A Coupling Calculation Method of Desorption Energy Distribution Applied to CO2 Capture by Chemical Absorption
Abstract
1. Introduction
2. Methodology
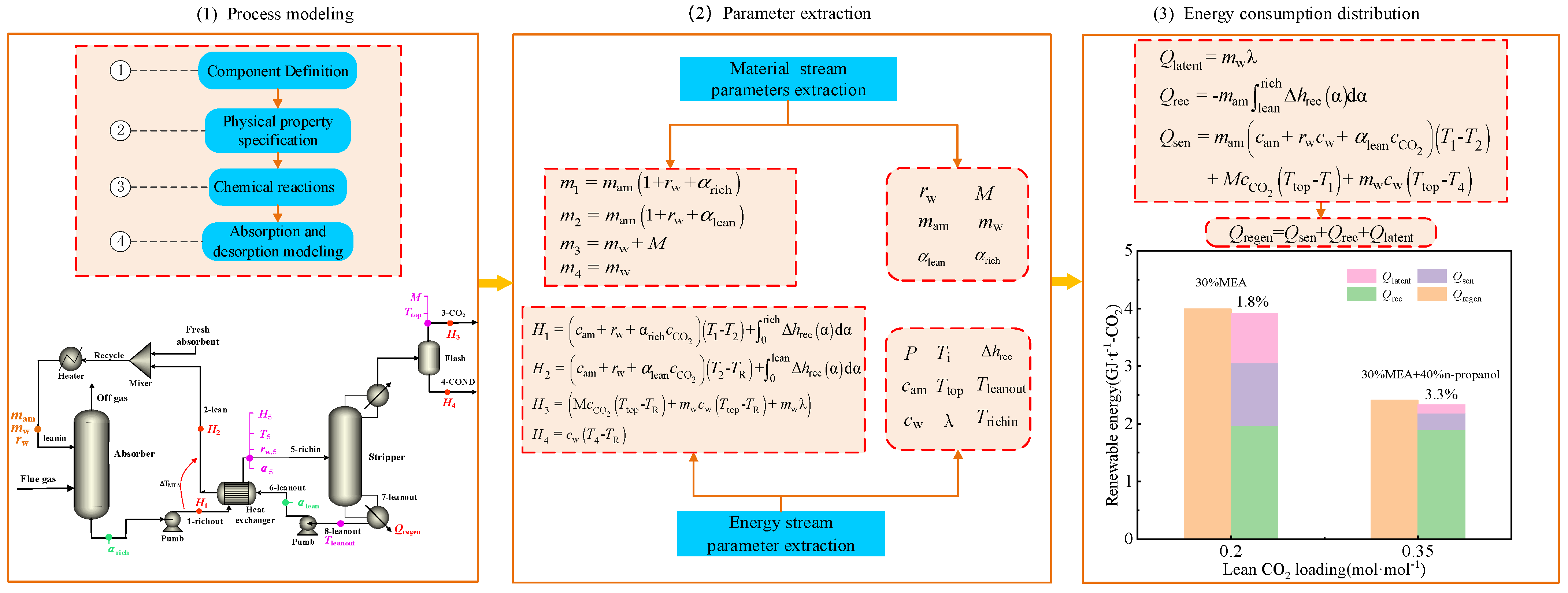
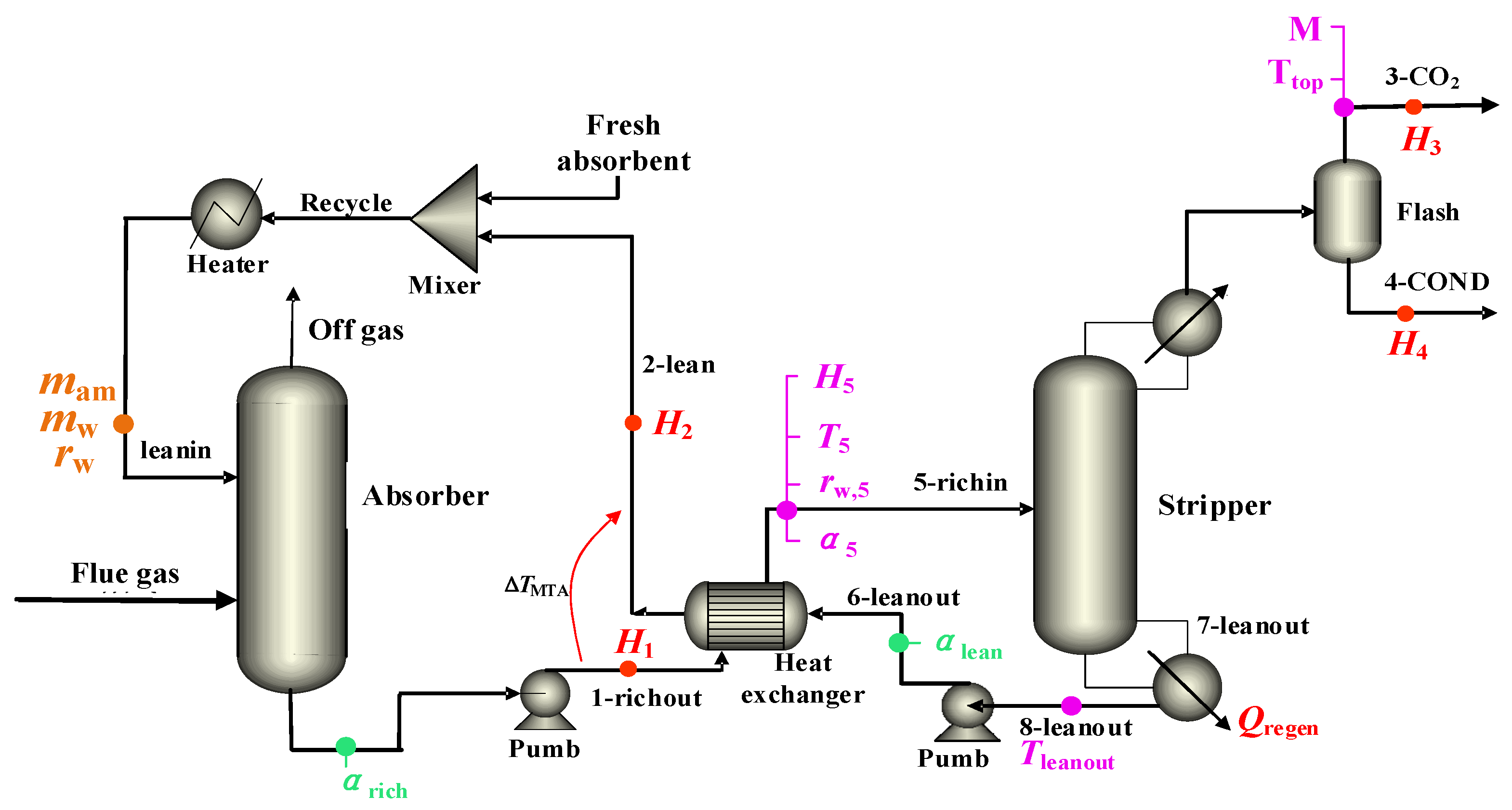
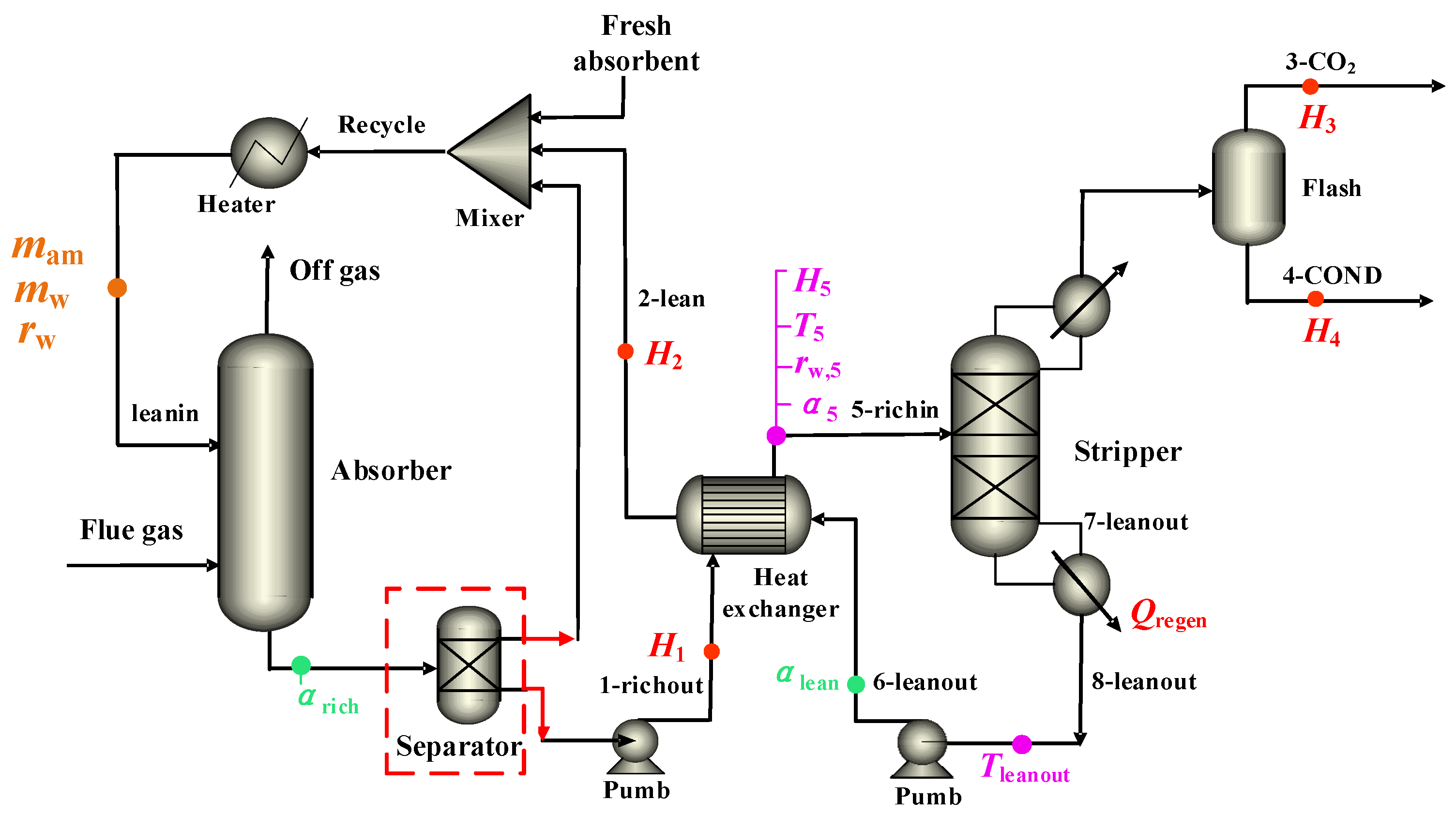
2.1. Process Modeling
2.2. Parameter Extraction
2.3. Energy Consumption Distribution
2.4. Determination of CO2 Loading
2.5. Case Analysis
2.5.1. Case I: CO2 Capture Process with 30% MEA Aqueous Solution
2.5.2. Case II: Phase-Change CO2 Capture Process
3. Results and Discussions
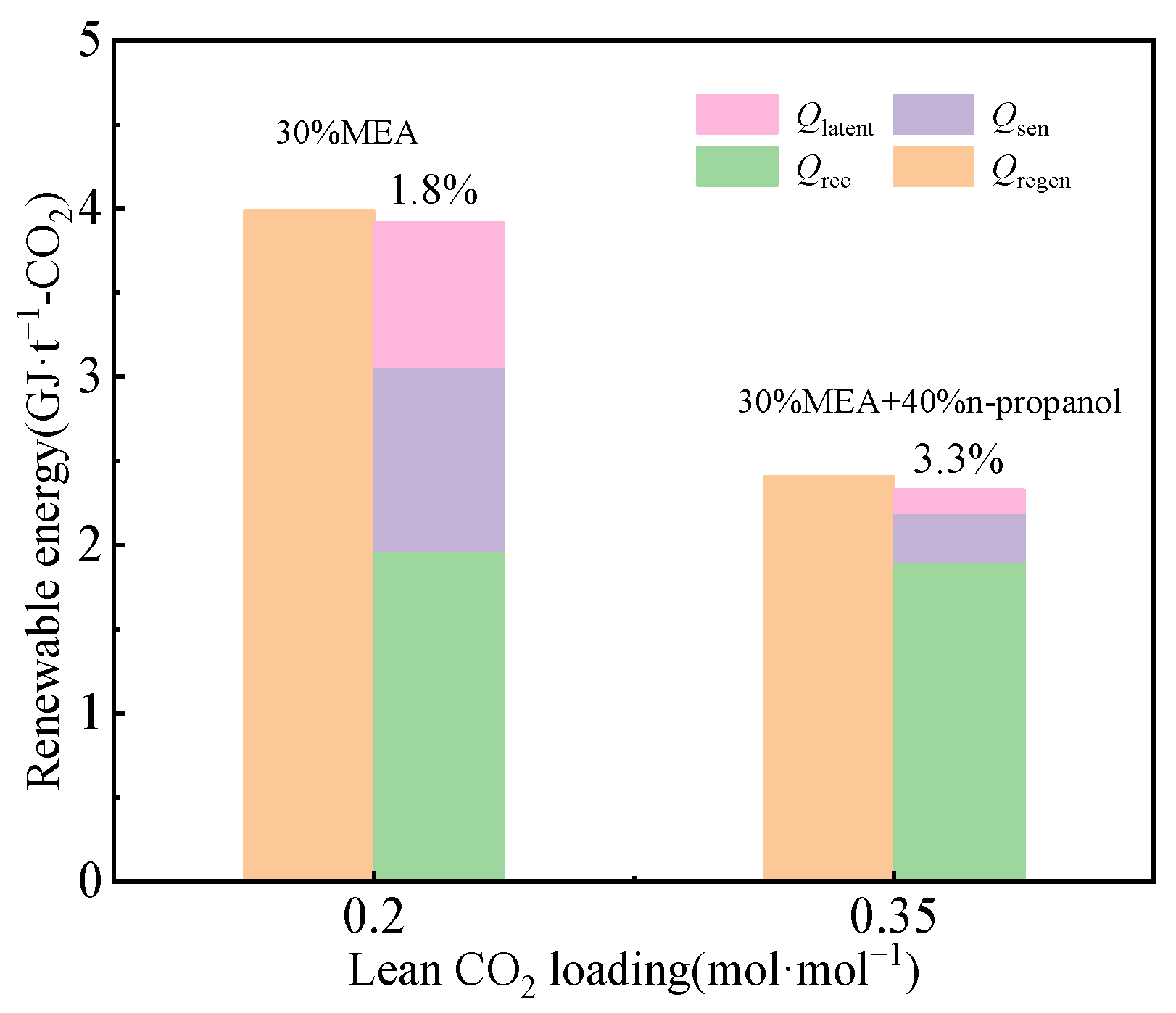
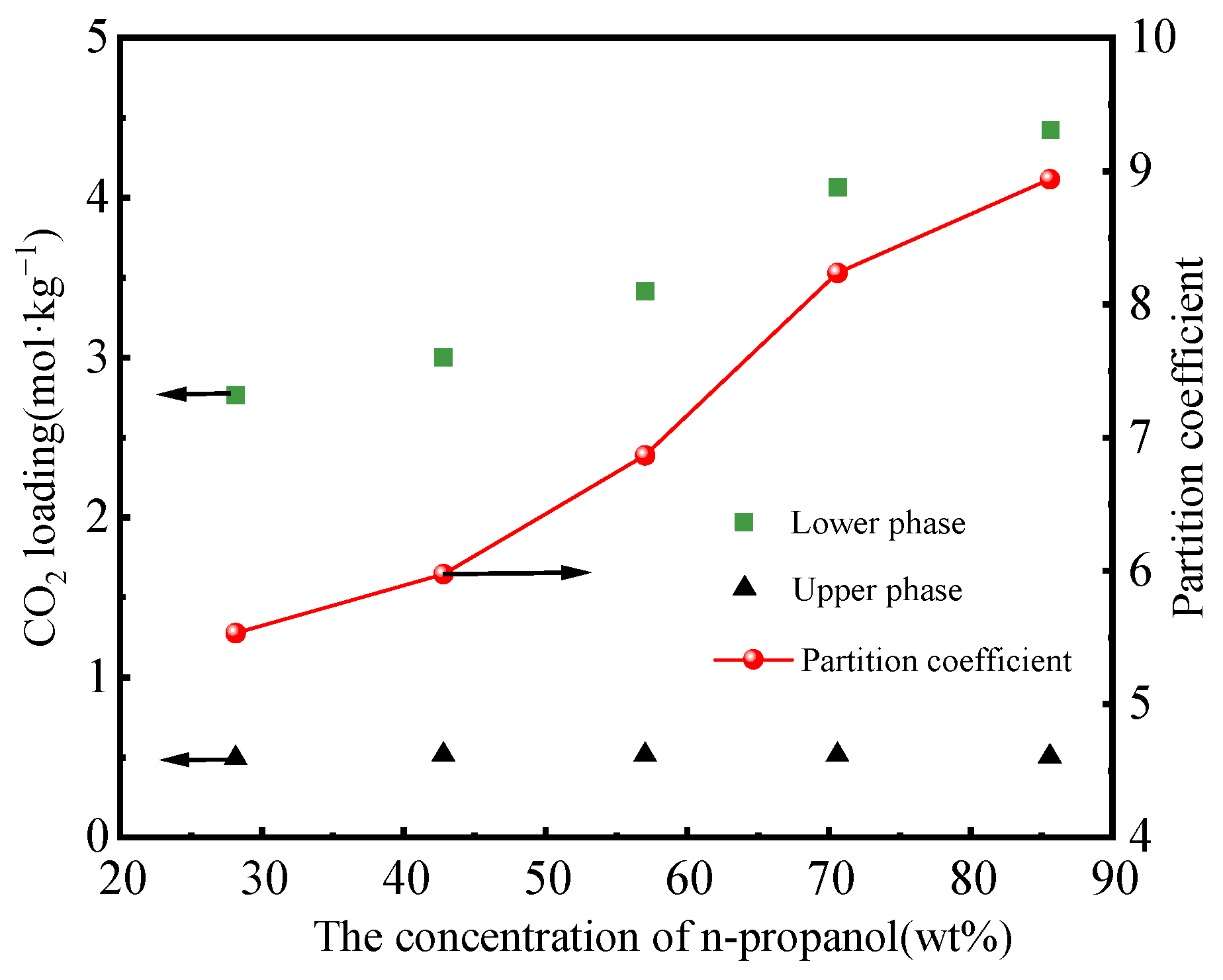
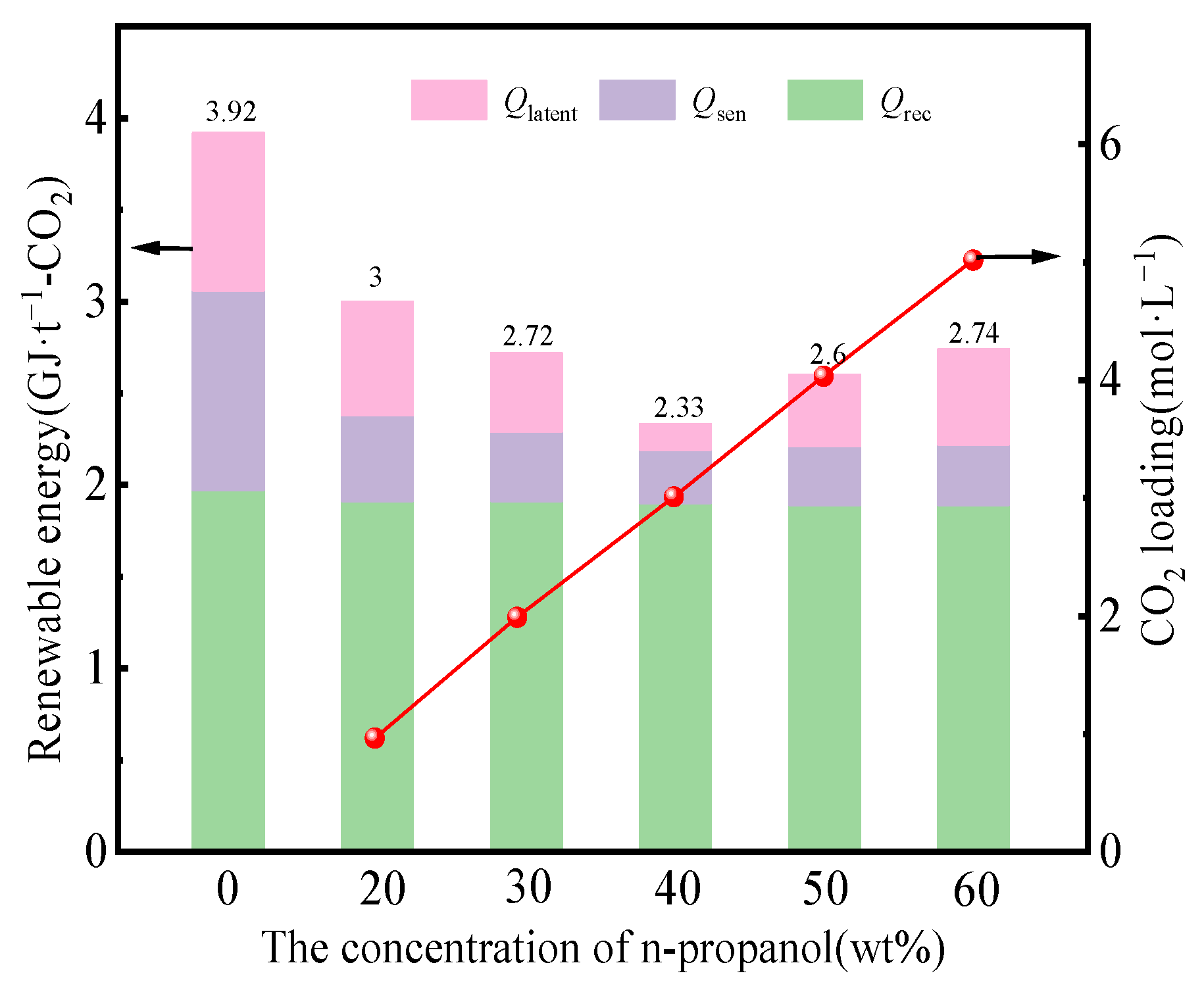
3.1. Impact of n-Propanol on Energy Consumption
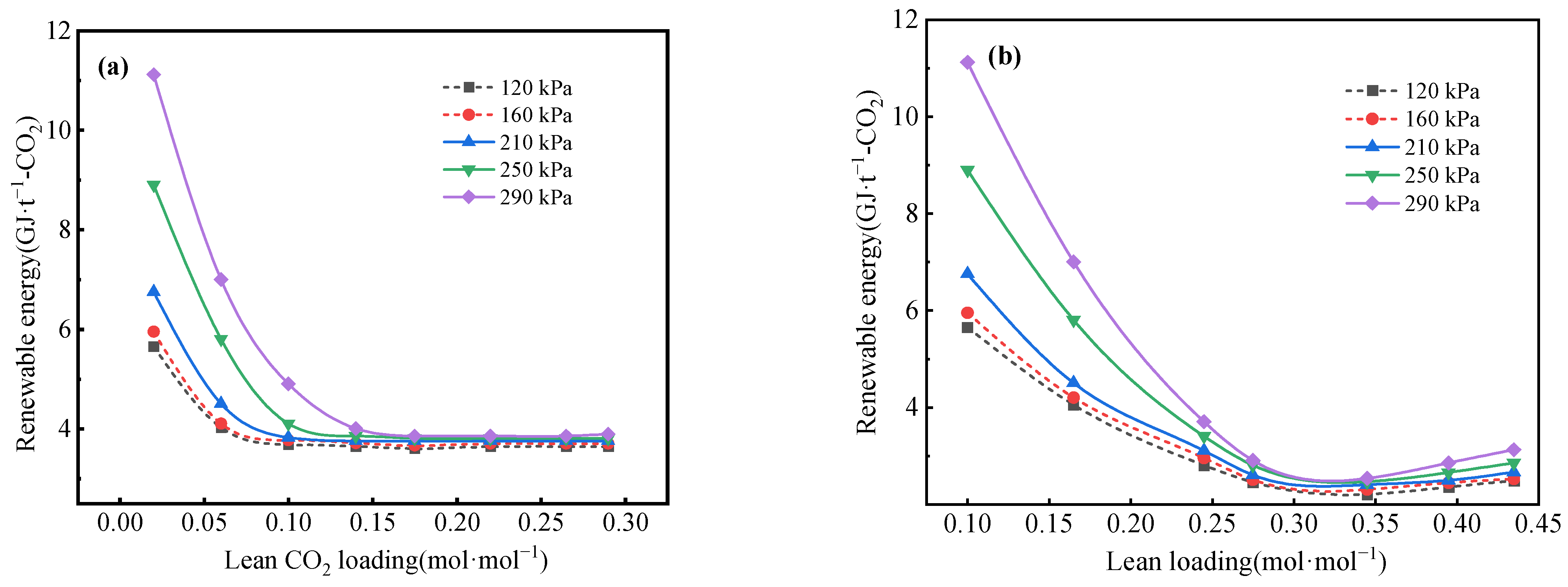
3.2. Impact of CO2 Loading in the Lean Liquid on Energy Consumption
3.3. Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AEEA | Ethylenediamine |
| AMP | 2-Amino-2-methyl-1-propanol |
| CCUS | Carbon dioxide capture, utilization and storage |
| COND | Condensate |
| CNY | Chinese Yuan |
| c | Heat capacity, kJ·mol−1·K−1 |
| DEEA | N,N-diethylethanolamine |
| EDA | Ethylenediamine |
| Hi | Mole enthalpy of amines in the ith stream for i = 1 and 2 and the stream itself for i = 3 and 4 Enthalpy per mole of amine in the stream and mole of the stream itself |
| [HDBU][Im] | 1,8-diazabicyclo[5,4,0]undec-7-en-imidazole |
| MDEA | N-methyldiethanolamine |
| MEA | Monoethanolamine |
| M | Molar amount of CO2 per ton of CO2 product, mol·t−1 CO2 |
| mi | Molar flow rate in the flow stream, mol·t−1 CO2 |
| mam | Molar amount of amine in the circulating solvent stream per ton of CO2 Product, mol·t−1 CO2 |
| mw | Molar amount of water vapor per ton of CO2 product, kJ·mol−1 |
| (T, α) | CO2 vapor pressure, kPa |
| PZ | Piperazine |
| Qlatent | Heat of evaporation of water, GJ·t−1-CO2 |
| Qrec | Heat t of desorption reaction, GJ·t−1-CO2 |
| Qregen | Energy consumption of the reboiler, GJ·t−1-CO2 |
| Qsen | Sensible heat required to warm the absorber, GJ·t−1-CO2 |
| rw | Molar ratio of water to amine in unloaded solvent |
| TETA | Triethylene tetramin |
| T | Absolute temperature, K |
| 1DMA2P | 1-dimethylamino-2-propanol |
| α | CO2 loading as moles of CO2 per mole of amine |
| αlean | Lean liquid CO2 loading as moles of CO2 per mole of amine |
| αrich | Rich liquid CO2 loading as moles of CO2 per mole of amine |
| λ | Latent heat of water, kJ·mol−1 |
| ΔTMTA | Minimum heat transfer temperature difference |
References
- Jiang, H.; Liu, Y.; Feng, Y. Analysis of power generation technology trend in 14th Five-Year Plan under the background of carbon peak and carbon neutrality. Power Gener. Technol. 2022, 43, 54–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Blunt, M. Advances in carbon capture, utilization and storage. Appl. Energy 2020, 278, 115627. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, Y.; Huang, C. Recent advances in amine-based solid sorbents for post-combustion CO2 capture system. Chem. Ind. Eng. Prog. 2018, 37, 610–620. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Wang, Q. Review on membrane wettability of membrane CO2 absorption method from coal-fired flue gas. Chem. Ind. Eng. Prog. 2019, 38, 3866–3873. [Google Scholar] [CrossRef]
- Liu, B.; Wu, L.; Dong, X. Experimental study on the recycling of a variety of organic amine absorbers. Sci. Technol. Chem. Ind. 2017, 25, 20–25. [Google Scholar]
- Lu, S.; Gong, Y.; Liu, L. Research status and future development direction of CO2absorption technology for organic amine. Clean. Coal Technol. 2022, 28, 44–54. [Google Scholar] [CrossRef]
- Josselyne, A.; Villarroel, A.; Alfredo, V.; Marvin, R. Kinetic and Thermodynamic Analysis of High-Pressure CO2 Capture Using Ethylenediamine: Experimental Study and Modeling. Energies 2021, 14, 6822. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, F.; Cui, Z. Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: Process improvement. Appl. Energy 2017, 185, 362–375. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Yang, Q. Analysis of the reduction of energy cost by using MEA-MDEA-PZ solvent for post-combustion carbon dioxide capture (PCC). Appl. Energy 2017, 205, 1002–1011. [Google Scholar] [CrossRef]
- Chen, Y. CO2 Absorption and Desorption Performance of BZA-DEEA/DMEA/1DMA2P /AMP Binary Blended Amines; Xiangtan University: Xiangtan, China, 2019. [Google Scholar]
- Sun, L.; Lian, S.; Wang, K. Research on aqueous TETA/AMP solution for CO2 capture. Clean. Coal Technol. 2020, 26, 58–63. [Google Scholar] [CrossRef]
- Wang, K.; Li, T.; Li, Y. Efficiently CO2capture by superbase ionic liquid-amine-water blending solvents. Chin. J. Chem. Eng. 2023, 23, 781–789. [Google Scholar]
- Lv, B.; Yang, K.; Zhou, X. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture. Appl. Energy 2020, 264, 114703. [Google Scholar] [CrossRef]
- Tao, M.; Gao, J.; Zhang, W. A novel phase-changing nonaqueous solution for CO2 capture with high capacity, thermostability, and regeneration efficiency. Ind. Eng. Chem. Res. 2018, 57, 9305–9312. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Wang, T.; Liu, F.; Fang, M. Research Progress in Biphasic Solvent for CO2 Capture Technology. Proc. CSEE 2021, 41, 1186–1196. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, W.; Wang, Q. Recent developments of phase-change absorption technology for CO2 capture from flue gas. Chem. Ind. Eng. Prog. 2022, 41, 2090–2101. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Tu, W. A novel CO2 phase change absorbent: MEA/1-propanol/H2O. Energy Fuels 2017, 31, 4273–4279. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wang, R. Advanced monoethanolamine absorption using sulfolane as a phase splitter for CO2 capture. Environ. Sci. Technol. 2018, 52, 14556–14563. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Parnas, R. The CO2 absorption and desorption performance of the triethylenetetramine+N,N-diethylethanolamine + H2O system. Chin. J. Chem. Eng. 2018, 26, 2351–2360. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Shao, P. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas. Environ. Sci. Technol. 2018, 52, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Kierzkowska-Pawlak, H.; Soabla, K. Heat of absorption of CO2 in aqueous solutions of DEEA and DEEA + MAPA blends—A new approach to measurement methodology. Int. J. Greenh. Gas. Control 2020, 100, 103102. [Google Scholar] [CrossRef]
- Mannisto, M.; Uusi-Kyyny, P.; Richon, D. Study of CO2 absorption into phase change solvents MAPA and DEEA. J. Chem. Eng. Data 2017, 62, 2261–2271. [Google Scholar] [CrossRef]
- Liu, F.; Fang, M.; Yi, N. Biphasic behaviors and regeneration energy of a 2-(diethylamino)-ethanol and 2-((2-aminoethyl) amino) ethanol blend for CO2 capture. Sustain. Energy Fuels 2019, 3, 3594–3602. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Q.; Wang, W. Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents. Energy Procedia 2013, 37, 1254–1261. [Google Scholar] [CrossRef]
- Chen, Z.; Jing, G.; Lu, B. An efficient solid-liquid biphasic solvent for CO2 capture: Crystalline powder product and low heat duty. ACS Sustain. Chem. Eng. 2020, 8, 14493–14503. [Google Scholar] [CrossRef]
- Abu-Zahra, M.; Schneiders, L.; Niederer, J. CO2 capture from power plants. Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas. Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Alie, C.; Backham, L.; Croiset, E.; Douglad, P. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- Jassim, M.; Rochelle, G. Innovative absorber/stripper configuration for CO2 capture by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2006, 45, 2465–2472. [Google Scholar] [CrossRef]
- Sigh, D.; Croiset, E. Techno-economic study of CO2 capture from an existing coal-fired power plant: MEA scrubbing vs. O2/CO2 recycle combustion. Energy Convers. Manag. 2003, 44, 3073–3091. [Google Scholar] [CrossRef]
- Meldon, J.H. Amine screening for flue gas CO2 capture at coal-fired power plants: Should the heat of desorption be high, low or in between? Curr. Opin. Chem. Eng. 2011, 1, 55–63. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Aroonwilas, A.; Veawab, A. Behavior of reboiler heat duty for CO2 capture plants using regenerable single and blended alkanolamines. Ind. Eng. Chem. Res. 2005, 44, 4465–4473. [Google Scholar] [CrossRef]
- Leites, I.; Sama, D.; Lior, N. The theory and practice of energy saving in the chemical industry: Some methods for reducing thermodynamic irreversibility in chemical technology processes. Energy 2003, 28, 55–97. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Wang, Y.; Li, Y. Analysis and calculation of the desorption energy consumption of CO2 capture process by chemical absorption method. Chem. Ind. Eng. Prog. 2013, 32, 3008–3014. [Google Scholar]
- Wang, D.; Xie, J.; Zhou, H. Parameters analysis and energy integration in flue gas SO2 capture process based on MDEA. CIESC J. 2021, 72, 1521–1528. [Google Scholar] [CrossRef]
- Rochekke, G. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, W.; Wang, D. A novel coal chemical looping gasification scheme for synthetic natural gas with low energy consumption for CO2 capture: Modelling, parameters optimization, and performance analysis. Energy 2021, 225, 120249. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Meng, W.; Jian, W. Integrated Process for Producing Glycolic Acid from Carbon Dioxide Capture Coupling Green Hydrogen. Processes 2022, 10, 1610. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, S.; Zhang, M. Research on low energy consumption CO capture technology and thermal economy of coal-fired units. Mod. Chem. Ind. 2021, 41, 210–214. [Google Scholar]
- Man, Y.; Yang, S.; Zhang, J.; Qian, Y. Conceptual design of coke-oven gas assisted coal to olefins process for high energy efficiency and low CO2 emission. Appl. Energy 2014, 133, 97–205. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Deng, S. Techno-economic Comparison of Solar-Assisted Power Plant for Emission Reduction. J. Eng. Thermophys. 2015, 36, 2547–2550. [Google Scholar]
- Adefarati, T.; Bansal, R. Reliability, economic and environmental analysis of a microgrid system in the presence of renewable energy resources. Appl. Energy 2019, 236, 1089–1114. [Google Scholar] [CrossRef]
- An, X.; Zuo, Y.; Zhang, Q. Methanol synthesis from CO2 hydrogenation with a Cu/Zn/Al/Zr fibrous catalyst. Chin. J. Chem. Eng. 2009, 17, 88–94. [Google Scholar] [CrossRef]
- Lim, H.; Park, M.; Kang, S.; Chae, H.; Bae, J.; Jun, K. Modeling of the kinetics for methanol synthesis using Cu/ZnO/Al2O3/ZrO2 catalyst: Influence of carbon dioxide during hydrogenation. Ind. Eng. Chem. Res. 2009, 48, 10448–10455. [Google Scholar] [CrossRef]
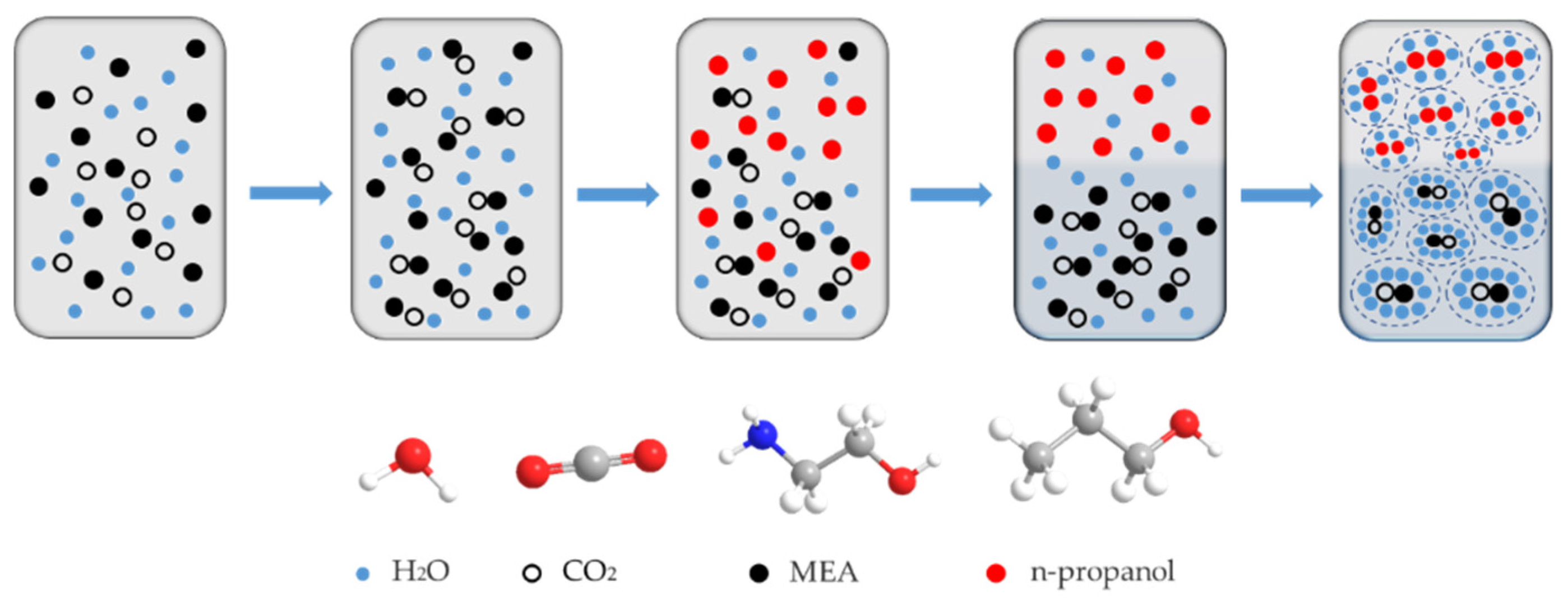

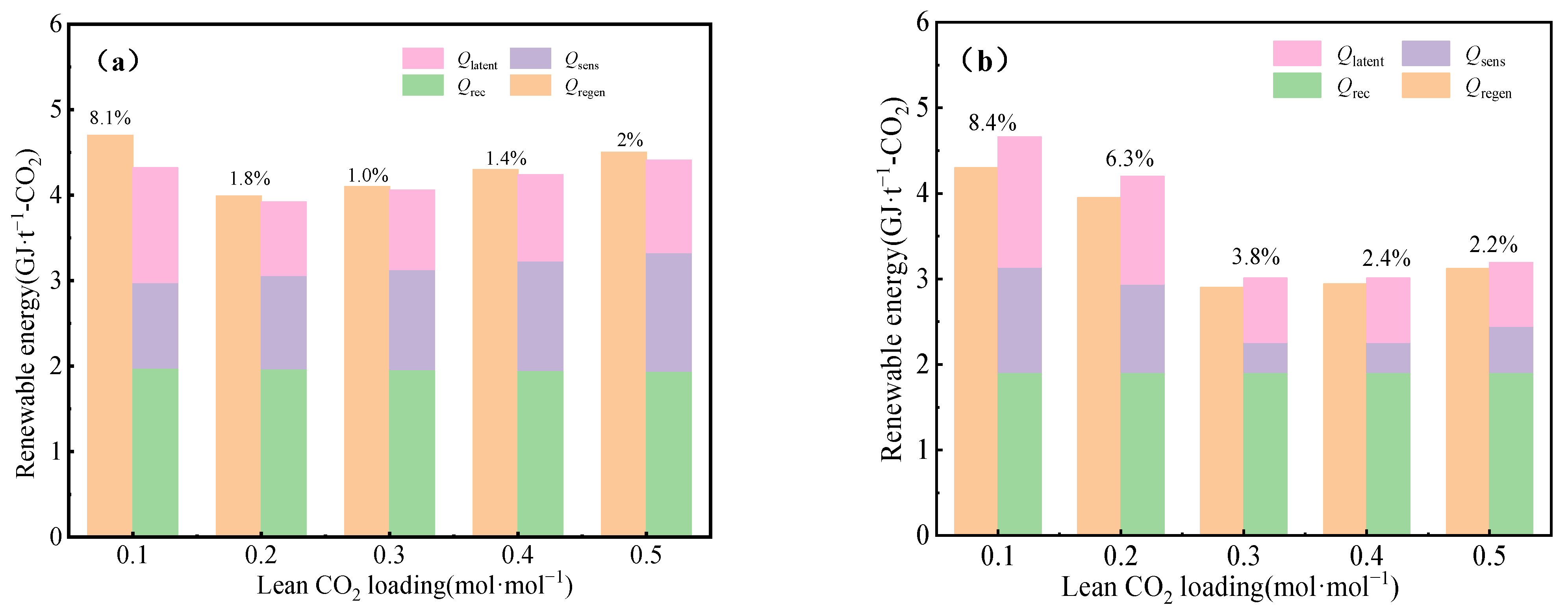
| Key Component | Absorption Condition | Absorption Rate | CO2 Loading Capacity | Ref. |
|---|---|---|---|---|
| MDEA + PZ | 308 K, 15 vol%CO2 | 1.2 × 10−3 mol·min−1 | 0.07 mol·mol−1 | [9] |
| DETA + PZ | 313 K | 9.5 µmol·L−1·s−1 | 0.65 mol·L−1 | [10] |
| AMP + TETA | 323 K, 12%CO2 | 0.02 g·min−1 | 0.056 g·g−1 | [12] |
| MDEA + carbonic anhydrase | 313 K | 0.63 mol·mol−1 | 1.2 mol·kg−1 | [13] |
| AMP + AEEA + NMP | 313 K | 1.65 mol·kg−1 | [14] | |
| TETA + PEG200 | 313 K, 15%CO2 | 16 mL·min−1 | 1.63 mol·mol−1 | [15] |
| MEA + 2ME | 313 K, 133%CO2 | 2.1 mol·kg−1 | [16] |
| Absorbent | Concentration | Characteristic | Energy Consumption (GJ·t−1-CO2) | Ref. |
|---|---|---|---|---|
| MEA | 30% | 4.22 | [18] | |
| MEA + n-propanol + H2O | 30% + 40% + 30% | Liquid–liquid phase separation | 2.40 | [19] |
| MEA + sulfolane | 4 mol·L−1 | Liquid–liquid phase separation | 2.67 | [20] |
| TETA + DEEA | 5 mol·L−1 | Liquid–liquid phase separation | 2.7 | [21] |
| TETA + DMCA | 4 mol·L−1 | Liquid–liquid phase separation | 2.6 | [22] |
| MAPA + DEEA | 7 mol·L−1 | Liquid–liquid phase separation | 2.2–2.4 | [23,24] |
| AEEA + DEEA | 20% + 60% | Liquid–liquid phase separation | 2.46 | [25] |
| DMCA + MCA + AMP | (3 + 1 + 1) mol·L−1 | Liquid–liquid phase separation | 2.0 | [26] |
| MEA + sulfolane + AMP | 20% + 50% + 30% | Liquid–liquid phase separation | 2.62 | [27] |
| Parameter | N2 (mol.%) | CO2 (mol.%) | O2 (mol.%) | H2O (mol.%) | Temperature (°C) | Pressure (kPa) | Molar Flow Rate (kmol∙h−1) |
|---|---|---|---|---|---|---|---|
| Value | 77.90 | 14.60 | 3.30 | 4.20 | 42.00 | 101.33 | 40,000.00 |
| Stream | Flue Gas | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 40 | 55 | 65 | 75 | 40 | 9 | 40 | 44 | 121 |
| Pressure (kPa) | 101 | 290 | 200 | 199 | 199 | 290 | 200 | 200 | 200 |
| Hi (kJ·mol−1) | −2928 | −6265 | −1722 | −1373 | −2927 | −12,931 | −12,209 | −3524 | |
| Molar flow rate (kmol∙h−1) | |||||||||
| H2O | 1680 | 45,846 | 62 | 27,946 | 829 | 45,846 | 62 | 62 | 62 |
| N2 | 31,160 | 0.21 | 31,160 | 0.00 | 0.00 | 0.21 | 0.00 | 0.00 | 0.00 |
| O2 | 1320 | 0.02 | 1320 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 |
| CO2 | 5480 | 4.00 | 0.00 | 4110 | 2.00 | 4.00 | 5257 | 5257 | 52,567 |
| MEA | 0.00 | 213 | 14 | 109 | 109 | 213 | 0.00 | 0.00 | 0.00 |
| C3H8O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MEA+ | 0.00 | 5855 | 0.00 | 5668 | 5668 | 5855 | 0.00 | 0.00 | 0.00 |
| MEACOO− | 0.00 | 5214 | 0.00 | 5155 | 5155 | 5214 | 0.00 | 0.00 | 0.00 |
| HCO3− | 0.00 | 602.69 | 0.00 | 509.45 | 509.45 | 602.69 | 0.00 | 0.00 | 0.00 |
| CO32− | 0.00 | 18.97 | 0.00 | 8.76 | 8.76 | 18.97 | 0.00 | 0.00 | 0.00 |
| Mass flow rate (kg∙h−1) | |||||||||
| H2O | 30,240 | 825,942 | 2437 | 503,032 | 45.36 | 825,942 | 2430 | 2430 | 2430 |
| N2 | 872,480 | 6.00 | 872,474 | 0.00 | 0.00 | 6.00 | 0.00 | 0.00 | 0.00 |
| O2 | 42,240 | 0.64 | 42,239 | 0.00 | 0.00 | 0.64 | 0.00 | 0.00 | 0.00 |
| CO2 | 241,120 | 194.06 | 0.00 | 180,840 | 0.19 | 194.04 | 0.58 | 0.58 | 0.58 |
| MEA | 0.00 | 13,021 | 870 | 6625 | 6625 | 13,025 | 0.00 | 0.00 | 0.00 |
| C3H8O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MEA+ | 0.00 | 363,517 | 0.00 | 351,934 | 5668 | 363,484 | 0.00 | 0.00 | 0.00 |
| MEACOO− | 0.00 | 542,701 | 0.00 | 536,581 | 5155 | 542,874 | 0.00 | 0.00 | 0.00 |
| HCO3− | 0.00 | 36,771 | 0.00 | 31,082 | 510 | 36,800 | 0.00 | 0.00 | 0.00 |
| CO32− | 0.00 | 1128.72 | 0.00 | 523.31 | 8.76 | 1128.72 | 0.00 | 0.00 | 0.00 |
| Parameter | Numerical Value | Parameter | Numerical Value |
|---|---|---|---|
| mam (kmol·kg−1) | 0.2493 | Δhrec (kJ·mol−1 CO2) | 83.00 |
| mw (kmol·kg−1) | 21.0780 | cam (kJ·mol−1·K−1) | 169.58 |
| αlean (mol·mol−1) | 0.2000 | cw (kJ·mol−1·K−1) | 27.45 |
| αrich (mol·mol−1) | 0.4800 | cCO2 (kJ·mol−1·K−1) | 125.17 |
| α7 (mol·mol−1) | 0.2543 | rw | 2.33 |
| M (kmol·kg−1) | 0.2272 | rw,5l | 22.23 |
| mw,R (mol·t−1 CO2) | 1.7100 | λ (kJ·mol−1) | 40.80 |
| mCO2 (kmol·kg−1) | 1.1700 | m8 (kmol·kg−1) | 2.77 |
| Stream | Flue Gas | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 40 | 55 | 65 | 49 | 48 | 90 | 121 | 122 | 122 |
| Pressure (kPa) | 101 | 101 | 113 | 100 | 100 | 101 | 113 | 113 | 113 |
| Hi (kJ·mol−1) | −2798 | −6245 | −1332 | −1153 | −2797 | −6245 | −4647 | −1093 | |
| Molar flow rate (kmol∙h−1) | |||||||||
| H2O | 1680 | 504 | 1680 | 129 | 633 | 504 | 1680 | 1680 | 1680 |
| N2 | 31,160 | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| O2 | 1320 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CO2 | 5480 | 0.00 | 4.40 | 3836 | 0.00 | 52 | 241 | 241 | 241 |
| MEA | 0.00 | 21 | 1680 | 1563 | 0.00 | 21 | 1680 | 1680 | 1680 |
| C3H8O | 0.00 | 1310 | 2240 | 0.00 | 0.00 | 1310 | 2240 | 2240 | 2240 |
| MEA+ | 0.00 | 20.20 | 99.00 | 71.48 | 71.48 | 20.20 | 99.00 | 99.00 | 99,300 |
| MEACOO− | 0.00 | 89.90 | 14.00 | 0.30 | 0.30 | 89.90 | 14.00 | 14.00 | 14.00 |
| HCO3− | 0.00 | 3.80 | 0.56 | 0.03 | 0.00 | 3.80 | 0.56 | 0.56 | 0.56 |
| CO32− | 0.00 | 0.05 | 1.60 | 71.43 | 0.00 | 0.05 | 1.60 | 1.60 | 1.60 |
| Mass flow rate (kg∙h−1) | |||||||||
| H2O | 30,240 | 9072 | 30,240 | 2322 | 11,394 | 9072 | 30,240 | 30,240 | 30,240 |
| N2 | 872,480 | 0.00 | 5.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| O2 | 42,240 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CO2 | 241,120 | 0.00 | 194 | 1,168,784 | 0.00 | 2288 | 10,604 | 10,604 | 10,604 |
| MEA | 0.00 | 1250 | 102,614 | 95,455 | 0.00 | 1250 | 102,614 | 102,614 | 102,614 |
| C3H8O | 0.00 | 78,610 | 134,422 | 0.00 | 0.00 | 78,610 | 134,422 | 134,422 | 134,422 |
| MEA+ | 0.00 | 1254 | 6147 | 4439 | 4438 | 1254 | 6147 | 6147 | 6147 |
| MEACOO− | 0.00 | 9357 | 1457 | 31 | 31 | 9357 | 1457 | 1457 | 1457 |
| HCO3− | 0.00 | 231.59 | 231.59 | 1.83 | 0.00 | 231.59 | 34.17 | 34.17 | 34.17 |
| CO32− | 0.00 | 2.95 | 94.48 | 4217.94 | 0.00 | 2.95 | 94.48 | 94.48 | 94.48 |
| Parameter | Numerical Value | Parameter | Numerical Value |
|---|---|---|---|
| mam (kmol·kg−1) | 0.1905 | Δhrec (kJ·mol−1 CO2) | 84.00 |
| mw (kmol·kg−1) | 3.4904 | cam (kJ·mol−1·K−1) | 169.58 |
| αlean (mol·mol−1) | 0.3500 | cw (kJ·mol−1·K−1) | 27.45 |
| αrich (mol·mol−1) | 0.6000 | cCO2 (kJ·mol−1·K−1) | 125.17 |
| α7 (mol·mol−1) | 0.1210 | rw | 1.33 |
| M (mol·t−1 CO2) | 0.2272 | rw,5l | 4.26 |
| mw,R (kmol·kg−1) | 0.1925 | λ (kJ·mol−1) | 40.80 |
| mCO2 (kmol·kg−1) | 0.0346 | m8 (kmol·kg−1) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, L.; Xie, J.; Yang, Y.; Zhou, H.; Fan, X. A Coupling Calculation Method of Desorption Energy Distribution Applied to CO2 Capture by Chemical Absorption. Processes 2024, 12, 187. https://doi.org/10.3390/pr12010187
Wang D, Liu L, Xie J, Yang Y, Zhou H, Fan X. A Coupling Calculation Method of Desorption Energy Distribution Applied to CO2 Capture by Chemical Absorption. Processes. 2024; 12(1):187. https://doi.org/10.3390/pr12010187
Chicago/Turabian StyleWang, Dongliang, Li Liu, Jiangpeng Xie, Yong Yang, Huairong Zhou, and Xueying Fan. 2024. "A Coupling Calculation Method of Desorption Energy Distribution Applied to CO2 Capture by Chemical Absorption" Processes 12, no. 1: 187. https://doi.org/10.3390/pr12010187
APA StyleWang, D., Liu, L., Xie, J., Yang, Y., Zhou, H., & Fan, X. (2024). A Coupling Calculation Method of Desorption Energy Distribution Applied to CO2 Capture by Chemical Absorption. Processes, 12(1), 187. https://doi.org/10.3390/pr12010187









