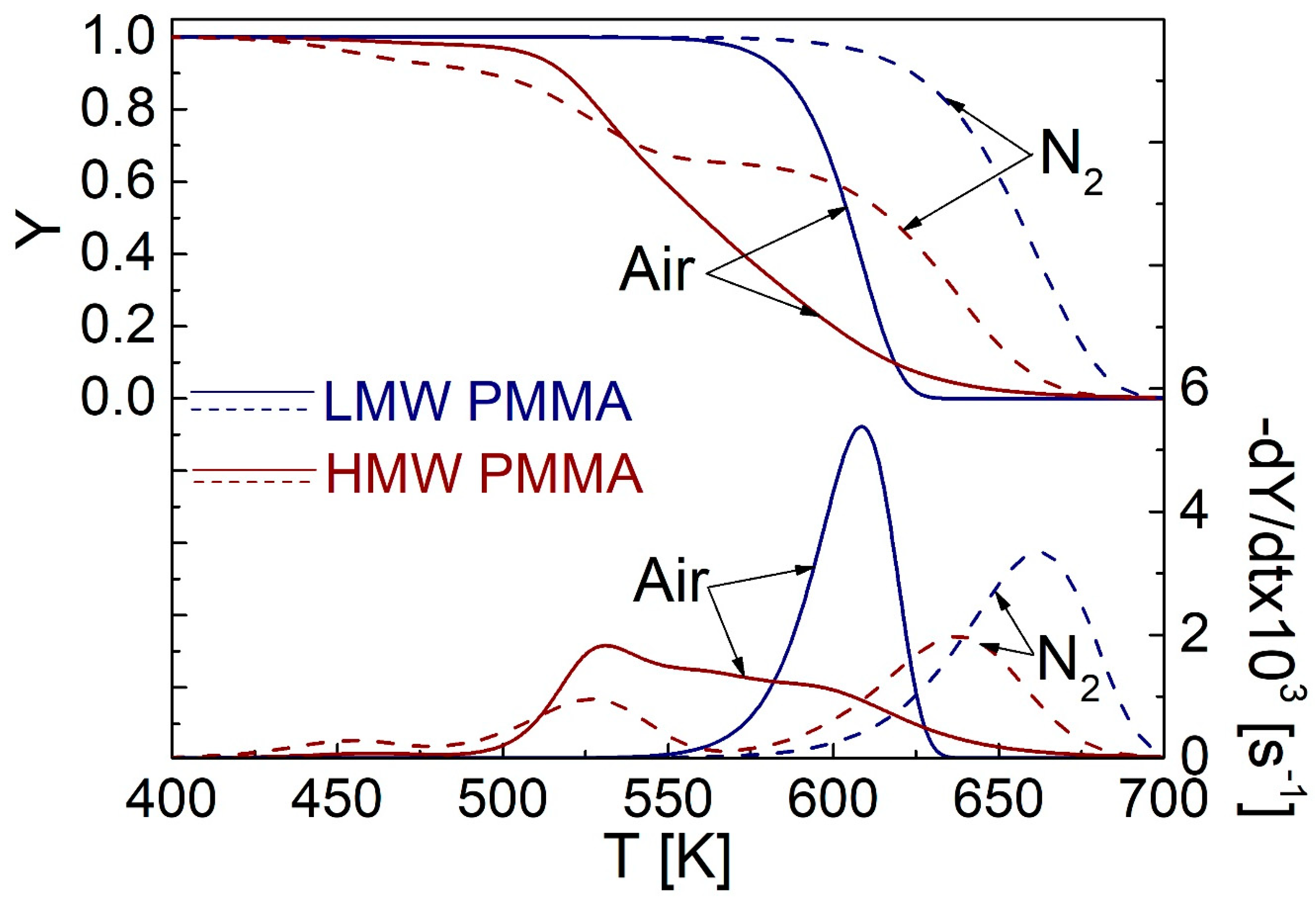
2. Decomposition Kinetics of the Selected Materials
3. Mathematical Model of Piloted and Spontaneous Ignition
4. Results and Discussion
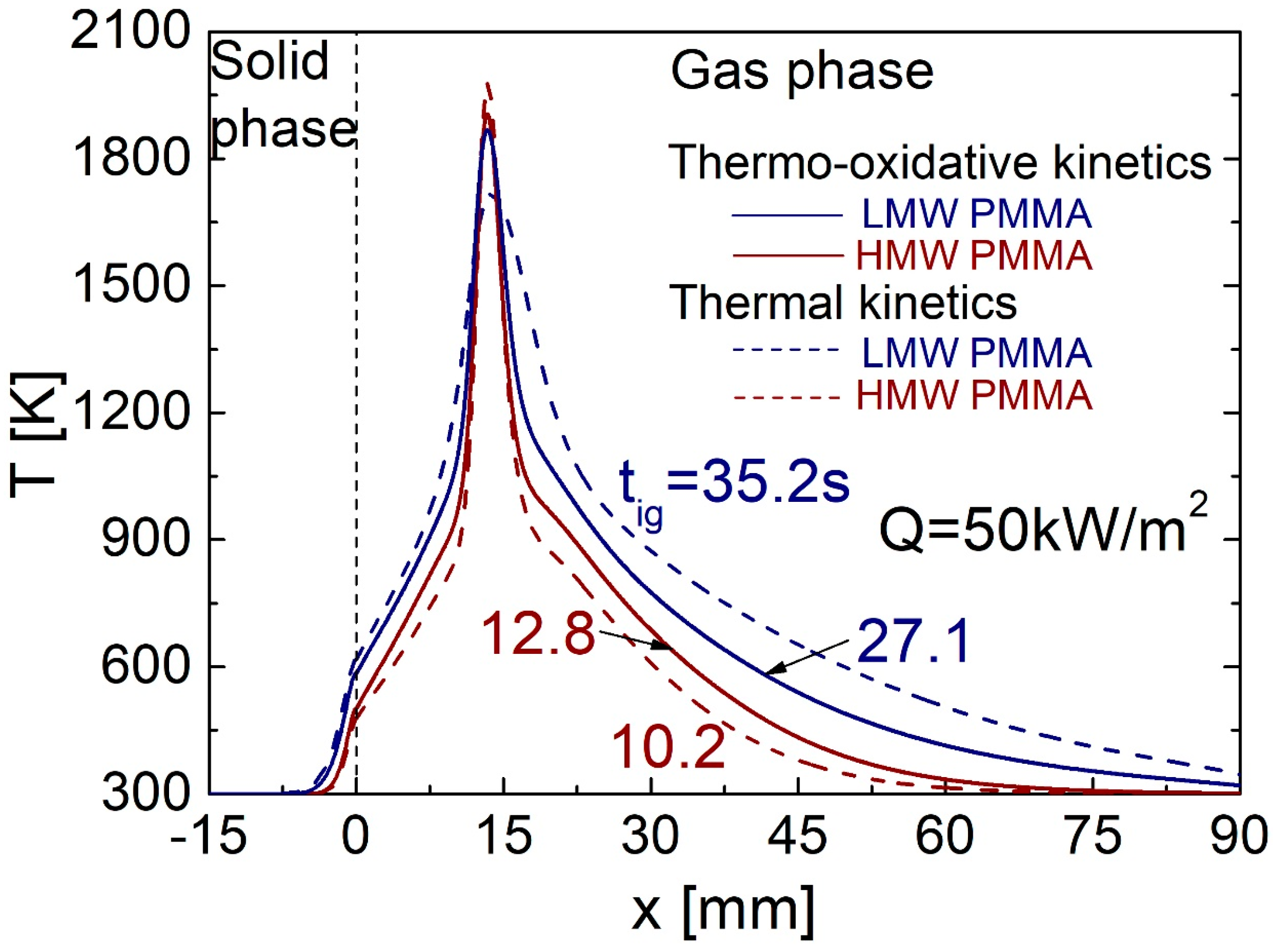
4.1. Piloted Ignition Characteristics for Q = 50 kW/m2
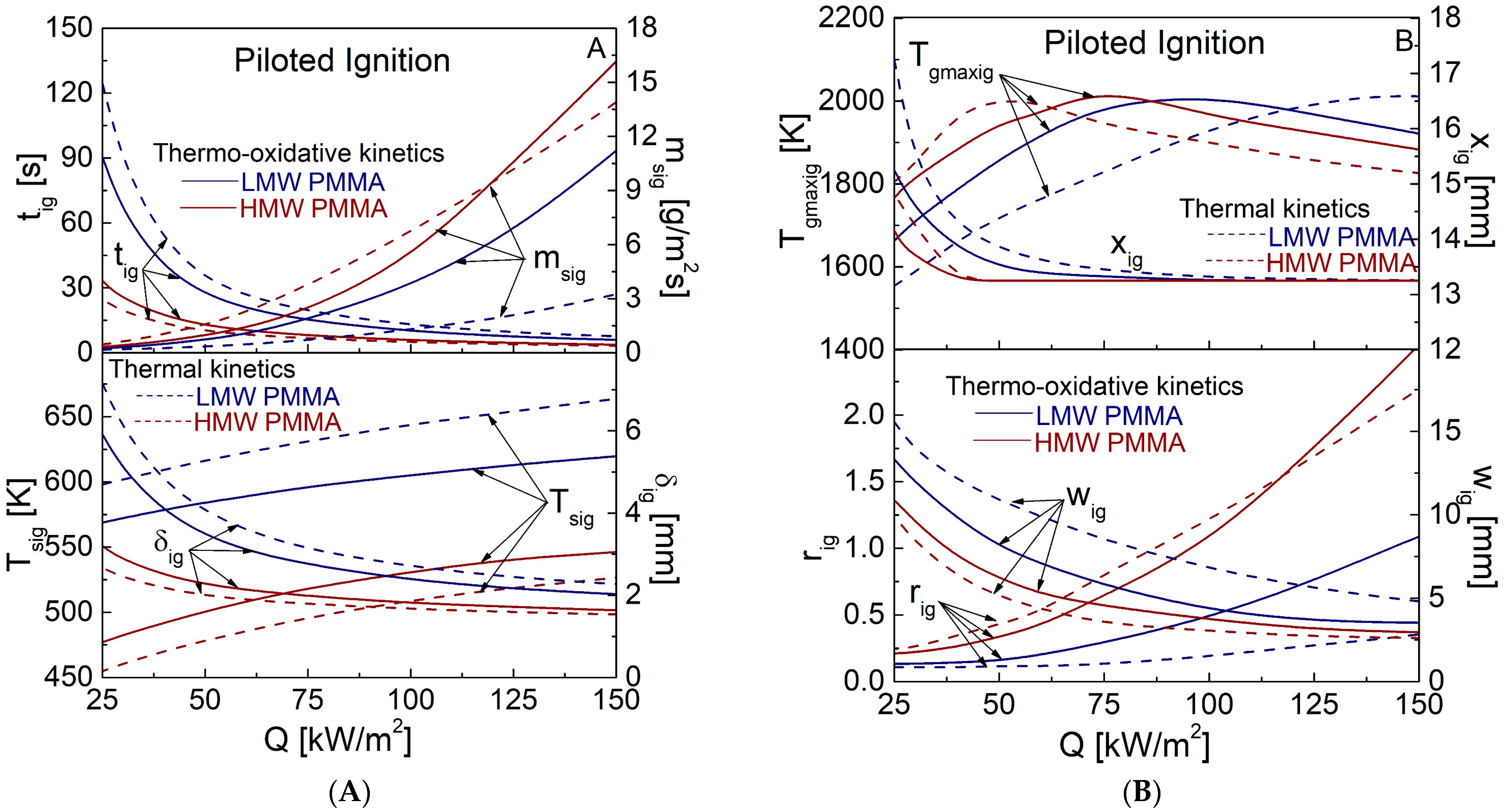
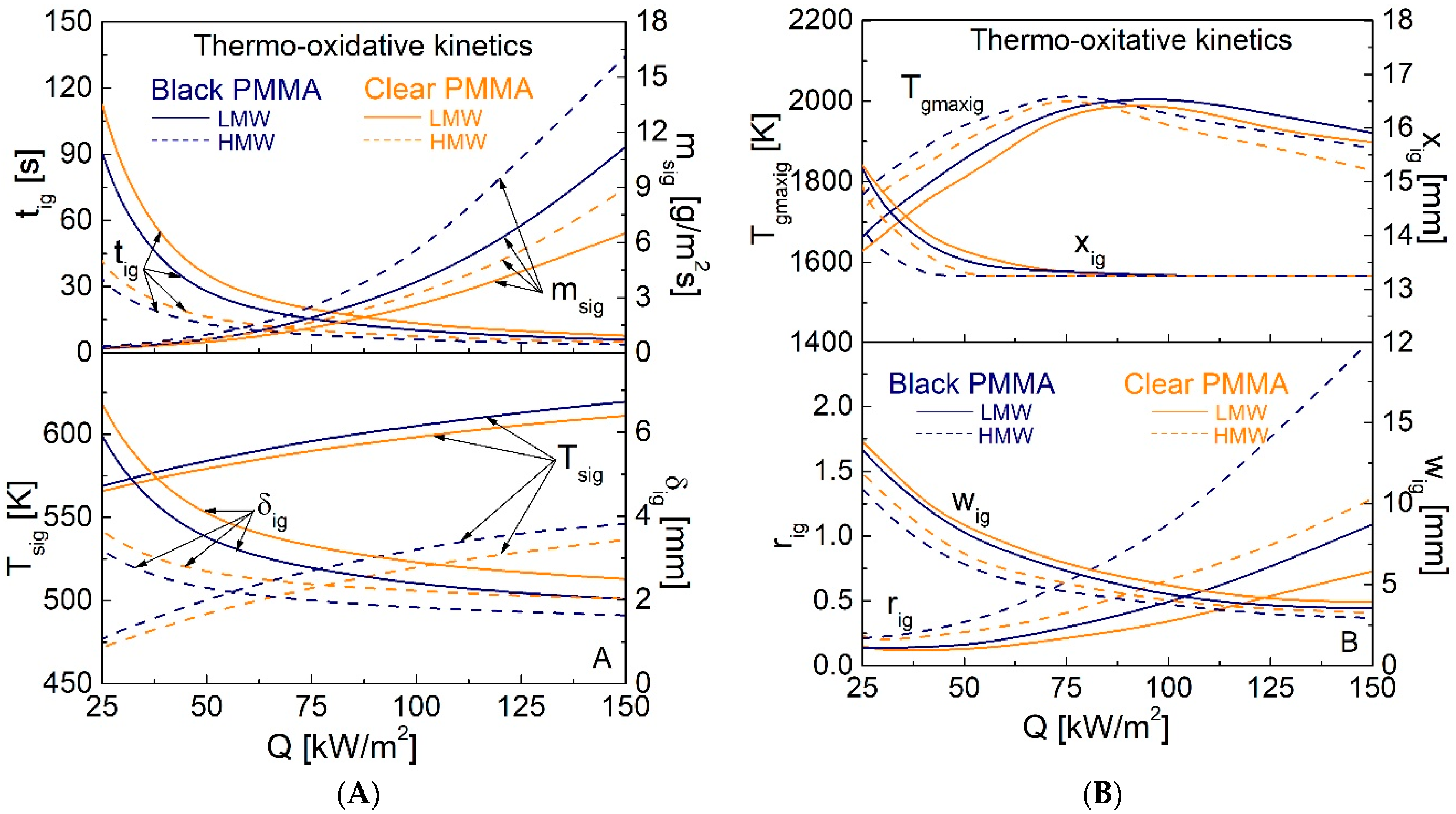
4.2. Piloted Ignition Characteristics for Q Values in the Range of 25–150 kW/m2
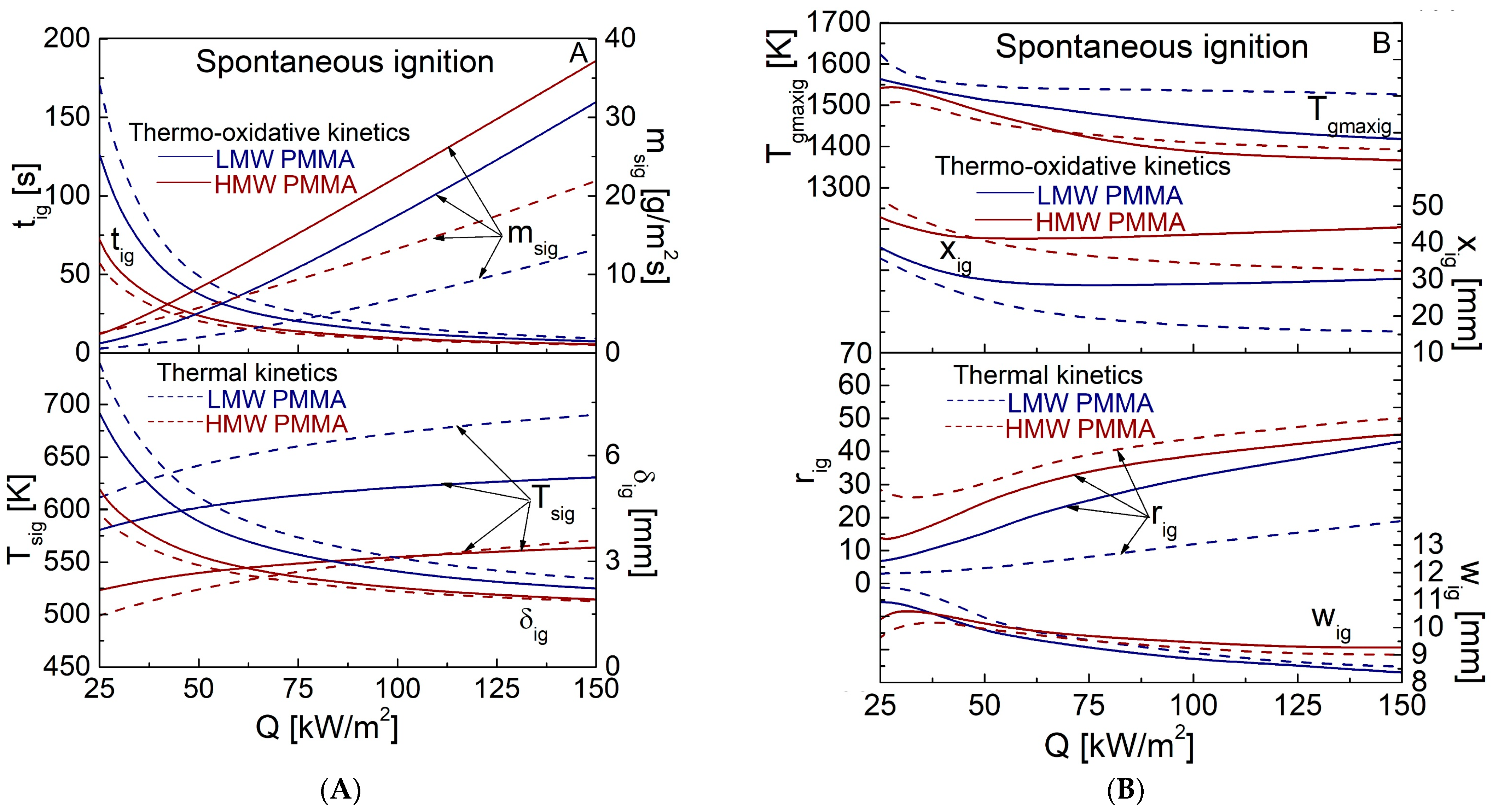
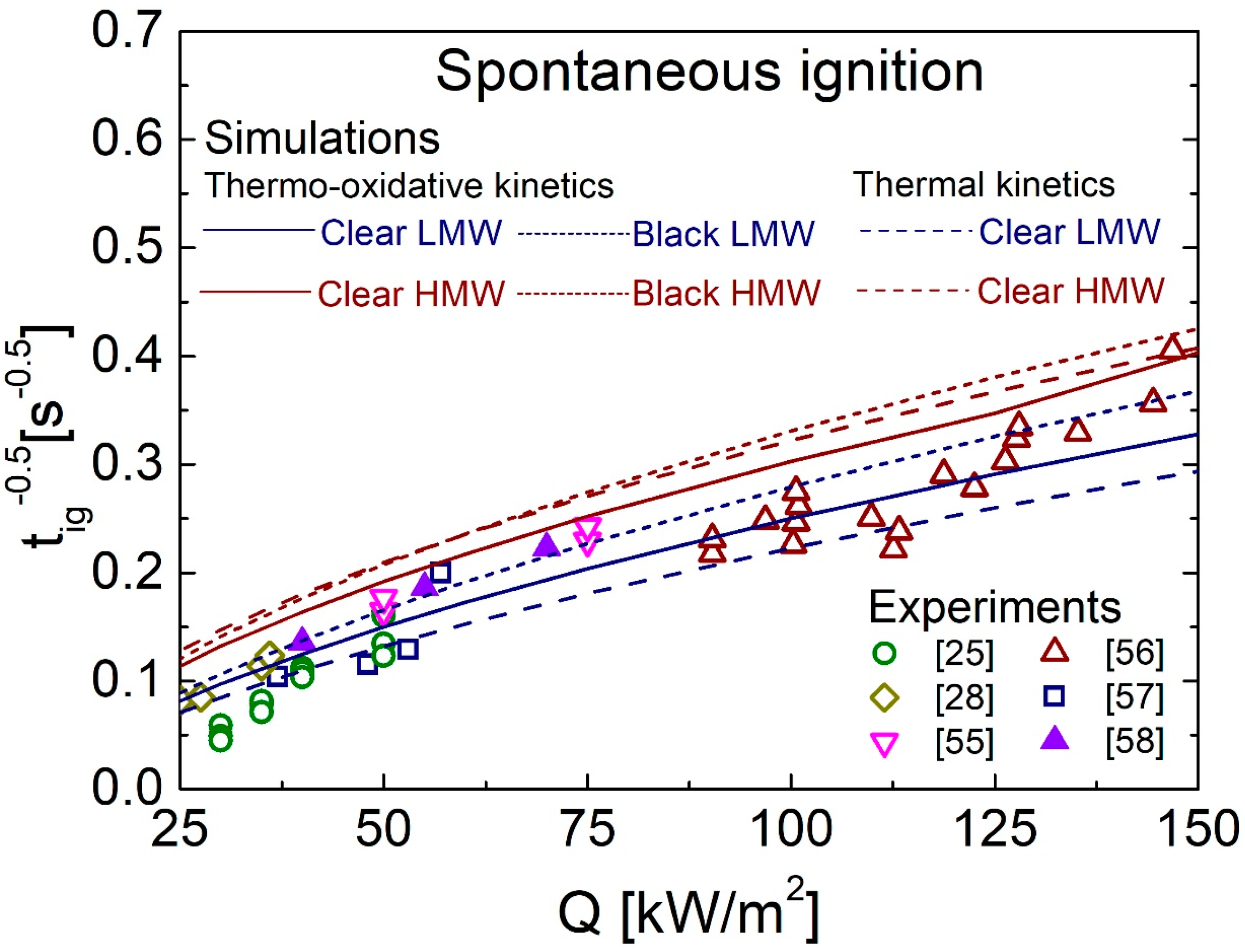
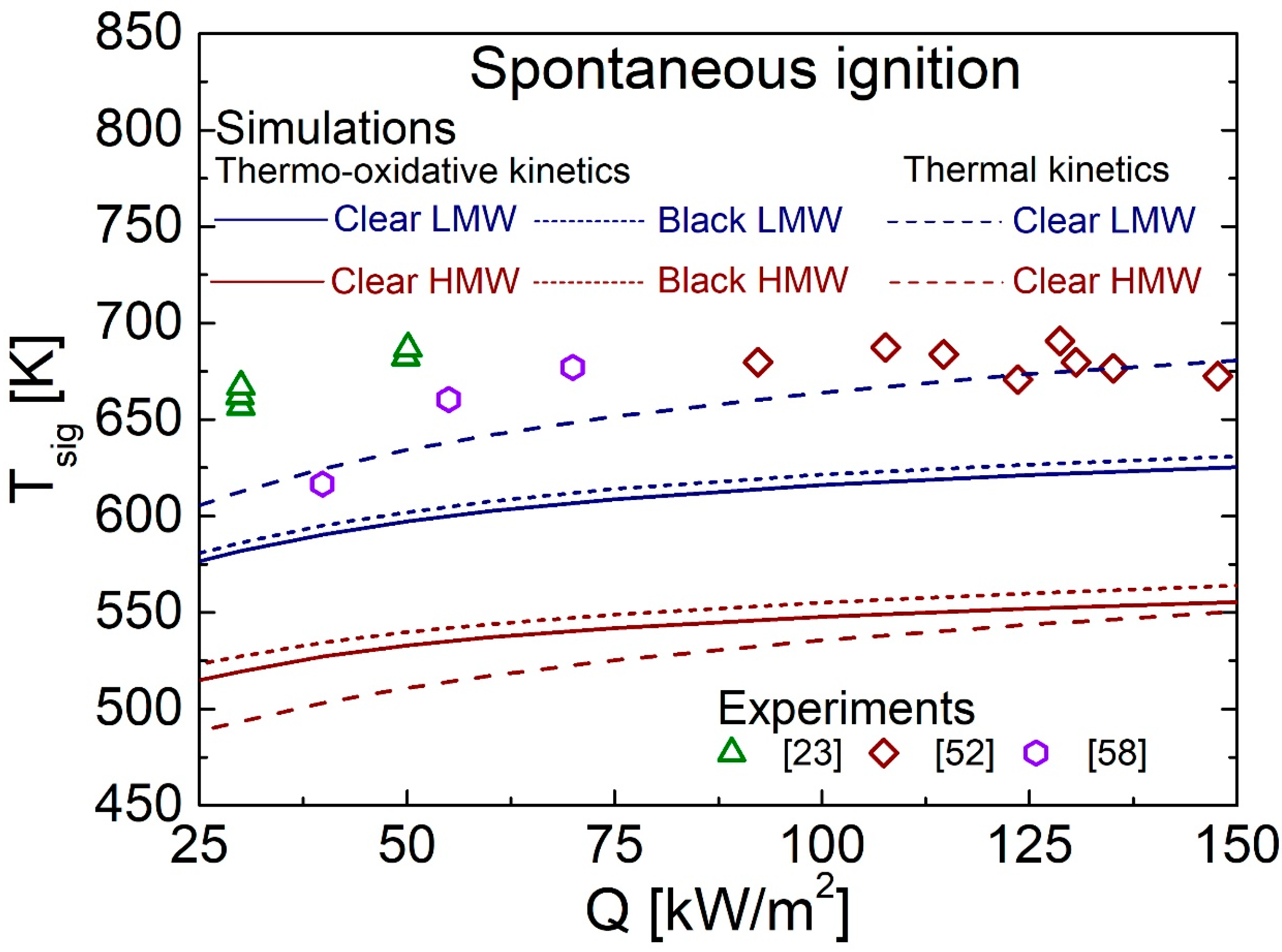
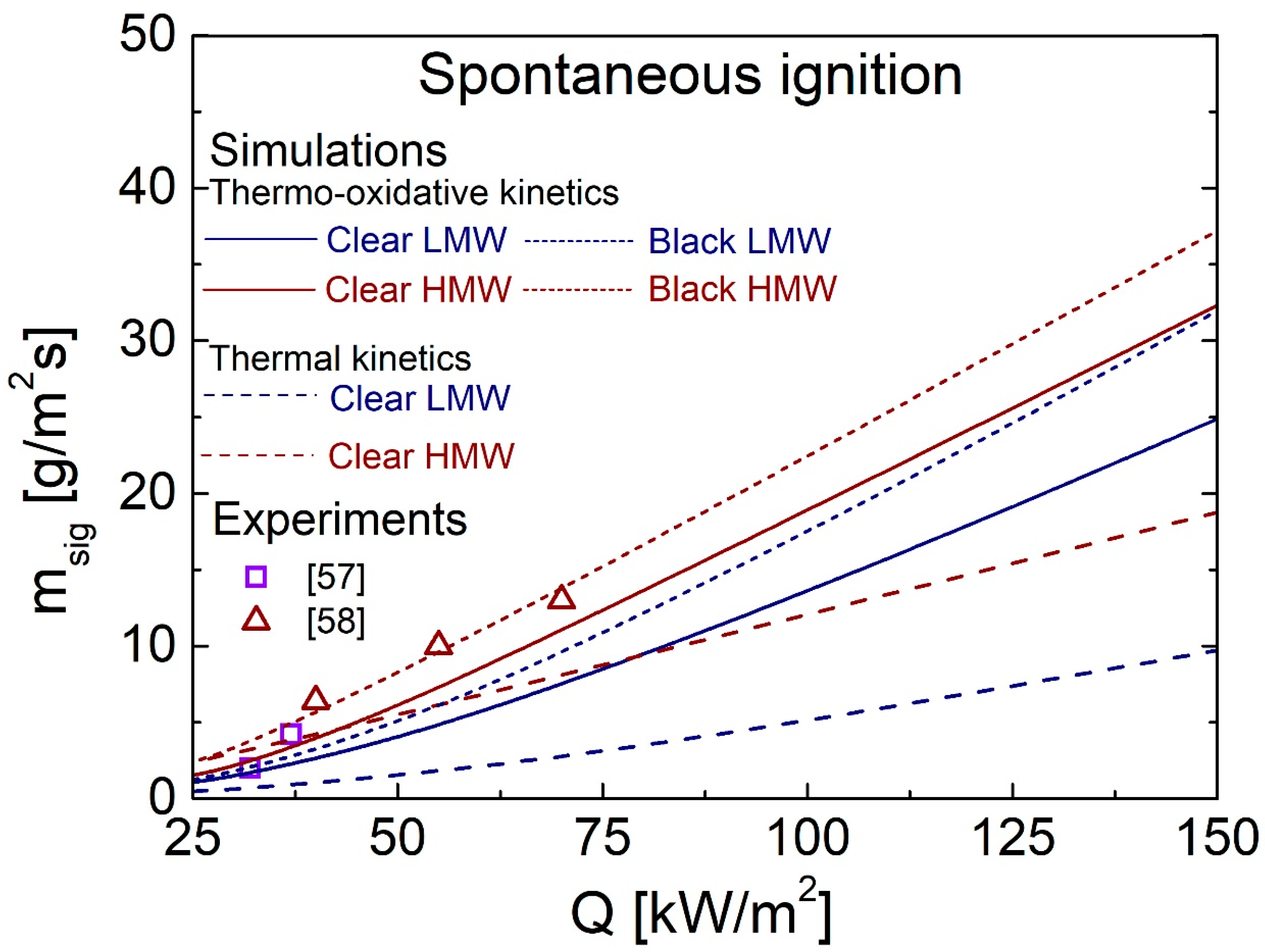
4.3. Spontaneous Ignition Characteristics for Q in the Range of 25–150 kW/m2
4.4. Effects of the Optical Properties
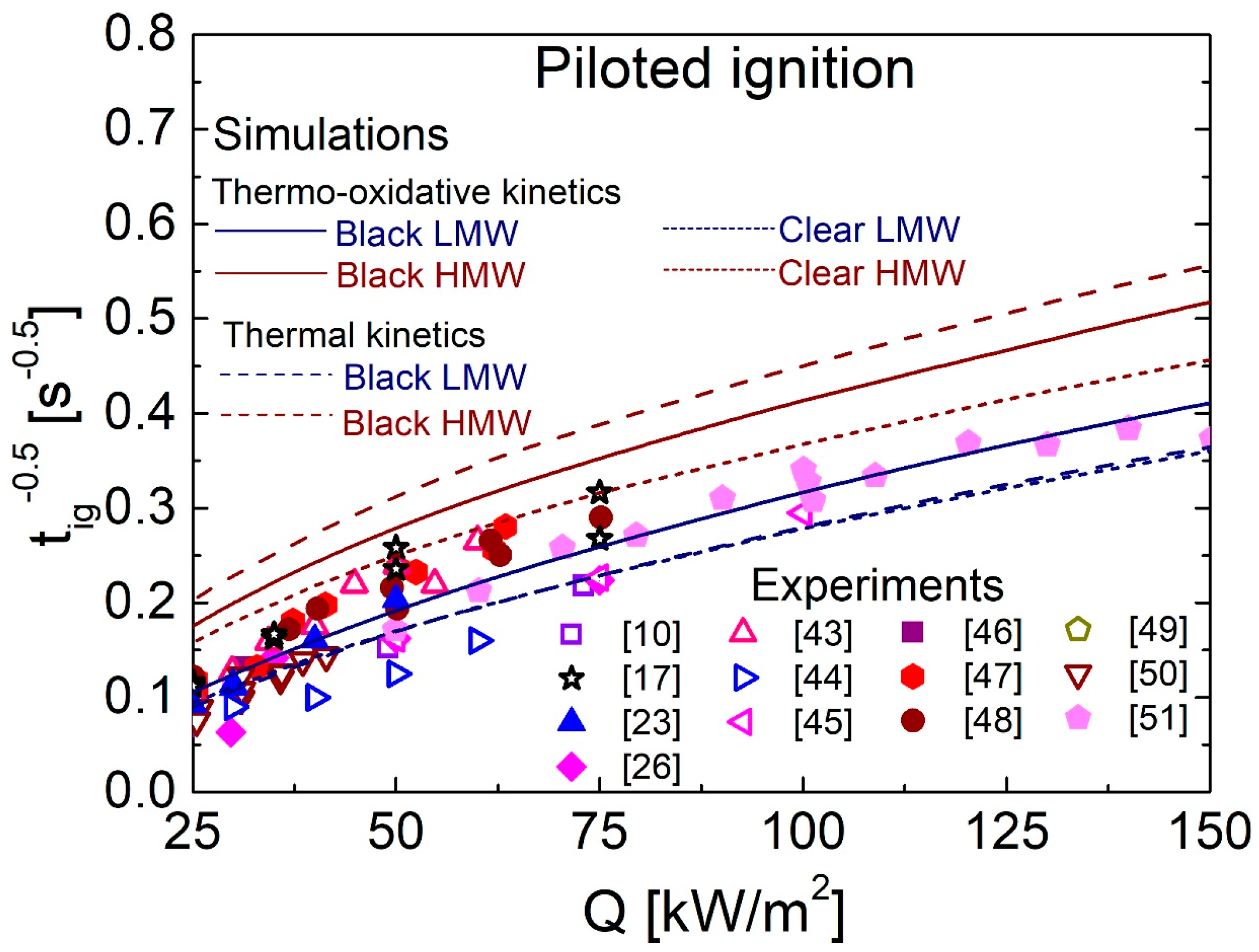
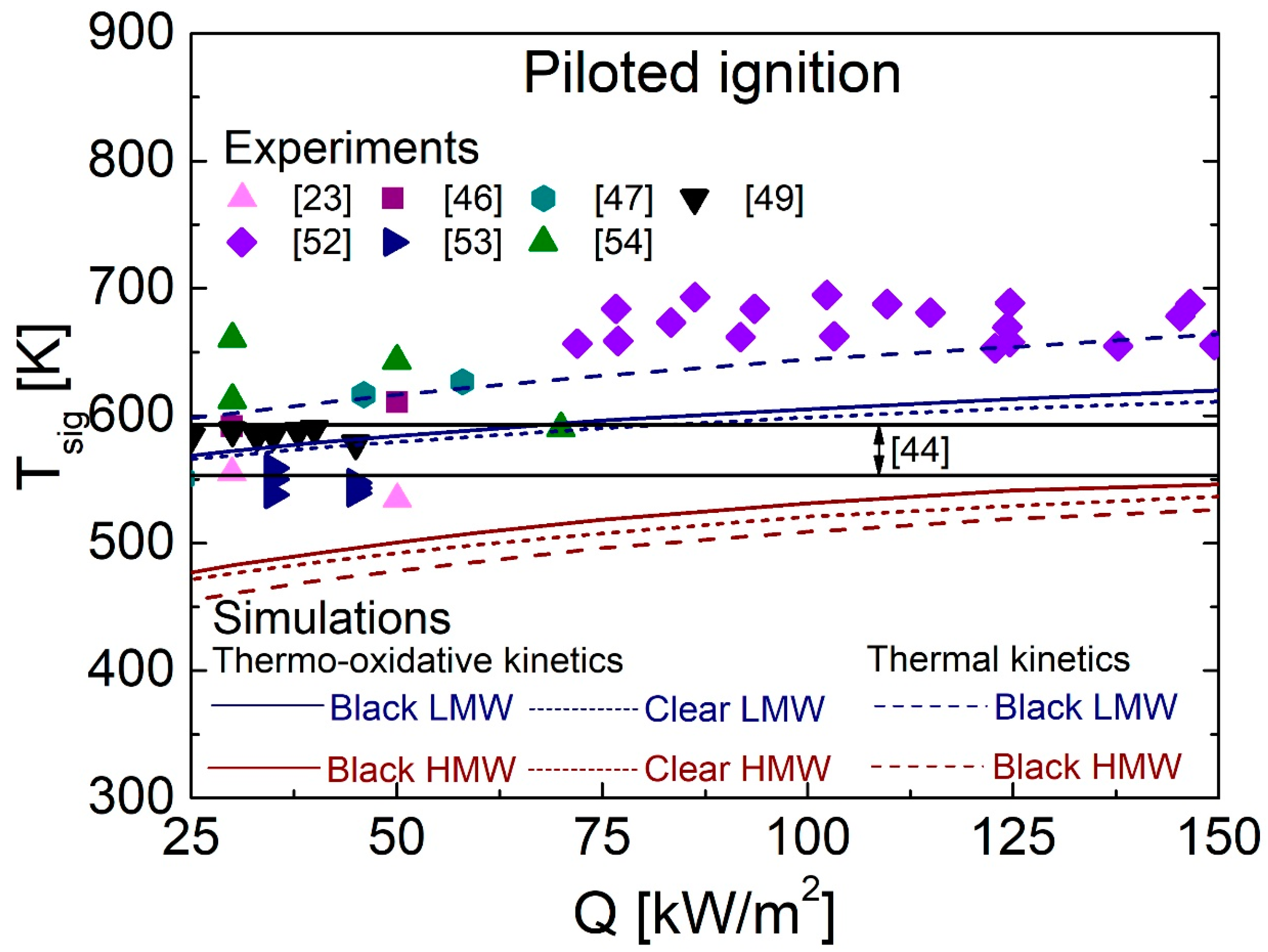
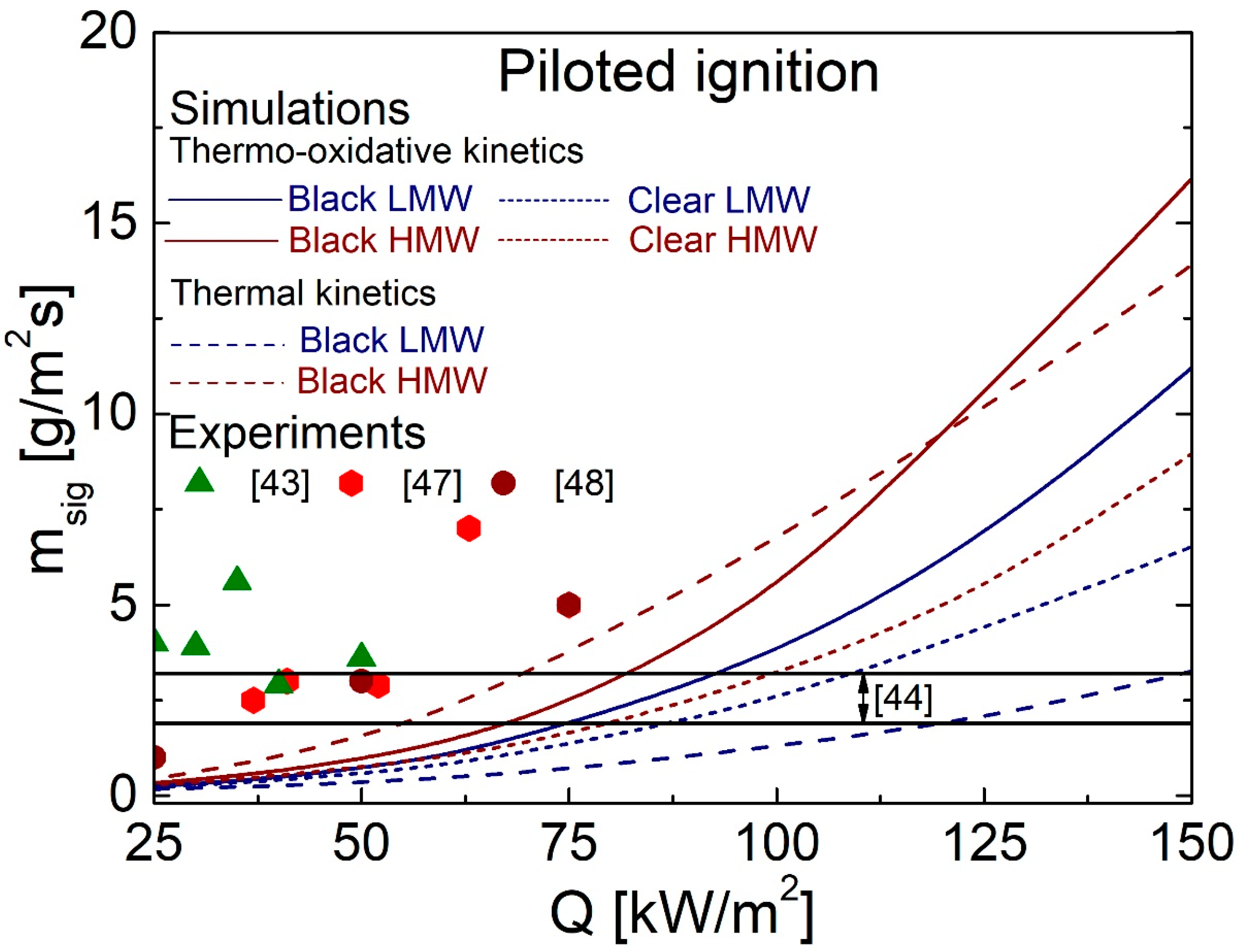
4.5. Comparison between Predictions and Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, N.; Sivanathan, K.; Mhaskar, P. Polymethyl Methacrylate Quality Modeling with Missing Data Using Subspace Based Model Identification. Processes 2021, 9, 1691. [Google Scholar] [CrossRef]
- Jayarama Krishna, J.V.; Srivatsa Kumar, S.; Korobeinichev, O.P.; Vinu, R. Detailed kinetic analysis of slow and fast pyrolysis of poly(methylmethacrylate)-Flame retardant mixtures. Thermochim. Acta 2020, 687, 178545. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, W.; Zhang, X.; Han, D.; Liu, W.; Ji, J. Pyrolysis and combustion behavior study of PMMA waste from micro-scale to bench-scale experiments. Fuel 2022, 319, 123717. [Google Scholar] [CrossRef]
- Yang, C.; Shang, H.; Li, J.; Fan, X.; Sun, J.; Duan, A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes 2023, 11, 1487. [Google Scholar] [CrossRef]
- Slavu, N.; Dinca, C. Clean Energy from Poplar and Plastic Mix Valorisation in a Gas Turbine with CO2 Capture Process. Processes 2023, 11, 2922. [Google Scholar] [CrossRef]
- Di Blasi, C. The state of the art of transport models for charring solid degradation. Polym. Int. 2000, 49, 1133–1146. [Google Scholar] [CrossRef]
- Galgano, A.; Di Blasi, C. One-dimensional modeling approaches for the piloted ignition of poly(methyl methacrylate). Fire Safety J. 2022, 129, 103566. [Google Scholar] [CrossRef]
- Galgano, A.; Di Blasi, C. Assessment of the predictive capabilities of a solid-gas model for poly (methyl methacrylate) ignition. Fire Safety J. 2024, 142, 104041. [Google Scholar] [CrossRef]
- van Blijderveen, M.; Bramer, E.A.; Brem, G. Modelling piloted ignition of wood and plastics. Waste Manag. 2012, 32, 1659–1668. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Lyon, R.E.; Linteris, G.P. Prediction of the burning rates of non-charring polymers. Combust. Flame 2009, 156, 1068–1083. [Google Scholar] [CrossRef]
- Bal, N.; Rein, G. Numerical investigation of the ignition delay time of a translucent solid at high radiant heat fluxes. Combust. Flame 2011, 158, 1109–1116. [Google Scholar] [CrossRef]
- Patel, A.; Hull, R.R.; Stec, A.A.; Lyon, R.E. Influence of physical properties on polymer flammability in the cone calorimeter. Polym. Advan Technol. 2011, 22, 1100–1107. [Google Scholar] [CrossRef]
- Linteris, G.T. Numerical simulations of polymer pyrolysis rate: Effect of property variations. Fire Mater. 2011, 35, 463–480. [Google Scholar] [CrossRef]
- Leventon, I.T.; Jing, L.; Stoliarov, S.I. A flame spread simulation based on a comprehensive solid pyrolysis model coupled with a detailed empirical flame structure representation. Combust. Flame 2015, 162, 3884–3895. [Google Scholar] [CrossRef]
- Vermesi, I.; Roenner, N.; Pironi, P.; Hadden, R.M.; Rein, G. Pyrolysis and ignition of a polymer by transient irradiation. Combust. Flame 2016, 163, 31–41. [Google Scholar] [CrossRef]
- Gong, J.; Chen, Y.; Jiang, J.; Yang, L.; Li, J. A numerical study of thermal degradation of polymers: Surface and in-depth absorption. Appl. Therm. Eng. 2016, 106, 1366–1379. [Google Scholar] [CrossRef]
- Galgano, A.; Branca, C.; Di Blasi, C.; Vollaro, P.; Milella, E. Modeling the ignition of poly(methyl methacrylate)/carbon nanotube nanocomposites. Polym. Degrad. Stabil. 2017, 144, 344–353. [Google Scholar] [CrossRef]
- Snegirev, A.; Kuznetsov, E.; Markus, E. Coupled analytical approach to predict piloted flaming ignition of non-charring polymers. Fire Saf. J. 2017, 93, 74–83. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, M.; Zhai, C.; Yang, L.; Wang, Y.Z.Z. Experimental, analytical and numerical investigation on auto-ignition of thermally intermediate PMMA imposed to linear time-increasing heat flux. Appl. Therm. Eng. 2020, 172, 115137. [Google Scholar] [CrossRef]
- Kashiwagi, T. A radiative ignition model of a solid fuel. Combust. Sci. Technol. 1974, 8, 225–236. [Google Scholar] [CrossRef]
- Di Blasi, C.; Crescitelli, S.; Russo, G.; Cinque, G. Numerical model of ignition processes of polymeric materials including gas phase absorption of radiation. Combust. Flame 1991, 83, 333–344. [Google Scholar] [CrossRef]
- Brescianini, C.P.; Yeoh, G.H.; Chandrasekaran, V.; Yuen, R. A numerical model for pilot ignition of PMMA in a cone calorimeter. Combust. Sci. Technol. 1997, 129, 321–345. [Google Scholar] [CrossRef]
- Tsai, T.H.; Li, M.J.; Shih, I.Y.; Jih, R.; Wong, S.C. Experimental and numerical study of autoignition and pilot ignition of PMMA plates in a cone calorimeter. Combust. Flame 2001, 124, 466–480. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kashiwagi, T. Effects of sample orientation on nonpiloted ignition of thin poly(methyl methacrylate) sheet by a laser. 1. Theoretical prediction. Combust. Flame 2005, 141, 149–169. [Google Scholar] [CrossRef]
- Esfahani, J.A.; Kashani, A. One-dimensional numerical model for degradation and combustion of polymethyl methacrylate. Heat Mass. Transfer 2006, 42, 569–576. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Cui, J.; Wang, X.; Zhang, J. Theoretical calculation on the piloted ignition of PMMA. J. Macromol. Sci. B 2007, 46, 475–485. [Google Scholar] [CrossRef]
- Fereres, S.; Lautenberger, C.; Fernandez-Pello, A.C.; Urban, D.L.; Ruff, G.A. Understanding ambient pressure effects on piloted ignition through numerical modeling. Combust. Flame 2012, 159, 3544–3553. [Google Scholar] [CrossRef]
- Peng, F.; Zhou, X.D.; Zhao, K.; Wu, Z.B.; Yang, L.Z. Experimental and numerical study on effect of sample orientation on auto-ignition and piloted ignition of poly(methyl methacrylate). Materials 2015, 8, 4004–4021. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, T.; Chen, Q.; Wang, J. Numerical study on the effect of oxygen concentration on the piloted ignition of PMMA in reduced pressure atmospheres. Int. J. Numer. Method. Heat Fluid Flow 2020, 30, 3903–3917. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of thermal decomposition of PMMA at different heating rates and in a wide temperature range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Chang, T.C.; Yu, P.Y.; Hong, Y.S.; Wu, T.R.; Chiu, Y.S. Effect of phenolic phosphite antioxidant on the thermo-oxidative degradation of PMMA. Polym. Degrad. Stabil. 2002, 77, 29–34. [Google Scholar] [CrossRef]
- Di Blasi, C.; Galgano, A.; Branca, C. Modeling the thermal degradation of poly(methyl methacrylate)/carbon nanotube nanocomposites. Polym. Degrad. Stabil. 2013, 98, 266–275. [Google Scholar] [CrossRef]
- Kang, B.S.; Kim, S.G.; Kim, J.S. Thermal degradation of poly(methyl methacrylate) polymers: Kinetics and recovery of monomers using a fluidized bed reactor. J. Anal. Appl. Pyrol. 2008, 8, 7–13. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stabil. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Hirata, T.; Kashiwagi, T.; Brown, J.E. Thermal and oxidative degradation of poly(methylmethacrylate): Weight loss. Macromolecules 1985, 18, 1410–1418. [Google Scholar] [CrossRef]
- Fina, A.; Camino, G. Ignition mechanisms in polymers and polymer nano-composites. Polym. Adv. Technol. 2011, 22, 1147–1155. [Google Scholar] [CrossRef]
- Manring, L.E.; Sogah, D.Y.; Cohen, G.M. Thermal degradation of poly(methyl-methacrylate. 3. Polymer with head-to-head linkages. Macromolecules 1989, 22, 4652–4654. [Google Scholar] [CrossRef]
- Amos, B.; Fernandez-Pello, A.C. Model of the ignition and flame development on a vaporizing combustible surface in a stagnation point flow: Ignition by vapor fuel radiation absorption. Combust. Sci. Technol. 1988, 62, 331–343. [Google Scholar] [CrossRef]
- Wilke, C.R.; Lee, C.Y. Estimation of diffusion coefficients for gases and vapors. Ind. Eng. Chem. 1955, 47, 1253–1257. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry's Chemical Engineers' Handbook, 6th ed.; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Stoliarov, S.I.; Walters, R.N. Determination of the heats of gasification of polymers using differential scanning calorimetry. Polym. Degrad. Stabil. 2008, 93, 422–427. [Google Scholar] [CrossRef]
- Linteris, G.; Zammarano, M.; Wilthan, B.; Hanssen, L. Absorption and reflection of infrared radiation by polymers in fire-like environments. Fire Mater. 2012, 36, 537–553. [Google Scholar] [CrossRef]
- Luche, J.; Rogaume, T.; Richard, F.; Guillame, E. Characterization of thermal properties and analysis of combustion behaviour of PMMA in a cone calorimeter. Fire Saf. J. 2011, 46, 451–461. [Google Scholar] [CrossRef]
- Lyon, R.E.; Quintiere, J.G. Criteria for piloted ignition of combustible solids. Combust. Flame 2007, 151, 551–559. [Google Scholar] [CrossRef]
- Babrauskas, V.; Parker, W. Ignitability measurements with cone calorimeter. Fire Mater. 1987, 11, 31–43. [Google Scholar] [CrossRef]
- Thompson, H.E.; Drysdale, D.D. Effect of sample orientation on the piloted ignition of PMMA. In Proceedings of the 5th International Conference Interflam ‘90, Canterbury, UK, 3–6 September 1990. [Google Scholar]
- Rhodes, B.T. Burning Rate and Flame Heat Flux for PMMA in the Cone Calorimeter. Master’s Thesis, Grant/Contract Reports (NISTGCR), National Institute of Standards and Technology, University of Maryland, Gaithersburg, MD, USA, 1994. [Google Scholar]
- Hopkins, D., Jr.; Quintiere, J.G. Material fire properties and predictions for thermoplastics. Fire Safety J. 1996, 26, 241–268. [Google Scholar] [CrossRef]
- Cordova, J.L.; Walther, D.C.; Torero, J.L.; Fernandez-Pello, A.C. Oxidizer flow effects on the flammability of solid combustibles. Combust. Sci. Technol. 2001, 164, 253–278. [Google Scholar] [CrossRef]
- Dakka, S.M.; Jackson, G.S.; Torero, J.L. Mechanisms controlling the degradation of poly(methylmethacrylate) prior to piloted ignition. Proc. Combust. Inst. 2002, 29, 281–287. [Google Scholar] [CrossRef]
- Beaulieu, P.A.; Dembsey, N.A. Flammability characteristics at applied heat flux levels up to 200 kW/m2. Fire Mater. 2008, 32, 61–86. [Google Scholar] [CrossRef]
- Kashiwagi, T. Experimental observation of radiative ignition mechanisms. Combust. Flame 1979, 34, 231–244. [Google Scholar] [CrossRef]
- Long, R.T., Jr.; Torero, J.L.; Quintiere, J.G.; Fernandez-Pello, A.C. Scale and transport considerations on piloted ignition of PMMA. In Fire Safety Science—Proceedings of the 6th International Symposium; International Association for Fire Safety Science: London, UK, 1999. [Google Scholar]
- Beaulieu, P.A. Flammability Characteristics at Heat Flux Levels Up to 200 KW/m2 and the Effect of Oxygen on Flame Heat Flux. Ph.D. Thesis, Worcester Polytechnic Institute, MA, USA, 2005. [Google Scholar]
- Shi, L.; Chew, M.Y.L. Fire behaviors of polymers under autoignition conditions in a cone calorimeter. Fire Safety J. 2013, 61, 243–253. [Google Scholar] [CrossRef]
- Kashiwagi, T. Effects of sample orientation on radiative ignition. Combust. Flame 1982, 44, 223–245. [Google Scholar] [CrossRef]
- Zhou, Y.; Lizhong, Y.; Jiakun, D.; Yafei, W.; Zhihua, D. Radiation attenuation characteristics of pyrolysis volatiles of solid fuels and their effect for radiation ignition model. Combust. Flame 2010, 157, 167–175. [Google Scholar] [CrossRef]
- Huang, D.; Wang, C.; Shen, Y.; Lin, P.; Shi, L. Fire behaviors of vertical and horizontal polymethyl methacrylate slabs under autoignition conditions. Process Saf. Prog. 2020, 39, e12109. [Google Scholar] [CrossRef]

| LMW Air | LMW N2 | HMW Air | HMW N2 | ||
|---|---|---|---|---|---|
| 1st step | Ad1 [s−1] | 3.3 × 1020 | 6.02 × 1012 | 1.3 × 1012 | 1.3 × 1012 |
| Ed1 [kJ/mol] | 260.6 | 188 | 118.0 | 118.0 | |
| nd1 | 1.0 | 1.0 | 1.4 | 1.4 | |
| νd1 | 1.0 | 1.0 | 0.02 | 0.07 | |
| 2nd step | Ad2 [s−1] | 6.0 × 1027 | 8.2 × 108 | ||
| Ed2 [kJ/mol] | 290.0 | 112.2 | |||
| nd2 | 2.65 | 0.97 | |||
| νd2 | 0.32 | 0.14 | |||
| 3rd step | Ad3 [s−1] | 1.3 × 1012 | 1.0 × 1013 | ||
| Ed3 [kJ/mol] | 149.4 | 149.4 | |||
| nd3 | 1.2 | 1.12 | |||
| νd3 | 0.23 | 0.13 | |||
| 4th step | Ad4 [s−1] | 6.0 × 1013 | 2.2 × 1012 | ||
| Ed4 [kJ/mol] | 175.4 | 175.4 | |||
| nd4 | 2.1 | 1.25 | |||
| νd4 | 0.43 | 0.66 | |||
| Parameters | Values | References | |
|---|---|---|---|
| Ac | 1.6 × 1015 | [KgK2/m3s] | [38] |
| DMMA0 | 8.83 × 10−6 | [m2/s] | [39] |
| DO20 | 2.075 × 10−5 | [m2/s] | [40] |
| DP0 | 1.85 × 10−5 | [m2/s] | [40] |
| d | 0.005 | [s] | [23] |
| Ec | 180 | [kJ/mol] | [38] |
| f | 0.1 | [s] | [23] |
| MMMA | 100.1 | [kg/kmol] | [40] |
| Tspark | 1623 | [K] | [29] |
| Ttrans | 403 | [K] | [10] |
| xi | 0.013 | [m] | [23] |
| βg | 4.0 | [m−1] | [21] |
| Δhc | 2.592 × 107 | [J/kg] | [21] |
| Δhd | 8.7 × 105 | [J/kg] | [41] |
| ρp | 1200 | [kg/m3] | [10] |
| νc | 1.92 | [40] | |
| Clear PMMA | |||
| es | 0.86 | [32] | |
| βs | 1870 | [m−1] | [42] |
| Black PMMA | |||
| es | 0.945 | [11] | |
| βs | 2620 | [m−1] | [42] |
| Material | Decomposition Kinetics | tig [s] | Tsig [K] | msig [g/m2 s] | δig [mm] | xmax [mm] | Tgmaxig [K] | rig | wig [mm] |
|---|---|---|---|---|---|---|---|---|---|
| Black LMW PMMA | Thermal | 35.2475 | 616 | 0.35 | 4.04 | 13.8 | 1719 | 0.12 | 10.9 |
| Thermo-oxidative | 27.1059 | 584 | 0.73 | 3.47 | 13.2 | 1878 | 0.16 | 8.1 | |
| Black HMW PMMA | Thermal | 10.2063 | 478 | 1.56 | 2.0 | 13.2 | 2003 | 0.47 | 5.1 |
| Thermo-oxidative | 12.8059 | 501 | 0.97 | 2.27 | 13.2 | 1927 | 0.34 | 6.2 |
| Material | Decomposition Kinetics | tig [s] | Tsig [K] | msig [g/m2 s] | δig [mm] | xmax [mm] | Tgmaxig [K] | rig | wig [mm] |
|---|---|---|---|---|---|---|---|---|---|
| Clear LMW PMMA | Thermal | 44.2545 | 610 | 0.30 | 4.64 | 13.8 | 1689 | 0.098 | 11.4 |
| Thermo-oxidative | 34.5168 | 579 | 0.58 | 4.04 | 13.8 | 1810 | 0.13 | 8.8 | |
| Clear HMW PMMA | Thermal | 12.8053 | 470 | 1.18 | 2.37 | 13.2 | 1957 | 0.32 | 5.6 |
| Thermo-oxidative | 16.0071 | 492 | 0.75 | 2.67 | 13.2 | 1904 | 0.26 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, A.; Di Blasi, C. Effects of the PMMA Molecular Weight on the Thermal and Thermo-Oxidative Decomposition as the First Chemical Stage of Flaming Ignition. Processes 2024, 12, 219. https://doi.org/10.3390/pr12010219
Galgano A, Di Blasi C. Effects of the PMMA Molecular Weight on the Thermal and Thermo-Oxidative Decomposition as the First Chemical Stage of Flaming Ignition. Processes. 2024; 12(1):219. https://doi.org/10.3390/pr12010219
Chicago/Turabian StyleGalgano, Antonio, and Colomba Di Blasi. 2024. "Effects of the PMMA Molecular Weight on the Thermal and Thermo-Oxidative Decomposition as the First Chemical Stage of Flaming Ignition" Processes 12, no. 1: 219. https://doi.org/10.3390/pr12010219
APA StyleGalgano, A., & Di Blasi, C. (2024). Effects of the PMMA Molecular Weight on the Thermal and Thermo-Oxidative Decomposition as the First Chemical Stage of Flaming Ignition. Processes, 12(1), 219. https://doi.org/10.3390/pr12010219






