Simulation Experiment Design and Control Strategy Analysis in Teaching of Hydrogen-Electric Coupling System
Abstract
1. Introduction
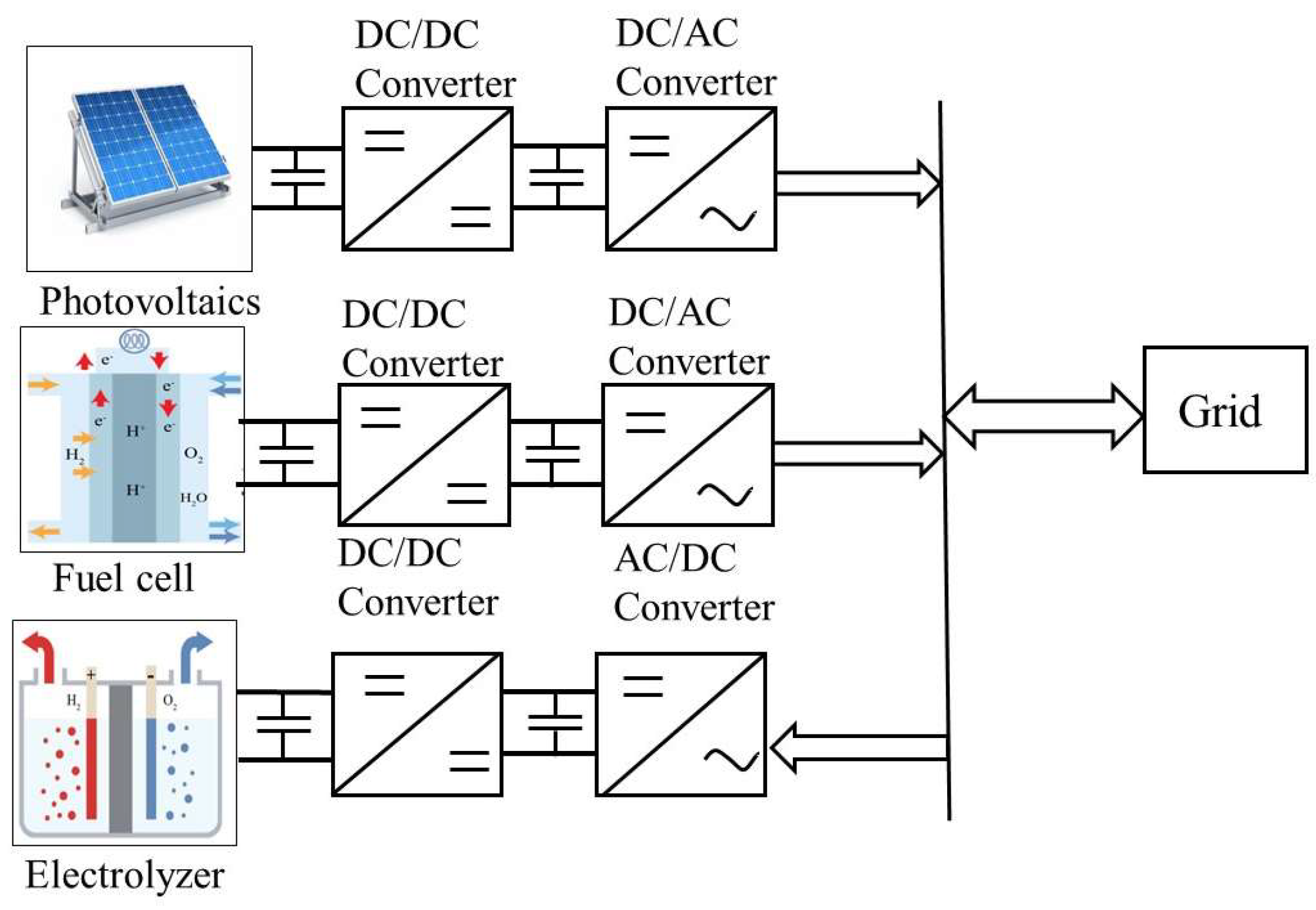
2. Typical Structure of Hydrogen-Electric Coupling System
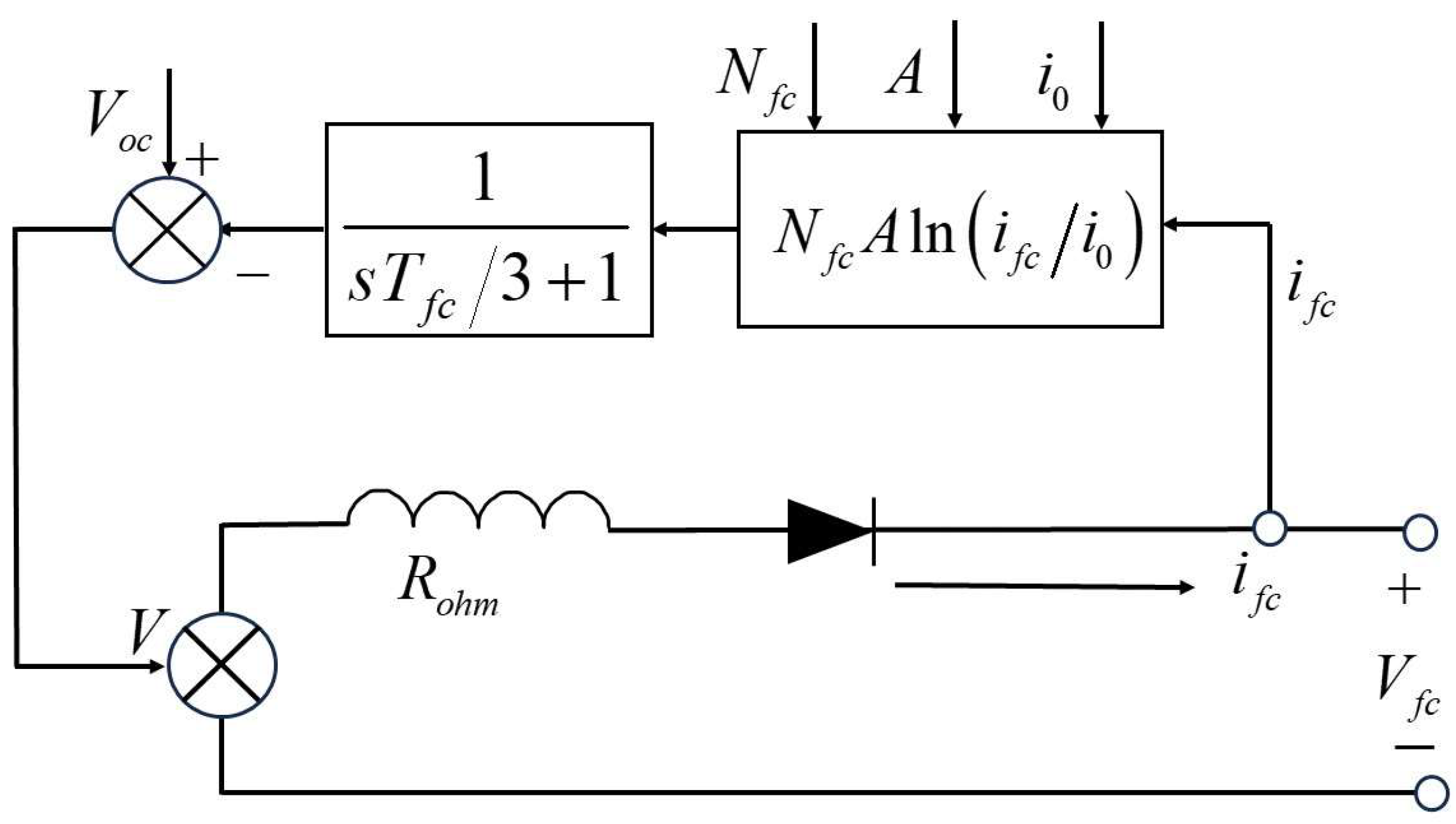
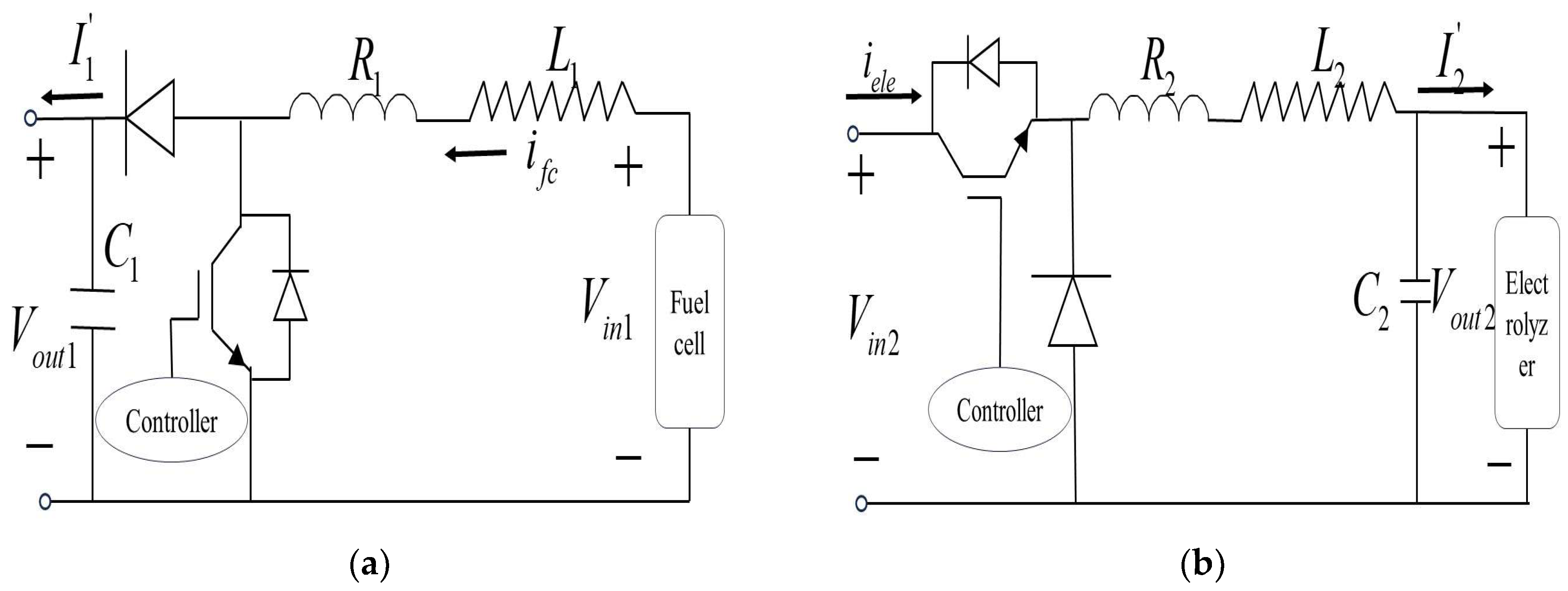
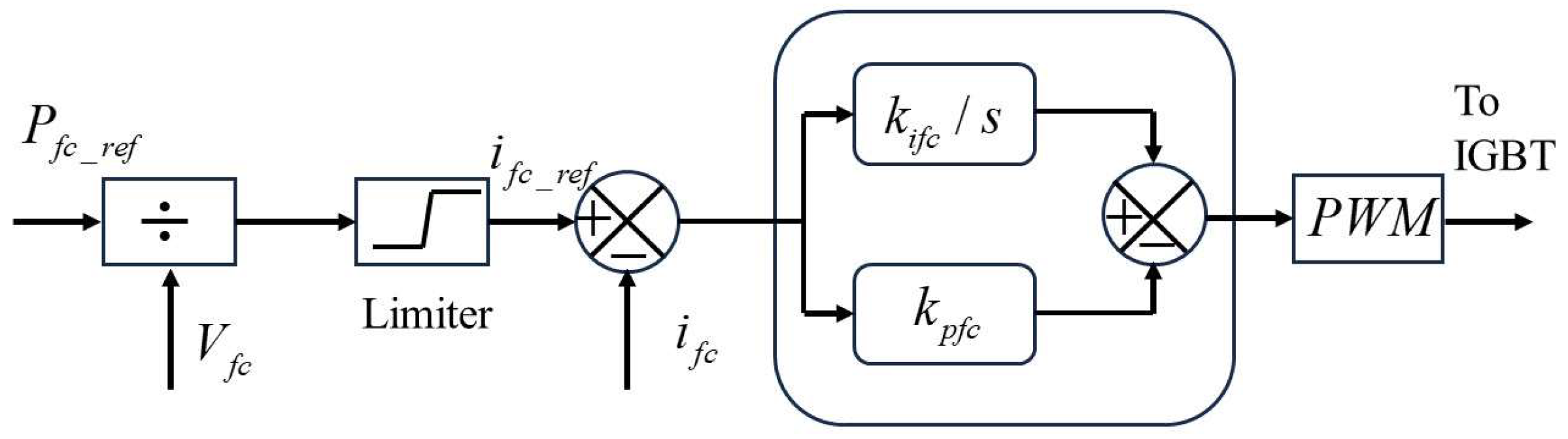
3. Simulation Model of Fuel Cell Power Generation
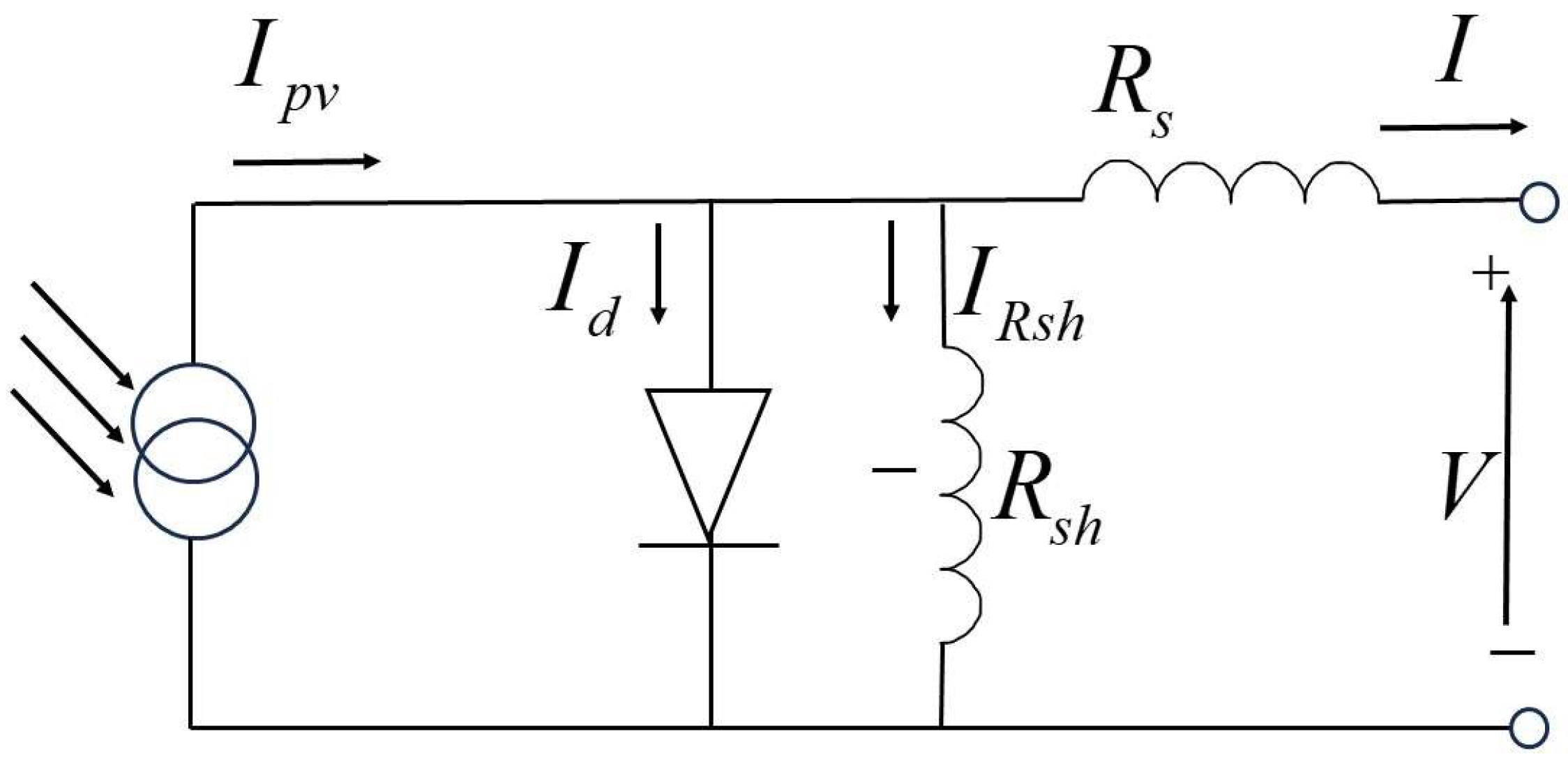
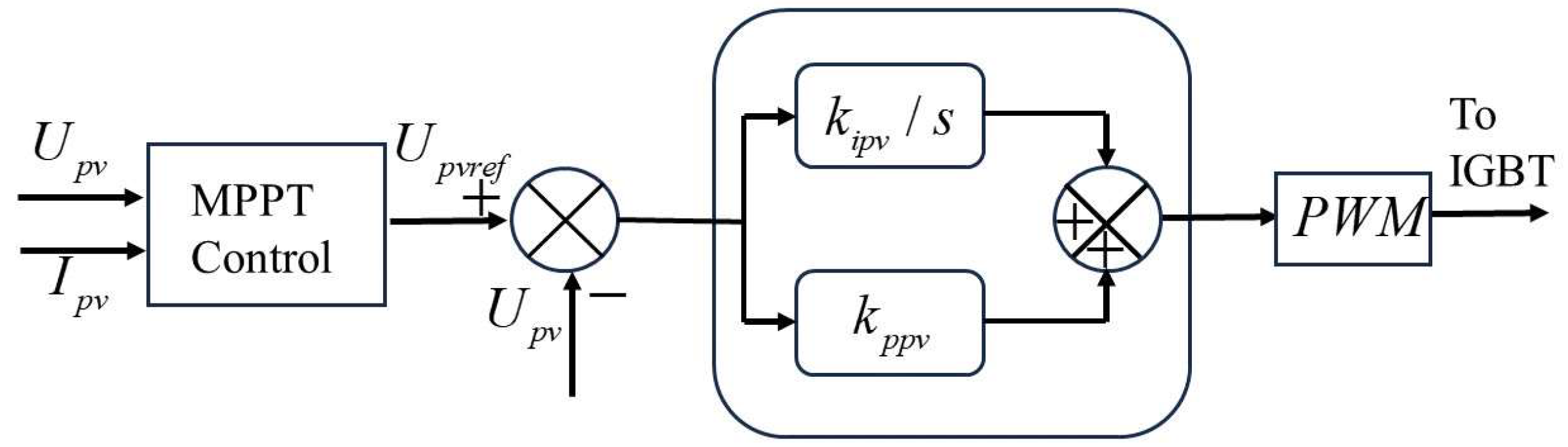
4. Simulation Model of Photovoltaic Power Generation
5. Simulation Model of Electrolytic Hydrogen Production
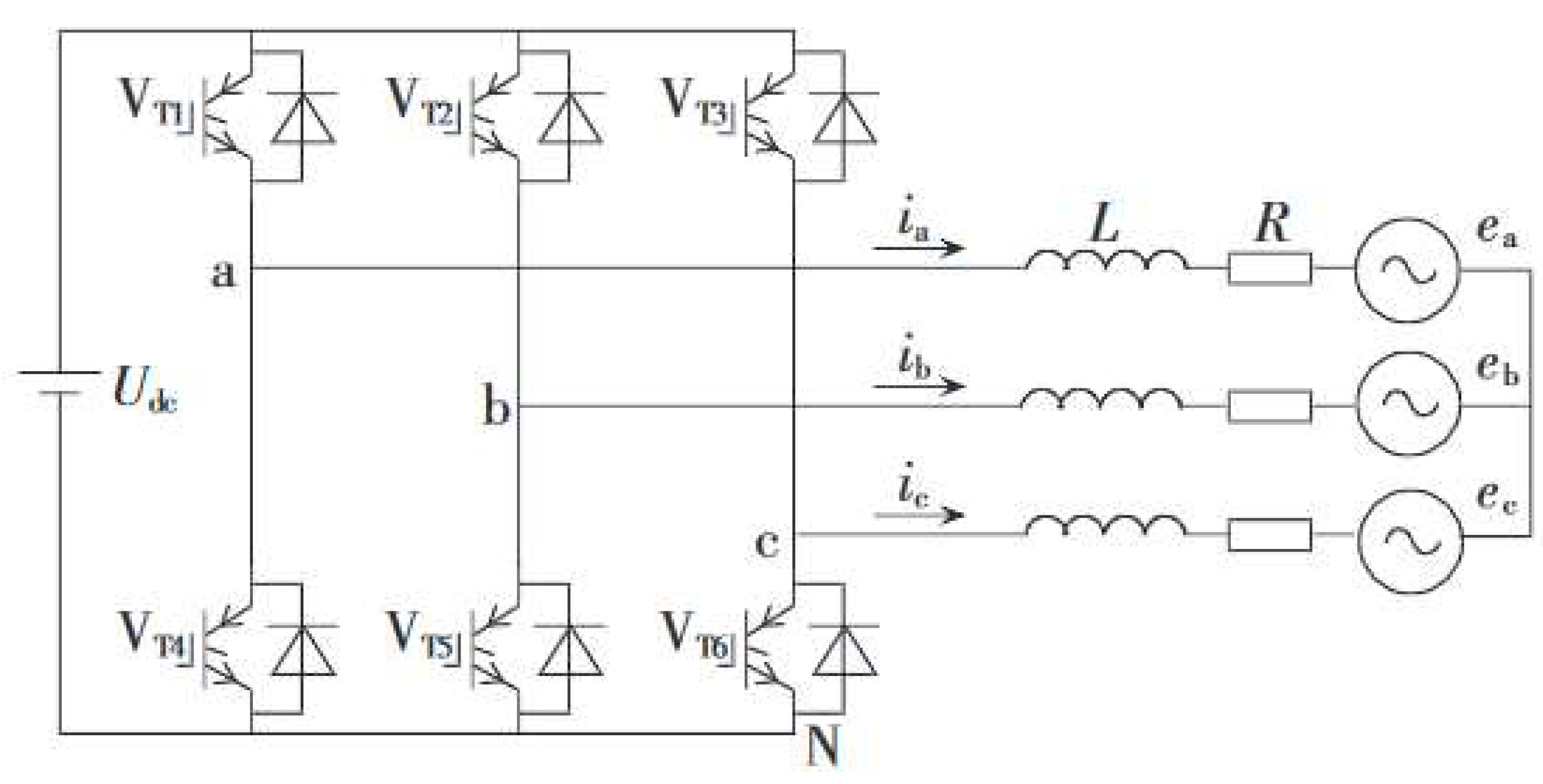
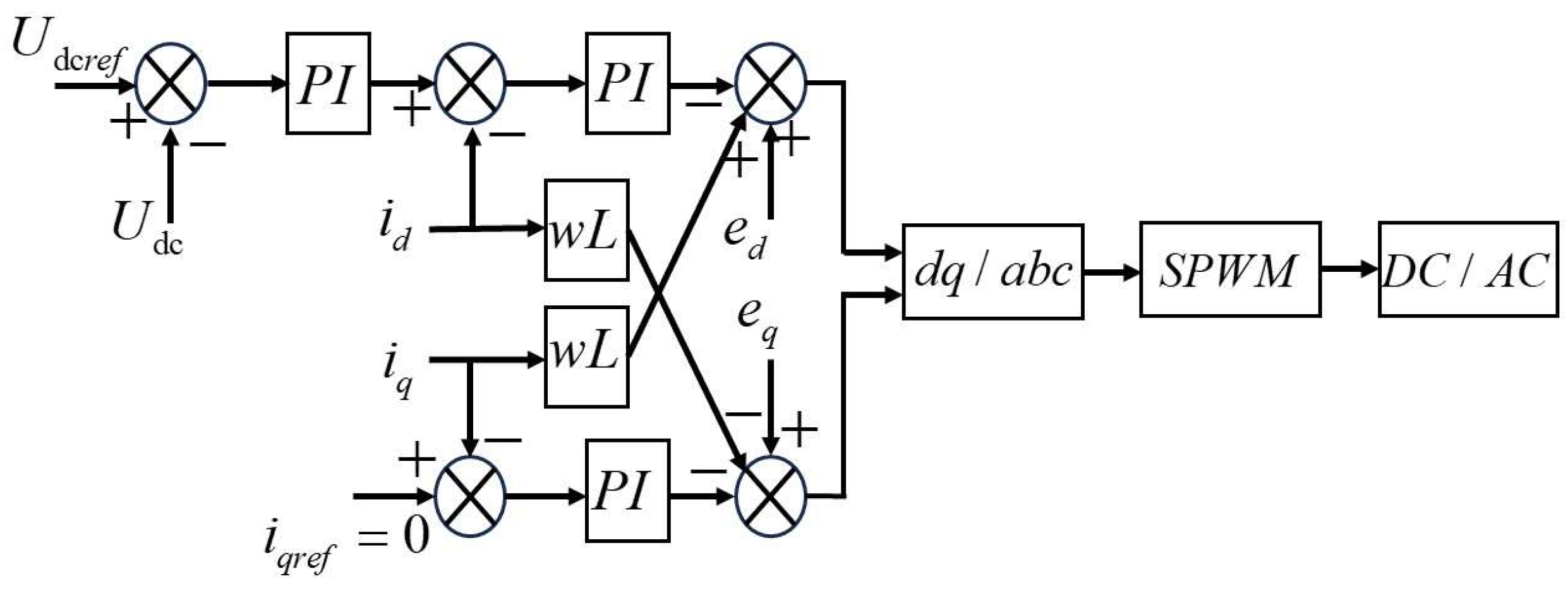
6. Modeling of the Power Electronic Converter
7. Simulation Scenarios and Control Strategy Design
7.1. Hydrogen-Electric Coupling System Simulation Scenario
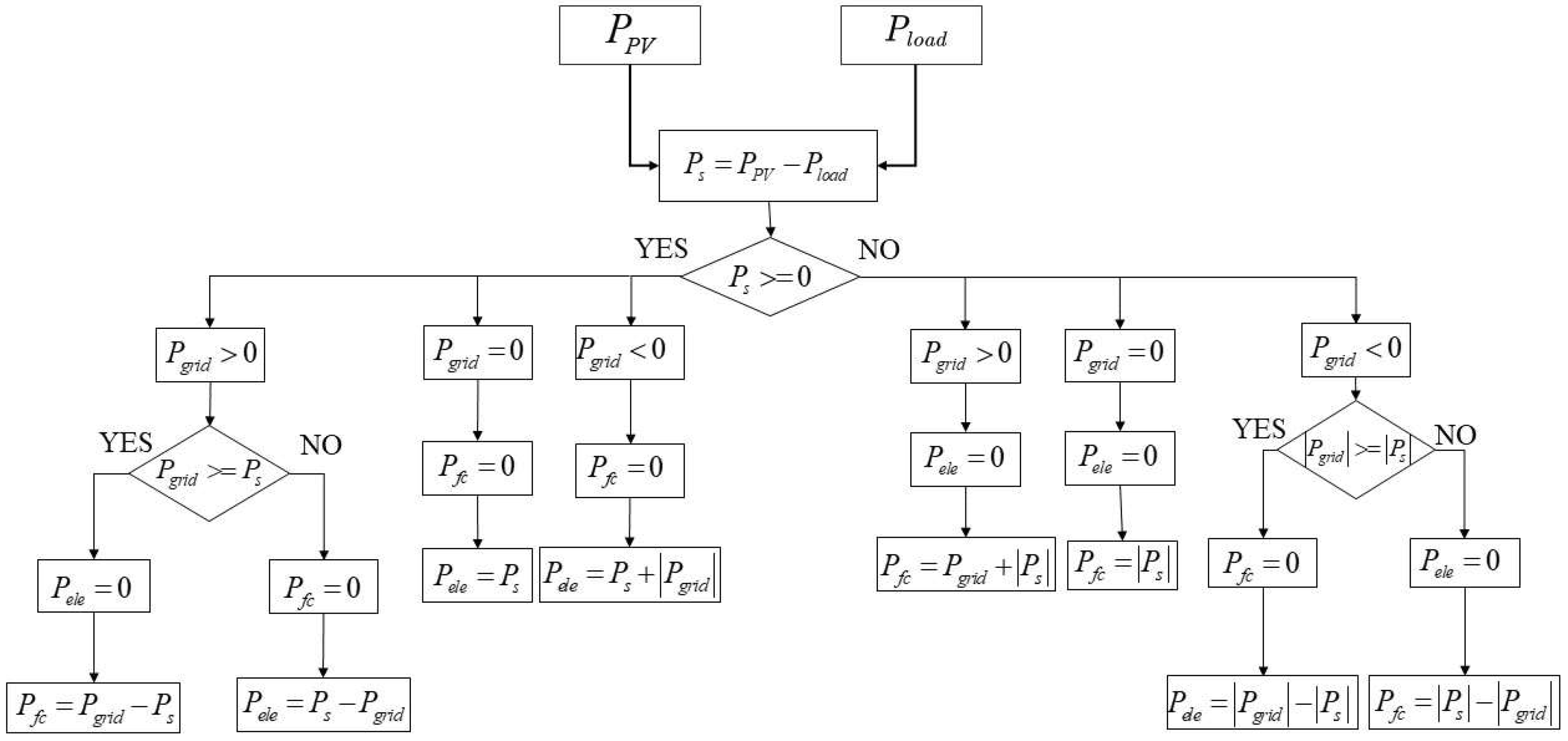
7.2. Cooperative Control Strategy of Fuel Cell and Photovoltaic Power Generation
7.3. The Collaborative Control Strategy for Electrolytic Hydrogen Production and Photovoltaic Power Generation
8. Simulation Analysis
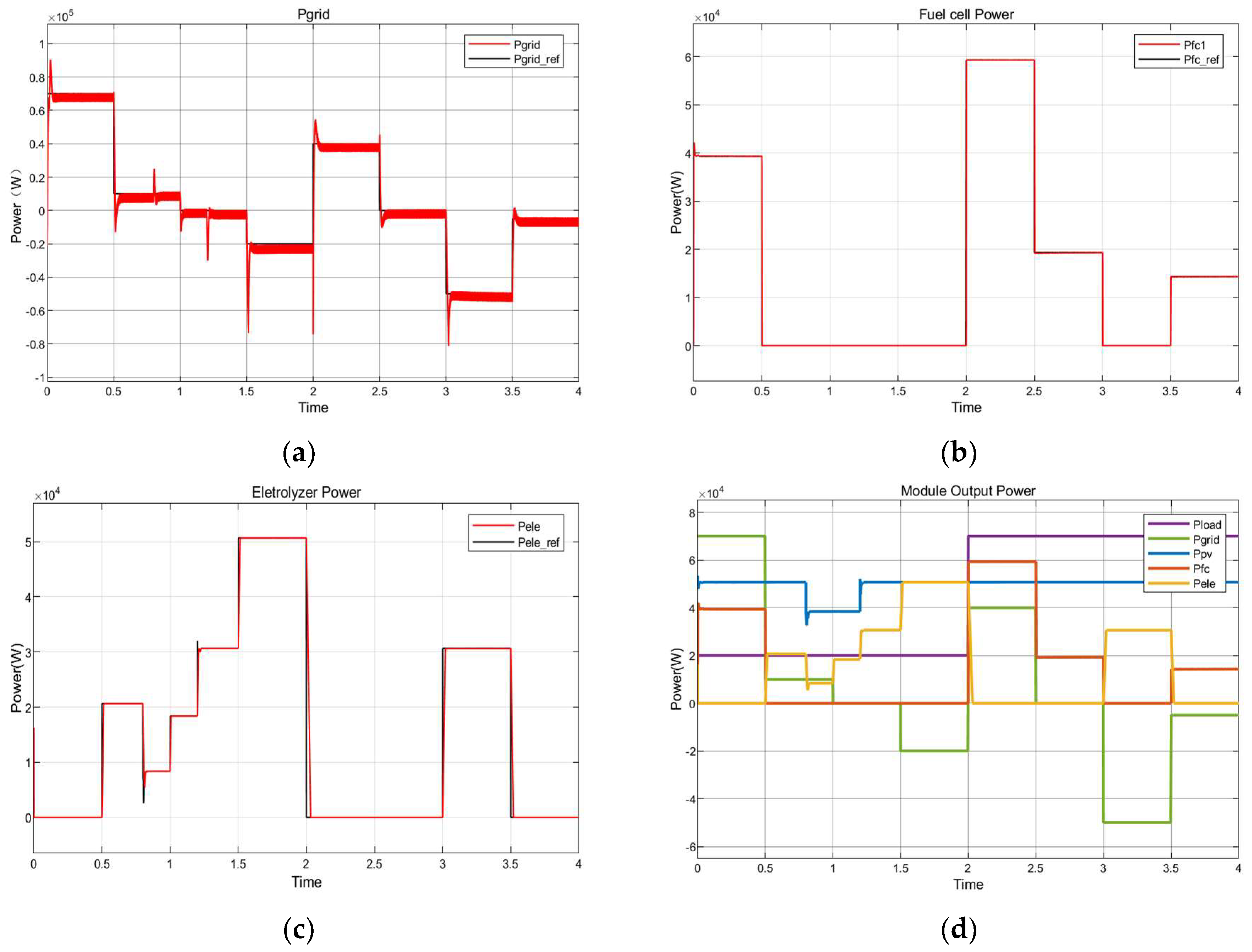
8.1. System Response Characteristics under Different Simulation Scenarios
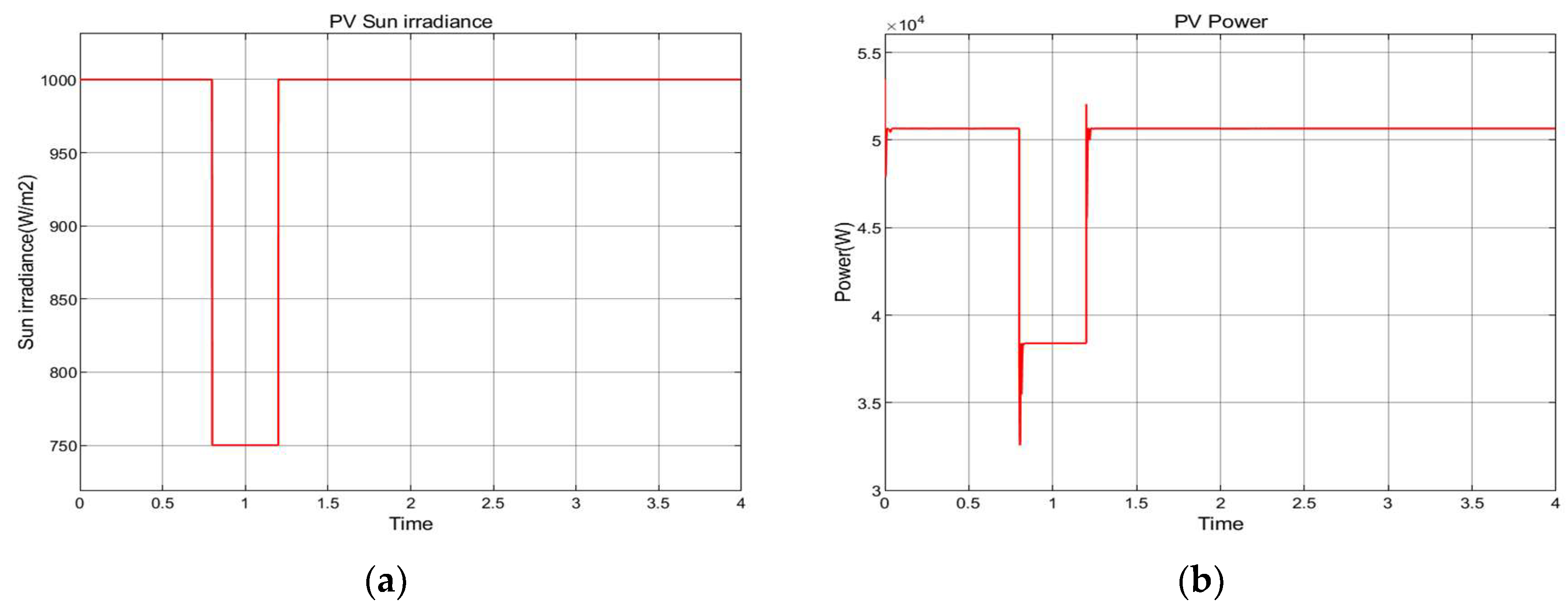
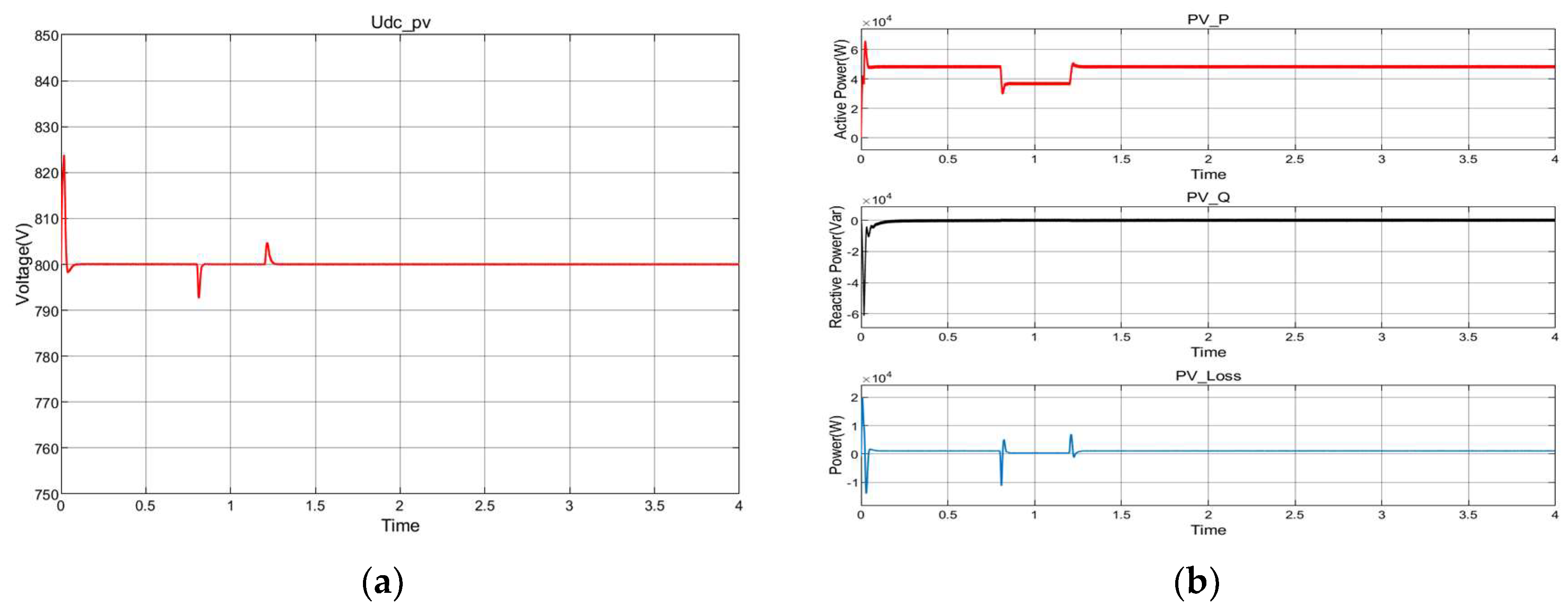
8.2. Photovoltaic Cells Energy Conversion
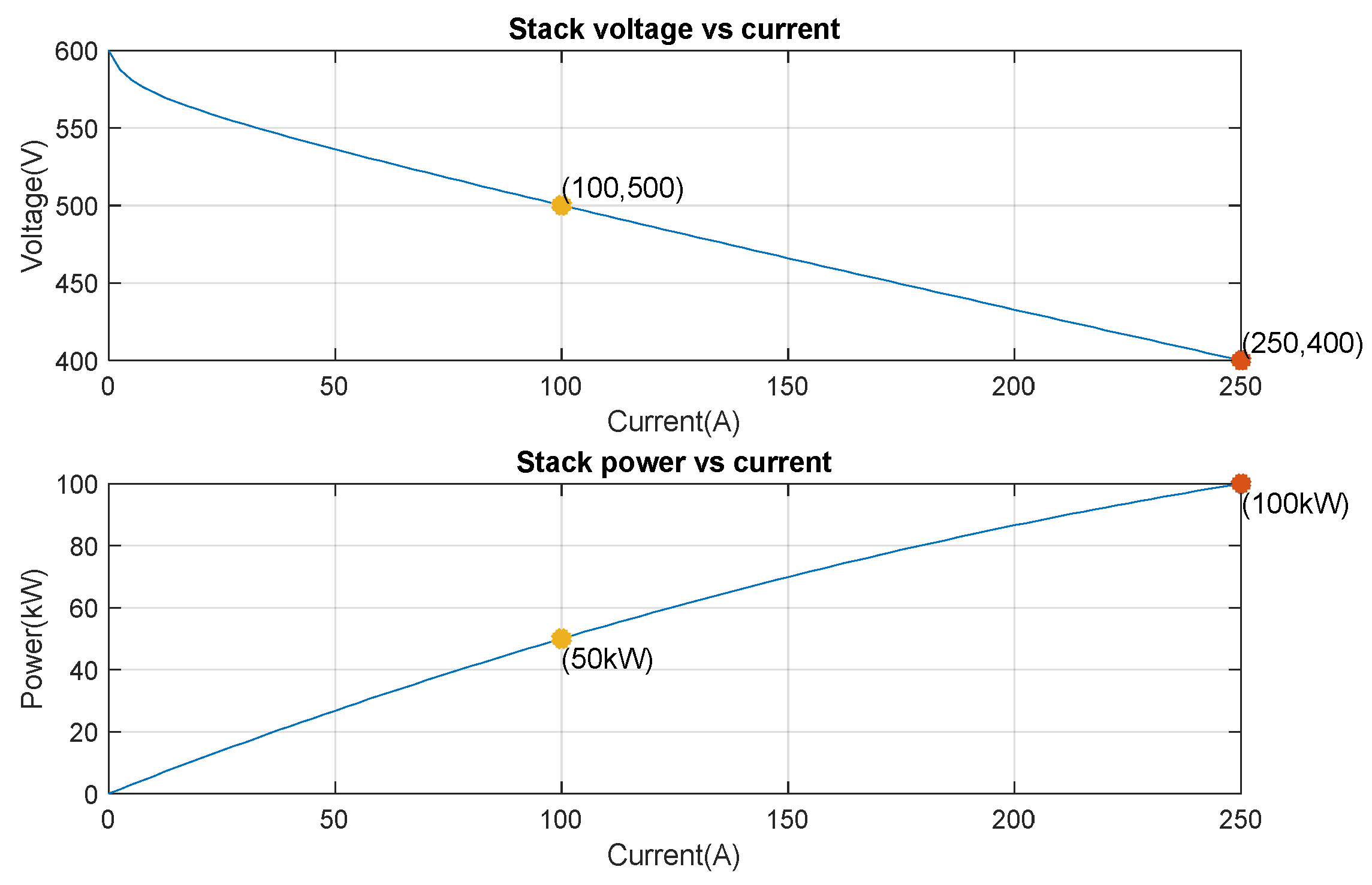
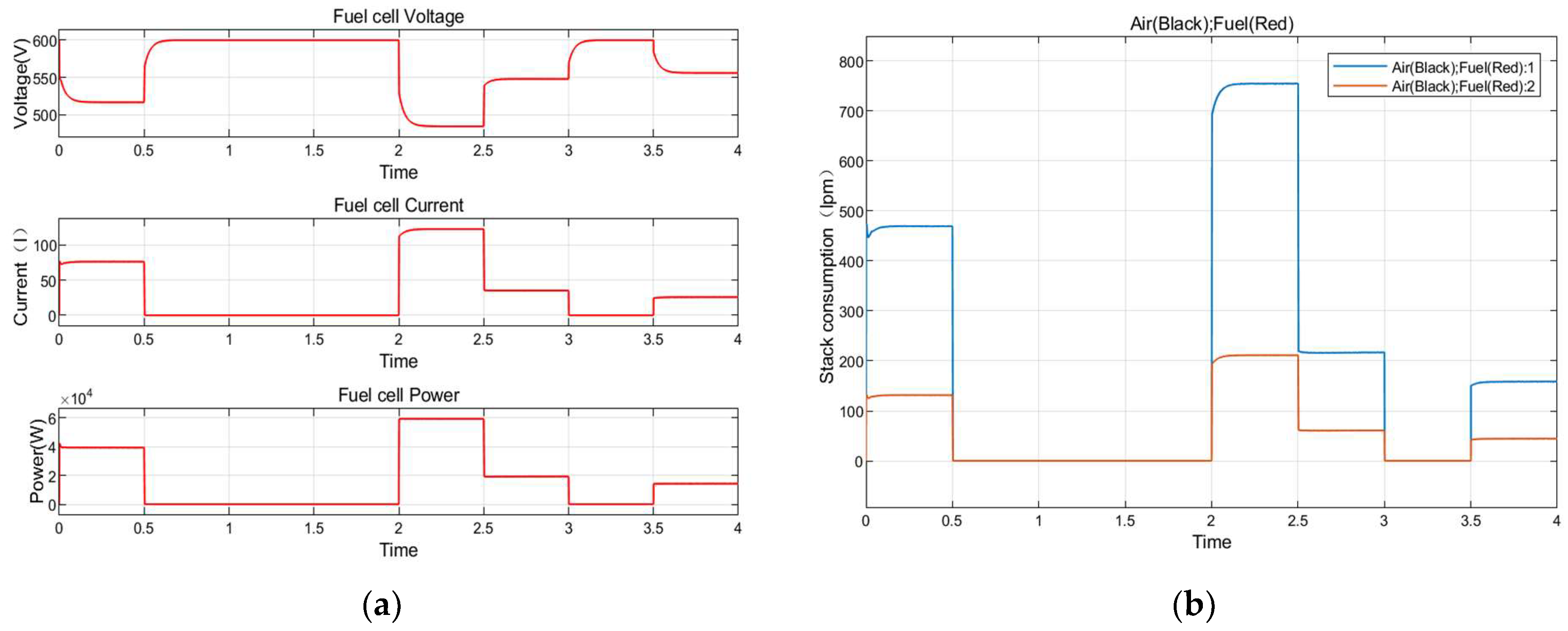
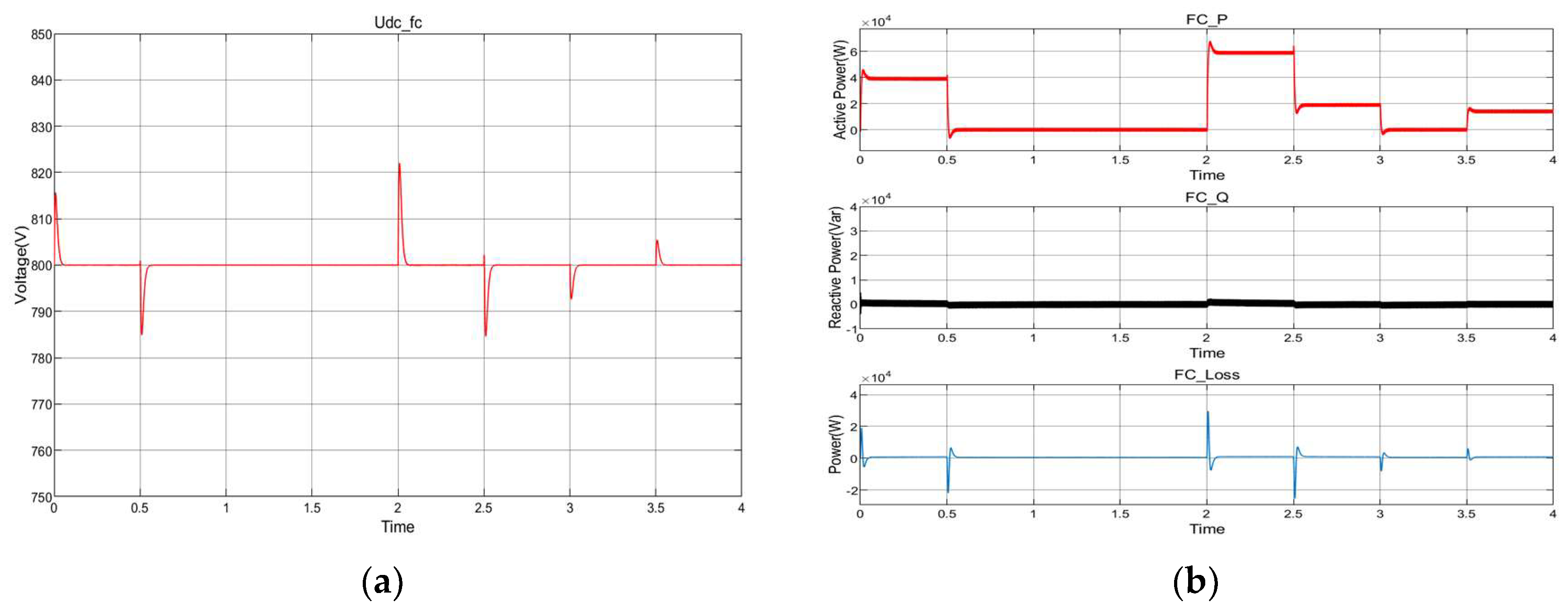
8.3. Fuel Cell Energy Conversion
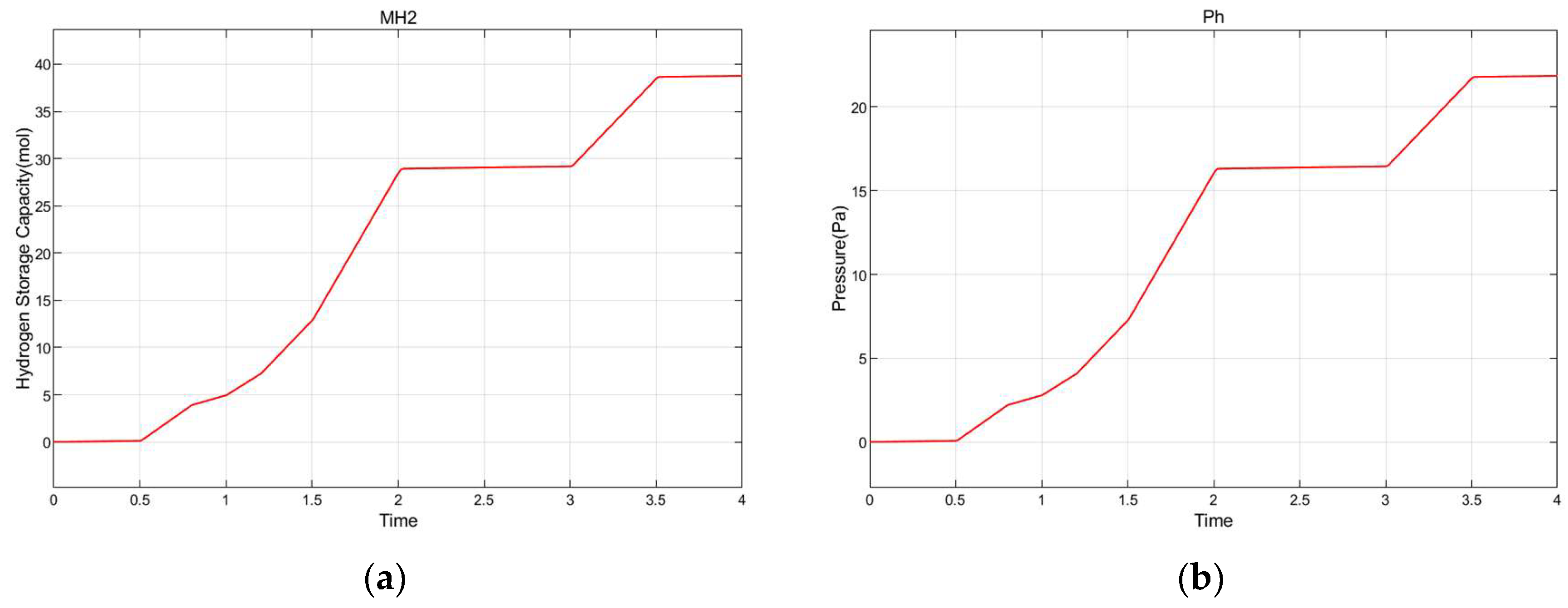
8.4. Electrolysis to Hydrogen Energy Conversion
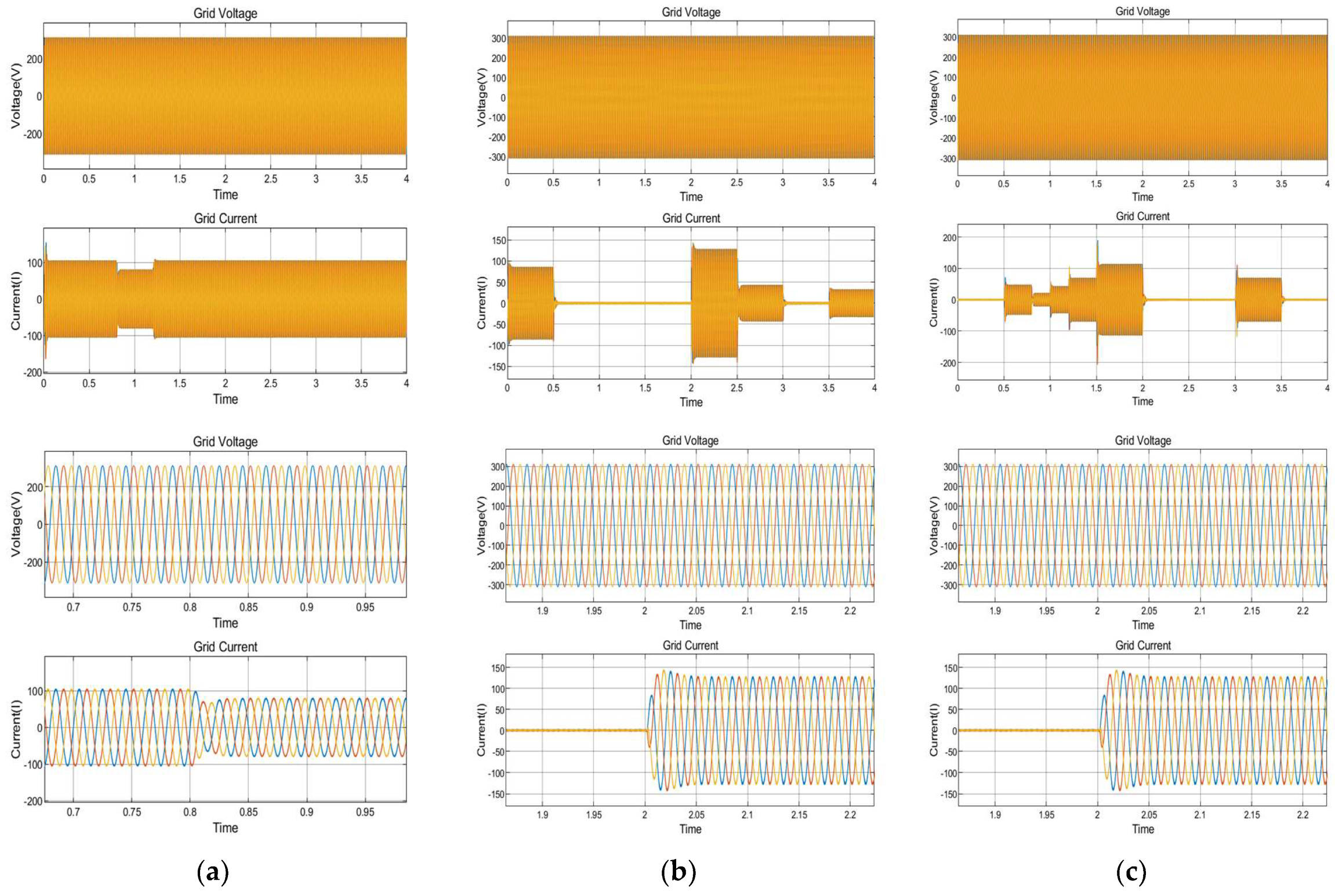
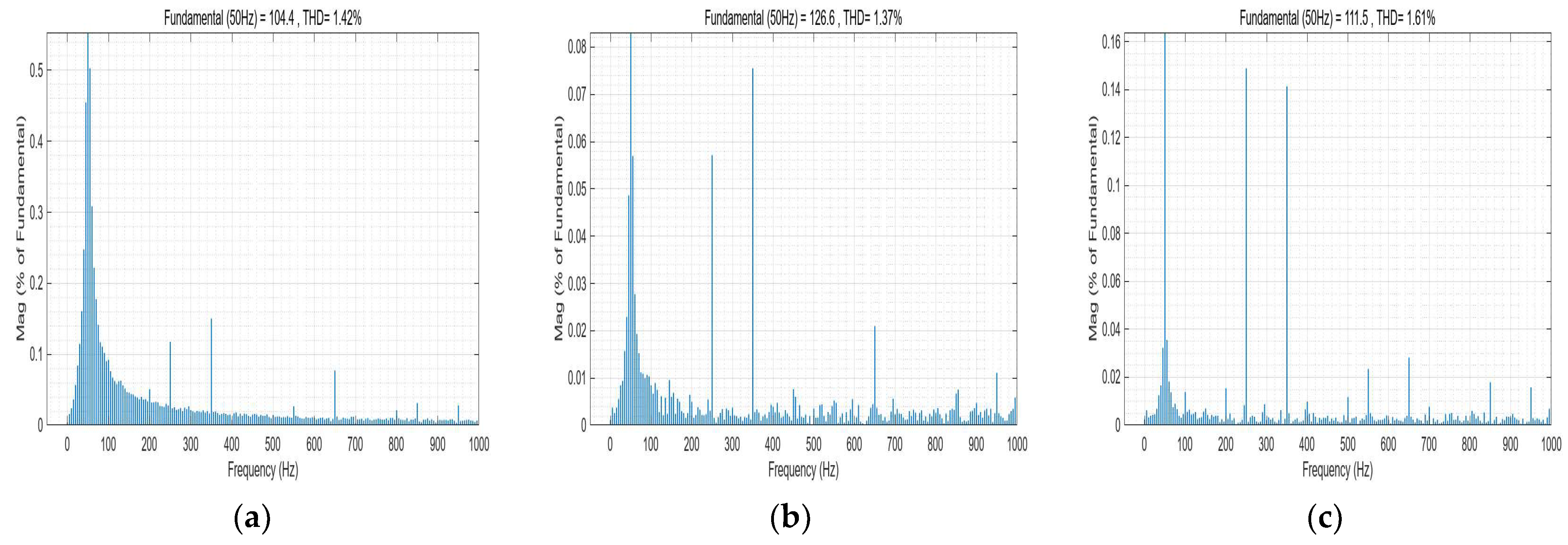
8.5. Dynamic Response Characteristics of the Power Electronic Converter
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A Technical and Environmental Comparison between Hydrogen and Some Fossil Fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Hossain, M.B.; Islam, M.R.; Muttaqi, K.M.; Sutanto, D.; Agalgaonkar, A.P. Advancement of Fuel Cells and Electrolyzers Technologies and Their Applications to Renewable-Rich Power Grids. J. Energy Storage 2023, 62, 106842. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Amatoul, F.Z.; Er-raki, M. Modeling and Simulation of Electrical Generation Systems Based on PEM Fuel Cell-Boost Converter Using a Closed Loop PI Controller. Energy Rep. 2023, 9, 296–308. [Google Scholar] [CrossRef]
- Sun, L.; Jin, Y.; Pan, L.; Shen, J.; Lee, K.Y. Efficiency Analysis and Control of a Grid-Connected PEM Fuel Cell in Distributed Generation. Energy Convers. Manag. 2019, 195, 587–596. [Google Scholar] [CrossRef]
- Rasekh, N.; Hosseinpour, M. LCL Filter Design and Robust Converter Side Current Feedback Control for Grid-Connected Proton Exchange Membrane Fuel Cell System. Int. J. Hydrogen Energy 2020, 45, 13055–13067. [Google Scholar] [CrossRef]
- Xue, F.; Su, J.; Li, P.; Zhang, Y. Application of Proton Exchange Membrane Electrolysis of Water Hydrogen Production Technology in Power Plant. IOP Conf. Ser. Earth Environ. Sci. 2021, 631, 012079. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Nguyen, T.; Abdin, Z.; Holm, T.; Mérida, W. Grid-Connected Hydrogen Production via Large-Scale Water Electrolysis. Energy Convers. Manag. 2019, 200, 112108. [Google Scholar] [CrossRef]
- Ismail, T.M.; Ramzy, K.; Elnaghi, B.E.; Abelwhab, M.N.; El-Salam, M.A. Using MATLAB to Model and Simulate a Photovoltaic System to Produce Hydrogen. Energy Convers. Manag. 2019, 185, 101–129. [Google Scholar] [CrossRef]
- Gaamouche, R.; Abbou, A.; Redouane, A.; Hasnaoui, A.E.; Belhorma, B. Modeling and Simulation of Direct Coupling of an Electrolyzer to PV System for Hydrogen Production. In Proceedings of the 2018 6th International Renewable and Sustainable Energy Conference (IRSEC), Rabat, Morocco, 5–8 December 2018; IEEE: Rabat, Morocco, 2018; pp. 1–5. [Google Scholar]
- Kuo, J.-K.; Wang, C.-F. An Integrated Simulation Model for PEM Fuel Cell Power Systems with a Buck DC–DC Converter. Int. J. Hydrogen Energy 2011, 36, 11846–11855. [Google Scholar] [CrossRef]
- İnci, M. Active/Reactive Energy Control Scheme for Grid-Connected Fuel Cell System with Local Inductive Loads. Energy 2020, 197, 117191. [Google Scholar] [CrossRef]
- Bizon, N.; Lopez-Guede, J.M.; Kurt, E.; Thounthong, P.; Mazare, A.G.; Ionescu, L.M.; Iana, G. Hydrogen Economy of the Fuel Cell Hybrid Power System Optimized by Air Flow Control to Mitigate the Effect of the Uncertainty about Available Renewable Power and Load Dynamics. Energy Convers. Manag. 2019, 179, 152–165. [Google Scholar] [CrossRef]
- Njoya, S.M.; Tremblay, O.; Dessaint, L.-A. A Generic Fuel Cell Model for the Simulation of Fuel Cell Vehicles. In Proceedings of the 2009 IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–11 September 2009; IEEE: Dearborn, MI, USA, 2009; pp. 1722–1729. [Google Scholar]
- Chen, W.; Han, Y.; Li, Q.; Liu, Z.; Peng, F. Design of Proton Exchange Membrane Fuel Cell Grid-Connected System Based on Resonant Current Controller. Int. J. Hydrogen Energy 2014, 39, 14402–14410. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Kou, Q.; Klein, S.A.; Beckman, W.A. A Method for Estimating the Long-Term Performance of Direct-Coupled PV Pumping Systems. Sol. Energy 1998, 64, 33–40. [Google Scholar] [CrossRef]
- Bensmail, S.; Rekioua, D.; Azzi, H. Study of Hybrid Photovoltaic/Fuel Cell System for Stand-Alone Applications. Int. J. Hydrogen Energy 2015, 40, 13820–13826. [Google Scholar] [CrossRef]
- Folgado, F.J.; González, I.; Calderón, A.J. Simulation Platform for the Assessment of PEM Electrolyzer Models Oriented to Implement Digital Replicas. Energy Convers. Manag. 2022, 267, 115917. [Google Scholar] [CrossRef]
- Koponen, J.; Ruuskanen, V.; Kosonen, A.; Niemela, M.; Ahola, J. Effect of Converter Topology on the Specific Energy Consumption of Alkaline Water Electrolyzers. IEEE Trans. Power Electron. 2019, 34, 6171–6182. [Google Scholar] [CrossRef]
- Milewski, J.; Guandalini, G.; Campanari, S. Modeling an Alkaline Electrolysis Cell through Reduced-Order and Loss-Estimate Approaches. J. Power Sources 2014, 269, 203–211. [Google Scholar] [CrossRef]
- Ursúa, A.; San Martín, I.; Barrios, E.L.; Sanchis, P. Stand-Alone Operation of an Alkaline Water Electrolyser Fed by Wind and Photovoltaic Systems. Int. J. Hydrogen Energy 2013, 38, 14952–14967. [Google Scholar] [CrossRef]
- Campos Da Paixão, I.; Cardozo, J.C.; Sales Monteiro, M.K.; Gondim, A.D.; Cavalcanti, L.N.; Fabiano De Santana Souza, D.; Martínez-Huitle, C.A.; Vieira Dos Santos, E. A Sustainable Solar-Driven Electrochemical Process for Reforming Lignocellulosic Biomass Effluent into High Value-Added Products: Green Hydrogen, Carboxylic and Vanillic Acids. RSC Adv. 2023, 13, 35755–35765. [Google Scholar] [CrossRef]
- Amores, E.; Rodríguez, J.; Carreras, C. Influence of Operation Parameters in the Modeling of Alkaline Water Electrolyzers for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 13063–13078. [Google Scholar] [CrossRef]
- Aboukalam Da Cruz, M.H.A.; Etancelin, M.; Marias, F.; Reneaume, J.-M.; Sochard-Reneaume, S.; Serra, S. Dynamic Modelling of an Alkaline Water Electrolysis System and Optimization of Its Operating Parameters for Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 12982–12999. [Google Scholar] [CrossRef]
- Tijani, A.S.; Yusup, N.A.B.; Rahim, A.H.A. Mathematical Modelling and Simulation Analysis of Advanced Alkaline Electrolyzer System for Hydrogen Production. Procedia Technol. 2014, 15, 798–806. [Google Scholar] [CrossRef]
- Panahandeh, B.; Bard, J.; Outzourhit, A.; Zejli, D. Simulation of PV–Wind-Hybrid Systems Combined with Hydrogen Storage for Rural Electrification. Int. J. Hydrogen Energy 2011, 36, 4185–4197. [Google Scholar] [CrossRef]
- Saravanan, S.; Ramesh Babu, N. Maximum Power Point Tracking Algorithms for Photovoltaic System—A Review. Renew. Sustain. Energy Rev. 2016, 57, 192–204. [Google Scholar] [CrossRef]
- Valdez-Resendiz, J.E.; Sanchez, V.M.; Rosas-Caro, J.C.; Mayo-Maldonado, J.C.; Sierra, J.M.; Barbosa, R. Continuous Input-Current Buck-Boost DC-DC Converter for PEM Fuel Cell Applications. Int. J. Hydrogen Energy 2017, 42, 30389–30399. [Google Scholar] [CrossRef]
- Guilbert, D.; Collura, S.M.; Scipioni, A. DC/DC Converter Topologies for Electrolyzers: State-of-the-Art and Remaining Key Issues. Int. J. Hydrogen Energy 2017, 42, 23966–23985. [Google Scholar] [CrossRef]
- Armghan, H.; Yang, M.; Ali, N.; Armghan, A.; Alanazi, A. Quick Reaching Law Based Global Terminal Sliding Mode Control for Wind/Hydrogen/Battery DC Microgrid. Appl. Energy 2022, 316, 119050. [Google Scholar] [CrossRef]
- Ansari, S.; Chandel, A.; Tariq, M. A Comprehensive Review on Power Converters Control and Control Strategies of AC/DC Microgrid. IEEE Access 2021, 9, 17998–18015. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mishra, S. Dynamic Power Management and Control of a PV PEM Fuel-Cell-Based Standalone Ac/Dc Microgrid Using Hybrid Energy Storage. IEEE Trans. Ind. Appl. 2018, 54, 526–538. [Google Scholar] [CrossRef]
- Esmaeilian, H.R.; Fadaeinedjad, R. Resolving Power Quality Issues Raised by Aerodynamic Aspects of Wind Turbine in Isolated Microgrids Using Fuel Cell/Electrolyzer System. IEEE Trans. Sustain. Energy 2016, 7, 1274–1283. [Google Scholar] [CrossRef]





| PEMFC Parameters | Value |
|---|---|
| Nominal operating point [Inom (A), Vnom (V)] | [100, 500] |
| Maximum operating point [Iend (A), Vend (V)] | [250, 400] |
| Number of cells | 300 |
| Nominal Power (kW) | 50 |
| Maximal Power (kW) | 100 |
| Exchange current (A) | 0.54669 |
| Nerst voltage of one cell (V) | 1.2041 |
| Fuel cell resistance (ohm) | 0.62238 |
| Nominal supply pressure (Fuel (bar)) | 1.5 |
| Nominal supply pressure (Air (bar)) | 1 |
| System temperature (Kelvin) | 338 |
| Capital cost ($/kW) | 400 |
| Lifetime (h) | 50,000 |
| Photovoltaic Parameters | Value |
|---|---|
| Parallel strings | 17 |
| Series-connected modules per string | 14 |
| Maximum Power (W) | 213.15 |
| Cells per module | 60 |
| Open circuit voltage (V) | 36.3 |
| Short-circuit current (A) | 7.84 |
| Voltage at maximum power point (V) | 29 |
| Current at maximum power point Imp (A) | 7.35 |
| Nominal temperature (°C) | 25 |
| Electrolysis Tank Parameters | Value |
|---|---|
| Reversible Voltage (V) | 1.23 |
| Area of Electrode (m2) | 0.1 |
| Number of cells | 200 |
| 0.185 | |
| 2.54 × 10−2 | |
| −0.158 | |
| 1.212 × 103 | |
| 8.232 × 10−5 | |
| −4.51 × 10−7 | |
| Nominal temperature (°C) | 35 |
| Capital cost ($/kW) | 100 |
| Lifetime (year) | 15 |
| Converter Parameters | Value |
|---|---|
| Boost converter inductor (H) | 0.01 |
| Boost converter capacitor (F) | 0.009 |
| Buck converter inductor (H) | 0.1 |
| Buck converter capacitor (F) | 0.001 |
| Resistor (ohms) | 0.1 |
| Filter inductor (H) | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Sheng, J.; Chen, Z.; Zhou, H. Simulation Experiment Design and Control Strategy Analysis in Teaching of Hydrogen-Electric Coupling System. Processes 2024, 12, 138. https://doi.org/10.3390/pr12010138
Shi T, Sheng J, Chen Z, Zhou H. Simulation Experiment Design and Control Strategy Analysis in Teaching of Hydrogen-Electric Coupling System. Processes. 2024; 12(1):138. https://doi.org/10.3390/pr12010138
Chicago/Turabian StyleShi, Tao, Jialin Sheng, Zhiqiang Chen, and Hangyu Zhou. 2024. "Simulation Experiment Design and Control Strategy Analysis in Teaching of Hydrogen-Electric Coupling System" Processes 12, no. 1: 138. https://doi.org/10.3390/pr12010138
APA StyleShi, T., Sheng, J., Chen, Z., & Zhou, H. (2024). Simulation Experiment Design and Control Strategy Analysis in Teaching of Hydrogen-Electric Coupling System. Processes, 12(1), 138. https://doi.org/10.3390/pr12010138








