Model and Parameter Study of Limestone Decomposition Reaction
Abstract
1. Introduction
2. Limestone Decomposition Reaction Model
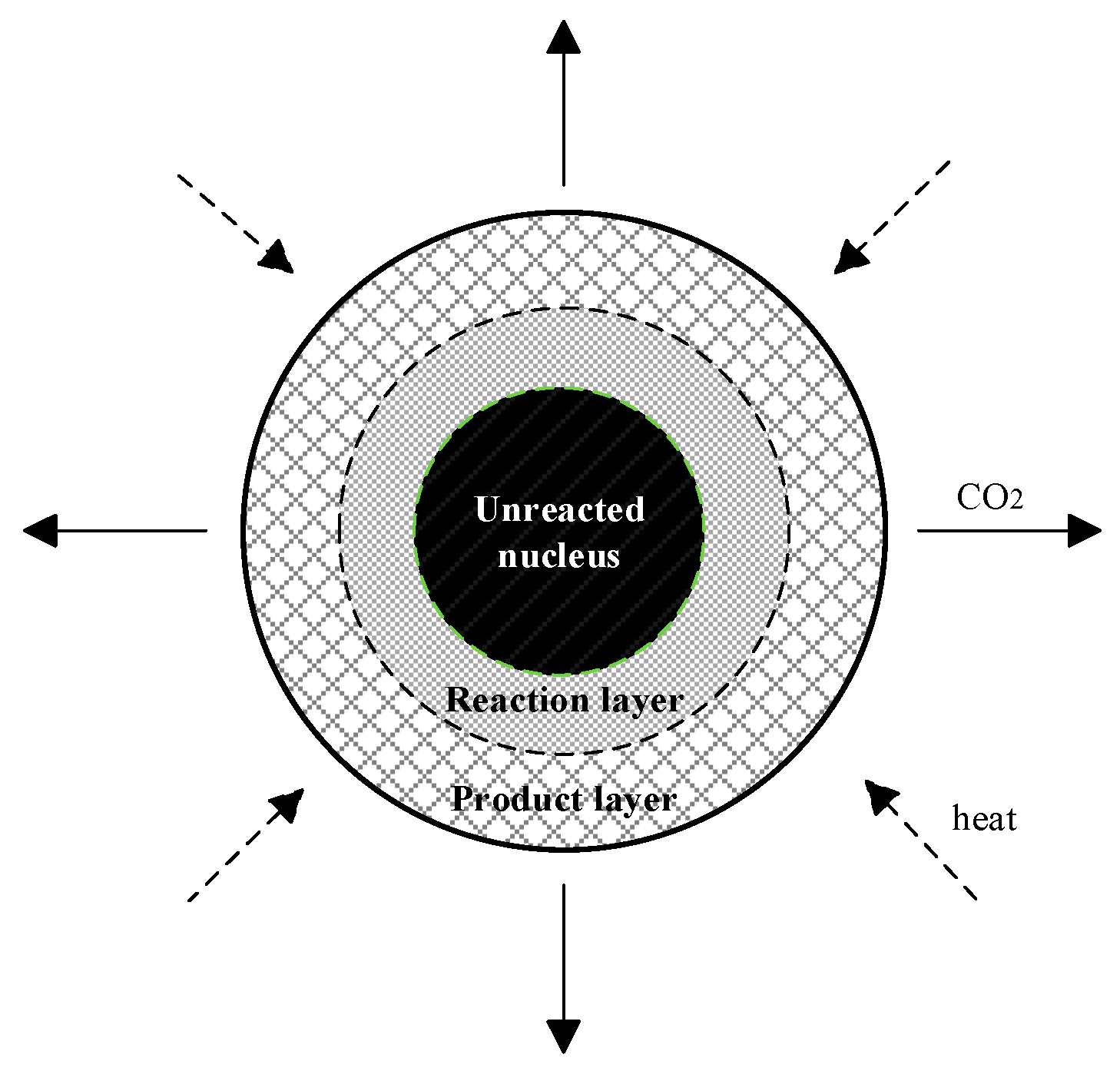
2.1. Physical and Chemical Process Analysis
2.2. Mathematical Model
2.2.1. Thermal Conduction Model
2.2.2. Decomposition Reaction Kinetics Model
Model 1
Model 2
3. Numerical Solution of the Model
3.1. Calculation Parameters
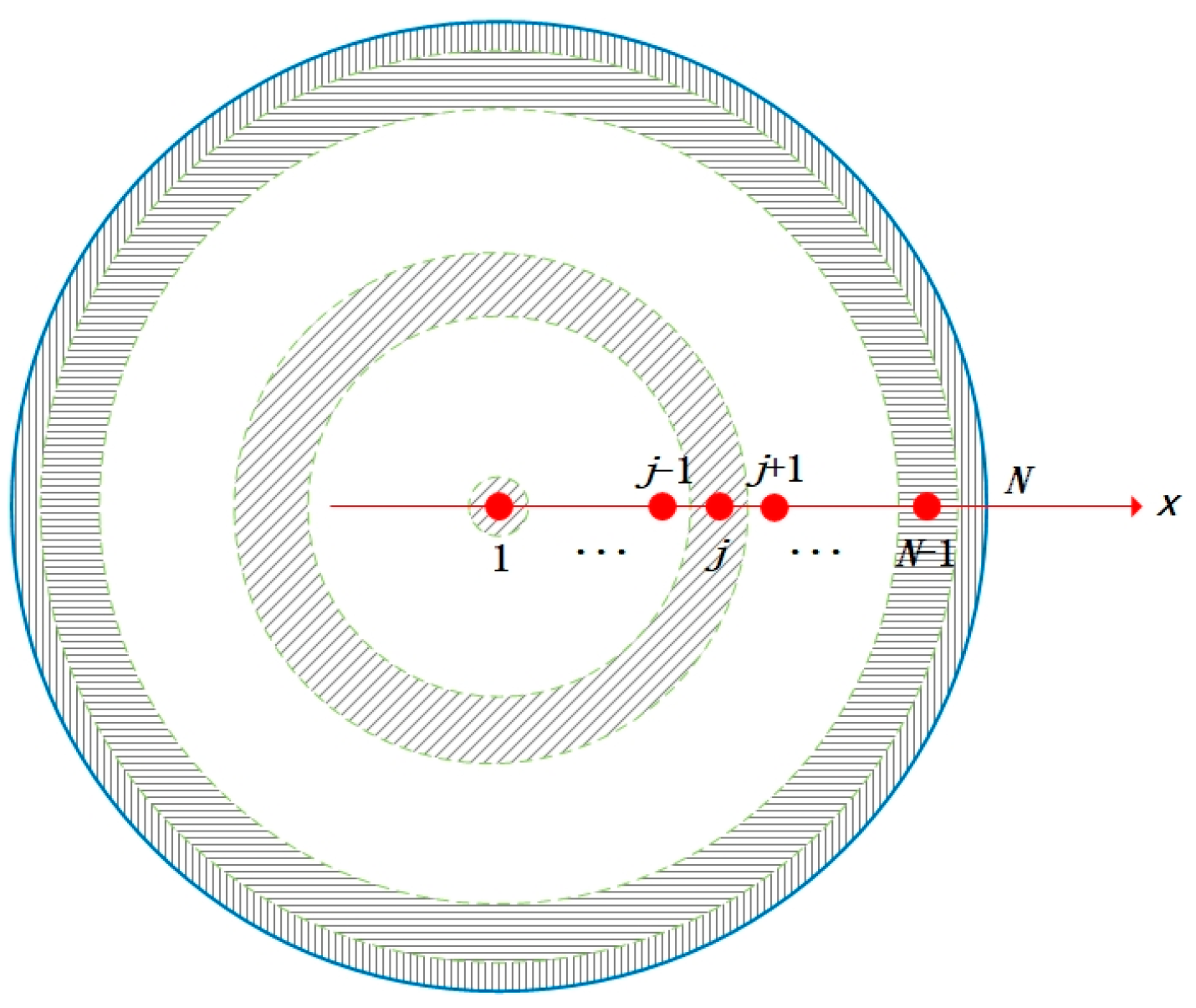
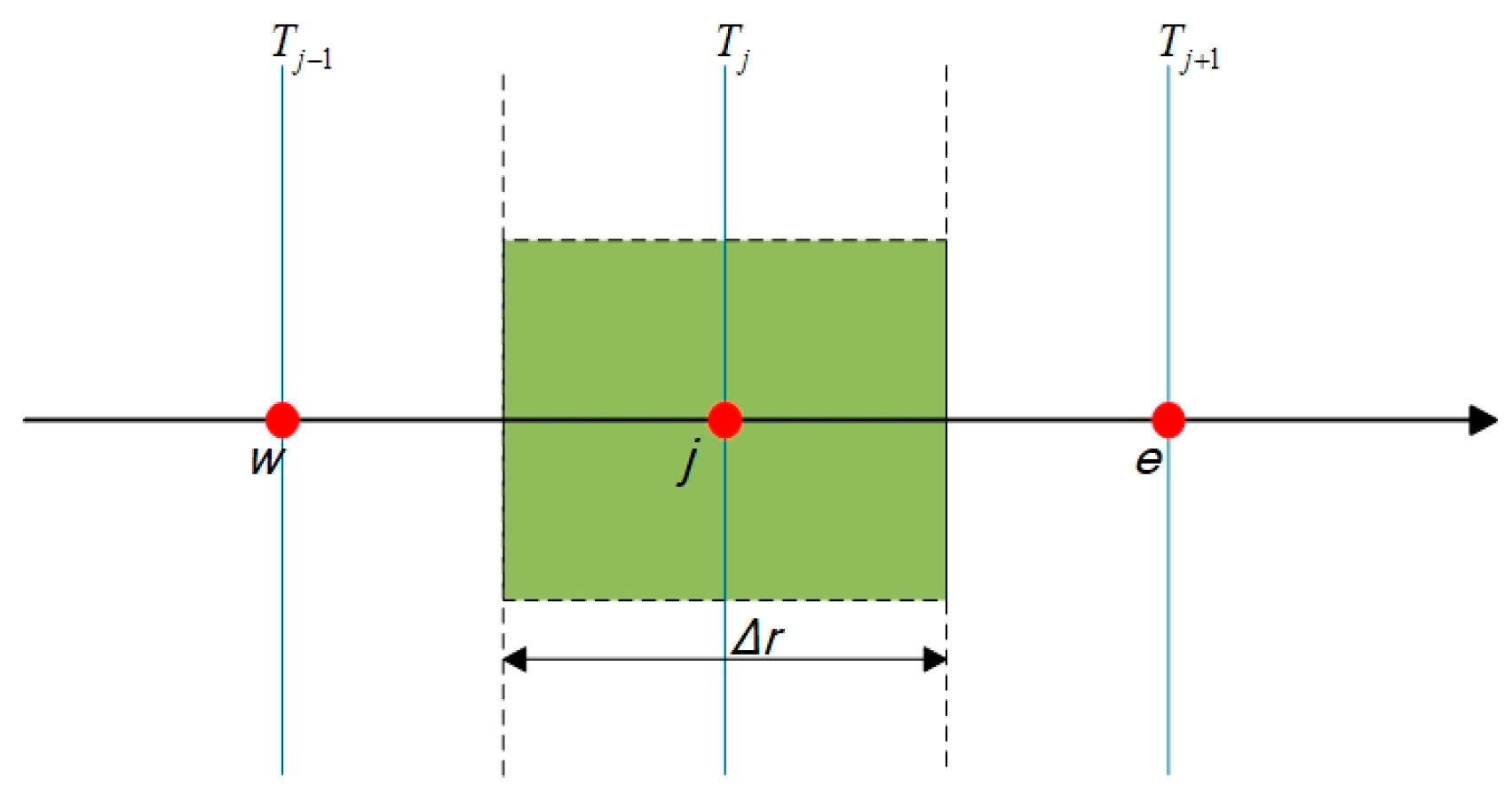
3.2. Discretization of Domain and Equations
- ①
- j = N (external grid points)
- ②
- j = 1 (spherical center point)
- ③
- j = 2,3, …, N − 1 (inner grid points)
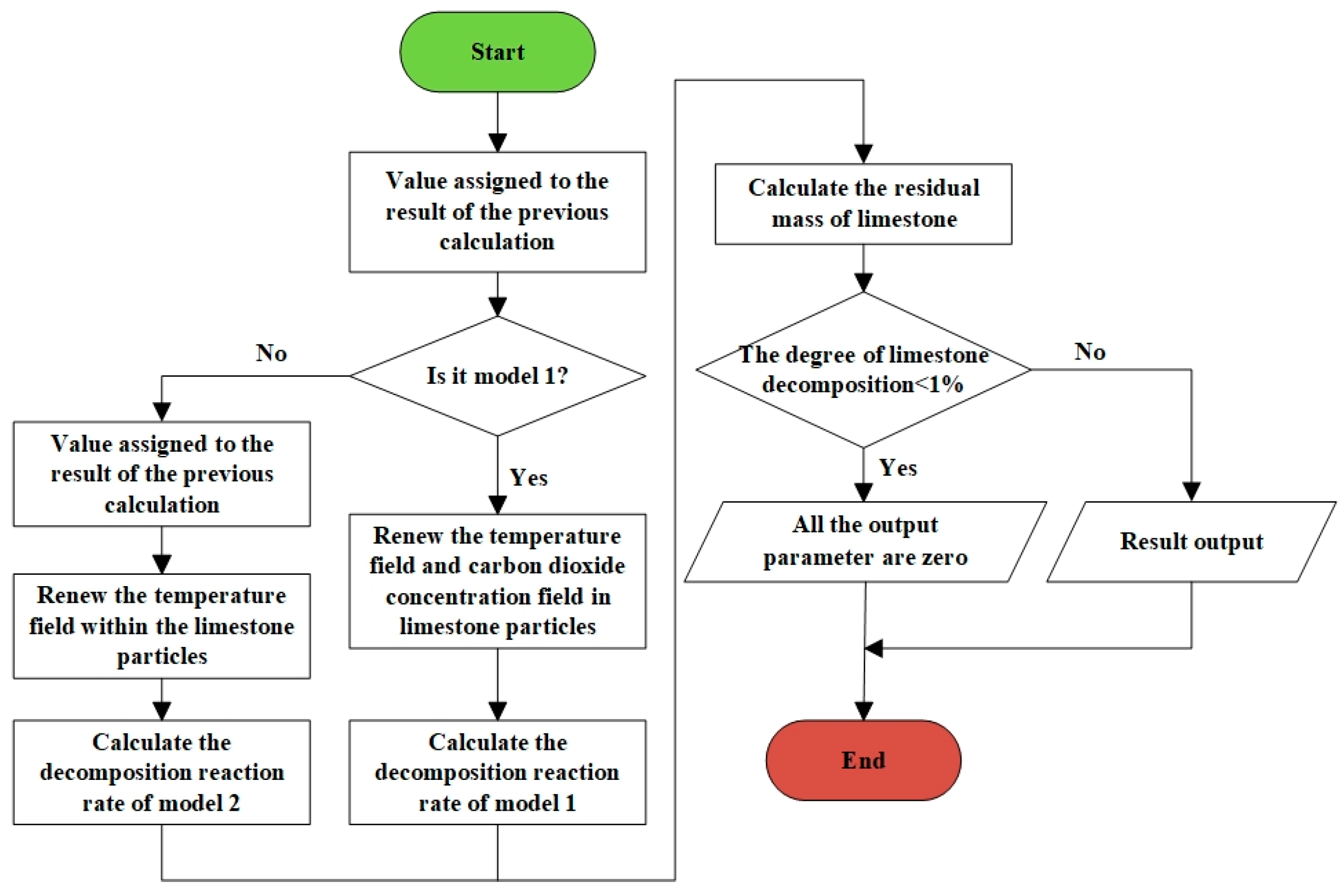
3.3. Numerical Solution Method and Calculation Flowchart
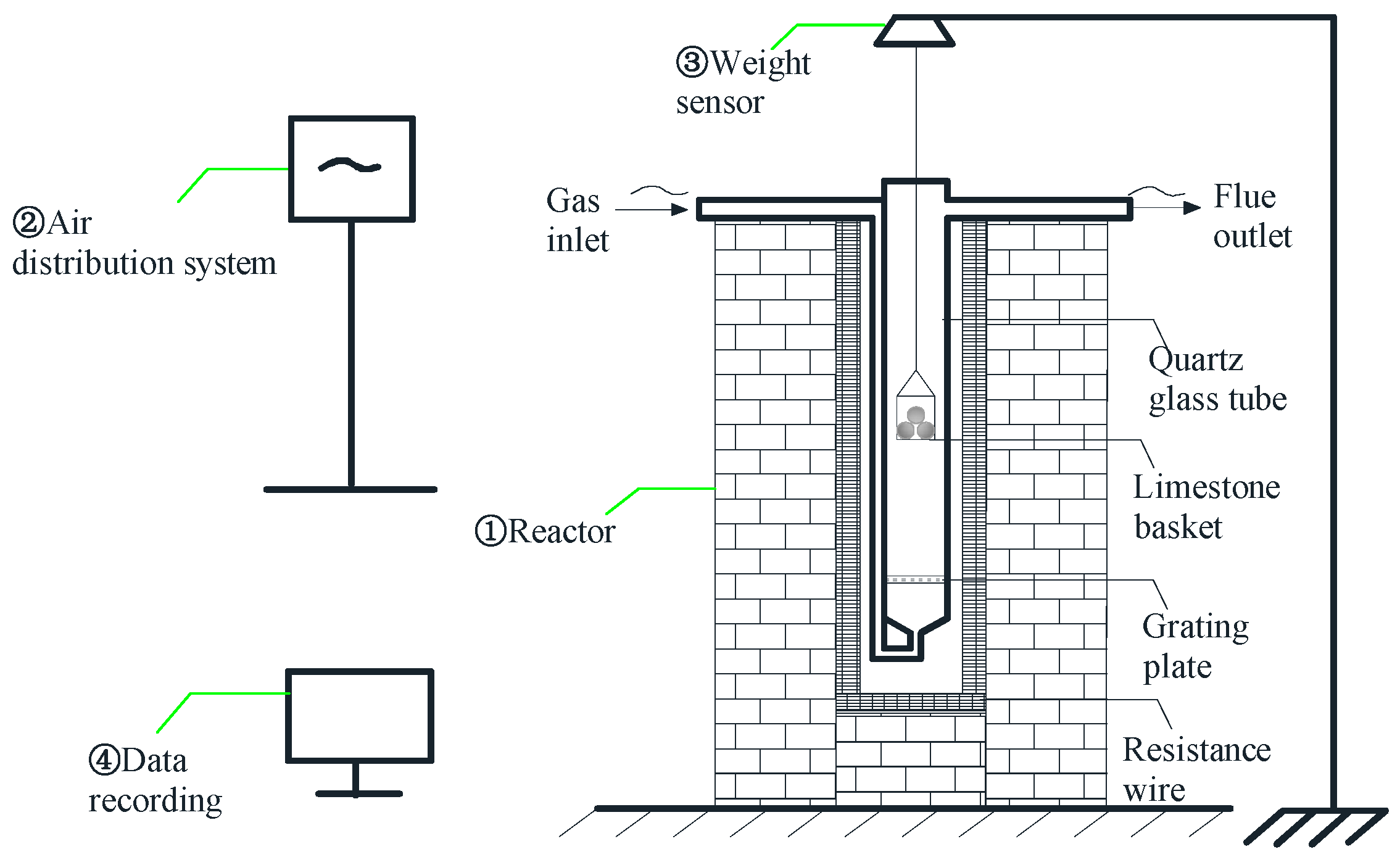
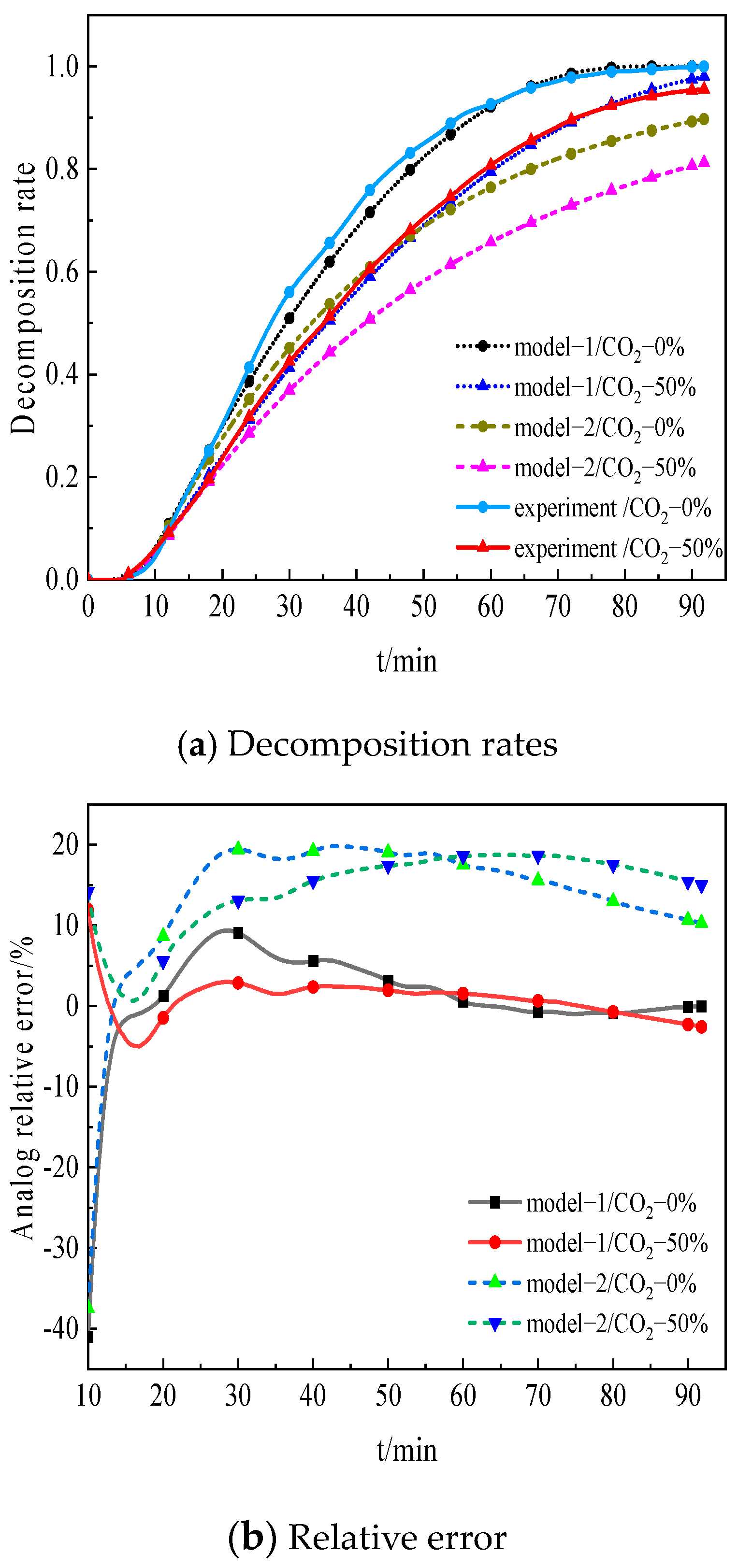
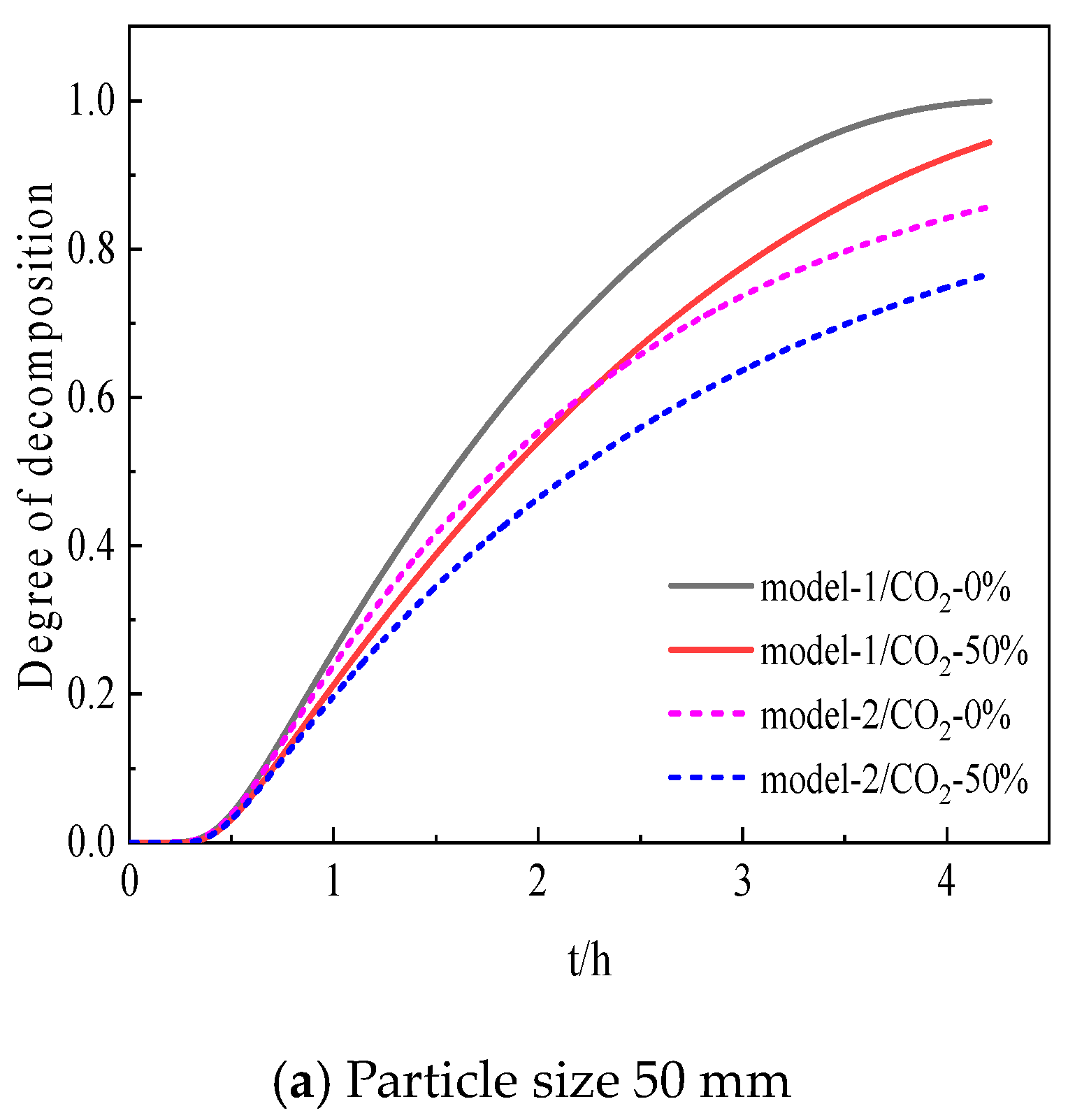
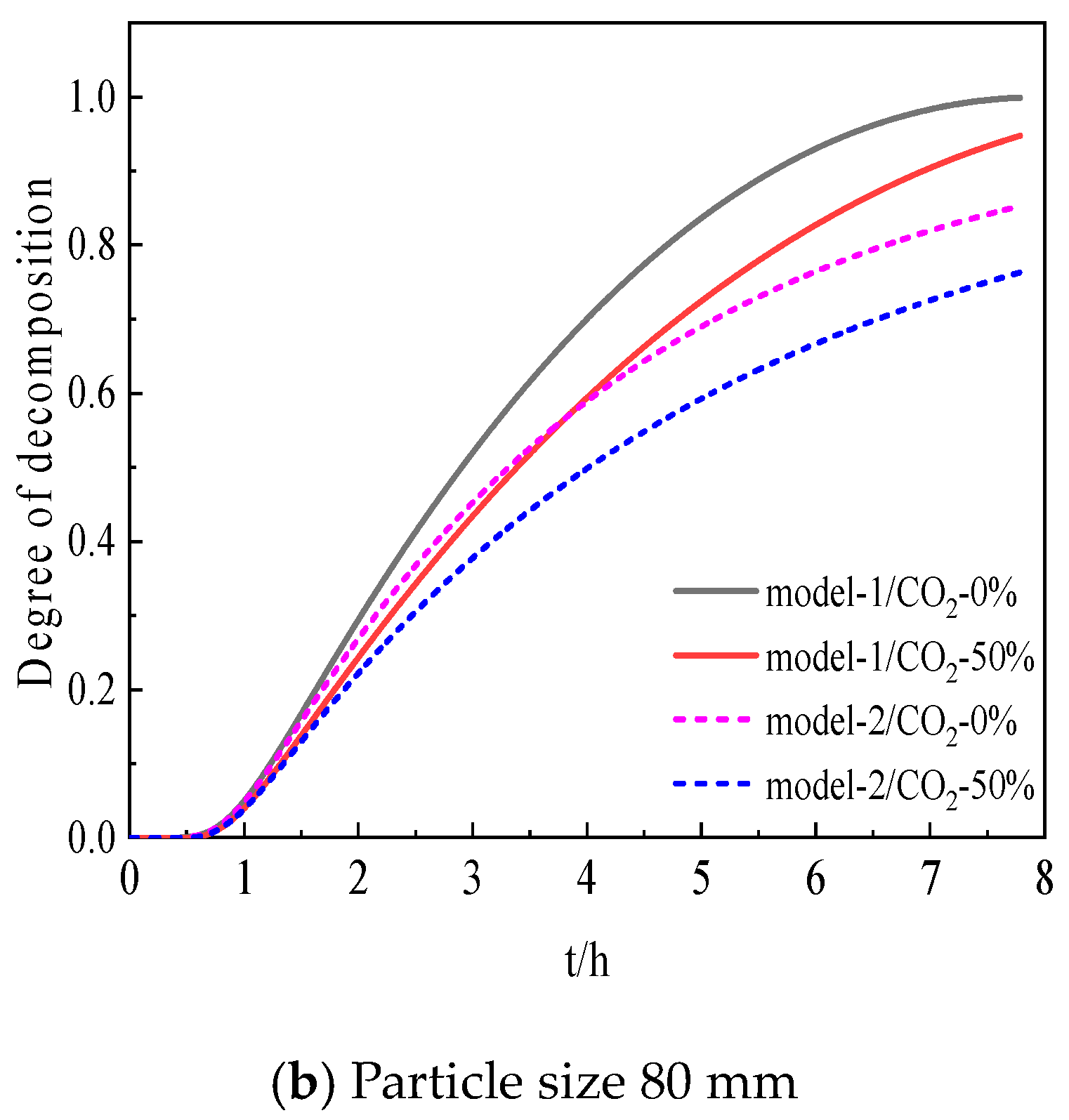
4. Model Validation of Limestone Decomposition
5. Parameter Study of Limestone Decomposition Model
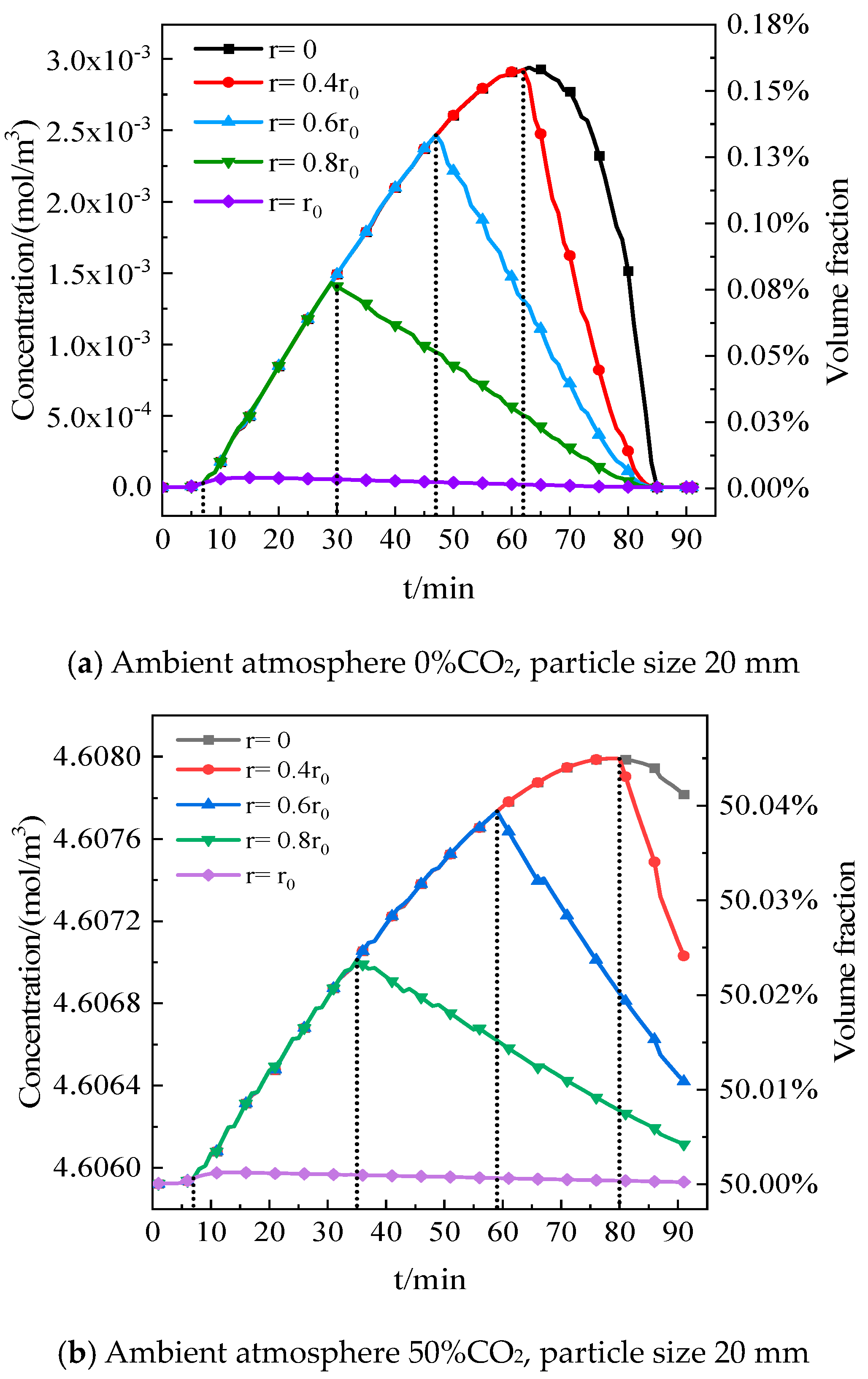
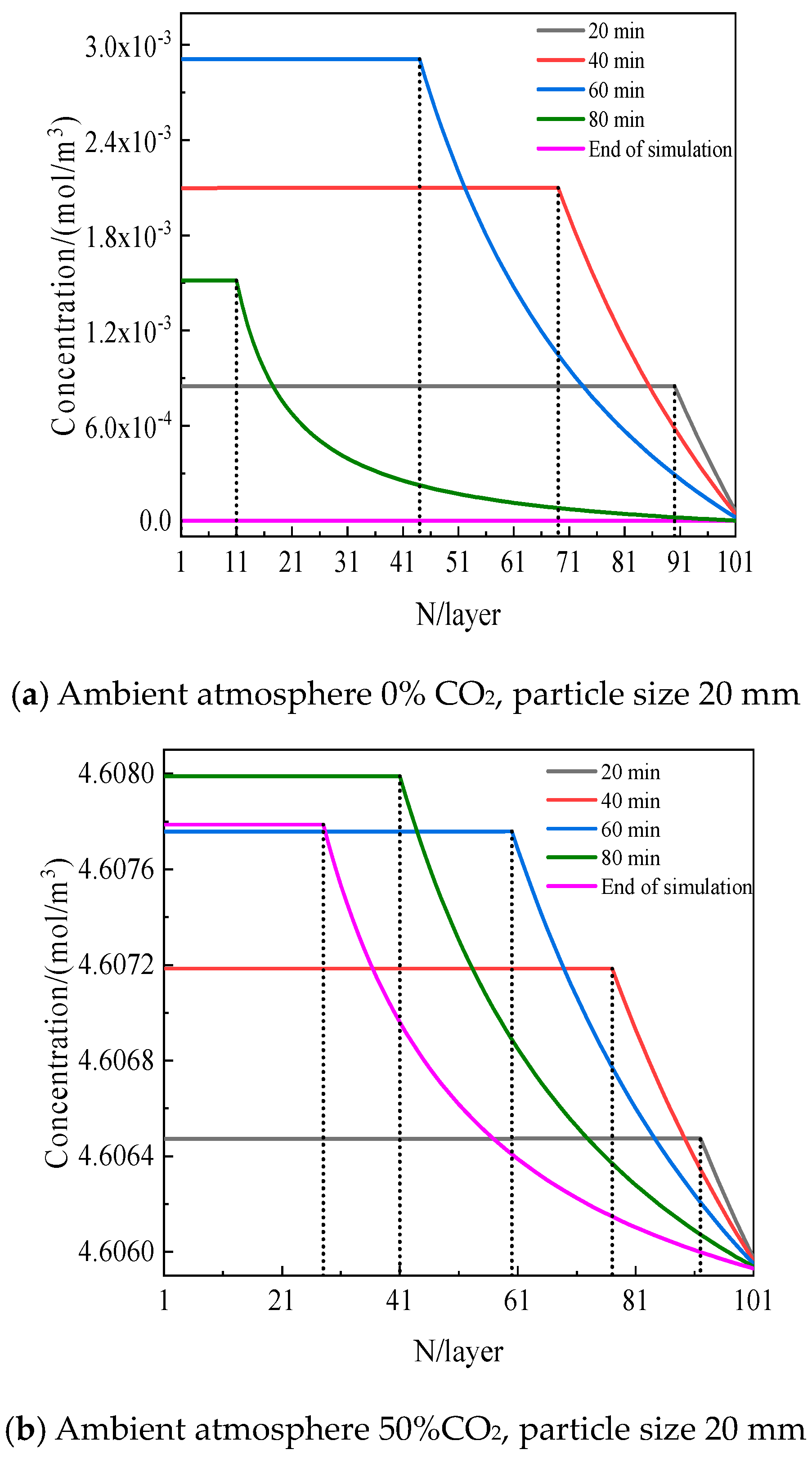
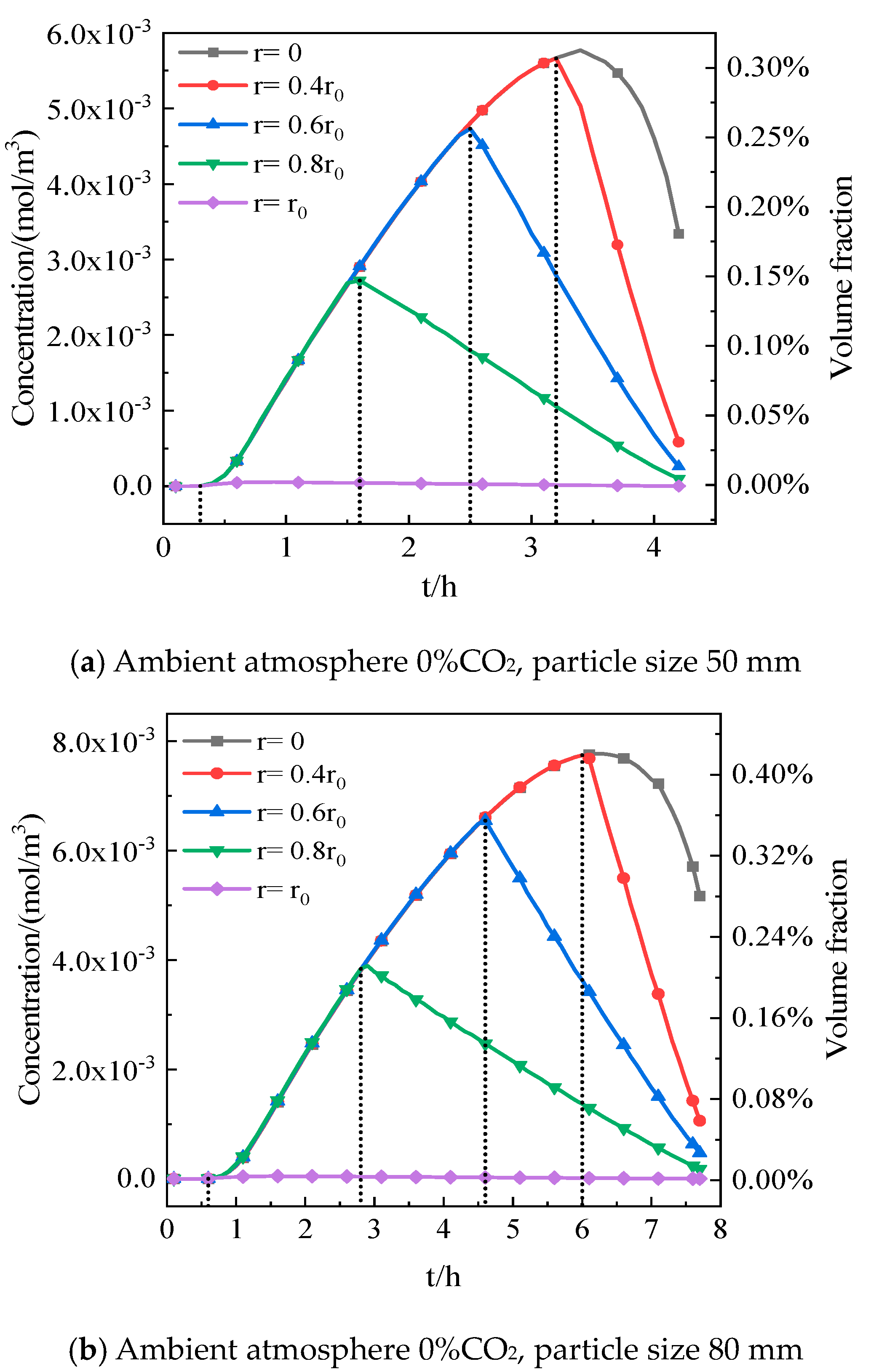
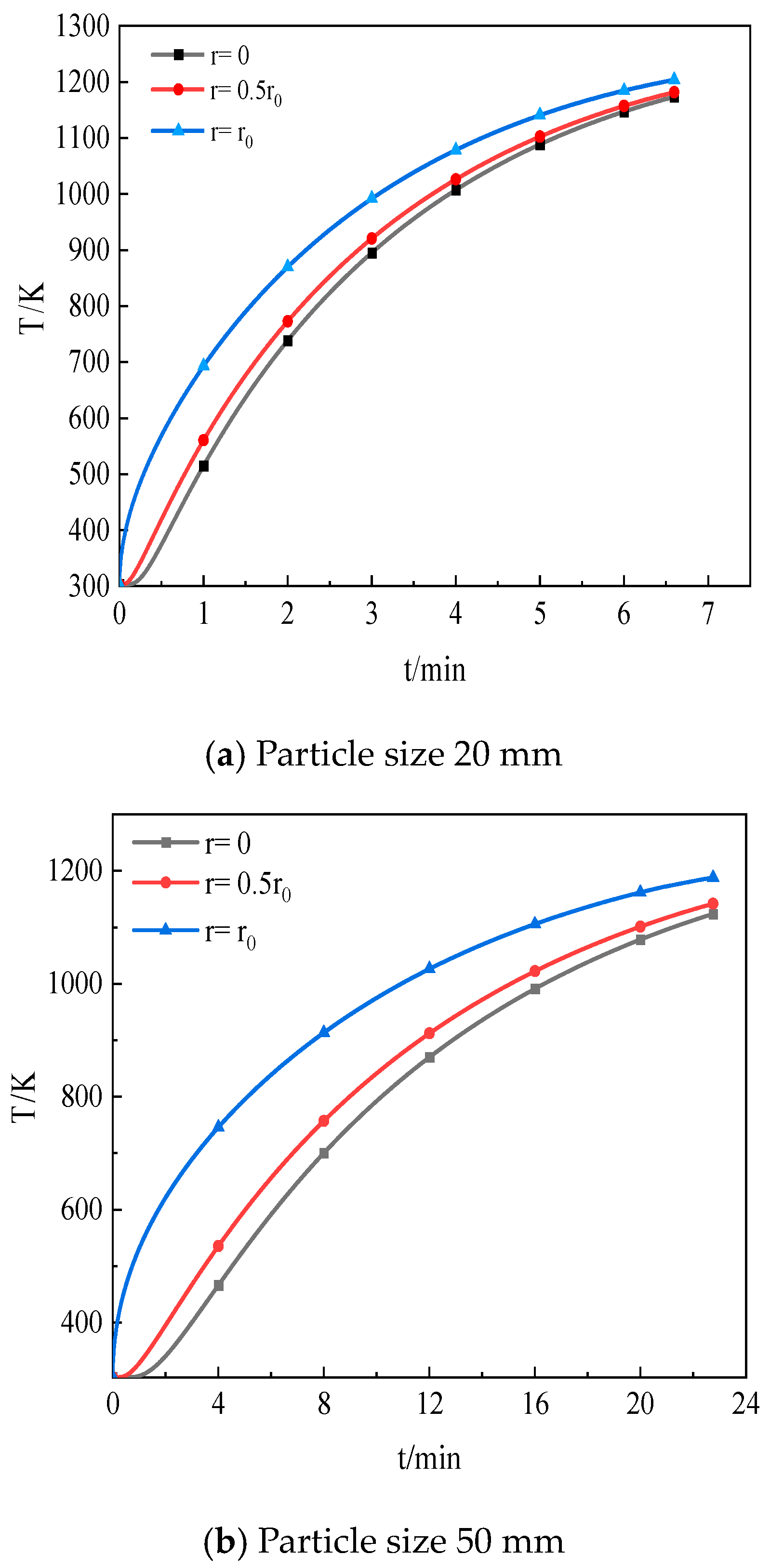
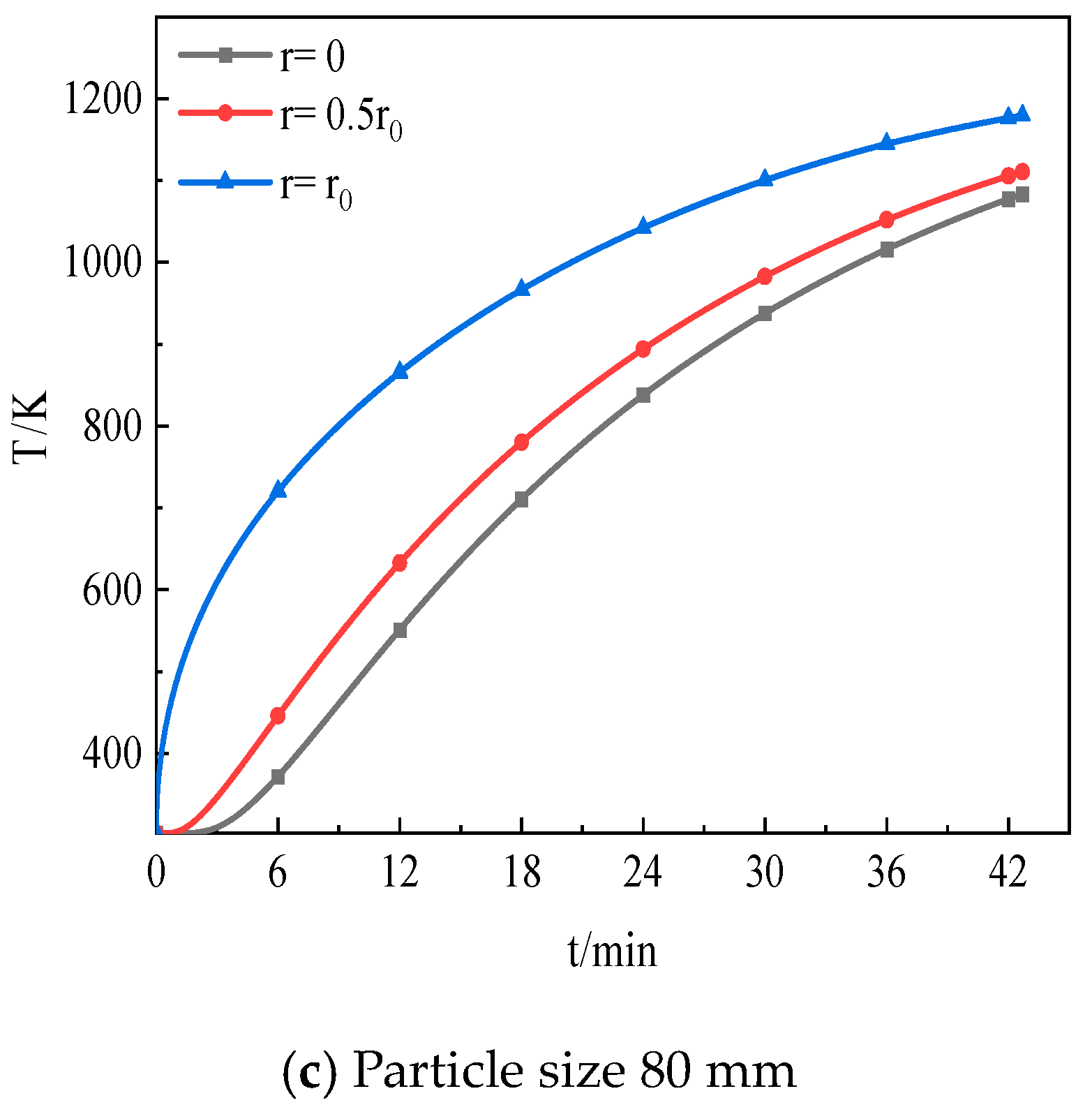
5.1. Effect of CO2 Distribution inside Particles on Decomposition
5.2. Influence of Limestone Particle Size on Decomposition
6. Conclusions and Outlook
- (1)
- The simulation results of model 1 show better agreement with the experimental data, with a relative error of less than 10% overall.
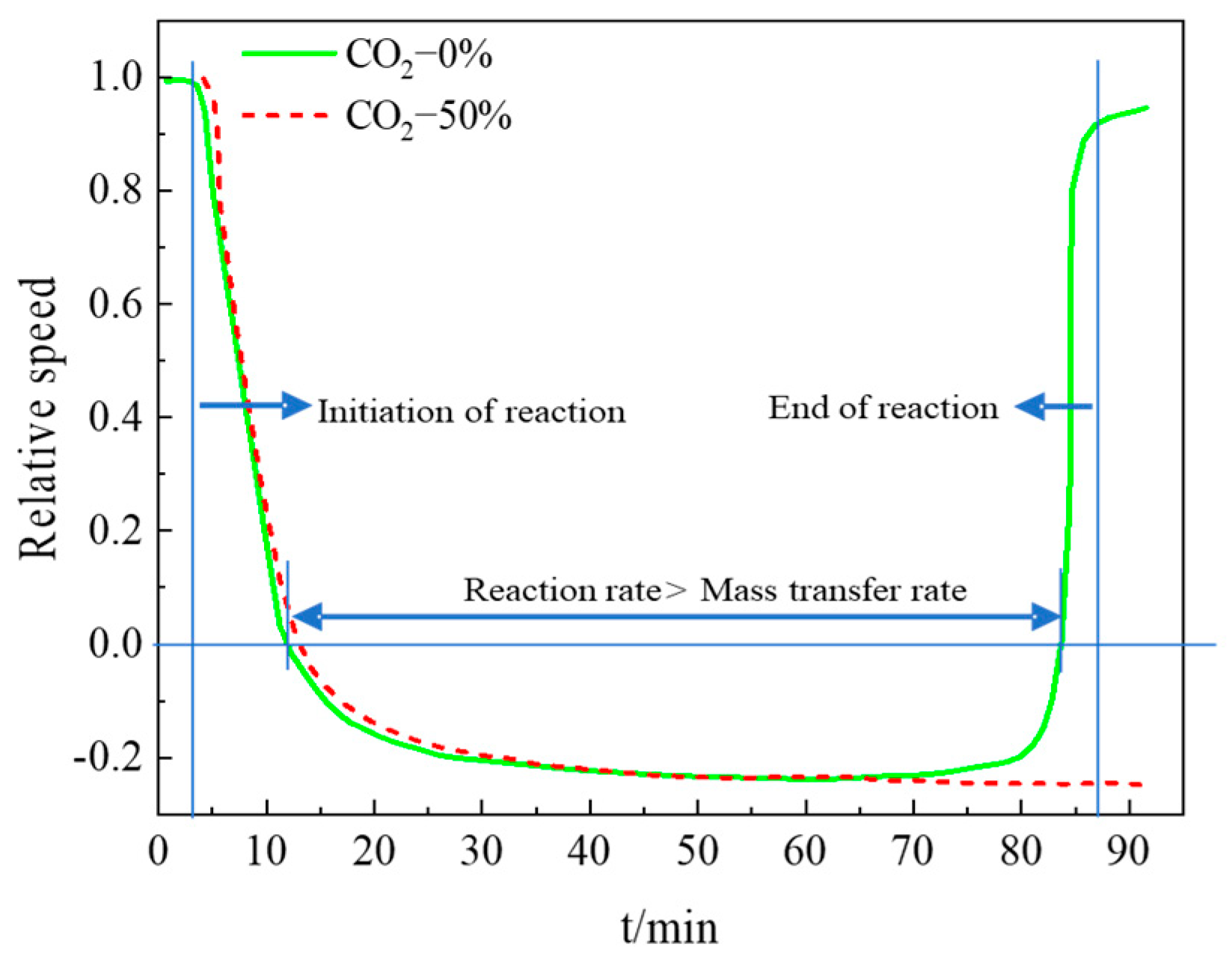
- (2)
- The average decomposition reaction rate is greater than the average mass transfer rate, and the average reaction rate is about 20% higher than the average mass transfer rate.
- (3)
- Compared to the ambient CO2 volume fraction outside the particle, the maximum increment of the CO2 volume fraction inside the particle approaches 0.42% as the particle size reaches 80 mm, indicating that the CO2 variation cannot be ignored for the decomposition of limestone.
- (4)
- Based on model 1, it was found that as the particle size of limestone increases, the time required for the limestone decomposition also increases. For every 10% increase in particle size, the time required for the decomposition increases by about 11.3%. Meanwhile, as the particle size increases from 20 mm to 80 mm, the time required for the temperature rise in the initial stage increases from 6.6 min to 42.7 min.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| molar concentration of CO2 in limestone, mol/m3 | |
| real-time concentration of CO2 at the interface of the reaction layer, mol/m3 | |
| molar concentration of CO2 in the hot gas stream, mol/m3 | |
| equilibrium concentration of CO2 at the reaction interface, mol/m3 | |
| molar concentration of CO2 in an ambient atmosphere, mol/m3 | |
| specific heat capacity of solid at constant pressure, J/(kg·K) | |
| thermals specific heat capacity, J/(kg·K) | |
| mass transfer coefficient, m2/s | |
| gas diffusion coefficient of CO2 through gas boundary layer, m2/s | |
| effective diffusion coefficient of CO2 through calcium oxide, m2/s | |
| diameter of limestone-calcium oxide interface, m | |
| internal pore diameter of solid particles, m | |
| initial diameter of limestone, m | |
| convective heat transfer coefficient, W/(m2·K) | |
| decomposition heat of limestone decomposition reaction, J/mol | |
| limestone decomposition equilibrium constant, Pa | |
| limestone decomposition reaction rate constant, mol/(m2·s) | |
| rate of CO2 formation in limestone reaction layer, mol/(m3·s) | |
| Nusselt number | |
| Prandtl number | |
| limestone decomposition reaction rate, mol/s | |
| Reynolds number | |
| gas constant, J/(mol·K) | |
| initial limestone particle radius, m | |
| calculated radius of limestone particle, m | |
| Sherwood number | |
| heat source term of limestone decomposition reaction, J/(m3·s) | |
| the temperature of the limestone reaction layer, K | |
| , | thermals and surface temperatures of limestone particles, K |
| volume of limestone reaction layer, m3 | |
| velocity of hot gas, m/s | |
| thermal conductivity of limestone particles, W/(m·K) | |
| thermal conductivity of hot air flow, W/(m·K) | |
| dynamic viscosity of thermals, Pa·s | |
| limestone particle density, kg/m3 | |
| density of thermals, kg/m3 | |
| porosity of the particles |
References
- Qu, B.H.; Ding, C.H.; Ning, S.L. Influence of limestone resources on iron and steel metallurgical industry. Refract. Lime 2022, 47, 37–41. [Google Scholar]
- Zhang, X.A.; Feng, P.; Xu, J.R.; Xu, J.A.; Feng, L.B.; Qing, S. Numerical research on combining flue gas recirculation sintering and fuel layered distribution sintering in the iron ore sintering process. Energy 2020, 192, 116660. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Emami, M.D.; Panjepour, M. The effects of kinetic parameters on combustion characteristics in a sintering bed. Energy 2014, 73, 160–176. [Google Scholar] [CrossRef]
- Huang, Z.Z. Application of limestone flour concrete in architectural engineering. J. Hunan City Univ. 2010, 19, 12–15. [Google Scholar]
- Jiang, L.Y.; Shao, J.N.; Zhou, J.; Hua, J.S. Discussion on limestone-gypsum desulfurization technology for ultra-low emission in coal-fired power plant. Mod. Chem. Ind. 2022, 42, 51–53. [Google Scholar]
- Wang, L.Y.; Xue, Z.L.; Chen, K.F.; Hu, B. High-temperature decomposition kinetics of large particle-size limestone. J. Chongqing Univ. 2020, 43, 32–46. [Google Scholar]
- Zhang, X.; Xu, J.; Wang, Z.B. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction. Inorg. Chem. Ind. 2023, 55, 79–84. [Google Scholar]
- Zhang, W.X. Study on Limestone Calcination Process under High CO2 Atmosphere. Master’s Thesis, Hebei University of Technology,, Tianjin, China, 2020. [Google Scholar]
- Li, H.; Zhang, L.L.; Duan, Y.H.; Mi, Y.G. Kinetics of thermal decomposition reaction of limestone at high carbon dioxide concentration. J. Chin. Ceram. Soc. 2013, 41, 637–643. [Google Scholar]
- Criado, J.; González, M.; Málek, J.; Ortega, A. The effect of the CO2 pressure on the thermal decomposition kinetics of calcium carbonate. Thermochim. Acta 1995, 254, 121–127. [Google Scholar] [CrossRef]
- Li, J.R. Study on Decomposition Characteristics and Reaction Kinetics of Limestone. Master’s Thesis, Institute of Engineering Thermophysics, Chinese Academy of Sciences, Beijing, China,, 2019. [Google Scholar]
- Zhang, L.N.; Yuan, Z.F.; Li, L.S.; Wu, Y.; Sui, D.P. Model research of thermal decomposition kinetics of limestone. Nonferrous Met. Sci. Eng. 2016, 7, 13–18. [Google Scholar]
- Zhang, W.X.; Liu, L.S.; Cao, H.J.; Wu, B.K.; Cheng, Z.P. Effect on kinetics of limestone decomposition under different CO2 atmospheres. Inorg. Chem. Ind. 2020, 52, 59–63. [Google Scholar]
- Jiang, T. Microstructure and Kinetic Analysis on Limestone Decomposition under Different Atmodphere. Master’s Thesis, South China University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Chen, J.T.; Chen, H.W.; Zhao, Z.H.; Wei, R.G. Analysis of variation characteristics of kinetics parameters on limestone calcination reaction in different temperature. Power Syst. Eng. 2012, 28, 10–12. [Google Scholar]
- Wang, L.Y. The Characteristics of the Lime Calcined Rapidly at Super High Temperature and Its Dissolution Behavior in Converter Slag. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2020. [Google Scholar]
- Cao, J.; Qiao, X.C.; Liu, C.L.; Zhang, J.S. A study on kinetics of limestone decomposition in air and CO2 atmospheres. Inorg. Chem. Ind. 2016, 48, 32–36. [Google Scholar]
- Chen, K.F.; Xue, Z.L.; Li, J.L. Kinetics of Thermal Decomposition Reaction of Limestone for Flash Heating of Limestone at High Temperature. J. Chin. Ceram. Soc. 2016, 44, 754–762. [Google Scholar]
- Hills, A.W.D. The mechanism of the thermal decomposition of calcium carbonate. Chem. Eng. Sci. 1968, 23, 297–320. [Google Scholar] [CrossRef]
- Bluhm-Drenhaus, T.; Simsek, E.; Wirtz, S.; Scherer, V. A coupled fluid dynamic-discrete element simulation of heat and mass transfer in a lime shaft kiln. Chem. Eng. Sci. 2010, 65, 2821–2834. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.H.; Zhou, H.Y.; Li, Q. Numerical simulation of lime calcination process based on nuclear shrinkage model. Ind. Furn. 2022, 44, 16–21. [Google Scholar]
- Wang, G.; Wen, Z.; Lou, G.; Dou, R.F.; Li, X.W.; Liu, X.L.; Su, F.Y. Mathematical modeling and combustion characteristic evaluation of a flue gas recirculation iron ore sintering process. Int. J. Heat Mass Transf. 2016, 97, 964–974. [Google Scholar] [CrossRef]
- Krause, B.; Liedmann, B.; Wiese, J.; Bucher, P.; Wirtz, S.; Piringer, H.; Scherer, V. 3D-DEM-CFD simulation of heat and mass transfer, gas combustion and calcination in an intermittent operating lime shaft kiln. Int. J. Therm. Sci. 2017, 117, 121–135. [Google Scholar] [CrossRef]
- Jiang, B.A.; Xia, D.H.; Yu, B.; Xiong, R.; Ao, W.Q.; Zhang, P.K.; Cong, L.A. An environment-friendly process for limestone calcination with CO2 looping and recovery. J. Clean. Prod. 2019, 240, 118147. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, S.; Liu, Q.; Yang, X.F.; Zhou, H.Y.; Chen, M.J.; Zhu, R.J.; Wu, D.L. Transient simulation of heat and mass transfer in a parallel flow regenerative lime kiln with pulverized coal injection. Appl. Therm. Eng. 2023, 227, 120412. [Google Scholar] [CrossRef]
- Zhou, A.L.; Zhang, Z.X.; Huang, Z.Q. Numerical simulation and experimental research on calcination of ultra-fine limestone. J. Chin. Soc. Power Eng. 2004, 24, 110–113. [Google Scholar]
- Chen, H.W.; Ji, Y.; Wang, C.B.; Li, Y.H.; Liao, D. Simulation and analysis on the calcinations of the limestone particle. Power Syst. Eng. 2004, 20, 12–14. [Google Scholar]
- Ji, J.J. Numerical Modeling and Optimal Design of Air Distribution and Furnace Arch for Coal-Burning Chain Boiler. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2008. [Google Scholar]
- Li, Y.J.; Zhao, C.S.; Qu, C.R.; Duan, L.B.; Li, Q.Z.; Liang, C. CO2 capture using CaO modified with ethanol/water solution during cyclic calcination/carbonation. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2008, 31, 237–244. [Google Scholar] [CrossRef]
- Jia, X.X.; Xu, M.H.; Hu, G.H.; Liu, J.; Lu, H.; Liang, Z. Numerical method for three-dimensional heat conduction in cylindrical and spherical coordinates. J. Chongqing Univ. Technol. 2014, 28, 33–37. [Google Scholar]
- Fu, D.; Tang, G.; Zhao, Y.; D’Alessio, J.; Zhou, C.Q. Modeling of iron ore reactions in blast furnace. Int. J. Heat Mass Transf. 2016, 103, 77–86. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Fu, L.; Liu, Q.; E, J.; Zhou, H. Model and Parameter Study of Limestone Decomposition Reaction. Processes 2024, 12, 150. https://doi.org/10.3390/pr12010150
Zhu R, Fu L, Liu Q, E J, Zhou H. Model and Parameter Study of Limestone Decomposition Reaction. Processes. 2024; 12(1):150. https://doi.org/10.3390/pr12010150
Chicago/Turabian StyleZhu, Rongjia, Liangyu Fu, Qian Liu, Jiaqiang E, and Haoyu Zhou. 2024. "Model and Parameter Study of Limestone Decomposition Reaction" Processes 12, no. 1: 150. https://doi.org/10.3390/pr12010150
APA StyleZhu, R., Fu, L., Liu, Q., E, J., & Zhou, H. (2024). Model and Parameter Study of Limestone Decomposition Reaction. Processes, 12(1), 150. https://doi.org/10.3390/pr12010150







